Abstract
The discovery that adults with Down syndrome (DS) have neuropathological features identical to individuals with sporadic Alzheimer's disease (AD) played a key role in the identification of the amyloid precursor protein gene on chromosome 21 and resulted in the amyloid cascade hypothesis. Individuals with DS have a lifetime risk for dementia in excess of 90%, and DS is now acknowledged to be a genetic form of AD similar to rare autosomal-dominant causes. Just as DS put the spotlight on amyloid precursor protein mutations, it is also likely to inform us of the impact of manipulating the amyloid pathway on treatment outcomes in AD. Ironically, however, individuals with DS are usually excluded from AD trials. This review will discuss primary and secondary prevention trials for AD in DS and the potential barriers and solutions to such trials and describe the Europe-wide Horizon21 Consortium to establish a DS-AD prevention clinical trials network.
Keywords: Alzheimer's disease, Down syndrome, Trisomy 21, Randomized controlled trials, Prevention, Dementia, APP, Amyloid, Biomarkers
1. Introduction
Alzheimer's disease (AD) due to amyloid-β (Aβ) neuropathology has become the most pressing health concern in aging populations, and there are currently no effective treatments available to reduce cognitive decline related to AD neuropathology. Drugs directed at reducing amyloid load or increasing Aβ clearance, including intervening at downstream targets using anti-amyloid antibodies and more upstream using BACE inhibition, have been proposed as the most rational approach to affect the course of AD, but their failure in recent clinical trials has been disappointing. It is now believed that treatments targeting amyloid may only be effective during the extended preclinical or prodromal phase of AD, driven by knowledge that the disease is typically associated with a prolonged presymptomatic period [1]. The current view is therefore that intervention during the early stages of the pathological process is the way forward, but such trials may be problematic in sporadic AD (sAD), given the difficulty to identify individuals most at risk of further decline. Though solutions are being put in place, these are substantial and resource-intensive initiatives [2]. There has therefore been increasing interest in trials of amyloid-targeting drugs in populations with rare genetic forms of AD, particularly autosomal-dominant AD (ADAD) due to fully penetrant mutations in the known AD genes amyloid precursor protein (APP), PSEN1, and PSEN2. So far, two major trials of this type being conducted in ADAD are the Alzheimer's Prevention Initiative and the Dominantly Inherited Alzheimer's Network-Trials Unit. The Dominantly Inherited Alzheimer's Network-Trials Unit enrolls mutation carriers as well as people at a 50% risk for ADAD, with nonmutation carriers allocated to the placebo arm, and all study drugs used to date directly or indirectly targeting Aβ as the therapeutic intervention [3]. Similar initiatives for sAD include the EPAD platform [2]. The possibility to conduct primary prevention studies in those at risk of Alzheimer's disease is now increasingly being considered, and both EMA and FDA guidance documents have been drafted to address the issues related to conducting such trials [4], [5].
The predictable development of AD pathology and high incidence of dementia in individuals with Down syndrome (DS; trisomy 21) suggests that this is another population in which trials in the preclinical or prodromal stage of AD to prevent or delay decline should be considered. This is especially so given this is the simplest cause of disease: overproduction of the APP gene. Ironically, individuals with DS are currently excluded from clinical trials of Aβ-targeting drugs, in part because of their comorbid intellectual impairment and other comorbidities, but also because until recently there has been a lack of biomarker data and validated measures of clinical progression in this population, and concern about tolerability of procedures. Intervention studies in people with DS depend upon determining the optimal age for treatments to be given, identifying or developing reliable outcome measures that are most sensitive to decline, and discovering biomarkers most closely related to disease progression.
In this position paper, we summarize recent progress to understand disease progression in DS at the clinical, neuroimaging, and biomarker levels and review the rationale for trials to prevent or delay AD-related decline in individuals with DS. We conclude that a collaborative approach is required to address remaining challenges, and we describe a European DS platform (Horizon21 Down Syndrome Consortium) that is developing a clinical trials network and feasible and acceptable protocols for such trials.
1.1. AD pathogenesis in DS
The discovery that adults with DS have neuropathology identical to individuals with sAD played a key role in the identification of Aβ contained within amyloid (senile) plaques and the subsequent identification of the APP gene on chromosome 21 [6]. Further evidence that three copies of APP lead to AD has been provided by familial cases with small duplications of the chromosome 21 region encoding APP who also develop early onset of AD [7]. Conversely, partial trisomy of chromosome 21 not involving APP does not lead to AD [8]. DS with APP triplication is thus a genetically determined form of AD, similar to the rare autosomal-dominant forms of AD (ADAD) [9].
An extra copy of APP results in excess Aβ production due to increased APP expression and through proteolytic processing. Some of the earliest AD neuropathological studies noted the exceptionally young age at which cases with DS may present with AD pathology [10]; it is now accepted that all older adults with full trisomy 21 have the neuropathological hallmarks of AD, consisting of a progressive build-up of extracellular Aβ plaques and intraneuronal hyperphosphorylated tau [11]. Recently, amyloid pathology in individuals with DS has been demonstrated with in vivo amyloid PET imaging studies [12] and tangle pathology has been shown with tau PET [13]. Fibrillar deposits of Aβ are increasingly prevalent with aging in DS brain tissue and correlate with the development of Alzheimer's dementia [14]. Similar to sAD and ADAD, postmortem studies imply that Aβ deposits are histopathologically detectable before PET imaging becomes “positive for amyloid.”
The amyloid cascade hypothesis posits that Aβ is the “toxic agent” responsible for initiating AD pathogenesis [15], and DS and ADAD both represent uniquely informative populations for clinical trials because of the almost certain risk of developing AD pathology related to amyloid deposition. More specifically, and unlike most ADAD forms (which are caused by mutations in several genes resulting in different mechanisms for Aβ deposition such as a change in ratio between Aβ1-40 to Aβ1-42, or Aβ that is more prone to aggregation), DS is genetically homogenous with a simple overproduction of Aβ as a cause for AD pathology (much like the ADAD-causative Swedish mutation in APP). Targeting this mechanism is further supported by the finding that an Icelandic mutation that reduces the production of amyloidogenic species via the processing of APP is protective against AD [16]. DS is therefore a critical population for clinical trials of antiamyloid drugs to prevent or delay onset of dementia.
