Abstract
Background
The prevalence of type 2 diabetes in youth is escalating rapidly. We aimed to evaluate the effects of liraglutide on beta-cell function, metabolic productions of oxidative stress, low grade inflammation compared with metformin in young patients with recent onset type 2 diabetes mellitus.
Methods
Sixty patients were randomly assigned to receive 8-week liraglutide or metformin treatment. Beta-cell function was assessed by modified beta cell function index (MBCI), early phase of insulin secretion index (ΔI30/ΔG30), proinsuin to insulin ratio (P/I) and the insulin area under the curve (AUCins). The expression of 8-OH-dG and 8-iso-PGF2α and hs-C-reactive protein (hs-CRP) were measured as indications of oxidative stress and low grade inflammation.
Results
After 8 weeks liraglutide treatment, MBCI, ΔI30/ΔG30, AUCins significantly increased, 8-OH-dG, 8-iso-PGF2α, P/I and hs-CRP remarkably reduced. The differences before and after 8-week liraglutide treatment in ΔMBCI (11.1 [2.81, 43.08] vs 0.00 [− 8.16, 10.47], P = 0.017), ΔLNΔI30/ΔG30 (0.44 [0.04, 0.85] vs − 0.09 [− 0.33, 0.36], P = 0.049), ΔAUCins (117 [− 8, 376] vs − 21 [− 314, 109] mIU/L, P = 0.013), ΔP/I (− 0.05 [− 0.09, − 0.03] vs − 0.02 [− 0.04, 0.01], P = 0.026)were remarkably enhanced compared to those of the metformin therapy. The expression of 8-OH-dG, 8-iso-PGF2α and hs-CRP also decreased after 8-week metformin treatment.
Conclusions
These data demonstrated that liraglutide administration was more effective on ameliorating beta-cell function than metformin treatment in young patients with new-onset type 2 diabetes mellitus. Both liraglutide and metformin could alleviate the level of oxidative stress and attenuate low grade inflammatory, we speculate this effect may not the main mechanism of beta-cell function improvement by liraglutide in diabetic patients.
Trial registration Chinese Clinical Trials registry, chiCTR1800018008, Registered 27 August 2018—retrospectively registered.
Electronic supplementary material
The online version of this article (10.1186/s13098-018-0392-8) contains supplementary material, which is available to authorized users.
Keywords: Liraglutide, Metformin, Type 2 diabetes mellitus, Beta-cell function, Oxidative stress, High sensitivity C-reactive protein
Background
The latest epidemiological surveys have documented that the prevalence rate of diabetes in adults over 18 years of age in China reaches to 10.9% [1], and exhibited the quickly increasing trend in young patients. Lifestyle changes such as higher fat intake and less physical activity are readily to suffer form T2DM in China, especially to young people. T2DM in east Asians is characterized primarily by beta-cell dysfunction, which is evident immediately after ingestion of glucose or mixed meal, less obesity and younger age of onset compared to Caucasians [2]. Reduced insulin secretory capacity and impaired beta-cell compensation are thought as the two major pathophysiological mechanism of beta-cell dysfunction in type 2 diabetes. At the last decade, incretin has received more and more attentions as a new treatment option for young patients with T2DM, and exerted greater glucose-lowering efficacy in East Asians [3]. Glucagon like peptide-1 (GLP-1) is an incretin hormone produced in the intestinal L cells, which stimulates glucose-dependent endogenous insulin release, decreases glucagon secretion, slows gastric motility and emptying, reduces appetite and food intake [4, 5]. Liraglutide, a long-acting GLP-1 receptor agonists, has been demonstrated that it could improve pancreatic beta cell mass and ameliorate insulin secretion capacity in the animal experiment and large prospective LEAD trial [6, 7]. However, the precise mechanisms behind this benefit effect of liraglutide remain unclear. This study aimed to investigate the effects of liraglutide versus metformin on islet beta-cell function, metabolic products of oxidative stress and C-reactive protein (CRP) in young patients with recent onset type 2 diabetes mellitus.
Methods
Subjects
Sixty subjects with type 2 diabetes were enrolled between April 2015 and December 2016 at Xiamen University Affiliated Zhongshan Hospital in China in the department of endocrinology and metabolism and physical examination center. Inclusion criteria for the initial selection were: the patients were initially diagnosed as type 2 diabetes according to World Health Organization criteria, who were 18–40 years of age, had a body mass index (BMI) of 25–35 kg/m2, had HbA1c between 6.5 and 9%, without therapy for diabetes including diet and exercise, antidiabetes agents prior to study. Exclusion criteria were set as follows: type 1 diabetes, recent acute complications including diabetic ketoacidosis and hyperglycaemic hyperosmolar coma, acute infection, impaired liver function, impaired renal function (creatinine clearance < 45 mL/min) [8], women in pregnancy or lactation, smoker. The study was approved by the ethics committee of Zhongshan Hospital Xiamen University and conducted in according to Helsinki Declaration [9]. Written informed consent was obtained for experimentation with each participant.
