Abstract
Introduction
The Age-Well clinical trial is an ongoing monocentric, randomized, controlled trial aiming to assess an 18-month preventive meditation-based intervention directly targeting the attentional and emotional dimensions of aging to promote mental health and well-being in elderly people.
Methods
One hundred thirty-seven cognitively unimpaired older adults are randomized to either an 18-month meditation-based intervention, a structurally matched foreign language training, or a passive control arm. The impact of the intervention and underlying mechanisms are assessed with detailed cognitive, behavioral, biological, neuroimaging and sleep examinations.
Results
Recruitment began in late 2016 and ended in May 2018. The interventions are ongoing and will be completed by early 2020.
Discussion
This is the first trial addressing the emotional and cognitive dimension of aging with a long-term nonpharmacological approach and using comprehensive assessments to investigate the mechanisms. Results are expected to foster the development of preventive strategies reducing the negative impact of mental conditions and disorders.
Keywords: Aging, Alzheimer's disease, Dementia, Prevention, Cognition, Reserve, Attention, Meditation, Mindfulness, Compassion, Foreign language training, Emotion, Lifestyle, Neuroimaging, Blood markers, Sleep
Highlights
-
•
Meditation or language training could improve mental health and well-being in aging.
-
•
Age-Well is a randomized controlled trial targeting mental health in aging.
-
•
Age-Well includes 18-month meditation and foreign language training in 137 elderly.
-
•
Age-Well interventions are expected to positively impact brain structure and function.
1. Introduction
As the number of older people grows, increasing healthy life years is a priority. The main drivers of decreased mental health and well-being in aging populations include dementia, depression, anxiety, insomnia, and even subclinical conditions such as stress, worry, sleep disturbances, and cognitive decline [1], [2]. Moreover, these conditions interact and promote each other. For instance, anxiety, depression, and sleep difficulties are associated with increased risk of Alzheimer's disease (AD).
The reduction of modifiable risk factors represents a true opportunity to prevent AD [3], [4]. Indeed, around a third of AD cases may be attributable to potentially modifiable risk factors, and the future prevalence of AD could be reduced by 8% to 15% if each of the main risk factors (e.g., cardiovascular risk factors, depression, physical, and cognitive inactivity) is reduced by 10 to 20% [3], [5]. Several lifestyle interventions in nondemented older adults have thus been investigated with mixed results before larger-scale trials with long-term (>1 year) follow-up and using multidomain interventions simultaneously targeting various risk factors were launched [6]. Examples of such multidomain prevention randomized controlled trials (RCTs) include the Multidomain Alzheimer Prevention Trial (MAPT) [7], the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) trial [8], and the Prevention of Dementia by Intensive Vascular Care (PreDIVA) trial [9]. Positive effects on cognitive function were found in Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability [10], which has evolved toward a worldwide consortium, World Wide Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability, including, for example, the U.S. equivalent U.S. POINTER (https://alz.org/us-pointer/). These trials target most of the main modifiable risk factors (cardiovascular risk factors, physical and cognitive inactivity). Yet although very important, psychoaffective risk factors have so far not been directly targeted.
A promising way of targeting psychoaffective risk factors consists in mental training for the reduction of stress, the regulation of attention and the cultivation of positive emotions through meditation practice. Such interventions might be beneficial to improve mental health and well-being in the aging population and reduce AD risks. Promising evidence exists that mindfulness meditation improves cognition in young adults (mainly attention, metacognition and memory, which are cognitive processes sensitive to aging and AD) [11], [12], [13], and reduces stress, anxiety, depression, insomnia [14], [15], [16], [17] and cardiovascular risk factors [18], [19]. Moreover, meditation has also been associated with brain structural and functional changes that persist beyond the time of actual practice and mainly impact frontal and limbic networks [20], [21], [22], [23]. In a recent cross-sectional pilot study, we showed that elderly expert meditators had higher gray matter volume and/or fluorodeoxyglucose metabolism compared with age-matched non-meditators in frontal, insula and posterior associative regions [24]. These findings suggest that meditation might have a beneficial effect in brain regions sensitive to aging and AD and subtending reserve processes, thereby reducing the risk or delaying the onset of dementia/AD.
To test this hypothesis, we need an RCT including an adequate active control comparison condition to estimate the specific effects of a meditation intervention. The duration of the intervention should exceed that of the commonly used 8-week mindfulness-based stress reduction program to demonstrate an effect not only on behavioral, but also on protracted age-related biological processes. Moreover, complementary outcome measures allowing to investigate the mechanisms of action and to assess the multidimensional aspect of aging should be used.
Medit-Ageing (public name: Silver Santé Study; www.silversantestudy.eu) is a European research project focusing on mental health and well-being in aging populations. It includes two independent clinical studies (SCD-Well and Age-Well) and the Age-Well study includes an RCT and an observational cross-sectional study on older expert long-term meditators. The present article will focus on the design and progress of the Age-Well RCT.