1.2. Opportunities for AD trials in DS
At the population level, adults with DS now have significantly increased life expectancy compared to their peers 50 years ago, and 40% of individuals with DS in Europe are currently aged 40 years and older [17]. These older individuals have an ultra-high risk for dementia; recent estimates suggest a lifetime risk of ∼90% [18]. AD is now the major cause of morbidity and mortality in older adults with DS, with a mean age of death of 60 years [19].
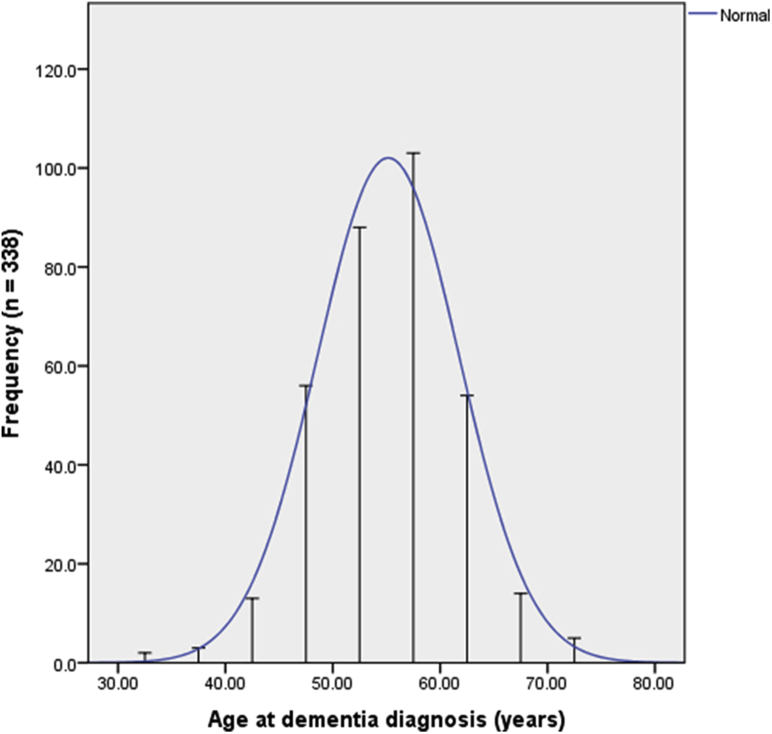
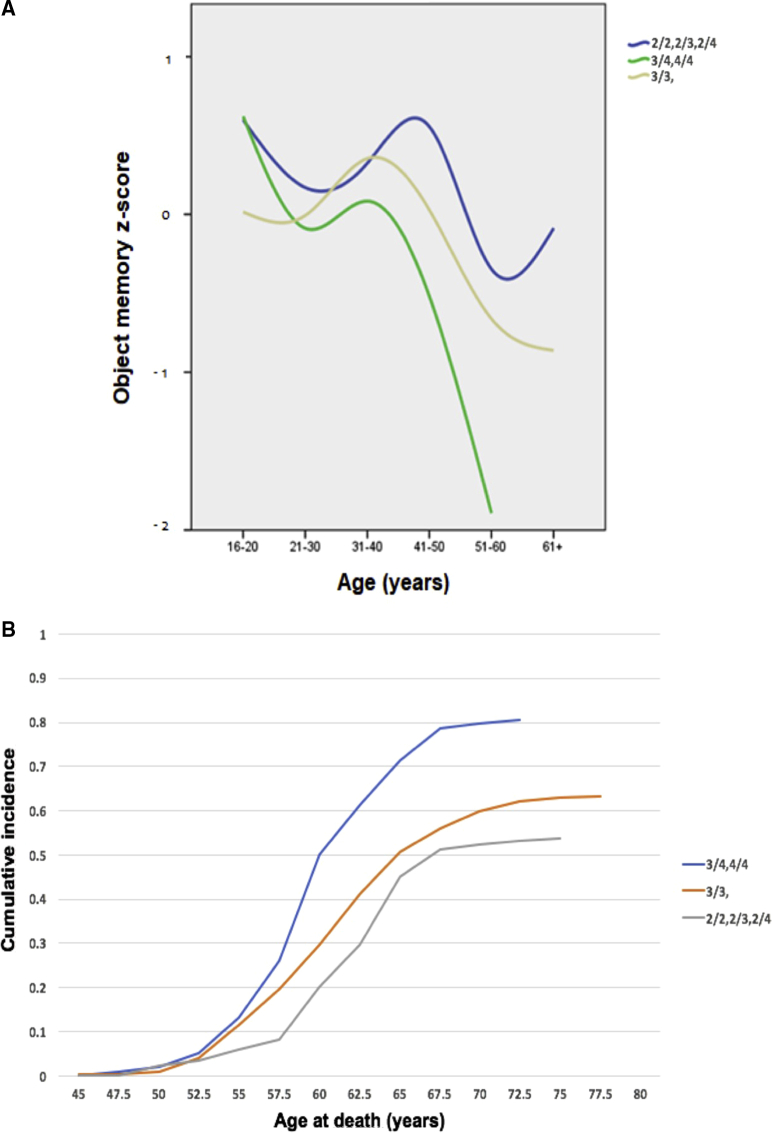
The prevalence of AD in DS increases from 9% to 23% between the ages of 35 and 49 years to 55% in those between 50 and 59 years, and 75% and 100% in persons above the age of 60 years [20], [21]. Dementia diagnosis in DS has a predictable relationship with age [19] (see Fig. 1). The average age of diagnosis is 55 years, while cognitive decline may already be observed after age 40 years, up to 15 years before the typical age of dementia diagnosis (see Fig. 2).
Fig. 1.
Cross-sectional data showing the distribution of age at dementia diagnosis in people with DS. Graph based on Sinai et al., 2018 [19]. Abbreviation: DS, Down syndrome.
Fig. 2.
(A) Cross-sectional data showing the performance (z-score) of adults with DS on object memory test across age, split by APOE status (2/2, 2/3; 3/3; 3/4, 4/4). Data from the London Down Syndrome Consortium (LonDownS), as yet unpublished. (B) Cumulative incidence of mortality, split by APOE status (2/2, 2/3, 2/4; 3/3; 3/4, 4/4). Data from the Rotterdam group, as yet unpublished. Abbreviation: DS, Down syndrome.
