Abstract
Background
This study evaluated the proportion of patients with atherosclerotic cardiovascular disease (ASCVD) and probable heterozygous familial hypercholesterolemia (HeFH) achieving ≥50% reduction in low-density lipoprotein cholesterol (LDL-C) or reaching the LDL-C ≤70 mg/dL threshold, after initiating or modifying statin, and/or ezetimibe therapy.
Materials and methods
Adult ASCVD patients with baseline LDL-C >70 mg/dL (index) and a subset of patients with probable HeFH (proxied by LDL-C ≥190 mg/dL) were identified between January 1, 2012, and August 31, 2014, from the IQVIA electronic medical record database. Patients were followed for 12 months pre-index to examine baseline lipid-lowering therapy (LLT) use, and 12 months post index to evaluate treatment modifications and post-treatment LDL-C levels, stratified by type of treatment received and LDL-C levels at baseline.
Results
Of the sample of ASCVD patients who initiated treatment post-index (n=111,147), only 7.6% patients achieved a ≥50% reduction from baseline LDL-C and 19.1% of patients reached the LDL-C ≤70 mg/dL threshold. Among treated ASCVD patients who modified therapy post-index (n=75,523), 5.6% achieved a ≥50% reduction in LDL-C, and proportion of patients achieving LDL-C ≤70 mg/dL ranged from 6.9% to 26.7%, depending on the baseline LDL-C levels. Approximately 50% of the untreated probable HeFH patients (n=3,064) initiated LLT; however, the mean (SD) post-treatment LDL-C remained high (136.2 [47.8] mg/dL), with only 4.4% reaching LDL-C ≤70 mg/dL. Of the treated probable HeFH patients (n=1,073), 41.5% modified treatment; 22.1% achieved a ≥50% reduction in LDL-C and 1.1% reached LDL-C ≤70 mg/dL.
Conclusion
This study found that most patients had suboptimal LDL-C responses after initiating or modifying standard LLT (statin and/or ezetimibe). More frequent and aggressive lipid management, including increasing statin intensity and alternative therapies, may be needed in patients with ASCVD and probable HeFH to reduce their cardiovascular risk.
Keywords: hyperlipidemia, ASCVD, HeFH, LDL-C, statin, lipid-lowering therapy
Introduction
Cardiovascular disease (CVD) is the leading cause of death in the US, with more than 600,000 related deaths annually.1 Atherosclerotic cardiovascular disease (ASCVD) is a form of heart disease characterized by plaque build-up in the arteries that can lead to stroke, myocardial infarction (MI), and other life-threatening vascular events.2,3 Heterozygous familial hypercholesterolemia (HeFH) is an inherited lipid disorder associated with premature CVD.4,5 Elevated low-density lipoprotein cholesterol (LDL-C) is associated with increased cardiovascular (CV) risk and adverse clinical outcomes in both ASCVD and HeFH patients.
The American College of Cardiology and American Heart Association (ACC/AHA) 2013 guidelines suggested a 50% reduction in LDL-C from baseline, while others, including prior version of the ACC/AHA guidelines, have suggested an LDL-C ≤70 mg/dL as a treatment target6 for ASCVD and HeFH patients. The 2013 ACC/AHA guidelines also recommended use of high-intensity statins for all patients with ASCVD or HeFH. Prior to the release of the 2013 guidelines, a number of studies reported that many patients failed to reach recommended LDL-C thresholds.7–11 Prior to the widespread availability of generic statins, 1 in 4 high-risk patients who were eligible for lipid-lowering therapy (LLT) remained untreated.7 It is not known if this has changed given the new guidelines, availability of generic statins, and expansion of insurance coverage with the Affordable Care Act.
The 2013 ACC/AHA cholesterol-lowering guidelines emphasize that treatment intensity should match the risk for adverse ASCVD events.6,10 This differs from previous guidelines that favored achieving LDL-C goal levels based on the estimated extent of CV risk.10,12 Statin agents are recommended as first-line therapy to manage ASCVD and HeFH; however, treatment with the cholesterol absorption inhibitor, ezetimibe, and/or a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor (such as evolocumab or alirocumab) may also be warranted in patients who cannot achieve adequate LDL-C control on a statin alone.4,13–15 Evidence supporting statin therapy augmentation finds that adherence to statin therapy is suboptimal and that discontinuation rates are higher among patients prescribed high-intensity statin doses (eg, those at high CV risk and those with suboptimal response to a moderate-intensity dose).11,16–18 In addition, despite receiving statin therapy, patients at high CV risk are found to have an elevated 1-year risk of ASCVD-related re-hospitalization.11 To this point, the 2016 ACC/AHA expert consensus decision pathways describe the role of non-statin therapies in lowering LDL-C.19
The objective of this study was to evaluate the proportion of patients with ASCVD and subset of patients with ASCVD and probable HeFH (ASCVD + HeFH, proxied by a baseline LDL-C >190 mg/dL) with a baseline LDL-C >70 mg/dL who reached an LDL-C threshold of ≤70 mg/dL and/or ≥50% reduction from baseline LDL-C after treatment initiation or modification.
Materials and methods
Data source
The patient sample was drawn from the IQVIA US ambulatory electronic medical record (EMR) database containing approximately 47 million patient records from an “opt-in” provider research network. This aggregated database is comprised of records collected across 40,000 physicians in large practices and physician networks. Approximately 40% of contributing physicians are primary care practitioners and the remainder are specialists. IQVIA ambulatory EMR database has been used in previous retrospective observational studies.20–22 This study used data from January 1, 2011, to August 31, 2015; information on demographics, patient care episodes, risk factors, laboratory tests, diagnoses, procedures, and written prescriptions was included.
Patient selection
The study population was patients with at least a valid LDL-C lab value (ranging from 20 to 500 mg/dL) between January 1, 2012, and August 31, 2014. The first valid LDL-C value was considered a patient’s baseline LDL-C and defined as their index date. Patients also had to have at least one recorded visit within the 12 months prior to their index date; evidence of ASCVD in the 12 months prior or on their index date; and at least 1 visit more than 12 months after their index date to ensure they were still active in the EMR. Patients with data quality issues, such as missing age, gender, or prescription data, were excluded. Eligible patients were followed for 12 months post-index.