The Age-Well RCT is the first trial addressing the emotional and cognitive dimension of aging with a long-term nonpharmacological approach and including both an active and a passive control conditions. A complete set of unique measurements will be used to investigate the mechanisms of action, including cognitive tests particularly focusing on memory and attention, scales and questionnaires assessing well-being, quality of life, psychoaffective factors and lifestyle, but also complementary neuroimaging measures of brain structural and functional integrity, emotion and attention-related neural activity, biological blood measures and gold-standard measures of sleep with polysomnography. Finally, on the level of social relations, measurements in participants' partners will allow to investigate their perception of the participant's changes and their own perceived social support. Objective biomarkers of brain integrity will be used as primary outcomes.
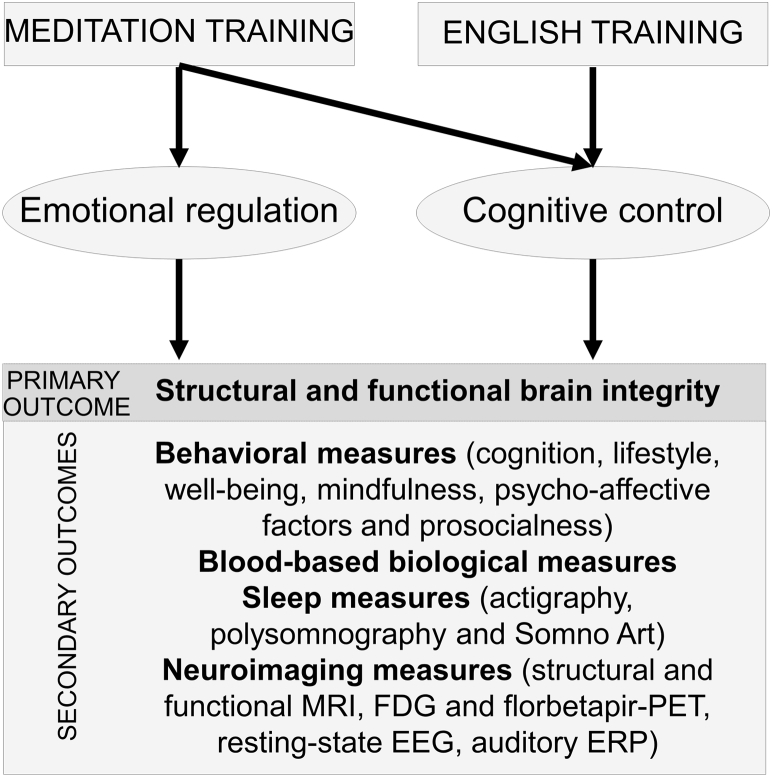
We hypothesize that meditation training will be associated with an increase in positive emotions and improved cognitive control which will in turn enhance health and well-being, and promote brain and cognitive reserve processes that are protective of dementia. Qualitative and quantitative differences are expected in the effects of meditation versus foreign language training interventions as they are thought to involve overlapping but partly distinct mechanisms (Fig. 1).
Fig. 1.
Hypothetical model of the expected effects and mechanisms of the meditation and foreign language training interventions included in the Age-Well RCT. Meditation training is thought to promote both emotional regulation/positive affect and cognitive control. Foreign language training is expected to act mainly through cognitive stimulation. Consequently, while both the meditation and foreign language training interventions are expected to have a positive impact on markers of mental health and well-being in aging, the nature and degree of these effects are expected to differ between both interventions. Abbreviations: EEG, electroencephalography; ERP, event-related potential; FDG, fluorodeoxyglucose; MRI, magnetic resonance imaging; PET, positron emission tomography.
2. Methods/design
2.1. Clinical trial setting and design
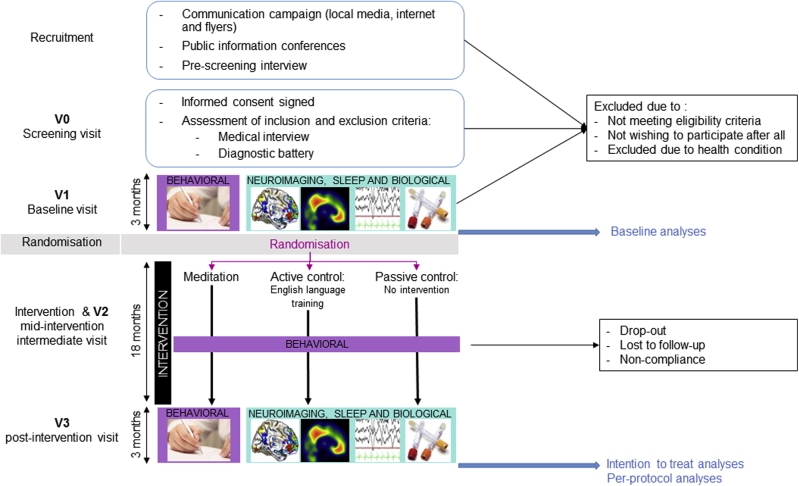
The Age-Well clinical trial is a monocentric, randomized, controlled superiority clinical trial with blinded endpoint assessment and with three parallel arms: an 18-month meditation arm, an 18-month foreign language (English) training intervention arm (the active control) and a no-intervention passive control arm. The Age-Well RCT includes 137 cognitively unimpaired older adults. Participants were recruited in three successive cohorts of 43, 50 and 44 participants, respectively, spaced about 6 months apart. The general design of the trial is depicted in Fig. 2 and the different steps are detailed in the Supplementary Material. In brief, participants were recruited from the general population, prescreened, and then invited to a screening visit (V0) at which the diagnostic battery depicted in Table 1 was performed. Participants fulfilling eligibility criteria (Table 2) were invited to the baseline pre-intervention visit (V1), then randomized to one of the three arms (groups) at a ratio of 1:1:1, and the 18-month intervention period starts. A mid-intervention intermediate visit (V2) is performed 9 months after the start of the intervention, and the post-intervention visit (V3) is performed at the end of the 18-month intervention period.