With an incidence of approximately 1 in 650 to 1000 live births, there is an estimated ∼500,000 individuals with DS in Europe (6.4/10,000), and ∼206,000 (6.7/10,000) in the United States [17], [22]. In addition to DS being much more common than ADAD, there is an ethical imperative for treatment trials that aim to reduce disease burden within this population. As a consequence of this, individuals with DS ought to be considered for trials of suitable treatments. Such trials may also serve as proof-of-concept (PoC) for other forms of AD, particularly where excess amyloid or Aβ is implicated as central mechanism, including sAD where preventive trials would require much larger numbers, take much longer to complete (and therefore be prohibitively expensive to undertake), and where amyloid-independent pathways may mask potential benefit in some individuals.
Because of the universal presence of amyloid neuropathology in all individuals with full trisomy 21 (which is the cause of DS in more than 95% of cases) from their 30s, all adults with DS over age 35 years can be considered to be in a preclinical AD state. This presents a unique opportunity to undertake primary prevention trials (i.e., to avoid or reduce amyloid overproduction and its subsequent detectable brain deposition and downstream pathological consequences) and secondary prevention trials (defined as delaying the onset of dementia after the presence of detectable brain amyloid deposition, but in the absence of criteria fulfilling features).
Despite the role of DS in the discovery of the basic mechanisms of AD and the obvious treatment strategy to reduce amyloid overproduction in individuals with DS, there are virtually no such trials currently being conducted. This is in contradiction to the United Nations Convention on the rights of persons with disabilities which encourages participation of persons with disabilities in biomedical research [23]. Furthermore, the United Nations Convention on the rights of persons with disabilities requires health professionals to “provide care of the same quality to persons with disabilities as to others (…)” [23]. Given the current lack of clinical data to support treatment strategies of AD in DS, such trials are imperative for clinicians to live up to the standards set by the UN.
1.3. Challenges to conducting AD trials in DS
There are ethical issues similar to those concerning prevention trials in sAD and ADAD populations, including starting treatments in a healthy population who may not develop dementia for several years. It is therefore vital to understand the “preclinical” (i.e., at risk but before development of signs and symptoms) and “prodromal” (i.e., above a threshold on certain biomarkers, with some cognitive impairment or behavioral changes, but before dementia diagnosis) stages of AD in DS to better predict when to start preventive treatment, as well as to provide data for efficient trial designs. Such data have, until recently, been lacking in DS; however, over the past 5 years, we have been focusing on exploiting clinical, cognitive, fluid biomarker, and imaging data from several DS longitudinal cohorts across Europe to address these gaps in knowledge (see section “The Horizon21 European Down Syndrome Consortium” below), while the NIH has recently funded two DS AD biomarker cohorts in the US (Neurodegeneration in Aging Down Syndrome and Alzheimer's Disease in Down Syndrome).
Individuals with DS are a particularly vulnerable population due to several factors including prevalence of chronic conditions, and the impact of these conditions on long-term functioning, as well as potential for adverse effects associated with particular treatments. This needs to be carefully considered before undertaking treatment trials. Furthermore, individuals with DS have premorbid cognitive impairments that may affect decision-making capacity. Many individuals with DS will not possess the capacity required to make decisions about whether to participate in research studies or not. In these cases, special ethical safeguards should be respected, and caregivers have a prominent role in helping to make decisions to participate or not, as well as to support individuals taking part in trials. These factors need to be considered when selecting potential targets and procedures and should inform trial design and recruitment strategies. However, DS research has moved from focusing predominantly on policy and care issues such as inclusion, to ensuring access to treatment, starting in the 1970s with surgical interventions for congenital heart disease. Clinical trials of medication treatments for dementia in DS are now both desirable and feasible, with recent examples including trials of vitamin E and memantine to prevent decline [24], [25].
Finally, given the relatively low prevalence of DS, it is ethically obligated to pool resources internationally to enable timely and representative recruitment across countries. This also requires harmonization of research procedures and protocols.
1.4. The Horizon21 European Down Syndrome Consortium
It is in this context that clinical research groups across Europe—focused on the clinical, biomarker, and genetic aspects of AD in DS—are collaborating in an ongoing effort to obtain the necessary data, tools, and infrastructure to enable PoC trials of treatment to delay or prevent AD in DS. We are leveraging a globally unique critical mass of several existing AD-DS cohorts (with a combined total of more than 1000 older participants with DS) to pool our data and bioresources to address current gaps in knowledge about AD in DS.
The Horizon21 DS Consortium consists of DS cohorts from the UK (the London Down Syndrome Consortium [LonDownS] and the Cambridge Dementia in Down's Syndrome [DiDS] cohort), Netherlands (the Rotterdam Down syndrome study and the Health Watch study), Germany (AD21 study group, Munich), France (TriAL21 for Lejeune Institute, Paris), and Spain (the Down Alzheimer Barcelona Neuroimaging Initiative (DABNI). We have developed a consortium model that is focused on delivering essential data on clinical progression of the early stages of AD in DS, while developing a clinical trials network. The consortium has as its starting point a DS trial-ready cohort that builds on existing studies to establish a large multinational cohort of individuals with DS with run-in data, which will also serve as a registry of potential trial participants. This is linked with a genomics project already underway, pooling DNA data to conduct genome-wide association studies (GWAS) of cognitive decline in DS to understand the genetic factors underlying variation in age of onset of disease. Another key initial aim of the consortium is to identify and refine cognitive outcome measures, alongside harmonization of assessments and procedures. We are also working to identify feasible interim markers of disease progression by exploring the relationship between fluid biomarkers, neuroimaging, and the development of cognitive decline in DS [26], [27], [28], [29], [30].
In the sections below, we describe progress made and highlight aspects of future work to establish clinical trials of treatments to prevent or delay AD in individuals with DS.
1.5. DS trial registry and cohorts with run-in data
Recruitment to clinical trials is potentially challenging, but enrollment benefits from access to existing registries of eligible participants. Furthermore, enrollment will likely be speeded up if information that is important for participant selection is already known, such as trisomy status, clinical diagnosis, and whether the individual already has symptoms suggestive of AD. Longitudinal assessment is also necessary to measure change against individual baselines, which overcomes the complexities related to variable premorbid expression of cognitive impairments, and has the added advantage of providing run-in data that have the potential to reduce sample sizes of trials [2].