The definition of ASCVD was based on the ACC/AHA definition and included MI, stroke, transient ischemic attack, peripheral artery disease, and stable or unstable angina. Patients with an ICD-9 diagnosis for any of these conditions prior to their first LDL-C assessment were categorized as having ASCVD. A subset of ASCVD patients were considered probable HeFH if the baseline LDL-C value was ≥190 mg/dL per the ACC/AHA 2016 expert consensus decision pathway.19
Cohort definition
Prior exposure to treatment was defined as a prescription order for a statin and/or ezetimibe. Patients were first categorized into one of four mutually exclusive baseline treatment cohorts based on their most recent prescriptions for statins or ezetimibe on or within 12 months before the index date: 1) untreated (no prescription order for a statin or ezetimibe); 2) statin only (≥1 prescription for statin and no prescription for ezetimibe); 3) ezetimibe only (≥1 prescription for ezetimibe and no prescriptions for a statin); and 4) statin + ezetimibe (≥1 prescription order for both a statin and ezetimibe, or ≥1 prescription order for a statin/ezetimibe combination product). This study assumed that if orders for a statin and ezetimibe were both observed within 12 months pre-index or at index, they were used concurrently, and the patient was classified into the “statin + ezetimibe” cohort. Use of other lipid-lowering agents including niacin and bile acid sequestrates was not evaluated.
Patients’ prior use of statins were further categorized and evaluated by statin dose intensity (low, moderate, high – see Table S1). The algorithm used to classify statin intensity was based on the 2013 ACC/AHA cholesterol guidelines.6
Measures
Patients were followed for 12 months post-index LDL-C assessment to evaluate their LDL-C outcomes, separately for those who initiated treatment and those who modified their prior LLT. Treatment initiation was use of any LLT in patients with no prior LLT treatment. Treatment modification was defined as switching statins, changing statin dose intensity, or augmenting current statin therapy with ezetimibe. “Switching” was defined as changing to a different statin while maintaining the same statin intensity level; “changing statin intensity” was defined as moving from one statin to another with a different intensity or changing the dose of their current statin where the new dose change resulted in a change in statin intensity; and “augmenting” was defined as adding ezetimibe to the statin regimen.
Demographic characteristics were measured on the index date including age, age group (<18, 18–44, 45–54, 55–64, and ≥65 years), gender, and geographic region (Northeast, South, Midwest, and West). Clinical characteristics included Charlson Comorbidity Index (CCI) score (Dartmouth-Manitoba adaptation), comorbidities (diabetes, hypertension, and dyslipidemia identified by using International Classification of Diseases, Ninth and Tenth Revision, Clinical Modification [ICD-9-CM and ICD-10 CM] diagnosis codes), baseline LDL-C, evidence of use of antidiabetic, antiarrhythmic, and antihypertensive therapy, body mass index, and specialty of treating physician (prescriber for baseline treatment or LDL-C lab test). Except for LDL-C, which was measured on the index date, clinical characteristics were assessed during the 12-month pre-index period, including the index date.
The primary outcome of interest was reaching LDL-C goal, defined as LDL-C of ≤70 mg/dL or a ≥50% reduction in LDL-C from baseline post-treatment initiation or modification. These measures were evaluated separately in patients who newly initiated statin and/or ezetimibe after index and in those who with prior statin or ezetimibe experienced a treatment modification during the post-index period. The first LDL-C value recorded 30 days to 12 months after treatment initiation or modification was used to determine attainment of LDL-C outcomes while allowing time for the new regimen to take effect. For all analyses, patients were further categorized into three groups by baseline LDL-C level: 71–100 mg/dL, 101–130 mg/dL, and >130 mg/dL.
All analyses were conducted using SAS v9.2 (SAS Institute Inc., Cary, NC, USA) and no statistical comparisons were performed for this descriptive study. Outcomes were stratified by treatment cohort or index LDL-C (where applicable), and conducted separately for ASCVD and ASCVD + probable HeFH groups. The IQVIA ambulatory EMR database is a proprietary and HIPAA-compliant database. All patient data are de-identified, and no direct subject contact or primary collection of individual human subject data occurred. In addition, study results were in tabular form and aggregate analyses omitted subject identification; therefore, informed consent, ethics committee, or IRB approval were not required.
Results
Study subgroups
A total of 4,431,936 patients with a valid LDL-C value during the study index period (January 1, 2012, to August 31, 2014) and a diagnosis of ASCVD in the pre-index period were initially identified in the database. Of these, 260,607 (5.9%) remained after applying other inclusion and exclusion criteria. For this study, the 71.6% (n=186,670) of patients with an uncontrolled baseline LDL-C >70 mg/dL (Table 1) were selected for analysis. Of those, 50.5% had an LDL-C 71–100 mg/dL, 29.2% had an LDL-C 101–130 mg/dL; 20.3% had an LDL-C >130 mg/dL; and 2.2% had an LDL-C ≥190 mg/dL (probable HeFH patients; Table 2).
Table 1.
All ASCVD sample: demographic and clinical characteristics
| Demographic and clinical characteristics | All ASCVD, N=186,670 | |||
|---|---|---|---|---|
| All ASCVD, index LDL-C value >70 mg/dL N=186,670 (100%) | Index LDL-C value 71–100 mg/dL n=94,323 (50.5%) | Index LDL-C value 101–130 mg/dL n=54,424 (29.2%) | Index LDL-C value >130 mg/dL n=37,923 (20.3%) | |
| Age in years, mean (SD) | 68.29 (11.45) | 69.66 (10.79) | 67.51 (11.85) | 66.02 (12.00) |
| Age groups, % | ||||
| <18 | 0.0 | 0.1 | 0.1 | 0.0 |
| 18–44 | 0.8 | 2.4 | 4.1 | 4.8 |
| 45–54 | 2.6 | 7.3 | 10.7 | 13.3 |
| 55–64 | 9.5 | 18.7 | 21.4 | 23.9 |
| >65 | 20.5 | 71.7 | 63.8 | 58.0 |
| Gender, % | ||||
| Male | 50.3 | 55.4 | 48.0 | 40.9 |
| Female | 49.7 | 44.6 | 52.1 | 59.1 |
| Geographic region, % | ||||
| Northeast | 26.9 | 27.4 | 26.4 | 26.2 |
| South | 36.8 | 35.8 | 37.4 | 38.5 |
| Midwest | 20.6 | 20.7 | 20.5 | 20.7 |
| West | 15.7 | 16.1 | 15.7 | 14.5 |
| CCI score, % | ||||
| 0 | 99.5 | 99.5 | 99.5 | 99.4 |
| >0 | 0.5 | 0.5 | 0.5 | 0.6 |
| Mean (SD) | 0.01 (0.08) | 0.01 (0.07) | 0.01 (0.07) | 0.01 (0.08) |
| Comorbidities, % | ||||
| Diabetes | 27.3 | 30.1 | 25.0 | 23.8 |
| Hypertension | 65.3 | 68.0 | 63.8 | 60.8 |
| Dyslipidemia | 68.1 | 71.1 | 63.9 | 66.5 |
| Coronary disease | 39.1 | 41.6 | 36.6 | 36.7 |
| Cerebrovascular disease | 17.4 | 16.1 | 18.5 | 19.1 |
| Peripheral vascular disease | 54.1 | 54.0 | 54.6 | 53.7 |
| Index LDL-C, mg/dL | ||||
| Mean (SD) | 108.12 (31.60) | 84.73 (8.52) | 113.81 (8.52) | 158.12 (26.89) |
| Number of LDL-C tests, % | ||||
| 1 (index test) | 43.5 | 43.6 | 44.6 | 41.6 |
| 2 | 31.2 | 31.7 | 30.5 | 31.1 |
| 3 | 14.6 | 14.5 | 14.4 | 15.3 |
| 4 | 6.5 | 6.4 | 6.4 | 7.1 |
| >4 | 4.1 | 3.8 | 4.2 | 5.0 |
| Medications, % | ||||
| Antidiabetic | 17.2 | 19.0 | 15.8 | 14.9 |
| Antiarrhythmic | 0.5 | 0.5 | 0.5 | 0.4 |
| Antihypertensive | 59.9 | 62.6 | 58.6 | 55.4 |
| Index BMI | ||||
| Patients with BMI, % | 91.0 | 90.9 | 91.1 | 91.0 |
| Mean (SD) | 29.46 (6.48) | 29.44 (6.41) | 29.43 (6.61) | 29.53 (6.47) |
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; CCI, Charlson Comorbidity Index; LDL-C, low-density lipoprotein cholesterol.