Fig. 2.
Flow chart of the Age-Well RCT participants. The different steps are detailed in the text. The boxes at V1 and V3 depict the types of measurements that are collected. Abbreviation: RCT, randomized controlled trial.
Table 1.
Tests included in the diagnostic battery performed during the screening visit V0
| Diagnostic battery | ||||
|---|---|---|---|---|
| Domains evaluated | Tests | Score(s) | References | Expected performances |
| Manual laterality | Edinburgh Questionnaire | Unique | [25] | Not applicable |
| Global cognitive functioning | MMSE | Unique | [26] | Norms according to age, sex, and education level |
| Depression | Montgomery and Asberg Depression Rating Scale | Unique | [27] | Score < 19 |
| Executive functions | Wisconsin Card Sorting Test | Multiple | [28] | Z score > −1.65 (norms according to age, sex, and education level) |
| Verbal episodic memory | RL-RI16 | Multiple | [29] | Z score > −1.65 (norms according to age, sex, and education level) |
| English test | Evaluation of oral and written comprehension. | Unique | Original test | Score <16/18 |
Table 2.
Inclusion and exclusion criteria for the Age-Well clinical trial
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
2.2. Measures collected at the baseline and follow-up visits (V1, V2, and V3)
V1 and V3 comprise a multidisciplinary assessment of a wide range of behavioral and biological measures collected within a maximum of 3 months before (V1) and after (V3) the start of the intervention. The detailed biological, behavioral, neuroimaging and sleep measures collected at the pre-intervention and post-intervention visits (V1 and V3) are listed in Table 3; the mid-intervention intermediate visit (V2) includes a selected set of the behavioral measures collected at V1 and V3 as indicated in Supplementary Table 1. Briefly, behavioral measures are neuropsychological tests assessing different cognitive functions (e.g. episodic memory, attention, executive function), scales and questionnaires assessing, for example, sleep quality, lifetime and current engagement in cognitive, social and physical activities; Mediterranean diet adherence, health-related behaviors such as self-medication, smoking and alcohol consumption, quality of life and well-being, psychoaffective factors such as anxiety and depression, and prosocialness (Supplementary Table 1 for details). Some of the questionnaires are also given to a participant's close relative or friend (subsequently referred to as the “partner”). Neuroimaging measures include a series of structural and functional (resting-state and task-related) MRI scans, fluorodeoxyglucose (Glucotep) and florbetapir (AV45, Amyvid) positron emission tomography scans, and resting-state electroencephalography and auditory event related potential recording (Supplementary Table 2 for details). Objective measures of sleep include actigraphy, an ambulatory monitoring device using heart rate and body movements to score sleep (SomnoArt) and polysomnography. Biological measures are obtained from blood sampling (Supplementary Table 3). All procedures for data acquisition were discussed and audited by experienced and skilled study staff to ensure standardization of the procedures.
Table 3.
List of collected measures and corresponding outcomes
| Measures collected at V1 and V3 (and V2 for a selected set of behavioral measures) | Outcomes |
|---|---|
| Behavioral measures (Supplementary Table 1 for details): Series of neuropsychological tests,scales and questionnaires selected as they are particularly sensitive to aging and AD (e.g., assessing episodicmemory, attention, executive functions and metacognition) and/or meditation practices (e.g., assessing mindfulness, compassion, and interoception), emotions (e.g., assessing anxiety, depression, empathy, emotion regulation, positive and negative emotions), or as they allow to assess different aspects of sleep quality, lifestyle, well-being, prosociality, loneliness, social support and quality of life. Questionnaires are also proposed to a partner (i.e., a participant's close relative or friend) to assess how the partner perceives the mindfulness, compassion, depression, anxiety, and prosocialness of the participant as well as questions on the social support and the role ofan informal carer of the partner. |
Composite scores and raw individual measures of cognitive performance, well-being, mindfulness and meta-cognition, emotion-related questionnaires, altruism, prosociality, sleep quality, lifestyle, and quality of life of the participants. Partner perception of the participant's mindfulness, compassion, depression, anxiety, and prosocialness as well as questions on the social support and the role of an informal carer of the partner. |
Neuroimaging measures (Supplementary Table 2 for details):
|
|
| Biological measures from blood (Supplementary Table 3 for details): Fasting sampling performed in the morning and after one day of diet excluding ich food (tomatoes, avocados, pineapple, chocolate, bananas…). 18 tubes (68 mL) of blood collected at V1 and 16 tubes (62 mL) at V3. |
|
Objective measures of sleep:
|
|
More details can be found in the Supplementary Material.