Research design
In this 8-week, randomized, active-control, parallel trial, sixty subjects with type 2 diabetes were randomly assigned (1:1) to receive subcutaneous liraglutide (Novo Nordisk company) or oral metformin (Sino American Shanghai Squibb Pharmaceutical Co.). Metformin were administered at a dose of 1–2 g/day for 8 weeks. Liraglutide started at once-daily dose of 0.6 mg/d for 1 week, increased up to 1.2 mg/day for 7 weeks. Before the study and after 8-week treatment, a 75 g oral glucose tolerance test (OGTT) was conducted for each participant. Blood samples were drawn before and 30, 60, 120 min after OGTT, respectively. At the same time, participant provided a clean-catch 24-h urine sample, which was immediately separated into 1.5 mL aliquots after collection and stored at − 80 °C until analysis. All subjects were given diet and exercise education by professional nurses. There was a follow-up visit once per month. The plasma glucose, body weight, waist circumference, hip circumference and blood pressure were measured and adverse events were monitored during the follow-up period. At the end of the trial, the clinical and laboratory indices were assessed, as previously described.
Clinical and laboratory measurement
Body weight, height, waist circumference (WC), hip circumference, systolic blood pressure (SBP), diastolic blood pressure (DBP) were collected by professional nurses. The body mass index (BMI) was calculated as the body weight in kilograms divided by the square of the patient’s height in metres. WC was measured midway between the lowest rib and the top of the iliac crest. Hip circumference was measured around the peak of the buttocks. Blood pressure was measured with a mercury manometer on the right arm, after taking rest of 5 min in the sitting position. After an overnight fast, blood samples were drawn for measurements of hemoglobin A1c (HbA1c), plasma glucose (PG), plasma insulin (INS), lipid profile, proinsulin and hs-CRP. Subsequently, a 75 g oral glucose tolerance test (OGTT) was performed, and plasma glucose and insulin were measured at 0 min and 30 min, 60 min, 120 min after OGTT. PG were measured by the hexokinase method. The plasma glucose, renal and liver functions, plasma lipids and lipoprotein concentrations including triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) were performed using an automated method (Roche cobas8000 automatic biochemical analyser). HbA1c was measured by HPLC (Bio-Rad, Inc., Hercules, CA, USA). Plasma insulin levels were measured using the electrochemiluminescence immunoassay (ECLI). Proinsuin concentrations were assessed by ELISA kit (Arigo bioaboratories Corporation, Enzyme immunoassay Hsinchu city 300, Taiwan). High-sensitivity C-reactive protein (hsCRP) concentrations were measured using immune turbidimetry.
Urine sample were taken for the determination of 8-hydroxy-2′-deoxy guanosine (8-OH-dG) and 8-isoprostane F2α (8-iso-PGF2α). Urinary 8-OH-dG concentrations were assayed using a competitive enzyme-linked immunosorbent assay (ELISA) kit (Japan institute for the control of aging, shizuoka pref. Japan) [10]. Urinary 8-iso-PGF2α concentrations were also assayed using competitive ELISA kit (Northwest life science specialities, LLC, Vancouver, Canada) [11]. The intra-assay and inter-assay coefficients of variation (CV) of the ELISA kits mentioned above were all less than 10%.
The formulas which we assessed beta-cell function were shown as follows:
Modified beta cell function index was calculated as MBCI = (INS0 × GLU0)/(GLU120 + GLU60 − 7). INS0 denotes fasting plasma insulin, GLU0 denotes fasting plasma glucose, GLU60 denotes plasma glucose level at 60 min after glucose load, and GLU120 denotes plasma glucose level at 120 min after glucose load [12].
Insulin area under the curve (AUCins) and glucose area under the curve (AUCGLU) during the OGTT were analysed using the trapezoidal method [13].
The early phase insulin secretion index was calculated as (ΔI30/ΔG30) = ([insulin at 30 min] − [fasting insulin])/([glucose at 30 min] − [fasting glucose]) [14].
Proinsuin to insulin ratio was abbreviated as P/I [15].
Deltas (Δ) are presented as the difference before and after treatment, which were suitable for the variables ΔMBCI, ΔAUCins, ΔLNΔI30/ΔG30, ΔP/I and ΔAUCGLU.
Statistical analysis
SPSS packages 21 (SPSS software, IBM Inc., USA) and GraphPad Prism version 5.0 (GraphPad software, Inc., La Jolla, CA, USA) was used for statistical analysis and cartography. Normally distributed data were expressed as mean ± standard deviation (SD). Unpaired t test was used to evaluate the relationship between groups before or after treatment. Paired t test was used to identify differences of baseline and post-treatment in the same group. Non normally distributed data were expressed as median (interquartile rang) and the Mann–Whitney U test or Wilcoxon signed rank test was performed. The Mann–Whitney U test was used to identify differences from baseline with post 8-week treatment for 8-OH-dG, 8-iso-PGF2α, hs-CRP, MBCI, ΔI30/ΔG30 and AUCins between the liraglutide and metformin group. Comparisons of ΔMBCI, ΔLNΔI30/ΔG30, ΔP/I and ΔAUCins after 8-week treatment between liraglutide and metformin group were analyzed using the Mann–Whitney U test. Covariance analysis was performed to determine the associations of Δ AUCGLU with baseline MBCI, LNΔI30/ΔG30, P/I and AUCins, it was also used to evaluate the relationship of ΔMBCI, ΔLNΔI30/ΔG30, ΔP/I and ΔAUCins with baseline levels of HbA1c, BMI and waist circumference (WC).
Data with the difference before and after treatment of early phase insulin secretion index (ΔI30/ΔG30) were logarithmically transformed prior to analysis. A two-tailed P < 0.05 was considered significant.
Results
Comparisons of clinical and laboratory characteristics of the study participants
Baseline characteristics of the study participants between two groups were not statistically significant (P > 0.05) (shown in Additional file 1: Table S1).