Table 2.
Probable HeFH sample: demographic and clinical characteristics
| Demographic and clinical characteristics | Probable HeFH patients |
|---|---|
| Index LDL-C value >190 mg/dL n=4,137 (2.2%) | |
| Age in years, mean (SD) | 65.32 (11.57) |
| Age groups, % | |
| <18 | 0.0 |
| 18–44 | 3.9 |
| 45–54 | 14.6 |
| 55–64 | 26.9 |
| >65 | 54.7 |
| Gender, % | |
| Male | 33.9 |
| Female | 66.1 |
| Geographic region, % | |
| Northeast | 25.7 |
| South | 40.4 |
| Midwest | 21.3 |
| West | 12.6 |
| CCI score, % | |
| 0 | 99.2 |
| >0 | 0.8 |
| Mean (SD) | 0.01 (0.09) |
| Comorbidities, % | |
| Diabetes | 26.0 |
| Hypertension | 62.7 |
| Dyslipidemia | 72.0 |
| Coronary disease | 40.2 |
| Cerebrovascular disease | 18.2 |
| Peripheral vascular disease | 52.5 |
| Index LDL-C, mg/dL | |
| Mean (SD) | 215.97 (30.92) |
| Number of LDL-C tests, % | |
| 1 (index test) | 38.0 |
| 2 | 31.3 |
| 3 | 16.7 |
| 4 | 8.1 |
| >4 | 5.9 |
| Medications, % | |
| Antidiabetic | 16.3 |
| Antiarrhythmic | 0.3 |
| Antihypertensive | 56.2 |
| Index BMI | |
| Patients with BMI, % | 90.8 |
| Mean (SD) | 29.63 (6.49) |
Abbreviations: BMI, body mass index; CCI, Charlson Comorbidity Index; HeFH, heterozygous familial hypercholesterolemia; LDL-C, low-density lipoprotein cholesterol.
Sample characteristics
Among the 186,670 ASCVD patients with index LDL-C values >70 mg/dL, the proportion of males ranged from 40.9% in the LDL-C >130 mg/dL group to 55.4% in the LDL-C 71–100 mg/dL group (Table 1). Mean (SD) ages among these subgroups decreased from 69.7 (10.8) years for the LDL-C 71–100 mg/dL group to 67.5 (11.9) years for the LDL-C 101–130 mg/dL group and 66.0 (12.0) years for the LDL-C >130 mg/dL group. The largest group of patients resided in the US southern region (36.8%). Aside from underlying ASCVD, the overall study population had few serious comorbidities with over 99% patients in all LDL-C subgroups having a CCI=0. However, a high proportion of patients in all LDL-C subgroups had comorbid hypertension (65.3%), and most patients used antihypertensives, ranging from 62.6% of those in the 71–100 mg/dL group to 55.4% of those in the >130 mg/dL group. Use of antidiabetic medication was observed in 27.3% of the study sample. Very few patients used antiarrhythmics (0.4%–0.5%; Table 1).
The ASCVD + probable HeFH subgroup had a mean (SD) age of 65.3 (11.6) years, and 66.1% of them were female (Table 2). The comorbidity profile of this subgroup suggested few serious comorbidities (mean [SD] CCI: 0.01 [0.09]; 99% with CCI=0); however, a high proportion of these patients had hypertension (62.7%). Most of these patients used antihypertensives (56.2%) but use of antidiabetics (16.3%) was lower. Few patients were on antiarrhythmics (0.3%). The mean (SD) index LDL-C for this subgroup was 215.97 (30.92; Table 2).
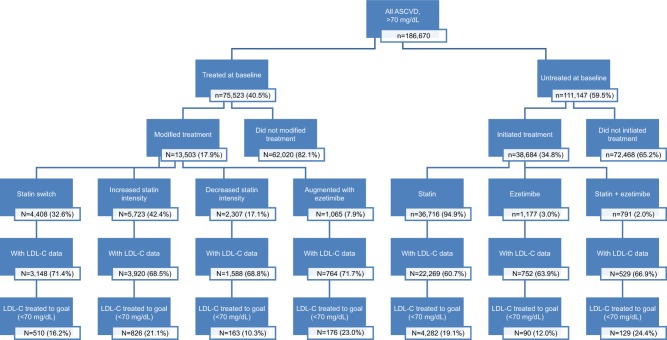
In terms of prescriptions for LLT, of the final study sample (n=186,670; LDL-C >70 mg/dL at baseline), only 40.5% (n=75,523) had prior LLT treatment at baseline (Figure 1); the majority (92.1%) received statins only; 2.4% were receiving ezetimibe only; and 5.5% were receiving statin + ezetimibe. Prior LLT treatment was also not common in patients with HeFH; of the 4,137 HeFH sample, only 1,073 (25.1%) were previously treated (Table 5).
Figure 1.
Summary of post-index treatment and goal achievement, by baseline treated vs not treated.