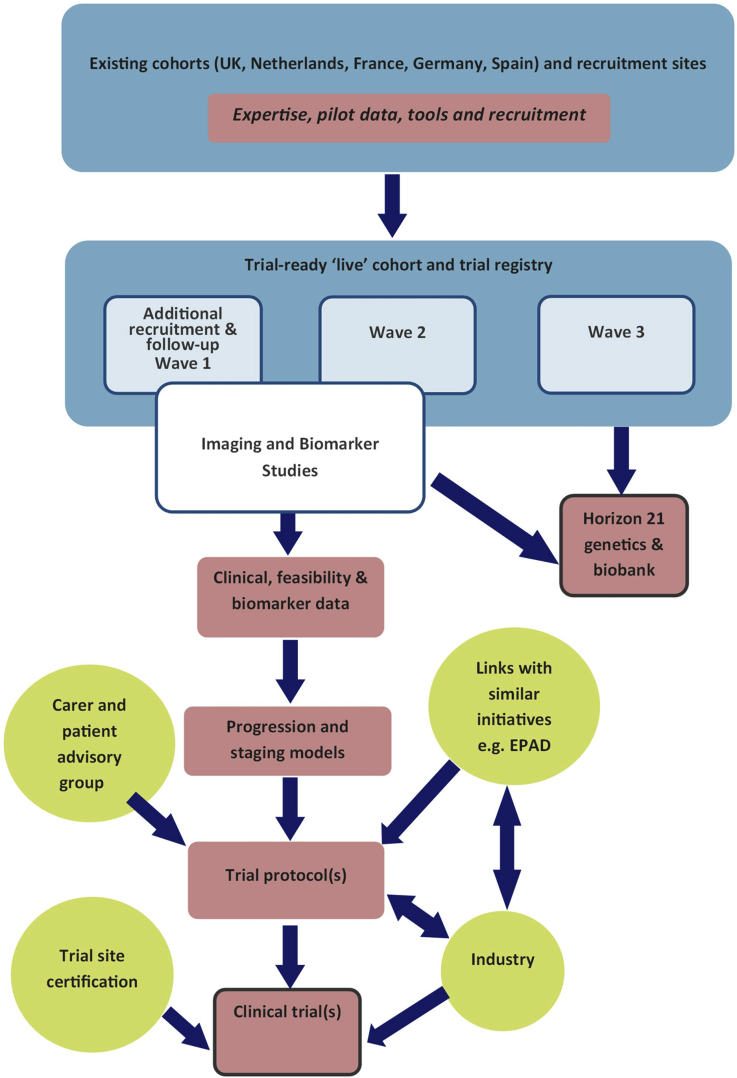
Horizon21 is developing a harmonized core assessment protocol, which will be used to follow our combined cohorts. Participants will undergo identical protocols for longitudinal clinical phenotyping. Participants will be asked to consent to inclusion in a coordinated participant registry, consisting of regularly updated contact details kept separately from clinical data, but with anonymized linkage to run-in data. At the most basic level, run-in variables will include age, apolipoprotein E (APOE) genotype, clinical diagnoses, cognitive test scores (particularly premorbid IQ or ability level), as well as information on potential exclusion criteria for trials. Participants will be followed with sequential assessments. Such longitudinal data will allow us to identify clinical markers of progression in cognitive abilities, using multivariate approaches and survival analyses to inform trial design. The first recruitment to a run-in cohort will start toward the end of 2018, with the first phase being focused on validating cognitive outcome measures (identified using the methods described below) and a harmonized assessment protocol, as well as to obtain baseline genetic and blood biomarker data. Subsequent waves of recruitment are planned to allow for a dynamic cohort of individuals within the age ranges suitable for both prevention and disease-modifying trials (see Fig. 3).
Fig. 3.
Flow diagram showing existing DS trial-ready cohorts. Abbreviation: DS, Down syndrome.
1.6. Genetic analyses to refine estimates of age at onset in DS
Genetic analysis could enable a clearer and more precise estimate of the age at which amyloid deposition and clinical dementia begin in DS to prepare for and to power clinical trials. We have pooled all available DNA for the Horizon21 DS genomics study, which now includes data from more than 1000 individuals with DS. Our data show that the APOE ε4 allele is an important determinant of clinical age at onset (see Fig. 2); this can potentially be used in staging models [31]. Preliminary analyses also suggest that other GWAS hits such as PICALM may have a role [32]. A current aim is to investigate whether incorporating other genetic markers improves predictions of onset age and could thus have value in sample size calculation for trials. A parsimonious hypothesis would be that GWAS hits for diploid AD will have effects on age at onset in DS, but one might also expect that other chromosome 21 loci may play a significant role or that, because APP expression is clearly a key, genes that have direct effects on APP processing may have a larger role in DS than in the general population. In addition, 2%–4% of DS cases are mosaic, and 2%–4% are due to translocations; these will be investigated to establish if they follow the general “rules” that apply to the majority of DS cases and to determine whether they should be included in PoC trials.
1.7. Developing cognitive outcome measures for AD trials in DS
Trials of AD treatments in DS require reliable clinical outcome measures. In individuals with DS, the development of dementia needs to be understood in the context of a complex cognitive phenotype that includes not only variable degrees of intellectual impairment but also specific (and variable) impairments in executive function, memory, language, and motor domains [33]. These preexisting impairments need to be distinguished from subsequent decline and, in combination with varying baseline abilities and limitations in speech abilities, can complicate the clinical diagnosis of dementia and interpretation of cognitive test data.
Cognitive tests of domains commonly affected in sAD have been adapted for use in individuals with DS, and there are some data to suggest that these can discriminate between those with and without dementia as well as to track cognitive decline [33], [34], [35]. However, floor effects are a concern, and approximately a third of older DS participants without dementia may be unable to perform complex cognitive tasks, affecting their suitability as clinical trial outcome measures. Furthermore, some tests are limited by their reliance on verbal language abilities, which is a particular issue for individuals with DS. Language-heavy tests also limit their applicability across multinational and multicultural settings.
In addition, there are no agreed definitions of prodromal stages of AD in DS when some symptoms may already be present, but before clinical diagnosis of dementia is possible (diagnosis is generally made at a relatively late stage in the process of pathology). Most previous studies of decline in DS were small scale and results were mixed; some identified early changes in memory [35], while others suggested changes in executive function, behavior, and personality occur before AD diagnosis [36]. Because a common approach is to use informant questionnaires to obtain detailed information on the development of behavioral or cognitive symptoms related to the onset of dementia, it is possible that some of these findings reflect the changes that are noticed first by caregivers and thus not necessarily representative of the underlying sequence of dementia-associated changes. Informant-rated tools may be more useful in trials targeting later stages of disease when prominent symptoms are present.