After 8-week liraglutide treatment, FPG (9.40 ± 2.32 vs 7.33 ± 2.06 mmol/L, P = 0.024), 30 min PG (15.43 ± 2.96 vs 11.46 ± 3.61 mmol/L, P = 0.003), 60 min PG (18.19 ± 3.60 vs 14.64 ± 3.86 mmol/L, P = 0.012), 120 min PG (17.68 ± 4.38 vs 12.16 ± 5.78 mmol/L, P = 0.002) significantly decreased. shown in Additional file 2: Table S2). At the same time, HbA1c (8.36 ± 0.55 vs 6.85 ± 0.71%, P = 0.001), BMI (28.63 ± 3.86 vs 27.67 ± 3.62 kg/m2, P = 0.001) and waist circumference (92 ± 12 vs 88 ± 11 cm, P = 0.001) significantly decreased.
Nevertheless after 8-week metformin treatment, only FPG (8.45 ± 1.57 vs 6.67 ± 1.26 mmol/L, P = 0.001) significantly decreased, there were no changes in 30 min PG, 60 min PG and 120 min PG before and after metformin treatment (P > 0.05) (shown in Additional file 2: Table S2). Both HbA1c (8.35 ± 0.55 vs 6.53 ± 0.65%, P = 0.001) and waist circumference (88 ± 8 vs 85 ± 8 cm, P = 0.002) notably reduced, but there were no changes in BMI (P > 0.05).
Liraglutide treatment ameliorated beta-cell function
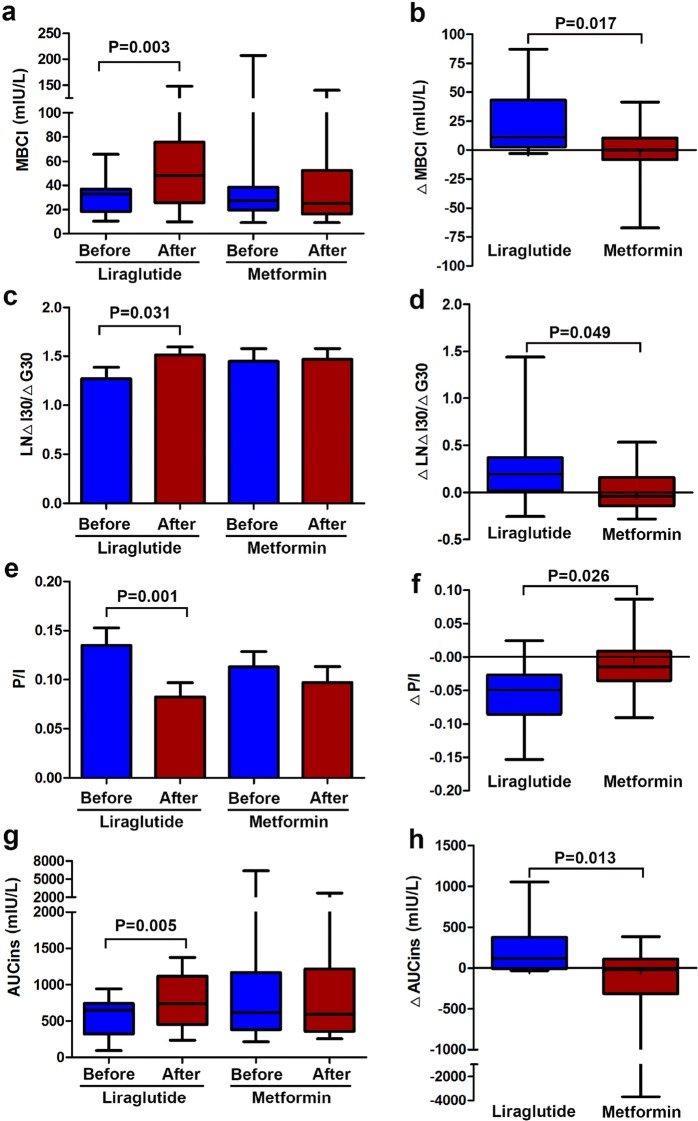
After 8 weeks liraglutide treatment, MBCI (32.76 [18.23, 36.91] vs 48.01 [25.70, 75.84], P = 0.003), ΔI30/ΔG30 (24.94 [7.78, 38.89] vs 31.13 [17.67, 59.09], P = 0.031), AUCins (648 [321, 742] vs 738 [451, 1118] mIU/L, P = 0.005) significantly increased, The levels of P/I (0.14 ± 0.07 vs 0.08 ± 0.06, P = 0.001) were remarkably inhibited (shown in Additional file 2: Table S2 and Fig. 1).
Fig. 1.
Effect of liraglutide and metformin on beta-cell function. a Comparison of modified beta cell function index (MBCI) before and after 8-week treatment. b Comparison of the difference of MBCI before and after treatment between two groups. c Comparison of log-transformed early phase of insulin secretion (ΔI30/ΔG30) before and after 8-week treatment. d Comparison of the difference of LNΔI30/ΔG30 before and after treatment between two groups. e Comparison of proinsulin to insulin ratio (P/I) before and after 8-week treatment. f Comparison of the difference of P/I before and after treatment between two groups. g Comparison of insulin area under the curve (AUCins) before and after 8-week treatment. h Comparison of the difference of AUCins before and after treatment between two groups
There were no significant changes in MBCI, ΔI30/ΔG30, AUCins and P/I before and after metformin treatment (P > 0.05)(shown in Additional file 2: Table S2, Fig. 1).