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; LDL-C, low-density lipoprotein cholesterol.
Table 5.
Goal achievement in probable HeFH patients who initiated or modified treatment post-index
| Probable HeFH patients (index LDL-C value ≥190 mg/dL) (untreated at index) | n=3,064 (2.8%) | Probable HeFH patients (index LDL-C value ≥190 mg/dL) (treated at index) | n=1,073 (1.4%) |
|---|---|---|---|
| Pts initiating LLT therapy, n (%) | 1,540 (50.3) | Pts modifying therapy, n (%) | 445 (41.5) |
| Pts with post-therapy LDL-C, n (%) | 896 (58.2) | Pts with post-therapy LDL-C, n (%) | 281 (63.2) |
| Pts with ≥50% LDL-C reduction, % (CI) | 31.1 (28.1–34.2) | Pts with ≥50% LDL-C reduction, % | 22.1 (17.2–26.9) |
| Pts with LDL-C ≤70 mg/dL, % (CI) | 4.4 (3.0–5.7) | Pts with LDL-C ≤70 mg/dL, % | 1.1 (-0.1–2.3) |
| LDL-C, mean (SD) | 136.21 (47.75) | LDL-C, mean (SD) | 151.87 (54.37) |
| Pts initiating statin only, n (%) | 1,410 (91.6) | Pts switching statin, n (%) | 153 (34.4) |
| Pts with post-therapy LDL-C, n (%) | 815 (57.8) | Pts with post-therapy LDL-C, n (%) | 81 (52.9) |
| Pts with ≥50% LDL-C reduction, % (CI) | 32.0 (28.8–35.2) | Pts with ≥50% LDL-C reduction, % | 22.2 (13.0–31.5) |
| Pts with LDL-C ≤70 mg/dL, % (CI) | 4.8 (3.3–6.3) | Pts with LDL-C ≤70 mg/dL, % | 0.0 |
| LDL-C, mean (SD) | 135.12 (48.04) | LDL-C, Mean (SD) | 147.40 (49.25) |
| Pts initiating ezetimibe only, n (%) | 90 (5.8) | Pts increasing statin intensity, n (%) | 170 (38.2) |
| Pts with post-therapy LDL-C, n (%) | 58 (64.4) | Pts with post-therapy LDL-C, n (%) | 109 (64.1) |
| Pts with ≥50% LDL-C reduction, % (CI) | 10.3 (2.3–18.4) | Pts with ≥50% LDL-C reduction, % | 26.6 (18.2–35.0) |
| Pts with LDL-C ≤70 mg/dL, % (CI) | 0.0 | Pts with LDL-C ≤70 mg/dL, % | 1.8 (−0.7–4.4) |
| LDL-C, mean (SD) | 110.93 (32.07) | LDL-C, mean (SD) | 152.55 (57.84) |
| Pts initiating statin + ezetimibe, n (%) | 40 (2.6) | Pts decreasing statin intensity, n (%) | 81 (18.2) |
| Pts with post-therapy LDL-C, n (%) | 23 (57.50) | Pts with post-therapy LDL-C, n (%) | 61 (75.3) |
| Pts with ≥50% LDL-C reduction, % (CI) | 52.2 (30.1–74.3) | Pts with ≥50% LDL-C reduction, % | 13.1 (4.4–21.8) |
| Pts with LDL-C ≤70 mg/dL, % (CI) | 0.0 | Pts with LDL-C ≤70 mg/dL, % | 0.0 |
| LDL-C, mean (SD) | 161.45 (38.89) | LDL-C, mean (SD) | 164.49 (57.70) |
Notes: Only patients with a recorded valid LDL-C measurement from 1 to 12 months after new treatment initiation are shown. The immediate test value post-treatment initiation was used (test values were limited to those observed between 1 and 12 months post-treatment initiation). Baseline statin intensity: among the 1,073 probable HeFH patients treated at baseline, 10.7% were on low statin intensity, 48.6% were on moderate statin intensity, 24.6% were on high statin intensity, and 16.0% were treated with other LLTs.
Abbreviations: HeFH, heterozygous familial hypercholesterolemia; LDL-C, low-density lipoprotein cholesterol; LLT, lipid-lowering therapy; Pts, patients.
LDL-C goal achievement in ASCVD patients
Untreated patients initiating post-index therapy
Of the overall study sample that were untreated at baseline (n=111,147), only 34.8% (n=38,684) initiated treatment in the post-index period, with the vast majority (94.9%, n=36,716) initiating statins alone, 3.0% (n=1,177) initiating ezetimibe alone, and only 2.0% (n=791) initiating ezetimibe in combination with a statin (Table 3, Figure 1).
Table 3.