These issues highlight the need to refine or develop more sophisticated neuropsychological tools to track cognitive decline in this population for use as outcome measures in clinical trials.
The Horizon21 DS consortium is using existing data from our ongoing longitudinal studies of AD in DS to identify the cognitive domains affected in the early (prodromal) stages of AD. We are applying statistical methods such as Least Absolute Shrinkage and Selection Operator (LASSO) regression [37]. This is an ideal method for variable selection when a set of predictors may be correlated, as with cognitive tests or clinical rating scales. It is a data-driven method to identify the variables in a data set that are most associated with an outcome, to identify the tests most related to the outcome of interest. Potentially, LASSO could be used to identify cognitive tests that are sensitive to cognitive change. In addition, we may use an event-based model (EBM), another data-driven model capable of estimating biomarker orderings and staging of participants. The EBM has been applied previously to imaging, CSF, and cognitive markers in sAD and to model more complex cognitive data sets in ADAD [38]. We have recently applied EBM to estimate the order of cognitive decline of AD in DS and assign participants to a disease stage, which suggests that tests of memory and attention are most sensitive to early AD decline [39]; however, it is necessary to confirm this longitudinally and across cohorts stratified by level of ID.
Within Europe, the CAMCOG-DS [40] is a composite measure often used in both clinical and research settings but has some limitations, being fairly long, with several potentially redundant items and few memory items, as well as relying on culturally sensitive concepts, pictures, and language. It has already been translated into several European languages. Horizon21 is working to improve and refine the CAMCOG-DS, using existing longitudinal data and item-level analysis. A revised version of the CAMCOG-DS will be validated in our longitudinal run-in cohort.
Finally, as definitions of mild cognitive impairment are difficult to apply in DS due to premorbid memory deficits and the potential for impoverished subjective reporting, we will use data on symptom development (obtained using the CAMDEX-DS informant questionnaire) from our existing longitudinal studies to identify symptoms that can be reliably used to define stages during the prolonged preclinical period of AD in DS, based on existing models of disease such as those of the International Working Group [1], or the staging system proposed in the draft FDA guideline [41], which will be validated alongside cognitive tests in a run-in cohort.
1.8. Patient stratification for clinical trials
Amyloid markers using either CSF biomarkers or amyloid PET scans are central in patient stratification. Individuals with DS who are amyloid “negative” will be candidates for primary prevention trials, in which the primary objective is likely to be an attenuation in the accumulation of amyloid. Those with “positive” results, but without AD-related cognitive impairment, would qualify for secondary prevention using disease-modifying drugs, in which on top of Aβ removal, other neurodegenerative markers or sensitive cognitive measures could constitute the primary outcomes. Neuroimaging is becoming an increasingly useful tool in understanding the pathogenesis of dementia development in relation to clinical symptoms and several Horizon21 groups are undertaking ongoing neuroimaging studies to identify the parameters required for patient stratification.
So far, studies in Europe and the United States have demonstrated that individuals with DS have amyloid-positive PET scans by the age of 50 [14], [42], with a conversion to amyloid positivity happening during a relatively narrow age range after 40, thus an opportunity for primary prevention, as defined using amyloid PET. Although amyloid load, as measured by PET, may not correlate well with cognitive function in adults who have DS in cross-sectional studies, longitudinal studies suggest a significant relationship [14].
CSF biomarkers, which can be used to contribute to AD diagnoses in the general population and allow for biomarker stratification (both for amyloid and tau pathologies, as well as neurodegeneration), are another option, but have been less studied in DS [43]. The safety of lumbar puncture in this population has been proven by members of our consortium [44].
1.9. Surrogate markers of efficacy and safety
Neuroimaging and blood biomarkers could be used as surrogate markers of efficacy. Blood draws are less costly and more acceptable than brain scans, and blood biomarkers would be ideal as method of stratification, but identification of such markers has become the holy grail of AD research. Recently, several studies have demonstrated that blood concentration of neurofilament light (NfL)—a component of axonal scaffolding proteins that is released after axonal damage—is increased in several neurological disorders associated with neurodegeneration. This includes both ADAD and sAD, where increased NfL has been associated with mild cognitive impairment in addition to cognitive, biochemical, and imaging hallmarks of AD [45], [46].
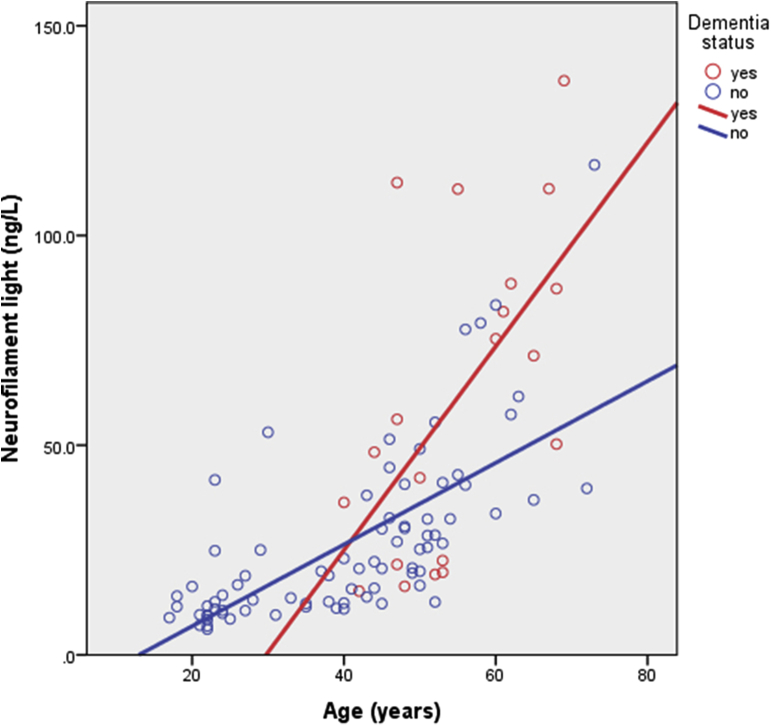
Members of the Horizon21 consortium have also identified NfL as a feasible blood biomarker of neurodegeneration associated with AD in DS [29], [47] (Fig. 4), which may offer an exciting opportunity for tracking response to treatment. However, it remains to be established how NfL relates to other biomarkers of disease progression in DS, including amyloid PET, MRI neuroimaging, and the development of clinical symptoms. Other biomarkers that have previously been associated with AD in DS include plasma amyloid and tau [48], plasma exosomes [28], and markers of oxidative stress and inflammation [26], [49], [50].