2.3. Interventions
The 18-month intervention period starts just after the randomization step for each of the three cohorts of 43, 50, and 44 participants, each subdivided in three groups (meditation, foreign language, control) of 14–17 participants.
During the study, participants are strongly encouraged not to practice the activity proposed in the other arms (groups). The meditation and the foreign language training interventions are structurally equivalent in overall course length, class time, and home activities and matched in administration, dosage, and duration. The number of teachers per class and their level of expertise are equal in both interventions. Participants are encouraged to participate in all those activities during the whole period of the intervention (i.e., 18 months).
In addition to being structurally equivalent, we actively tried to balance researcher allegiance to the two interventions as this might have significant impact on the findings [4], [5], [6], [32], [33]. A well-designed control should also include a rationale for the positive expectation for intervention success by both the teachers and participants [34]. We thus tried to keep all communications about the study fully balanced as regard to expected effects of both the meditation and the foreign language training interventions and underpinning the equipoise regarding their use. To assess participants' expectations regarding the intervention, a questionnaire was proposed to them at the beginning of the intervention (Credibility and Expectancy questionnaire, [35]).
For both the meditation-based and the foreign language training interventions, participants follow:
-
-
2-hour weekly group sessions,
-
-
daily home practice (at least 20 minutes per day),
-
-
one day of more intense practice during the intervention (5 hours during the day).
Both interventions have been fully described a priori in respective manuals.
The meditation intervention consists of an original secular program of meditation training labeled “The Silver Santé Study Meditation Programme” especially designed for this study based on pre-existing interventions (as detailed in the Supplementary Materials) with the objective of personal development and healthy aging, and is provided by expert meditator instructors in Caen. The objective of this 18-month intervention program is to develop mindfulness, kindness and compassion abilities as additional psychological resources to cope with challenges related with aging on physical, cognitive and psychological aspects. The first 9 months of the intervention are dedicated to the teaching of mindfulness meditation whereas the 9 following months are dedicated to the teaching of the meditation on loving kindness and compassion. Each session contains moments of group meditation, sitting, or walking and moments of sharing and teaching.
The foreign language training program is a cognitively stimulating intervention structurally matched to the meditation intervention and hypothesized to have no specific effect on emotional measures. It consists of English exercises designed to reinforce each participant's abilities in understanding, writing and speaking. Sessions are held by mixing oral comprehension and expression activities to work in priority, the acquisition of new vocabulary and new grammatical structures.
Participants in the passive control group are requested not to change their habit and continue living as they used to before engaging in the study and until the end of V3. They are specifically asked not to engage in meditation or foreign language training.
More details on the interventions can be found in the Supplementary Material.
2.4. Outcome measures
The main objective of the Age-Well RCT is to test, whether an 18-month meditation-based intervention in cognitively unimpaired older adults is superior to i) a passive control group on changes in volume and perfusion of the anterior cingulate cortex (ACC); ii) an 18-month foreign language training program on changes in volume and perfusion of the insula. Accordingly, the primary outcomes are the mean change in the volume and perfusion of both the ACC and the insula, as measured with structural T1-weighted MRI and early florbetapir positron emission tomography scan respectively from the baseline pre-intervention to the 18-month post-intervention follow-up visits.
The secondary objectives will focus on the effects of the interventions on cognition, well-being, quality of life, psychoaffective factors and lifestyle; complementary neuroimaging measures of brain integrity, emotion and attention-related neural activity; sleep quality with polysomnography; and biological blood measures in the aging population. Sex-specific effects of the interventions and effects on participants' partners (perception of participant's changes and their own perceived social support) will also be assessed, together with exploratory analyses unrelated to the clinical trial intervention (e.g., aiming to further understand the impact of lifestyle factors and the physiopathological mechanisms of AD). The secondary outcomes used to assess these questions are listed in Table 3. Adverse events and measures collected during the intervention by the teachers and self-report of participants will be used to assess safety, acceptance and adherence.
2.5. Statistical considerations
2.5.1. Sample size calculation
The comparison of the meditation versus passive control arms will focus on the mean change in (1) volume and (2) perfusion of the ACC from the baseline pre-intervention visit to the end of the 18-month intervention, with an expected relevant effect size of 0.75, as suggested by a meta-analysis of meditation effects on neuroimaging markers [20]. To demonstrate an effect size of 0.75 for each of the four comparisons, with 80% power and a two-sided type I error of 1.25% (Bonferroni correction for test multiplicity), 42 participants per arm (126 in total) need to be included. The total number of participants included in the Age-Well RCT (n = 137) is higher than this required minimum of 126.
2.5.2. Statistical analyses
The planned statistical analyses are detailed in a statistical analysis plan and summarized in the Supplementary Material. Briefly, statistical analyses related to the primary outcome will be conducted on an intent-to-treat principle and missing primary endpoint data will be handled with a “missing = failure” strategy. Additional analyses conducted on both primary and secondary outcomes will include sensitivity analyses, per-protocol analyses and analyses of exposure/dose effects.