Goal achievement in untreated ASCVD patients initiating treatment
| Cohort | ASCVD patients, untreated at index, N=111,147 | |||
|---|---|---|---|---|
| Index LDL-C value | All patients N=111,147 (100.0%) | 71–100 mg/dL n=47,882 (43.1%) | 101–130 mg/dL n=34,983 (31.5%) | >130 mg/dL n=28,282 (25.5%) |
| Pts initiating LLT therapy, n (%) | 38,684 (34.8) | 17,078 (35.7) | 10,561 (30.2) | 11,045 (39.1) |
| Pts with post-therapy LDL-C, n (%) | 23,550 (60.9) | 10,389 (60.8) | 6,474 (61.3) | 6,687 (60.5) |
| Pts with ≥50% LDL-C reduction, % (CI) | 7.6 (7.3–8.0) | 1.8 (1.6–2.1) | 6.8 (6.2–7.4) | 17.4 (16.5–18.3) |
| Pts with LDL-C ≤70 mg/dL, % (CI) | 19.1 (18.6–19.6) | 26.9 (26.0–27.7) | 16.4 (15.5–17.3) | 9.7 (8.9–10.4) |
| LDL-C, mean (SD) | 96.29 (32.30) | 83.61 (22.94) | 96.74 (28.02) | 115.54 (38.42) |
| Pts initiating statin only, n (%) | 36,716 (94.9) | 16,281 (95.3) | 10,051 (95.2) | 10,384 (94.1) |
| Pts with post-therapy LDL-C, n (%) | 22,269 (60.7) | 9,878 (60.7) | 6,132 (61.0) | 6,259 (60.3) |
| Pts with ≥50% LDL-C reduction, % (CI) | 7.8 (7.4–8.1) | 1.9 (1.6–2.1) | 6.9 (6.2–7.5) | 17.9 (17.0–18.9) |
| Pts with LDL-C ≤70 mg/dL, % (CI) | 19.1 (18.7–19.7) | 26.8 (25.9–27.7) | 16.5 (15.6–17.4) | 10.0 (9.3–10.7) |
| LDL-C, mean (SD) | 95.78 (31.93) | 83.62 (22.87) | 96.39 (27.94) | 114.37 (38.18) |
| Pts initiating ezetimibe only, n (%) | 1,177 (3.0) | 332 (1.9) | 339 (3.2) | 506 (4.6) |
| Pts with post-therapy LDL-C, n (%) | 752 (63.9) | 200 (60.2) | 224 (66.1) | 328 (64.8) |
| Pts with ≥50% LDL-C reduction, % (CI) | 4.8 (3.3–6.3) | 2.0 (0.0–4.0) | 6.3 (3.1–9.4) | 5.5 (3.0–8.0) |
| Pts with LDL-C ≤70 mg/dL, % (CI) | 12.0 (9.6–14.3) | 25.0 (18.9–31.1) | 13.8 (9.3–18.4) | 2.7 (1.0–4.5) |
| LDL-C, mean (SD) | 114.96 (38.64) | 87.31 (27.26) | 104.15 (28.38) | 139.20 (35.93) |
| Pts initiating statin + ezetimibe, n (%) | 791 (2.0) | 465 (2.72) | 171 (1.6) | 155 (1.4) |
| Pts with post-therapy LDL-C, n (%) | 529 (66.9) | 311 (66.88) | 118 (69.01) | 100 (64.52) |
| Pts with ≥50% LDL-C reduction, % (CI) | 4.9 (4.7–9.0) | 1.0 (−0.1–2.1) | 5.1 (1.1–9.1) | 27.0 (18.1–35.9) |
| Pts with LDL-C ≤70 mg/dL, % (CI) | 24.4 (20.7–28.1) | 31.8 (26.6–37.0) | 17.0 (10.1–23.8) | 10.0 (4.0–16.0) |
| LDL-C, mean (SD) | 91.19 (29.59) | 81.15 (21.86) | 100.71 (29.71) | 111.18 (36.07) |
Notes: Only patients with a recorded valid LDL-C measurement from 1 to 12 months after new treatment initiation are shown. The immediate test value post-treatment initiation was used (test values were limited to those observed between 1 and 12 months post-treatment initiation).
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; LDL-C, low-density lipoprotein cholesterol; LLT, lipid-lowering therapy; Pts, patients.
Among all patients initiating treatment post-index, 60.9% (n=23,550) had an LDL-C assessment post-treatment; among these, less than one fifth (19.1%, n=4,501) achieved an LDL-C ≤70 mg/dL after initiating treatment (Table 3). The rate of goal achievement (LDL-C ≤70 mg/dL) in patients with a baseline LDL-C 71–100 mg/dL ranged from 25.0% in patients initiating ezetimibe to 31.8% in patients initiating statins and ezetimibe together. Rate of goal achievement decreased with higher baseline LDL-C. For example, in patients initiating statins only, the rates ranged from 16.5% in patients with a baseline LDL-C 101–130 mg/dL to only 10.0% in those with a baseline LDL-C over >130 mg/dL. A similar trend was observed in patients initiating ezetimibe alone and in those initiating statin + ezetimibe combination (Table 3).
Overall, only 7.8% of patients achieved a ≥50% reduction in LDL-C post-treatment initiation, and the rate appeared to vary by baseline LDL-C level and the type of treatment initiated (from 1.0% in the subgroup of patients with index LDL-C 71–100 mg/dL and initiating statin + ezetimibe to 27.0% in the subgroup of patients with index LDL-C >130 mg/dL and initiating statin + ezetimibe) (Table 3).
Treated patients modifying therapy post-index
Among all treated ASCVD patients with LDL-C >70 mg/dL at index (n=75,523), 17.9% (n=13,503) received post-index treatment modification, with the most common modification being change in statin dose intensity (59.5%), followed by statin switching (32.6%), and augmentation of statin therapy (7.9%) post-index (Table 4, Figure 1). There was variation in the rates of treatment modifications across the three baseline LDL-C subgroups as described in Table 4, with modifications more common in patients with LDL-C >130 mg/dL (34.6%, n=3,340). Among patients modifying therapy post-index, LDL-C goal achievement can only be evaluated in the 69.8% (n=9,240) with an LDL-C assessment post-treatment modification.
Table 4.
Goal achievement in treated ASCVD patients with post-index therapy modification
| Cohort | ASCVD patients, treated at index N=75,523 | |||
|---|---|---|---|---|
| Index LDL-C value | All patients N=75,523 (100.0%) | 71–100 mg/dL N=46,441 (61.5%) | 101–130 mg/dL N=19,441 (25.7%) | >130 mg/dL N=9,641 (12.8%) |
| Pts modifying therapy, n (%) | 13,503 (17.9) | 5,723 (12.3) | 4,440 (22.8) | 3,340 (34.6) |
| Pts with post-therapy LDL-C, n (%) | 9,420 (69.8) | 4,044 (70.7) | 3,120 (70.3) | 2,256 (67.5) |
| Pts with ≥50% LDL-C reduction, % (CI) | 5.6 (5.1–6.0) | 1.8 (1.4–2.2) | 4.6 (3.9–5.4) | 13.6 (12.2–15.0) |
| Pts with LDL-C ≤70 mg/dL, % (CI) | 17.8 (17.0–18.6) | 26.7 (25.4–28.1) | 14.0 (12.8–15.3) | 6.9 (5.9–8.0) |
| LDL-C, mean (SD) | 99.03 (34.59) | 86.50 (26.39) | 99.28 (29.85) | 121.17 (41.76) |
| Pts switching statin, n (%) | 4,408 (32.6) | 2,012 (35.2) | 1,366 (30.8) | 1,030 (30.8) |
| Pts with post-therapy LDL-C, n (%) | 3,148 (71.4) | 1,462 (72.7) | 996 (72.9) | 690 (67.0) |
| Pts with ≥50% LDL-C reduction, % (CI) | 5.4 (4.6–6.2) | 2.0 (1.3–2.7) | 4.1 (2.9–5.4) | 14.6 (12.0–17.3) |
| Pts with LDL-C ≤70 mg/dL, % (CI) | 16.2 (14.9–17.5) | 23.3 (21.2–25.5) | 12.2 (10.1–14.2) | 7.0 (5.1–8.9) |
| LDL-C, mean (SD) | 99.47 (33.99) | 87.32 (25.09) | 101.53 (29.97) | 122.24 (42.47) |
| Pts increasing statin intensity, n (%) | 5,723 (42.4) | 2,156 (37.7) | 2,116 (47.7) | 1,451 (43.4) |
| Pts with post-therapy LDL-C, n (%) | 3,920 (68.5) | 1,485 (68.9) | 1,472 (69.6) | 963 (66.4) |
| Pts with ≥50% LDL-C reduction, % (CI) | 6.6 (5.8–7.3) | 2.1 (1.4–2.8) | 5.2 (4.1–6.4) | 15.5 (13.2–17.8) |