After 8-week liraglutide treatment, the differences in ΔMBCI (11.1 [2.81, 43.08] vs 0.00 [− 8.16, 10.47], P = 0.017), ΔLNΔI30/ΔG30 (0.44 [0.04, 0.85] vs − 0.09 [− 0.33, 0.36], P = 0.049), ΔAUCins (117 [− 8, 376] vs − 21 [− 314, 109] mIU/L, P = 0.013), ΔP/I (− 0.049 [− 0.086, − 0.027] vs − 0.015 [− 0.036, 0.009], P = 0.026) were remarkably enhanced compared to those of the metformin therapy (shown in Table 1 and Fig. 1). However, which were not significant with baseline levels of HbA1c, BMI and waist circumference (P > 0.05) (shown in Additional file 3: Table S3).
Table 1.
Comparisons of ΔMBCI, ΔLNΔI30/ΔG30, ΔP/I and ΔAUCins levels after 8-week treatment between liraglutide and metformin group
| Variable | Liraglutide | Metformin | Difference | P value |
|---|---|---|---|---|
| ΔMBCI | 11.1 (2.81, 43.08) |
0.00 (− 8.16, 10.47) |
6.53 (− 1.75, 14.02) |
0.017 |
| ΔLNΔI30/ΔG30 | 0.44 (0.04, 0.85) |
− 0.09 (− 0.33, 0.36) |
0.21 (− 0.15, 0.68) |
0.049 |
| ΔP/I | − 0.05 (− 0.09, − 0.03) |
− 0.02 (− 0.04, − 0.01) |
− 0.03 (− 0.06, − 0.01) |
0.026 |
| ΔAUCins (mIU/L) | 117 (− 8, 376) |
− 21 (− 341, 109) |
39 (− 33, 227) |
0.013 |
Data are expressed as median (interquartile rang). Deltas (Δ) are presented as the difference of variables before and after treatment
MBCI, modified B cell function index; ΔI30/ΔG30, [(insulin at 30 min) − (insulin at 0 min)]/[(glucose at 30 min) − (glucose at 0 min)]; P/I, proinsuin to insulin ratio; AUCins, insulin area under the curve; LN, log-transformed
In covariance analysis model, the reductions of AUCGLU (ΔAUCGLU) after liraglutide and metformin treatment were associated with the baseline MBCI (F = 8.041, P = 0.009), P/I (F = 12.72, P = 0.001), AUCins (F = 14.923, P = 0.001), and LNΔI30/ΔG30 (F = 6.080, P = 0.020) (shown in Table 2).
Table 2.
Covariate analysis in ΔAUCGLU with baseline MBCI, P/I, AUCins and LNΔI30/ΔG30
| Variables | Test statistics with ΔAUCGLU (F-value) |
P-value |
|---|---|---|
| MBCI | 8.041 | 0.009 |
| P/I | 12.72 | 0.001 |
| AUCins (mIU/L) | 14.923 | 0.001 |
| LNΔI30/ΔG30 | 6.080 | 0.020 |
Deltas (Δ) are presented as the difference of variables before and after treatment
AUCGLU, glucose area under the curve; MBCI, modified B cell function index; P/I, proinsulin to insulin ratio; AUCins, insulin area under the curve; ΔI30/ΔG30, [(insulin at 30 min) − (insulin at 0 min)]/[(glucose at 30 min) − (glucose at 0 min)]; LN, log-transformed
Liraglutide and metformin treatment inhibited oxidative stress and low grade inflammatory
The levels of 8-OH-dG (35.95 [29.30, 50.70] vs 18.74 [4.84, 24.20]ng/mL, P = 0.002), 8-iso-PGF2α (1345 [885, 1920] vs 288 [183, 472]ng/mL, P = 0.001), hs-CRP (1.96 [1.11, 3.89] vs 1.47 [0.53, 1.86]mg/L, P = 0.002)were remarkably inhibited after 8-week liraglutide treatment (shown in Table 3). The expression of 8-OH-dG (16.77 [9.71, 32.60] vs 7.86 [2.87, 23.31]ng/mL, P = 0.027), 8-iso-PGF2α (1180 [1025, 1765] vs 299 [228, 586] ng/mL, P = 0.001) and hs-CRP (1.88 [1.06, 3.69] vs 1.44 [0.67, 2.35] mg/L, P = 0.017) also decreased after 8-week metformin treatment (shown in Table 3).
Table 3.
Comparisons the levels of 8-OH-dG, 8-iso-PGF2α and hsCRP before and after 8-week treatment between two groups
| Variable | Liraglutide group | Metformin group | ||||
|---|---|---|---|---|---|---|
| Pre-treatment | Post-treatment | P-value | Pre-treatment | Post-treatment | P-value | |
| 8-OH-dG (ng/mL) | 35.95 (29.30, 50.70) |
18.74 (4.84, 24.20) |
0.002 | 16.77 (9.71, 32.60) |
7.86 (2.87, 23.31) |
0.027 |
| 8-iso-PGF2α (ng/mL) | 1345 (885, 1920) |
288 (183, 472) |
0.001 | 1180 (1025, 1765) |
299 (228, 586) |
0.001 |
| hsCRP (mg/L) | 1.96 (1.11, 3.89) |
1.47 (0.53, 1.86) |
0.002 | 1.88 (1.06, 3.69) |
1.44 (0.67, 2.35) |
0.017 |
Data are expressed as median (interquartile rang)
8-OH-dG, 8-hydroxy-2′-deoxyguanosine; 8-iso-PGF2α, 8-isoprostane F2α; hsCRP, high sensitivity C-reactive protein
Discussion
Our data show the human GLP-1 analogue liraglutide ameliorates beta-cell function and insulin secretion capacity compared with 8-week metformin treatment in young patients with new-onset type 2 diabetes mellitus. In this study, we combined the modified beta cell function index (MBCI), early phase of insulin secretion (ΔI30/ΔG30), fasting proinsulin to insulin ratio (P/I) with the insulin area under the curve (AUCins) to assess the beta-cell function and insulin secretion capacity. We found the levels of MBCI, ΔI30/ΔG30, AUCins increased by 47%, 25% and 14% respectively, the ratio of P/I remarkably reduced 43% compared with baseline after 8-week liraglutide treatment. However, no statistical changes of MBCI, P/I, ΔI30/ΔG30 and AUCins were achieved in the metformin treatment group.