2.6. Ethics, safety, and study monitoring
The Age-Well RCT was approved by the ethics committee (Comité de Protection des Personnes CPP Nord-Ouest III, Caen; trial registration number: EudraCT: 2016-002441-36; IDRCB: 2016-A01767-44; ClinicalTrials.gov Identifier: NCT02977819) and adheres to Standard Protocol Items: Recommendations for Interventional Trials guidelines for clinical trial protocols [36].
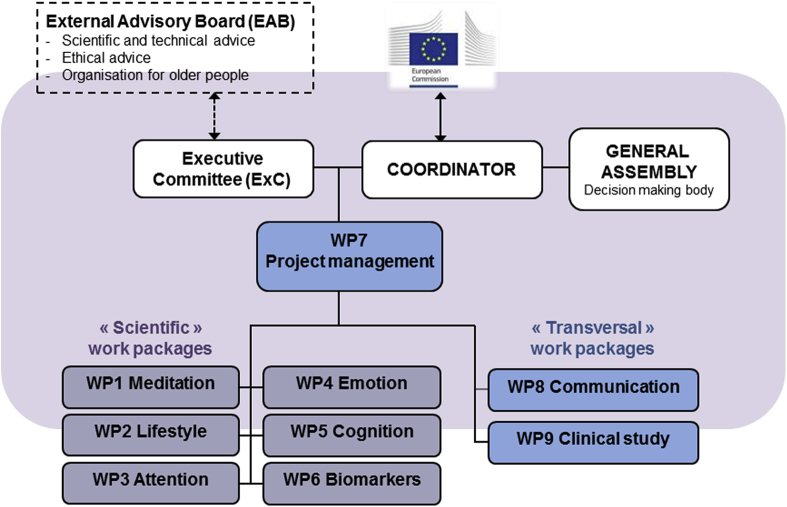
The management structure of Medit-Ageing is illustrated in Fig. 3 and includes the coordinator (G.C.), the executive committee and 9 work packages (including the management work package). In addition, the sponsor (Inserm) has established a trial steering committee in line with Good Clinic Practice guidelines, and an external Data and Safety Monitoring Board, independent of the sponsor, was appointed.
Fig. 3.
Management structure of Medit-Ageing.
More details on ethics and safety, study governance and monitoring, as well as study progress can be found in the Supplementary Material.
2.7. Clinical trial progress
From May 2016 to May 2018, about 900 individuals came to public conferences and filled in the online prescreening questionnaire. Among those, 157 participants were screened. Thirteen did not fulfill the inclusion criteria (the main reason being abnormal performance in the diagnostic battery), 6 participants were excluded during V1 because of artifacts or claustrophobia attack during the MRI scan or high level of glycemia and 1 participant withdrew. Finally, 137 participants (40/60% men/women) were randomized to the three experimental groups. Participants from the first and second cohort had their 9-month intermediate follow-up visit—at that time only 1 participant dropped out (death).
The 18-month follow-up post-intervention visits will end in early 2020. Electronic data entry, monitoring and processing are currently ongoing.
3. Discussion
Age-Well is the first RCT to comprehensively assess the long-term efficacy of meditation on well-being and aging through a multidisciplinary approach. The strength and originality of the Age-Well RCT relies, first, in the nature, dose and duration of the proposed interventions. Thus, this meditation intervention is especially designed to target not only cognitive control via the regulation of attention, but also psychoaffective factors through the reduction of stress and the cultivation of positive emotions. This is crucial as stress, anxiety and depression significantly contribute to reduced quality of life and increased risk for dementia in older adults [2]. Current lifestyle preventive trials tend to include multidomain interventions as recommended [6] but most often do not focus on the emotional dimension of aging; thus they target the main risk factors for dementia (cardiovascular risk, diet, cognitive and physical activity) except the psychoaffective ones (e.g., depression). Moreover, interventions in Age-Well include weekly 2-h group practice monitored by highly experienced teachers and daily individual home practice collected over the 18 months of the interventions. They might have the potential to induce long-term effects on brain and biological markers of healthy aging.
Second, the Age-Well RCT assesses a wide range of complementary outcomes including cognitive tests, scales and questionnaires assessing well-being, quality of life, psychoaffective factors and lifestyle, but also complementary MRI and positron emission tomography neuroimaging measures of brain structural and functional integrity, emotion and attention-related neural activity, biological blood measures and gold-standard measures of sleep with polysomnography. In addition to being useful to provide a comprehensive overview of the effects and to monitor the effectiveness of the intervention [6], this multidisciplinary approach will allow for the investigation of mechanisms underlying the possible effects of the intervention. A better understanding of the mechanisms of action of meditation will facilitate sensitivity to intervention analysis and help refine and tailor future meditation-based interventions. The Age-Well RCT is monocentric thus avoiding intersite variability, which often limits the inclusion of certain biomarkers in multisite clinical trials.