| Pts with LDL-C ≤70 mg/dL, % (CI) | 21.1 (19.8–22.3) | 34.5 (32.1–36.9) | 16.0 (14.1–17.8) | 8.2 (6.5–9.9) |
| LDL-C, mean (SD) | 94.79 (33.10) | 80.56 (23.52) | 94.52 (27.00) | 117.13 (41.02) |
| Pts decreasing statin intensity, n (%) | 2,307 (17.1) | 1,146 (20.0) | 604 (13.6) | 557 (16.7) |
| Pts with post-therapy LDL-C, n (%) | 1,588 (68.8) | 794 (69.3) | 402 (66.6) | 392 (70.4) |
| Pts with ≥50% LDL-C reduction, % (CI) | 3.2 (2.3–4.0) | 0.5 (0.0–1.0) | 4.2 (2.3–6.2) | 7.4 (4.8–10.0) |
| Pts with LDL-C ≤70 mg/dL, % (CI) | 10.3 (8.8–11.8) | 13.6 (11.2–16.0) | 10.5 (7.4–13.5) | 3.3 (1.5–5.1) |
| LDL-C, mean (SD) | 108.70 (36.50) | 97.70 (29.28) | 111.25 (34.53) | 128.37 (42.49) |
| Pts augmenting with ezetimibe, n (%) | 1,065 (7.9) | 409 (7.2) | 354 (8.0) | 302 (9.0) |
| Pts with post-therapy LDL-C, n (%) | 764 (71.7) | 303 (74.1) | 250 (70.6) | 211 (69.9) |
| Pts with ≥50% LDL-C reduction, % (CI) | 6.0 (4.3–7.7) | 3.0 (1.0–4.9) | 3.6 (1.3–5.9) | 13.3 (8.7–17.9) |
| Pts with LDL-C ≤70 mg/dL, % (CI) | 23.0 (20.0–26.0) | 39.6 (34.1–45.1) | 16.0 (11.4–20.6) | 7.6 (4.0–11.2) |
| LDL-C, mean (SD) | 98.92 (36.35) | 82.23 (28.04) | 99.06 (31.01) | 122.73 (39.46) |
Notes: Only patients with a recorded valid LDL-C measurement from 1 to 12 months after new treatment initiation are shown. The immediate test value post-treatment initiation was used (test values were limited to those observed between 1 and 12 months post-treatment initiation). Baseline statin intensity: among the 75,523 ASCVD patients treated at baseline, 12.18% were on low statin intensity, 58.4% were on moderate statin intensity, 20.6% were on high statin intensity, and 8.8% were treated with other lipid-lowering agents.
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; LDL-C, low-density lipoprotein cholesterol; Pts, patients.
LDL-C outcomes (rates of >50% reduction in LDL-C and LDL-C <70 mg/dL) were also found to be suboptimal in treated patients who modified therapy post-index as described in Table 4. The most effective treatment modification overall was adding ezetimibe. Among patients with baseline LDL-C 71–100 mg/dL, 39.6% achieved the LDL-C ≤70 mg/dL threshold after adding ezetimibe, compared to only 16.0% and 7.5% in patients with baseline LDL-C 101–130 mg/dL and >130 mg/dL, respectively. Rates of goal achievement were similar for patients who increased their statin intensity, but lower for patients increasing the dose of the index statin. The proportion of patients achieving a ≥50% reduction in baseline LDL-C were highest in those with baseline LDL-C >130 mg/dL, and ranged from 7.4% (decreased statin intensity) to 15.5% (increased statin intensity) (Table 4).
LDL-C goal achievement in probable HeFH patients
Untreated patients initiating post-index therapy
In the probable HeFH subgroup of patients untreated at baseline (n=3,064), half of the patients (50.3%, n=1,540) initiated treatment post-index. The mean (SD) post-treatment LDL-C was 136.2 (47.8) mg/dL and post-treatment LDL-C was lowest for those initiating ezetimibe (110.9 [32.1] mg/dL). Overall, only 4.8% of these patients initiating statin achieved the LDL-C ≤70 mg/dL threshold. None of the ASCVD + probable HeFH patients initiating ezetimibe only, or statin + ezetimibe achieved goal LDL-C threshold. Approximately 31.1% of these patients had a ≥50% reduction in LDL-C after initiating therapy; 50% reduction occurred most frequently in patients initiating statin + ezetimibe (52.2%) (Table 5).
Treated patients modifying therapy post-index
Only 1,073 probable HeFH patients treated at baseline and a large majority received statin alone. Of those treated, 41.5% (n=445) modified treatment. Based on the 281 patients with available post-treatment LDL-C assessment, overall 62 patients (22.1%) achieved a ≥50% reduction in LDL-C, but only 3 (1.1%) patients reached a LDL-C of ≤70 mg/dL. The highest ≥50% LDL-C reduction was observed in the patients increasing statin intensity at 26.61% (Table 5).
Discussion
In the current study, a majority (59.5%) of the patients with a diagnosis of ASCVD and an LDL-C >70 mg/dL were not previously or currently receiving statin or ezetimibe therapy at the time the baseline abnormal LDL-C level was observed. As we indexed patients to their first LDL-C test during the index window, it is possible that for some patients the baseline LDL-C assessment was their first abnormal one. Nevertheless, this finding of many untreated patients corroborates the observation by other researchers who postulated that despite evidence showing statins and ezetimibe to reduce the risk of ASCVD-related events, many patients do not receive treatment per established guidelines.10,23,24 It has been suggested that provider confusion regarding the most appropriate guidelines for a patient and attempting to implement recommendations that are evolving may contribute to this treatment gap.10
The rate of treatment initiation among previously untreated patients was generally under 40%. In patients with an LDL-C >190 mg/dL (probable HeFH patients), the treatment initiation rate was higher, but still only at approximately 50%. Rates of treatment modification after elevated index LDL-C values were also low overall, ranging from a low of 12.3% in patients with a baseline LDL-C 71–100 mg/dL to 34.6% in patients with a baseline LDL-C >130 mg/dL. An earlier study entailing a survey of health care providers also indicated that it was not uncommon for LLT to remain unchanged, even when the patient is not at goal. That survey found that while the need for lower LDL-C and CV risk was considered important in these patients by the health care providers, physicians’ treatment choices were still substantially less aggressive than guideline recommendations.25
Although there was an overall decrease in the mean LDL-C value among previously untreated ASCVD patients initiating therapy post-index, the majority (80.9%) did not achieve the LDL-C threshold of ≤70 mg/dL. This should not be interpreted as a reason not to initiate treatment, but rather to titrate patients to higher intensity statins, and add ezetimibe per guidelines, to increase the chance of achieving an LDL-C ≤70 mg/dL. If still not successful in achieving treatment goals, initiating alternative agents such as PCSK9 inhibitors may be warranted.