Fig. 4.
Neurofilament light concentration (ng/L) by age and dementia status of adults with DS. Graph based on Strydom et al., 2018 [29]. Abbreviation: DS, Down syndrome.
MRI markers of neurodegeneration have been associated with Aβ+ status in DS [51], and thus sequential MRI can be used to establish the sequence of brain changes concurrent with, or after conversion to Aβ+ on PET scans. The consortium includes several groups that are currently obtaining longitudinal MRI data for this purpose. In subjects with negative amyloid PET scans, changes in the rate of change of amyloid accumulation could be used as a surrogate measure for amyloid targeting drugs.
Finally, anti-amyloid therapies are associated with adverse events, including amyloid-related imaging abnormalities. These phenomena have also been observed in controls in trials for sAD. We have shown that MRI markers of cerebral amyloid angiopathy (including white matter hyperintensities) are more frequently found in DS than in sporadic AD [44] but their clinical relevance still needs to be established, and we therefore have ongoing longitudinal MRI studies to establish this.
2. Conclusions
Trisomy of chromosome 21, with an extra copy of the APP gene, has been critically important to understand the pathophysiology associated with Aβ production and deposition, but despite an ultra-high risk for developing AD, individuals with DS have yet to be included in clinical trials of treatments targeting the amyloid mechanism. DS is much more common than all of the autosomal-dominant causes of AD combined, and it is thus surprising that such trials are not considered in this population. The ethical imperative for treatment trials that aim to reduce the disease burden in DS remains unfulfilled.
Although there has, until recently, been a lack of knowledge about in vivo markers of progression of disease, several studies have demonstrated the similarity in amyloid pathology and neurodegeneration between DS and most types of ADAD using neuropathological, amyloid PET, and MRI neuroimaging studies. Furthermore, there is a growing literature on blood biomarkers associated with the development of AD in DS, and pilot data from Horizon21 study groups on CSF biomarkers suggest similar associations to age and AD status as those observed in studies in ADAD populations. Although some questions about tolerability of procedures remain, we have demonstrated that many DS individuals, particularly younger individuals in the preclinical stages of AD, can tolerate procedures such as lumbar punctures, PET, and MRI imaging and can participate in detailed cognitive phenotyping studies. We have also made progress in identifying potential cognitive outcomes that are sensitive to early decline, and development of reliable cognitive test measures is well underway. A concerted, collaborative approach is now required to overcome remaining issues, provide essential data for trials in DS, and to establish the infrastructure and networks required to run large-scale trials. This will require collaboration with clinical experts, industry, caregivers, and participants to design appropriate trial procedures, refine outcome measures, and deliver efficient trial designs.
The Horizon21 project has identified three main areas to focus on: to create a trial-ready cohort across countries with run-in data to enable efficient recruitment to trials, which will also help to establish the necessary infrastructure and expertise across sites; to develop and validate clinical trial outcome measures; and to improve on predementia disease models through the accumulation of clinical and biomarker data that is necessary to enable trials of treatment to prevent dementia in DS.
The consortium will engage with key stakeholders, including patients with DS and cognitive decline, their caregivers, and other experts and industry partners, to develop protocols for AD Prevention Trials. These protocols will be conceptualized to deliver evidence of the impact of an intervention on a biomarker and/or early cognitive decline as intermediate phenotype(s) to provide initial evidence of clinical benefit before being tested in confirmatory trials.
Research in Context.
-
1.
Systematic review and Interpretation: Intervention during the early stages of the pathological process of Alzheimer's disease may hold the key to prevention of clinical progression, but this may be problematic in sporadic Alzheimer's disease as it is not yet possible to identify high-risk individuals. There has therefore been considerable interest in conducting prevention trials in individuals with genetic forms of Alzheimer's disease, where all individuals are at risk, such as those with autosomal dominant mutations. Individuals with Down syndrome also invariably develop Alzheimer's disease pathology, but until recently they have not been the focus of much research.
-
2.
Future directions: We summarize recent progress in clinical research on cognitive and biomarker changes associated with the development of dementia in Down syndrome to highlight the potential for undertaking primary and secondary prevention trials in this population.
Acknowledgments
The Horizon21 project has received funding from the Jérôme Lejeune Foundation. A.S. and J.H. are funded by the Wellcome Trust Strategic Award (grant number: 098330/Z/12/Z) conferred upon The London Down Syndrome (LonDownS ) Consortium. The authors also acknowledge the input of Dr Sarah Hamburg for editing.
References
- 1.Dubois B., Hampel H., Feldman H.H., Scheltens P., Aisen P., Andrieu S. Preclinical Alzheimer's disease: Definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12:292–323. doi: 10.1016/j.jalz.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ritchie K., Ropacki M., Albala B., Harrison J., Kaye J., Kramer J. Recommended cognitive outcomes in preclinical Alzheimer's disease: Consensus statement from the European Prevention of Alzheimer's Dementia project. Alzheimers Dement. 2017;13:186–195. doi: 10.1016/j.jalz.2016.07.154. [DOI] [PubMed] [Google Scholar]
- 3.Bateman R.J., Benzinger T.L., Berry S., Clifford D.B., Duggan C., Fagan A.M. The DIAN-TU Next Generation Alzheimer's prevention trial: Adaptive design and disease progression model. Alzheimers Dement. 2017;13:8–19. doi: 10.1016/j.jalz.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Medicines Agency. Guideline on the clinical investigation of medicines for the treatment of Alzheimer's disease 2018.
- 5.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Early Alzheimer's Disease: Developing Drugs for Treatment Guidance for Industry 2018.