A further strength of this study to estimate specific meditation effects is the ability to use the foreign language intervention as an active control condition. Many of the previous studies of mindfulness-based interventions have suffered from a lack of an adequate comparison condition. As mindfulness-based interventions contain a number of non-specific elements, such as social interaction, light exercise, or the provision of treatment expectancies, the use of an active control condition is important. Foreign language training was selected as the active control condition for several reasons. Like meditation, it involves cognitive mental training. It can easily be matched structurally to the meditation intervention (e.g., group sessions and daily practices with audio and video supports). Positive effects are expected given that the learning of a new language has been shown to impact on cognitive functions and brain structures sensitive to aging and AD [33], [34]. In addition to being useful as an active control condition, this intervention has its own scientific interest as the first RCT to date on language training in older adults over 18 months and with multidisciplinary outcomes including multimodal neuroimaging. Note that, while both interventions are expected to have a positive impact in aging populations, they are expected to target distinct aspects of mental health and well-being and to involve distinct mechanisms (Fig. 1). In particular, foreign language training is not expected to directly impact on emotional states, contrary to meditation training. It will thus be interesting to compare the relative effects of both interventions on the different outcome measures.
As for the choice of the primary endpoint, most previous long-term preventive trials used clinical/cognitive measures. Here we think that the use of neuroimaging biomarker is more appropriate as we are interested in the earliest stages where clinical and cognitive decline is not expected to be significant within 18 months, while neuroplasticity is likely to occur and be translated in measurable brain changes. Neuroimaging biomarkers are increasingly used in ongoing trials, especially in preventive trials or early disease stages and when the goal is to show an effect on the pathophysiology of the disease [37]. More specifically, the ACC and insula emerge as the most appropriate endpoints for the following reasons: the ACC is both i) a brain area with known relevance to maintain cognitive function in older people [36], [37], [38], [39] and ii) one of the brain regions most sensitive to meditation [20], [22]. The insula has been selected to assess the effects of the meditation intervention compared with the foreign language training intervention as it is a region related to interoceptive awareness, emotional and empathic processing, also most frequently involved in meditation studies [20], [22] but less likely to be involved in cognitive training and to be impacted by the foreign language training intervention.
A side effect of most clinical studies and especially those that are highly demanding (in terms of examinations and intervention), is that individuals interested in participating are likely to be particularly active and educated. They might thus not be representative of the general population. Future studies should develop specific strategies to stimulate enrollment of under-represented populations in such clinical trials and increase generality of findings. In addition, the Age-Well RCT is interested in assessing the effects of the interventions on brain structure and function and investigating the mechanisms underlying these effects. Future studies could focus on estimating impact on clinical outcomes such as conversion to mild cognitive impairment or dementia using comparative trials with a sufficient duration of follow-up.
The results of the Age-Well RCT are expected to further the understanding of the factors preventing and delaying age-related diseases and disabilities to promote healthy aging and older adults’ well-being and to propose innovative therapeutic approaches. Our objectives are expected to reduce stress, to improve the maintenance of cognitive abilities and the regulation of emotion in older adults through meditation practice, to establish a preventive strategy favoring the emotional dimension of healthy aging and to reduce the negative impact of mental conditions and disorders. The Age-Well RCT, and Medit-Ageing at large, should help shape and optimize future lifestyle-based and meditation-based clinical trials and facilitate the integration of meditation practice into existing and future prevention programs and clinical interventions in older people. Future trials would be needed to confirm the long-term clinical impact on aging populations.
Research in Context.
-
1.
Systematic review: We reviewed previously published literature and existing randomized controlled trials (RCTs) in the fields of aging, prevention of dementia or Alzheimer's disease, and nonpharmacological interventions from PubMed and clinicaltrials.gov. Previous RCT-evaluated individualized programs of physical activities or technology-based solutions to improve the quality of life of older people. The largest ongoing nonpharmacological prevention RCT use multidomain interventions simultaneously targeting various vascular and lifestyle-related risk factors.
-
2.
Interpretation: Age-Well is the first RCT in aging to propose a long-term intervention addressing the plasticity of emotional and cognitive dimensions during aging. It will assess the effects and mechanisms of a long-term meditation-based intervention compared with a foreign language training and a passive control condition on behavioral, neuroimaging, sleep and biological blood markers of mental health and well-being in the aging population.
-
3.
Future directions: The Age-Well clinical trial might facilitate the integration of meditation practice and foreign language learning into existing and future preventive programs in older people and contribute to the design of larger multinational prevention RCTs.
Acknowledgments
The authors would like to thank all the contributors listed in the Medit-Ageing Research Group, as well as Gwendoline Le Du, Valérie Lefranc, Aurélia Cognet, Clarisse Gaubert, Sylvie Brucato, Dr Laurence Michel, Marine Faure, Jeanne Lepetit, Rhonda Smith, Charlotte Reid, Marie Saville, Dr Alain Manrique, Inserm administrative financial and legal departments, Euclid team, the sponsor (Pôle de Recherche Clinique at Inserm), Inserm Transfert (Delphine Smagghe), the Cyceron staff and the participants in the Age-Well RCT.
The Age-Well RCT is part of the Medit-Ageing project funded through the European Union's Horizon 2020 research and innovation programme related to the call PHC22 “Promoting mental well-being in the aging population” and under grant agreement N°667696. Inserm, Région Normandie, Fondation d'entreprise MMA des Entrepreneurs du Futur also contribute to fund parts of the Medit-Ageing project not initially included in the initial grant (not covered by the European Union funding). Funding sources are not involved in the study design, data acquisition, data analysis, data interpretation or manuscript writing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.trci.2018.10.011.