As we known, type 2 diabetes (T2D) is a progressive disease characterized by both beta-cell deficit and insulin resistance. Previous reports have shown that beta-cell volume decreased by 63% in obese T2DM patients due to increasing threefold beta-cell apoptosis [16], which suggested that improvement of beta-cell dysfunction may be an important therapeutic strategy for the treatment of T2DM. GLP-1 is an incretin hormone secreted by intestinal epithelial L cells that promotes glucose-dependent insulin secretion, decreases glucagon secretion, stimulates beta-cell proliferation, suppresses apoptosis, and restores the function of islet beta-cells [17–19]. It is widely recognized that T2DM in East Asians is characterized primarily by beta-cell dysfunction, which is evident immediately after ingestion of glucose or meal, and less adiposity compared to the disease in Caucasians [20]. Interestingly, the glucose-lowering efficacy of glucagon-like peptide-1 receptor agonists was reported to be greater in Asians than in non-Asians. The difference in the GLP-1 treatment responses could be ascribed to a different pathophysiology of type 2 diabetes, namely, lower insulin secretory function and less insulin resistance, lower body mass index, different genetic makeups, preserved incretin effect and different food compositions in East Asians compared with other ethnic groups [21]. We have also documented that the reductions of AUCGLU (ΔAUCGLU) after liraglutide or metformin treatment were associated with the baseline MBCI, P/I, AUCins, and LNΔI30/ΔG30 by covariance analysis, in other words, HbA1c-lowering effects of liraglutide depends on remaining beta-cell function.
Liraglutide protected against reductions of beta-cells in a glucokinase–independent fashion and increased glucokinase protein expression, which was correlated to beta-cell threshold sensitivity to glucose [22]. Liraglutide also improved the proliferation and insulin secretion of beta-cell in high FFAs condition, which enhanced pancreatic and duodenal homeobox 1 (PDX-1) and MafA and NeuroD expressions, down-regulated of p27, Bax expressions, induced the phosphorylation of FoxO1 by activation of PI3K/Akt signalling pathway [23].
Degn et al. reported that beta-cell function in the fasting state, as assessed by HOMA-B analysis, was increased by 30%, first-phase insulin response after the intravenous glucose bolus was increased by 60% after 1 week of liraglutide administration. The proinsulin/insulin ratio was reduced by 40–50%, mean insulin concentration was increased by 2- to 3.5- fold, mean circulating glucagon concentration was reduced by 20% during the hyper-glycemic clamp. Our findings are generally consistent with previous literature [24], document that liraglutide efficiently improves beta-cell function and insulin secretion capacity, which were not correlated with baseline levels of HbA1c, BMI and waist circumference. Our results suggest that improvement of beta-cell function was independent of the basal values on glucose and weight.
We demonstrated in this study that liraglutide and metformin treatment significantly reduced the expression of urinary 8-OH-DG and 8-iso-PGF2α.than those of baseline. At the same time, we also demonstrated that liraglutide treatment inhibited the expression of sVCAM-1 and hs-CRP [25]. 8-Hydroxy-2′-deoxyguanosine (8-OHdG), produced by oxidation of the nucleoside deoxyguanosine and subsequently excreted directly into urine, has been considered as a sensitive marker for oxidative DNA damage [26]. 8-iso-PGF2α derived from arachidonic acid, which was formed non-enzymatically through oxygen radicals, induced peroxidation of membrane phospholipids [27]. Urinary 8-OH-dG and 8-iso-PGF2α levels have been validated as sensitive biomarkers of oxidative stress in large-scale human studies [28].
Increased levels of oxidative stress exerted deleterious effect on beta-cell function, impaired glucose tolerance and ultimately leading to T2DM. Beta cells are particularly sensitive to ROS because there are relatively low levels of antioxidant enzymes, then oxidative stress should damage mitochondria and markedly blunt insulin secretion, specifically for early phase of insulin secretion [29, 30]. Oxidative stress impaired insulin action through an increase in intracellular calcium concentration or a reduction in nitric oxide availability [31, 32].