- 6.Goldgaber D., Lerman M.I., McBride W.O., Saffiotti U., Gajdusek D.C. Isolation, characterization, and chromosomal localization of human brain cDNA clones coding for the precursor of the amyloid of brain in Alzheimer's disease, Down's syndrome and aging. J Neural Transm Suppl. 1987;24:23–28. [PubMed] [Google Scholar]
- 7.McNaughton D., Knight W., Guerreiro R., Ryan N., Lowe J., Poulter M. Duplication of amyloid precursor protein (APP), but not prion protein (PRNP) gene is a significant cause of early onset dementia in a large UK series. Neurobiol Aging. 2012;33:426.e13–426.e21. doi: 10.1016/j.neurobiolaging.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prasher V.P., Farrer M.J., Kessling A.M., Fisher E.M., West R.J., Barber P.C. Molecular mapping of Alzheimer-type dementia in Down's syndrome. Ann Neurol. 1998;43:380–383. doi: 10.1002/ana.410430316. [DOI] [PubMed] [Google Scholar]
- 9.Zis P., Strydom A. Clinical aspects and biomarkers of Alzheimer's disease in Down syndrome. Free Radic Biol Med. 2018;114:3–9. doi: 10.1016/j.freeradbiomed.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Struwe F. Histopathologische Untersuchungen fiber Entstebung und Wesen der senilen Plaques. Z Ges Neurol Psychiatry. 1929 [Google Scholar]
- 11.Mann D.M. Alzheimer's disease and Down's syndrome. Histopathology. 1988;13:125–137. doi: 10.1111/j.1365-2559.1988.tb02018.x. [DOI] [PubMed] [Google Scholar]
- 12.Rafii M.S., Wishnek H., Brewer J.B., Donohue M.C., Ness S., Mobley W.C. The down syndrome biomarker initiative (DSBI) pilot: proof of concept for deep phenotyping of Alzheimer's disease biomarkers in down syndrome. Front Behav Neurosci. 2015;9:239. doi: 10.3389/fnbeh.2015.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rafii M.S., Lukic A.S., Andrews R.D., Brewer J., Rissman R.A., Strother S.C. PET Imaging of Tau Pathology and Relationship to Amyloid, Longitudinal MRI, and Cognitive Change in Down Syndrome: Results from the Down Syndrome Biomarker Initiative (DSBI) J Alzheimers Dis. 2017;60:439–450. doi: 10.3233/JAD-170390. [DOI] [PubMed] [Google Scholar]
- 14.Hartley S.L., Handen B.L., Devenny D., Mihaila I., Hardison R., Lao P.J. Cognitive decline and brain amyloid-β accumulation across 3 years in adults with Down syndrome. Neurobiol Aging. 2017;58:68–76. doi: 10.1016/j.neurobiolaging.2017.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardy J. The discovery of Alzheimer-causing mutations in the APP gene and the formulation of the “amyloid cascade hypothesis”. FEBS J. 2017;284:1040–1044. doi: 10.1111/febs.14004. [DOI] [PubMed] [Google Scholar]
- 16.Jonsson T., Atwal J.K., Steinberg S., Snaedal J., Jonsson P.V., Bjornsson S. A mutation in APP protects against Alzheimer's disease and age-related cognitive decline. Nature. 2012;488:96–99. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- 17.de Graaf G., Buckley F., Skotko B.G. Estimation of the number of people with Down syndrome in the United States. Genet Med. 2017;19:439–447. doi: 10.1038/gim.2016.127. [DOI] [PubMed] [Google Scholar]
- 18.McCarron M., McCallion P., Reilly E., Mulryan N. A prospective 14-year longitudinal follow-up of dementia in persons with Down syndrome. J Intellect Disabil Res. 2014;58:61–70. doi: 10.1111/jir.12074. [DOI] [PubMed] [Google Scholar]
- 19.Sinai A., Mokrysz C., Bernal J., Bohnen I., Bonell S., Courtenay K. Predictors of Age of Diagnosis and Survival of Alzheimer's Disease in Down Syndrome. J Alzheimers Dis. 2018;61:717–728. doi: 10.3233/JAD-170624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCarron M., McCallion P., Reilly E., Dunne P., Carroll R., Mulryan N. A prospective 20-year longitudinal follow-up of dementia in persons with Down syndrome. J Intellect Disabil Res. 2017;61:843–852. doi: 10.1111/jir.12390. [DOI] [PubMed] [Google Scholar]
- 21.Cosgrave M.P., Tyrrell J., McCarron M., Gill M., Lawlor B.A. A five year follow-up study of dementia in persons with Down's syndrome: early symptoms and patterns of deterioration. Ir J Psychol Med. 2000;17:5–11. [Google Scholar]
- 22.Alexander M., Ding Y., Foskett N., Petri H., Wandel C., Khwaja O. Population prevalence of Down's syndrome in the United Kingdom. J Intellect Disabil Res. 2016;60:874–878. doi: 10.1111/jir.12277. [DOI] [PubMed] [Google Scholar]
- 23.UN General Assembly, Convention on the Rights of Persons with Disabilities. Resolution adopted by the General Assembly, 24 January 2007, A/RES/61/106 2007.
- 24.Hanney M., Prasher V., Williams N., Jones E.L., Aarsland D., Corbett A. Memantine for dementia in adults older than 40 years with Down's syndrome (MEADOWS): a randomised, double-blind, placebo-controlled trial. Lancet. 2012;379:528–536. doi: 10.1016/S0140-6736(11)61676-0. [DOI] [PubMed] [Google Scholar]
- 25.Sano M., Aisen P.S., Andrews H.F., Tsai W.-Y., Lai F., Dalton A.J. Vitamin E in aging persons with Down syndrome: A randomized, placebo-controlled clinical trial. Neurology. 2016;86:2071–2076. doi: 10.1212/WNL.0000000000002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coppus A.M.W., Fekkes D., Verhoeven W.M.A., Evenhuis H.M., van Duijn C.M. Neopterin and the risk of dementia in persons with Down syndrome. Neurosci Lett. 2009;458:60–64. doi: 10.1016/j.neulet.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 27.Dekker A.D., Coppus A.M.W., Vermeiren Y., Aerts T., van Duijn C.M., Kremer B.P. Serum MHPG strongly predicts conversion to Alzheimer's disease in behaviorally characterized subjects with Down syndrome. J Alzheimers Dis. 2015;43:871–891. doi: 10.3233/JAD-140783. [DOI] [PubMed] [Google Scholar]