Contributor Information
Géraldine Poisnel, Email: poisnel@cyceron.fr.
Gaël Chételat, Email: chetelat@cyceron.fr.
Supplementary data
References
- 1.Jané-Llopis E., Gabilondo A. European Communities; Luxembourg: 2008. Mental Health in Older People. Consensus paper. [Google Scholar]
- 2.Marchant N.L., Howard R.J. Cognitive debt and Alzheimer's disease. J Alzheimers Dis JAD. 2015;44:755–770. doi: 10.3233/JAD-141515. [DOI] [PubMed] [Google Scholar]
- 3.Yaffe K. Modifiable Risk Factors and Prevention of Dementia: What Is the Latest Evidence? JAMA Intern Med. 2018;178:281–282. doi: 10.1001/jamainternmed.2017.7299. [DOI] [PubMed] [Google Scholar]
- 4.Livingston G., Sommerlad A., Orgeta V., Costafreda S.G., Huntley J., Ames D. Dementia prevention, intervention, and care. Lancet Lond Engl. 2017;390:2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 5.Norton S., Matthews F.E., Barnes D.E., Yaffe K., Brayne C. Potential for primary prevention of Alzheimer's disease: an analysis of population-based data. Lancet Neurol. 2014;13:788–794. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- 6.Wang J., Tan L., Yu J.-T. Prevention Trials in Alzheimer’s Disease: Current Status and Future Perspectives. J Alzheimers Dis JAD. 2016;50:927–945. doi: 10.3233/JAD-150826. [DOI] [PubMed] [Google Scholar]
- 7.Carrié I., van Kan G.A., Gillette-Guyonnet S., Andrieu S., Dartigues J.-F., Touchon J. Recruitment strategies for preventive trials. The MAPT study (MultiDomain Alzheimer Preventive Trial) J Nutr Health Aging. 2012;16:355–359. doi: 10.1007/s12603-012-0046-8. [DOI] [PubMed] [Google Scholar]
- 8.Kivipelto M., Solomon A., Ahtiluoto S., Ngandu T., Lehtisalo J., Antikainen R. The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER): study design and progress. Alzheimers Dement J Alzheimers Assoc. 2013;9:657–665. doi: 10.1016/j.jalz.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Richard E., Van den Heuvel E., Moll van Charante E.P., Achthoven L., Vermeulen M., Bindels P.J. Prevention of dementia by intensive vascular care (PreDIVA): a cluster-randomized trial in progress. Alzheimer Dis Assoc Disord. 2009;23:198–204. doi: 10.1097/WAD.0b013e31819783a4. [DOI] [PubMed] [Google Scholar]
- 10.Ngandu T., Lehtisalo J., Solomon A., Levälahti E., Ahtiluoto S., Antikainen R. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet Lond Engl. 2015;385:2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- 11.Gard T., Hölzel B.K., Lazar S.W. The potential effects of meditation on age-related cognitive decline: a systematic review. Ann N Y Acad Sci. 2014;1307:89–103. doi: 10.1111/nyas.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lutz A., Jha A.P., Dunne J.D., Saron C.D. Investigating the phenomenological matrix of mindfulness-related practices from a neurocognitive perspective. Am Psychol. 2015;70:632–658. doi: 10.1037/a0039585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newberg A.B., Serruya M., Wintering N., Moss A.S., Reibel D., Monti D.A. Meditation and neurodegenerative diseases. Ann N Y Acad Sci. 2014;1307:112–123. doi: 10.1111/nyas.12187. [DOI] [PubMed] [Google Scholar]
- 14.Chen K.W., Berger C.C., Manheimer E., Forde D., Magidson J., Dachman L. Meditative therapies for reducing anxiety: a systematic review and meta-analysis of randomized controlled trials. Depress Anxiety. 2012;29:545–562. doi: 10.1002/da.21964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galante J., Galante I., Bekkers M.-J., Gallacher J. Effect of kindness-based meditation on health and well-being: a systematic review and meta-analysis. J Consult Clin Psychol. 2014;82:1101–1114. doi: 10.1037/a0037249. [DOI] [PubMed] [Google Scholar]
- 16.Innes K.E., Selfe T.K. Meditation as a therapeutic intervention for adults at risk for Alzheimer’s disease - potential benefits and underlying mechanisms. Front Psychiatry. 2014;5:40. doi: 10.3389/fpsyt.2014.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuyken W., Warren F.C., Taylor R.S., Whalley B., Crane C., Bondolfi G. Efficacy of mindfulness-based cognitive therapy in prevention of depressive relapse: An individual patient data meta-analysis from randomized trials. JAMA Psychiatry. 2016;73:565–574. doi: 10.1001/jamapsychiatry.2016.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fredrickson B.L., Cohn M.A., Coffey K.A., Pek J., Finkel S.M. Open hearts build lives: positive emotions, induced through loving-kindness meditation, build consequential personal resources. J Pers Soc Psychol. 2008;95:1045–1062. doi: 10.1037/a0013262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider R.H., Grim C.E., Rainforth M.V., Kotchen T., Nidich S.I., Gaylord-King C. Stress reduction in the secondary prevention of cardiovascular disease: randomized, controlled trial of transcendental meditation and health education in Blacks. Circ Cardiovasc Qual Outcomes. 