LDL-C treatment outcomes in treated patients who modified treatment were also not optimal. Current analysis observed a general trend that patients with higher baseline LDL-C values had greater post-modification LDL-C reductions. In addition, post-modification benefits were more common in patients who increased statin dose intensity than in those who switched or augmented therapy. However, even in these subgroups of patients who showed greater post-treatment LDL-C reduction, the majority of them were still not at goal (≤70 mg/dL, or 50% reduction) based on the first LDL-C test post-treatment change. These findings are consistent with other published reports.26,27 The EUROASPIRE surveys found that in the past years, although the use of LLTs, including high-intensity statins, increased, the LDL-C treatment outcome is still suboptimal.26,27
This study underscores the challenges of managing LDL-C levels in patients with ASCVD and probable HeFH and provides further evidence of the need for more aggressive treatment, including high-intensity statin and alternative therapies, for this population. In addition, barriers such as utilization management that require patients and providers to navigate payer-imposed utilization management tasks related to step-therapy or prior authorization may inadvertently delay patients in receiving therapies that may support achieving therapeutic goals.
Limitations
These findings should be interpreted in the context of specific limitations of the study. The use of EMR data may have introduced some information bias as only data from physicians contributing to the EMR network were available. In addition, EMR data are not created for research purposes, and their quality is subject to a tradeoff between data entry and patient care. Patient visits conducted by other health care providers not included in the EMR data network were not captured, which may have led to the underreporting of LDL-C measurements and/or treatment with LLTs. Treatment exposure to statin and/or ezetimibe was based on observation of a valid prescription recorded in the EMR database, which does not guarantee that the patient filled the prescription or used the medication. Incomplete LDL-C data capture in the EMR system may also limit the generalizability of LDL-C treatment outcomes observed in this study. In addition, age at initial ASCVD diagnosis cannot be confirmed in this database, thus limiting the interpretation of the LLT treatment patterns and outcomes observed in a prevalent population. This study defined probable HeFH as with baseline LDL-C level ≥190 mg/dL, not by confirmed clinical diagnosis or genetic tests; this may lead to false positive/negative HeFH in identification of these patients in our study sample. Finally, this was a descriptive retrospective analysis that evaluates the associations between exposures and outcomes, but no causal relationships can be established from this observational study.
Conclusion
This study substantiates previous reports of under-prescribing of LLTs to ASCVD patients with elevated LDL-C levels. Moreover, for most of the patients with ASCVD and probable HeFH, standard treatment regimens or modifications of recommended treatments failed to produce >50% reduction in LDL-C and/or achieve LDL-C values of <70 mg/dL for most patients, regardless of their baseline LDL-C levels. More frequent and aggressive lipid management, including increasing statin intensity and alternative therapies, may be needed in these patients to reduce their CV risk. Given this and other evidence such as findings of high discontinuation rates for statin therapy, particularly for high-intensity statin doses, new health care system-based interventions and therapeutic paradigms are needed to address the unmet need in ASCVD and probable HeFH patients with elevated LDL-C levels.
Supplementary material
Table S1.
Stain dose intensity categories (low, moderate, and high)
| Statin therapy | Daily dose | |||
|---|---|---|---|---|
| Low intensity | Moderate intensity | High intensity | Intensity classifications of atypical doses | |
| Atorvastatin | <10 mg/day | 10 to <40 mg/day | ≥40 mg/day | 30 mg/day = moderate |
| Fluvastatin | <80 mg/day | 80 mg/day | NA | 10 mg/day = low |
| Lovastatin | <40 mg/day | ≥40 mg/day | NA | 10 mg/day = low 80 mg/day = moderate |
| Pitavastatin | <2 mg/day | ≥2 mg/day | NA | |
| Pravastatin | <40 mg/day | ≥40 mg/day | NA | <10 mg/day = low |
| Rosuvastatin | <5 mg/day | 5 to <20 mg/day | ≥20 mg/day | <5 mg/day = low 15 mg/day = moderate |
| Simvastatin | <20 mg/day | 20 to <80 mg/day | ≥80 mg/day | <20 mg/day = low >40 to <80 mg/day = moderate ≥80 mg/day = high |
Abbreviation: NA, not applicable.
Acknowledgments
Jin Wu (Statistical Programmer, IQVIA) provided analytic support. Xin Wang (consultant, IQVIA) provided editorial support for this manuscript. This study was sponsored by Amgen Inc.
Footnotes
Disclosure
C. C. Chen, D.M. Hines, and R.L. Wade are employees of IQVIA. IQVIA was hired by Amgen Inc. to conduct this study. P.B. Rane, J. Patel, J., and D. J Harrison are employees and stockholders of Amgen Inc.
References
- 1.Centers for Disease Control and Prevention, National Center for Health Statistics [webpage on the Internet] Underlying Cause of Death 1999–2014: Multiple Cause of Death Files (1999–2014) 2015. [Accessed November 2, 2016]. Available from: https://wonder.cdc.gov/ucd-icd10.html.