However, the precise mechanisms behind the effects of liraglutide on the signalling pathways that attenuate oxidative stress and anti-inflammation are not fully elucidated, although several hypotheses have been proposed. First, in diabetic db/db mice, liraglutide treatment for 2 weeks significantly increased the expression of genes involved in anti-oxidative stress (Cat and Gpx) and reduced endoplasmic reticulum stress in beta-cells, by binding with GLP-1 receptors, which activates adenylate cyclase and the cyclic AMP/protein kinase A (PKA) signalling pathway. Liraglutide also activates phosphoinositide 3-kinase (PI3K), p42 mitogen-activated protein kinase (MAPK) and the epidermal growth factor receptor [33]. Second, liraglutide time-dependent increased phosphorylation of the pro-survival kinase AKT, which was completely inhibited by the PI3K inhibitor wortmannin, demonstrated that phosphorylation of AKT was PI3K dependent [34]. Third, on a rat stroke model, wistar rats received occlusion of the middle cerebral artery for 90 min, liraglutide or saline was administered intraperitoneally at 1 h after reperfusion, liraglutide-treatment significantly reduced the level of derivatives of reactive oxygen metabolites (d-ROMs), compared with that of control, which demonstrated administration of GLP-1 suppressed glucose-stimulated inducible nitric oxide synthase (iNOS) activity and expression and its stimulation of insulin release in pancreatic islet cells at least partly through PKA signalling [35, 36]. Fourth, upon TNF-α-induced injury of the human umbilical vein endothelial cells (HUVECs), liraglutide inhibited rapid translocation of PKC-α into membrane, inhibited NF-κB signaling activation and NADPH oxidase, inhibited apoptosis of HUVEC and expression of Pentraxin-3, increased the levels of SOD-2, catalase and GPx, liraglutide exerts marked anti-oxidative and anti-inflammatory effects [37].
The strengths of the current study include the randomized, active controlled design and consistent baseline with few interference factors. To the best of our knowledge, this is the first study to combine four indexes with MBCI, P/I, Δ I30/Δ G30 and AUCins at the same time to evaluate the protective effects of liraglutide on beta-cell function. In addition, we collected 24 h of urine, not random urine tests, to assess the levels of urinary 8-OH-dG and 8-iso-PGF2α, which was more reliable to confirm the anti-oxidative capacity. Despite of our efforts to plan and complete the whole research, there are still some limitations. First, compared with the large longitudinal study, it has a non-blinded design, lacks a blank control group, has relatively small sample size and comparatively short study period. Second, further studies are needed to reveal the relevant signalling pathways by which liraglutide exerts beneficial influence on islet beta-cell function against oxidative stress and inflammation.
Conclusions
our findings indicate liraglutide administration was more effective on ameliorating beta-cell function than metformin treatment in young patients with new-onset type 2 diabetes mellitus. Both liraglutide and metformin could reduce the level of oxidative stress and attenuate low grade inflammatory, we speculate this effect may not the main mechanism of beta-cell function improvement by liraglutide in diabetic patients.
Additional files
Additional file 1: Table S1. Baseline characteristics of the study participants.
Additional file 2: Table S2. Comparisons of plasma glucose and insulin secretion capacity before and after 8-week treatment between two groups.
Additional file 3: Table S3. Covariate analysis on the changes of beta-cell function with baseline HbA1c, BMI and WC.
Authors’ contributions
XMC conceived and designed the research. LFW, XMC, WQZ, YT, CCC and CMQ performed the experiments. WQZ and XMC analysed the data. WQZ, YT and XMC drafted the manuscript. XMC provided critical revisions to the manuscript. All authors contributed to the writing of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We thank all the research subjects for their participation and acknowledge the skillful work of the entire medical staff at the Department of Endocrinology and Metabolism, Zhongshan Hospital Xiamen University. We are very grateful to Yu‑bing Yan (Zhongshan Hospital Xiamen University, Xiamen, P. R. China) for the help of statistical analysis.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All data generated or analyzed during the current study are included in this published article and its additional files.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study was conducted in according to Helsinki Declaration, approved by the ethics committee of Zhongshan Hospital Xiamen University and registered at registered at Chinese Clinical Trials registry, (chiCTR1800018008). Written informed consent was obtained for each participant.
Funding
This study was supported by the program from the Science and Technology Benefit Fund of Xiamen Technology Bureau (3502Z20154027). The funder didn’t participate in trial design, data collection and analysis.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- T2DM
type 2 diabetes mellitus
- BMI
body mass index
- WC
waist circumference
- TC
total cholesterol
- TG
triglycerides
- HDL-C
high-density lipoprotein cholesterol
- LDL-C
low-density lipoprotein cholesterol
- HbA1c
glycated haemoglobin
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- FPG
fasting plasma glucose
- FINS
fasting insulin
- AUCins
insulin area under the curve
- MBCI
modified B cell function index
- ΔI30/ΔG30
[(insulin at 30 min) − (insulin at 0 min)]/[(glucose at 30 min) − (glucose at 0 min)]
- P/I
proinsuin to insulin ratio
- hsCRP
high sensitivity C-reactive protein
- 8-OH-dG
8-hydroxy-2′-deoxyguanosine
- 8-iso-PGF2α
8-isoprostane F2α
- LN
log-transformed
- OGTT
oral glucose tolerance test
- GLP-1
glucagon-like peptide-1
- LRG
liraglutide
- MET
metformin
- sVCAM-1
soluble vascular cell adhesion molecule-1
- ELISA
enzyme-linked immunosorbent assay
- HPLC
high performance liquid chromatography
- ECLI
electrochemiluminescence immunoassay
- CV
coefficients of variation
- SD
standard deviation
- PDX-1
pancreatic and duodenal homeobox 1
- ROS
reactive oxygen species
- AMP
activated protein kinase
- AMPK
adenosine monophosphate activated protein kinase
- PKA
protein kinase A
- PI3K
phosphoinositide 3-kinase
- d-ROMs
derivatives of reactive oxygen metabolites
- iNOS
inducible nitric oxide synthase
- TNF-α
tumor necrosis factor-α
- HUVEC
human umbilical vein endothelial cells
- MAPK
mitogen-activated protein kinase
- NF-kB
nuclear factor kappa-light-chain-enhancer of activated B cells
Contributor Information
Wen-qiang Zhang, Email: nfmzwq@126.com.