2012;5:750–758. doi: 10.1161/CIRCOUTCOMES.112.967406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox K.C.R., Nijeboer S., Dixon M.L., Floman J.L., Ellamil M., Rumak S.P. Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neurosci Biobehav Rev. 2014;43:48–73. doi: 10.1016/j.neubiorev.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 21.Marciniak R., Sheardova K., Cermáková P., Hudeček D., Sumec R., Hort J. Effect of meditation on cognitive functions in context of aging and neurodegenerative diseases. Front Behav Neurosci. 2014;8:17. doi: 10.3389/fnbeh.2014.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang Y.-Y., Hölzel B.K., Posner M.I. The neuroscience of mindfulness meditation. Nat Rev Neurosci. 2015;16:213–225. doi: 10.1038/nrn3916. [DOI] [PubMed] [Google Scholar]
- 23.Valk S.L., Bernhardt B.C., Trautwein F.-M., Böckler A., Kanske P., Guizard N. Structural plasticity of the social brain: Differential change after socio-affective and cognitive mental training. Sci Adv. 2017;3:e1700489. doi: 10.1126/sciadv.1700489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chételat G., Mézenge F., Tomadesso C., Landeau B., Arenaza-Urquijo E., Rauchs G. Reduced age-associated brain changes in expert meditators: a multimodal neuroimaging pilot study. Sci Rep. 2017;7:10160. doi: 10.1038/s41598-017-07764-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 26.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.Montgomery S.A., Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry J Ment Sci. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 28.Grant D.A., Berg E.A. A behavioral analysis of degree of reinforcement and ease of shifting to new responses in a Weigl-type card-sorting problem. J Exp Psychol. 1948;38:404–411. doi: 10.1037/h0059831. [DOI] [PubMed] [Google Scholar]
- 29.Van der Linden M., Coyette F., Poitrenaud J., Kalafat M., Calicis F., Wyns C. et les membres du GREMEM. L’épreuve de rappel libre/rappel indicé à 16 items (RL/RI-16) In: Van der Linden M., Adam S., Agniel A., editors. et les membres du GREMEM, L’évaluation des troubles de la mémoire. Présentation de quatre tests de mémoire épisodique (avec leur étalonnage) Solal; Marseille: 2004. pp. 25–47. [Google Scholar]
- 30.Braver T.S., Barch D.M., Gray J.R., Molfese D.L., Snyder A. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb Cortex N Y N 1991. 2001;11:825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- 31.Babiloni C., Del Percio C., Caroli A., Salvatore E., Nicolai E., Marzano N. Cortical sources of resting state EEG rhythms are related to brain hypometabolism in subjects with Alzheimer's disease: an EEG-PET study. Neurobiol Aging. 2016;48:122–134. doi: 10.1016/j.neurobiolaging.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 32.Luborsky L., Diguer L., Luborsky E., Singer B., Dickter D., Schmidt K.A. Basic Books; New York, NY, US: 1993. The Efficacy of Dynamic Psychotherapies: Is it True that “Everyone Has Won and All Must Have Prizes?”; pp. 497–516. Psychodyn. Treat. Res. Handb. Clin. Pract. [Google Scholar]
- 33.Imel Z.E., Wampold B.E., Miller S.D., Fleming R.R. Distinctions without a difference: direct comparisons of psychotherapies for alcohol use disorders. Psychol Addict Behav. 2008;22:533–543. doi: 10.1037/a0013171. [DOI] [PubMed] [Google Scholar]
- 34.Mohr D.C., Spring B., Freedland K.E., Beckner V., Arean P., Hollon S.D. The selection and design of control conditions for randomized controlled trials of psychological interventions. Psychother Psychosom. 2009;78:275–284. doi: 10.1159/000228248. [DOI] [PubMed] [Google Scholar]
- 35.Devilly G.J., Borkovec T.D. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiatry. 2000;31:73–86. doi: 10.1016/s0005-7916(00)00012-4. [DOI] [PubMed] [Google Scholar]
- 36.Chan A.-W., Tetzlaff J.M., Altman D.G., Dickersin K., Moher D. SPIRIT 2013: new guidance for content of clinical trial protocols. Lancet Lond Engl. 2013;381:91–92. doi: 10.1016/S0140-6736(12)62160-6. [DOI] [PubMed] [Google Scholar]
- 37.Andrieu S., Coley N., Lovestone S., Aisen P.S., Vellas B. Prevention of sporadic Alzheimer's disease: lessons learned from clinical trials and future directions. Lancet Neurol. 2015;14:926–944. doi: 10.1016/S1474-4422(15)00153-2. [DOI] [PubMed] [Google Scholar]
- 38.Antoniou M., Wright S.M. Uncovering the mechanisms responsible for why language learning may promote healthy cognitive aging. Front Psychol. 2017;8:2217. doi: 10.3389/fpsyg.2017.02217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mårtensson J., Eriksson J., Bodammer N.C., Lindgren M., Johansson M., Nyberg L. Growth of language-related brain areas after foreign language learning. NeuroImage. 2012;63:240–244. doi: 10.1016/j.neuroimage.2012.06.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.