- 2.Kullo IJ, Trejo-Gutierrez JF, Lopez-Jimenez F, et al. A perspective on the New American College of Cardiology/American Heart Association guidelines for cardiovascular risk assessment. Mayo Clin Proc. 2014;89(9):1244–1256. doi: 10.1016/j.mayocp.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 3.Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur Heart J. 2016;37(39):2999–3058. doi: 10.1093/eurheartj/ehw272. [DOI] [PubMed] [Google Scholar]
- 4.Najam O, Ray KK. Familial hypercholesterolemia: a review of the natural history, diagnosis, and management. Cardiol Ther. 2015;4(1):25–38. doi: 10.1007/s40119-015-0037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reiner Ž. Management of patients with familial hypercholesterolaemia. Nat Rev Cardiol. 2015;12(10):565. doi: 10.1038/nrcardio.2015.92. [DOI] [PubMed] [Google Scholar]
- 6.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Foody JM, Sajjan SG, Hu XH, et al. Loss of early gains in low-density lipoprotein cholesterol goal attainment among high-risk patients. J Clin Lipidol. 2010;4(2):126–132. doi: 10.1016/j.jacl.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Nichols GA, Nag S, Chan W. Intensity of lipid-lowering therapy and low-density lipoprotein cholesterol goal attainment among the elderly before and after the 2004 National Cholesterol Education Program Adult Treatment Panel III update. Am Heart J. 2007;154(3):554–560. doi: 10.1016/j.ahj.2007.04.037. [DOI] [PubMed] [Google Scholar]
- 9.Foody JM, Toth PP, Tomassini JE, et al. Changes in LDL-C levels and goal attainment associated with addition of ezetimibe to simvastatin, atorvastatin, or rosuvastatin compared with titrating statin monotherapy. Vasc Health Risk Manag. 2013;9:719–727. doi: 10.2147/VHRM.S49840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris PB, Ballantyne CM, Birtcher KK, Dunn SP, Urbina EM. Review of clinical practice guidelines for the management of LDL-related risk. J Am Coll Cardiol. 2014;64(2):196–206. doi: 10.1016/j.jacc.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 11.Lin I, Sung J, Sanchez RJ, et al. Patterns of Statin Use in a Real-World Population of Patients at High Cardiovascular Risk. J Manag Care Spec Pharm. 2016;22(6):685–698. doi: 10.18553/jmcp.2016.22.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 13.Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1500–1509. doi: 10.1056/NEJMoa1500858. [DOI] [PubMed] [Google Scholar]
- 14.Marrett E, Zhao C, Zhang NJ, et al. Limitations of real-world treatment with atorvastatin monotherapy for lowering LDL-C in high-risk cardiovascular patients in the US. Vasc Health Risk Manag. 2014;10:237–246. doi: 10.2147/VHRM.S54886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1489–1499. doi: 10.1056/NEJMoa1501031. [DOI] [PubMed] [Google Scholar]
- 16.Phan K, Gomez YH, Elbaz L, Daskalopoulou SS. Statin treatment non-adherence and discontinuation: clinical implications and potential solutions. Curr Pharm Des. 2014;20(40):6314–6324. doi: 10.2174/1381612820666140620162629. [DOI] [PubMed] [Google Scholar]
- 17.Caspard H, Chan AK, Walker AM. Compliance with a statin treatment in a usual-care setting: retrospective database analysis over 3 years after treatment initiation in health maintenance organization enrollees with dyslipidemia. Clin Ther. 2005;27(10):1639–1646. doi: 10.1016/j.clinthera.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Virani SS, Woodard LD, Akeroyd JM, Ramsey DJ, Ballantyne CM, Petersen LA. Is high-intensity statin therapy associated with lower statin adherence compared with low- to moderate-intensity statin therapy? Implications of the 2013 American College of Cardiology/American Heart Association Cholesterol Management Guidelines. Clin Cardiol. 2014;37(11):653–659. doi: 10.1002/clc.22343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Writing Committee. Lloyd-Jones DM, Morris PB, et al. 2016 ACC Expert Consensus Decision Pathway on the Role of Non-Statin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk: A Report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2016;68(1):92–125. doi: 10.1016/j.jacc.2016.03.519. [DOI] [PubMed] [Google Scholar]
- 20.Duke JD, Ryan PB, Suchard MA, et al. Risk of angioedema associated with levetiracetam compared with phenytoin: Findings of the observational health data sciences and informatics research network. Epilepsia. 2017;58(8):e101–e106. doi: 10.1111/epi.13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shafrin J, Tebeka MG, Price K, Patel C, Michaud K. The economic burden of ACPA-positive status among patients with rheumatoid arthritis. J Manag Care Spec Pharm. 2018;24(1):4–11. doi: 10.18553/jmcp.2017.17129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosnaim G, Ariely R, Depietro M. Clinical Outcomes in Patients with Persistent Asthma by Attainment of Healthcare Effectiveness and Data Information Set (HEDIS) Measures; Poster presented at: AAE Annual Conference; July 20–22 2018; Phoenix, AZ. [Google Scholar]
- 23.Somma K, Bhatt D, Fonarow G. Guideline adherence after ST-segment elevation versus non-ST segment elevation myocardial infarction. Circ Cardiovasc Interv. 2013;5(5):654–661. doi: 10.1161/CIRCOUTCOMES.111.963959. [DOI] [PubMed] [Google Scholar]
- 24.Sachdeva A, Cannon CP, Deedwania PC, et al. Lipid levels in patients hospitalized with coronary artery disease: an analysis of 136,905 hospitalizations in Get With The Guidelines. Am Heart J. 2009;157(1):111–117. doi: 10.1016/j.ahj.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Krempf M, Simpson RJ, Ramey DR, et al. Patient and physician factors influence decision-making in hypercholesterolemia: a questionnaire-based survey. Lipids Health Dis. 2015;14:45. doi: 10.1186/s12944-015-0037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kotseva K. Lessons from Euroaspire I, II, and III surveys. Heart Metab. 2011;50:32–35. [Google Scholar]
- 27.Kotseva K, EUROASPIRE Investigators The EUROASPIRE surveys: lessons learned in cardiovascular disease prevention. Cardiovasc Diagn Ther. 2017;7(6):633–639. doi: 10.21037/cdt.2017.04.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Stain dose intensity categories (low, moderate, and high)
| Statin therapy | Daily dose | |||
|---|---|---|---|---|
| Low intensity | Moderate intensity | High intensity | Intensity classifications of atypical doses | |
| Atorvastatin | <10 mg/day | 10 to <40 mg/day | ≥40 mg/day | 30 mg/day = moderate |
| Fluvastatin | <80 mg/day | 80 mg/day | NA | 10 mg/day = low |
| Lovastatin | <40 mg/day | ≥40 mg/day | NA | 10 mg/day = low 80 mg/day = moderate |
| Pitavastatin | <2 mg/day | ≥2 mg/day | NA | |
| Pravastatin | <40 mg/day | ≥40 mg/day | NA | <10 mg/day = low |
| Rosuvastatin | <5 mg/day | 5 to <20 mg/day | ≥20 mg/day | <5 mg/day = low 15 mg/day = moderate |
| Simvastatin | <20 mg/day | 20 to <80 mg/day | ≥80 mg/day | <20 mg/day = low >40 to <80 mg/day = moderate ≥80 mg/day = high |
Abbreviation: NA, not applicable.