Yuan Tian, Email: 411954353@qq.com.
Xiao-min Chen, Email: chenxiaomin0517@sina.com.
Li-fen Wang, Email: 670298752@qq.com.
Chan-chan Chen, Email: 2513429464@qq.com.
Chuan-mei Qiu, Email: 2476921372@qq.com.
References
- 1.Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, et al. Prevalence and ethnic pattern of diabetes and prediabetes in china in 2013. JAMA. 2017;317:2515–2523. doi: 10.1001/jama.2017.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yabe D, Seino Y. Type 2 diabetes via β-cell dysfunction in east Asian people. Lancet Diabetes Endocrinol. 2016;4(1):2–3. doi: 10.1016/S2213-8587(15)00389-7. [DOI] [PubMed] [Google Scholar]
- 3.Seino Y, Kuwata H, Yabe D. Incretin-based drugs for type 2 diabetes: focus on east Asian perspectives. J Diabetes Invest. 2016;7(Suppl 1):102–109. doi: 10.1111/jdi.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kondo Y, Satoh S, Osada UN, Terauchi Y. Early liraglutide treatment improves β-cell function in patients with type 2 diabetes: a retrospective cohort study. Endocr J. 2015;62(11):971–980. doi: 10.1507/endocrj.EJ15-0206. [DOI] [PubMed] [Google Scholar]
- 5.Meier JJ. GLP 1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8(12):728–742. doi: 10.1038/nrendo.2012.140. [DOI] [PubMed] [Google Scholar]
- 6.Tamura K, Minaml K, Kudo M, Lemoto K, Takahashi H, Seino S. Liraglutide improves pancreatic beta cell mass and function in alloxan-induced diabetic mice. PLoS ONE. 2015;10(5):e0126003. doi: 10.1371/journal.pone.0126003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garber A, Henry RR, Ratner R, Hale P, Chang CT, Bode B, LEAD-3 (Mono) Study Group Liraglutide, a once-daily human glucagon-like peptide 1 analogue, provides sustained improvements in glycaemic control and weight for 2 years as monotherapy compared with glimepiride in patients with type 2 diabetes. Diabetes Obes Metab. 2011;13(4):348–356. doi: 10.1111/j.1463-1326.2010.01356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bell S, Farran B, McGurnaghan S, McCrimmon RJ, Leese GP, Petrie JR, et al. Risk of acute kidney injury and survival in patients treated with Metformin: an observational cohort study. BMC Nephrol. 2017;18(1):163. doi: 10.1186/s12882-017-0579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Medical Association Declaration of Helsinki: recommendations guiding physicians in biomedical research involving human subjects. JAMA. 1997;277:925–926. doi: 10.1001/jama.1997.03540350075038. [DOI] [PubMed] [Google Scholar]
- 10.Myoren T, Kobayashi S, Oda S, Nanno T, Ishiguchi H, Murakami W, et al. An oxidative stress biomarker, urinary 8-hydroxy-2 ‘-deoxyguanosine, predicts cardiovascular-related death after steroid therapy for patients with active cardiac sarcoidosis. Int J Cardiol. 2016;212:206–213. doi: 10.1016/j.ijcard.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Pouvreau C, Dayre A, Butkowski EG, Jong BD, Jelinek HF. Inflammation and oxidative stress markers in diabetes and hypertension. J Inflamm Res. 2018;11:61–68. doi: 10.2147/JIR.S148911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng S, Zhou H, Han T, Li Y, Zhang Y, Liu W, Hu Y. Clinical characteristics and beta cell function in Chinese patients with newly diagnosed type 2 diabetes mellitus with different levels of serum triglyceride. BMC Endocr Disord. 2015;15:21. doi: 10.1186/s12902-015-0018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Mello VD, Lindström J, Eriksson J, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Sundvall J, et al. Insulin secretion and its determinants in the progression of impaired glucose tolerance to type 2 diabetes in impaired glucose-tolerant individuals: the Finnish Diabetes Prevention Study. Diabetes Care. 2012;35(2):211–217. doi: 10.2337/dc11-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen J, Chen Z, Chen C, Zhu X, Han Y. Impact of incretin on early-phase Insulin secretion and glucose excursion. Endocrine. 2013;44(2):403–410. doi: 10.1007/s12020-012-9867-9. [DOI] [PubMed] [Google Scholar]
- 15.Saisho Y, Maruyama T, Hirose H, Saruta T. Relationship between proinsulin-to-insulin ratio and advanced glycation endproducts in Japanese type 2 diabetic subjects. Diabetes Res Clin Pract. 2007;78(2):182–188. doi: 10.1016/j.diabres.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52(1):102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 17.Santilli F, Simeone PG, Guagnano MT, Leo M, Maccarone MT, Di Castelnuovo A. Effects of liraglutide on weight loss, fat Distribution, and β-cell function in obese subjects with prediabetes or early type 2 diabetes. Diabetes Care. 2017;40(11):1556–1564. doi: 10.2337/dc17-0589. [DOI] [PubMed] [Google Scholar]