- 28.Hamlett E.D., Goetzl E.J., Ledreux A., Vasilevko V., Boger H.A., LaRosa A. Neuronal exosomes reveal Alzheimer's disease biomarkers in Down syndrome. Alzheimers Dement. 2017;13:541–549. doi: 10.1016/j.jalz.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strydom A., Heslegrave A., Startin C.M., Mok K.Y., Hardy J., Groet J. Neurofilament light as a blood biomarker for neurodegeneration in Down syndrome. Alzheimers Res Ther. 2018;10:39. doi: 10.1186/s13195-018-0367-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zis P., McHugh P., McQuillin A., Praticò D., Dickinson M., Shende S. Memory decline in Down syndrome and its relationship to iPF2alpha, a urinary marker of oxidative stress. PLoS One. 2014;9:e97709. doi: 10.1371/journal.pone.0097709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coppus A.M.W., Evenhuis H.M., Verberne G.-J., Visser F.E., Arias-Vasquez A., Sayed-Tabatabaei F.A. The impact of apolipoprotein E on dementia in persons with Down's syndrome. Neurobiol Aging. 2008;29:828–835. doi: 10.1016/j.neurobiolaging.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 32.Jones E.L., Mok K., Hanney M., Harold D., Sims R., Williams J. Evidence that PICALM affects age at onset of Alzheimer's dementia in Down syndrome. Neurobiol Aging. 2013;34:2441.e1–2441.e5. doi: 10.1016/j.neurobiolaging.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Startin C.M., Hamburg S., Hithersay R., Davies A., Rodger E., Aggarwal N. The LonDownS adult cognitive assessment to study cognitive abilities and decline in Down syndrome. Wellcome Open Res. 2016;1:11. doi: 10.12688/wellcomeopenres.9961.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sinai A., Hassiotis A., Rantell K., Strydom A. Assessing Specific Cognitive Deficits Associated with Dementia in Older Adults with Down Syndrome: Use and Validity of the Arizona Cognitive Test Battery (ACTB) PLoS One. 2016;11:e0153917. doi: 10.1371/journal.pone.0153917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krinsky-McHale S.J., Devenny D.A., Silverman W.P. Changes in explicit memory associated with early dementia in adults with Down's syndrome. J Intellect Disabil Res. 2002;46:198–208. doi: 10.1046/j.1365-2788.2002.00365.x. [DOI] [PubMed] [Google Scholar]
- 36.Dekker A.D., Strydom A., Coppus A.M.W., Nizetic D., Vermeiren Y., Naudé P.J.W. Behavioural and psychological symptoms of dementia in Down syndrome: Early indicators of clinical Alzheimer's disease? Cortex. 2015;73:36–61. doi: 10.1016/j.cortex.2015.07.032. [DOI] [PubMed] [Google Scholar]
- 37.Tibshirani R. Regression shrinkage and selection via the lasso: a retrospective. J R Stat Soc Ser B Stat Methodol. 2011 [Google Scholar]
- 38.Oxtoby N.P., Young A.L., Cash D.M., Benzinger T.L.S., Fagan A.M., Morris J.C. Data-driven models of dominantly-inherited Alzheimer's disease progression. Brain. 2018;141:1529–1544. doi: 10.1093/brain/awy050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Firth NC, Startin CM, Hithersay R, Hamburg S, Wijeratne PA, Mok KY, et al. Sequence of cognitive changes associated with development of Alzheimer's disease in Down syndrome - data driven analysis 2018. 10.1101/263095. [DOI] [PMC free article] [PubMed]
- 40.Hon J., Huppert F.A., Holland A.J., Watson P. Neuropsychological assessment of older adults with Down's syndrome: an epidemiological study using the Cambridge Cognitive Examination (CAMCOG) Br J Clin Psychol. 1999;38:155–165. doi: 10.1348/014466599162719. [DOI] [PubMed] [Google Scholar]
- 41.Jack C.R., Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B. NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Annus T., Wilson L.R., Acosta-Cabronero J., Cardenas-Blanco A., Hong Y.T., Fryer T.D. The Down syndrome brain in the presence and absence of fibrillar β-amyloidosis. Neurobiol Aging. 2017;53:11–19. doi: 10.1016/j.neurobiolaging.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dekker A.D., Fortea J., Blesa R., De Deyn P.P. Cerebrospinal fluid biomarkers for Alzheimer's disease in Down syndrome. Alzheimers Dement (Amst) 2017;8:1–10. doi: 10.1016/j.dadm.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carmona-Iragui M., Balasa M., Benejam B., Alcolea D., Fernández S., Videla L. Cerebral amyloid angiopathy in Down syndrome and sporadic and autosomal-dominant Alzheimer's disease. Alzheimers Dement. 2017;13:1251–1260. doi: 10.1016/j.jalz.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mattsson N., Andreasson U., Zetterberg H., Blennow K., Alzheimer's Disease Neuroimaging Initiative Association of Plasma Neurofilament Light With Neurodegeneration in Patients With Alzheimer Disease. JAMA Neurol. 2017;74:557–566. doi: 10.1001/jamaneurol.2016.6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weston P.S.J., Poole T., Ryan N.S., Nair A., Liang Y., Macpherson K. Serum neurofilament light in familial Alzheimer disease: A marker of early neurodegeneration. Neurology. 2017;89:2167–2175. doi: 10.1212/WNL.0000000000004667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fortea J., Carmona-Iragui M., Benejam B., Fernández S., Videla L., Barroeta I. Plasma and CSF biomarkers for the diagnosis of Alzheimer's disease in adults with Down syndrome: a cross-sectional study. Lancet Neurol. 2018;17:860–869. doi: 10.1016/S1474-4422(18)30285-0. [DOI] [PubMed] [Google Scholar]
- 48.Tatebe H., Kasai T., Ohmichi T., Kishi Y., Kakeya T., Waragai M. Quantification of plasma phosphorylated tau to use as a biomarker for brain Alzheimer pathology: pilot case-control studies including patients with Alzheimer's disease and down syndrome. Mol Neurodegener. 2017;12:63. doi: 10.1186/s13024-017-0206-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zis P., Dickinson M., Shende S., Walker Z., Strydom A. Oxidative stress and memory decline in adults with Down syndrome: longitudinal study. J Alzheimers Dis. 2012;31:277–283. doi: 10.3233/JAD-2012-120073. [DOI] [PubMed] [Google Scholar]
- 50.Naudé P.J.W., Dekker A.D., Coppus A.M.W., Vermeiren Y., Eisel U.L.M., van Duijn C.M. Serum NGAL is Associated with Distinct Plasma Amyloid-β Peptides According to the Clinical Diagnosis of Dementia in Down Syndrome. J Alzheimers Dis. 2015;45:733–743. doi: 10.3233/JAD-142514. [DOI] [PubMed] [Google Scholar]
- 51.Lao P.J., Handen B.L., Betthauser T.J., Mihaila I., Hartley S.L., Cohen A.D. Longitudinal changes in amyloid positron emission tomography and volumetric magnetic resonance imaging in the nondemented Down syndrome population. Alzheimers Dement (Amst) 2017;9:1–9. doi: 10.1016/j.dadm.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]