- 18.Garber AJ. Incretin effects on β-cell function, replication, and mass: the human perspective. Diabetes Care. 2011;34(Suppl 2):S258–S263. doi: 10.2337/dc11-s230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Liu X, Fang Q, Ding M, Li C. Liraglutide attenuates atherosclerosis via inhibiting ER-induced macrophage derived microvesicles production in T2DM rats. Diabetol Metab Syndr. 2017;9:94. doi: 10.1186/s13098-017-0289-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yabe D, Seino Y, Fukushima M, Seino S. βcell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in east Asians. Curr Diab Rep. 2015;15:36. doi: 10.1007/s11892-015-0602-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho YM. Incretin physiology and pathophysiology from an Asian perspective. J Diabetes Invest. 2015;6(5):495–507. doi: 10.1111/jdi.12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shirakawa J, Tanami R, Togashi Y, Tajima K, Orime K, Kubota N. Effects of liraglutide on β-cell-specific glucokinase-deficient neonatal mice. Endocrinology. 2012;153(7):3066–3075. doi: 10.1210/en.2012-1165. [DOI] [PubMed] [Google Scholar]
- 23.Shao S, Nie M, Chen C, Chen X, Zhang M, Yuan G, et al. Protective action of liraglutide in beta cells under lipotoxic stress via PI3K/Akt/FoxO1 pathway. J Cell Biochem. 2014;115(6):1166–1175. doi: 10.1002/jcb.24763. [DOI] [PubMed] [Google Scholar]
- 24.Degn KB, Juhl CB, Sturis J, Jakobsen G, Brock B, Chandramouli V, et al. One week’s treatment with the long-acting glucagon-like peptide 1 derivative liraglutide (NN2211) markedly improves 24-h glycemia and alpha- and beta-cell function and reduces endogenous glucose release in patients with type 2 diabetes. Diabetes. 2004;53(5):1187–1194. doi: 10.2337/diabetes.53.5.1187. [DOI] [PubMed] [Google Scholar]
- 25.Chen XM, Zhang WQ, Tian Y, Wang LF, Chen CC, Qiu CM. Liraglutide suppresses non-esterified free fatty acids and soluble vascular cell adhesion molecule-1 compared with metformin in patients with recent-onset type 2 diabetes. Cardiovasc Diabetol. 2018;17(1):53. doi: 10.1186/s12933-018-0701-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishikawa T, Sasahara T, Kiritoshi S, Sonoda K, Senokuchi T, Matsuo T, et al. Evaluation of urinary 8- hydroxydeoxy-guanosine as a novel biomarker of macrovascular complications in type 2 diabetes. Diabetes Care. 2003;26:1507–1512. doi: 10.2337/diacare.26.5.1507. [DOI] [PubMed] [Google Scholar]
- 27.Roberts LJ, Morrow JD. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med. 2000;28:505–513. doi: 10.1016/S0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- 28.Ilyasova D, Scarbrough P, Spasojevic I. Urinary biomarkers of oxidative status. Clin Chim Acta. 2012;413(19–20):1446–1453. doi: 10.1016/j.cca.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiu C, Hevner K, Abetew D, Enquobahrie DA, Williams MA. Oxidative DNA damage in early pregnancy and risk of gestational diabetes mellitus: a pilot study. Clin Biochem. 2011;44(10–11):804–808. doi: 10.1016/j.clinbiochem.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. 2004;24:816–823. doi: 10.1161/01.ATV.0000122852.22604.78. [DOI] [PubMed] [Google Scholar]
- 31.Paolisso G, Giugliano D. Oxidative stress and insulin action: is there a relationship? Diabetologia. 1996;39:357–363. doi: 10.1007/BF00418354. [DOI] [PubMed] [Google Scholar]
- 32.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress activated signaling apathways mediators of insulin resistance and beta-cell dysfunction? Diabetes. 2003;52:1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- 33.Shimoda M, Kanda Y, Hamamoto S, Tawaramoto K, Hashiramoto M, Matsuki M, Kaku K. The human glucagon-like peptide-1 analogue liraglutide preserves pancreatic beta cells via regulation of cell kinetics and suppression of oxidative and endoplasmic reticulum stress in a mouse model of diabetes. Diabetologia. 2011;54(5):1098–1108. doi: 10.1007/s00125-011-2069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kapodistria K, Tsilibary EP, Kotsopoulou E, Moustardas P, Kitsiou P. Liraglutide, a human glucagon-like peptide-1 analogue, stimulates AKT-dependent survival signalling and inhibits pancreaticβ-cell apoptosis. J Cell Mol Med. 2018;22(6):2970–2980. doi: 10.1111/jcmm.13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sato K, Kameda M, Yasuhara T, Agari T, Baba T, Wang F, et al. Neuroprotective effects of Liraglutide for stroke model of rats. Int J Mol Sci. 2013;14(11):21513–21524. doi: 10.3390/ijms141121513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jimenez-Feltstrom J, Lundquist I, Salehi A. Glucose stimulates the expression and activities of nitric oxide synthases in incubated rat islets: an effect counteracted by GLP-1 through the cyclic AMP/PKA pathway. Cell Tissue Res. 2005;319(2):221–230. doi: 10.1007/s00441-004-1013-4. [DOI] [PubMed] [Google Scholar]
- 37.Shiraki A, Oyama J, Komoda H, Asaka M, Komatsu A, Sakuma M, et al. The glucagon-like peptide 1 analog liraglutide reduces TNF-α-induced oxidative stress and inflammation in endothelial cells. Atherosclerosis. 2012;221(2):375–382. doi: 10.1016/j.atherosclerosis.2011.12.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Baseline characteristics of the study participants.
Additional file 2: Table S2. Comparisons of plasma glucose and insulin secretion capacity before and after 8-week treatment between two groups.
Additional file 3: Table S3. Covariate analysis on the changes of beta-cell function with baseline HbA1c, BMI and WC.
Data Availability Statement
All data generated or analyzed during the current study are included in this published article and its additional files.