Abstract
Introduction
Hepatocellular carcinoma (HCC) is one of the known major health problems across the globe, and is sixth ranked among all cancer, due to its high mortality rate. Polyunsaturated fatty acids (PUFAs) play an important role in the formation of a cell membrane, along with the fluidity of the membrane and proteins. Gamma linolenic acid (GLA) is member of the ω-6 family of PUFAs and converts into the arachidonic acid via a series of elongation and desaturation reactions. The aim of the current investigation was to scrutinize the effect of GLA on mitochondrial mediated apoptosis and anti-inflammatory pathway against diethylnitrosamine (DEN) induced HCC.
Materials and methods
Chemical carcinogenesis in Wistar rats was introduced by an intra-peritoneal dose of DEN (200 mg/kg). The rats received the various doses of GLA for 22 weeks. The progressions of serum biomarkers and histopathology components of hepatic tissue were used to access the prophylactic effects. The antioxidant parameters, cancer preventive agent status, and apoptosis mechanism were reviewed to scrutinize the possible mechanism.
Results
Dose-dependent treatment of GLA significantly (P<−0.001) modulated the hepatic nodules, hepatic, body weight, antioxidant, and non-hepatic parameters. Curiously, the Real-time polymerase chain reaction (RT-PCR) and immunoblotting showed the GLA altered reduced the hypoxic microenvironment, mitochondrial mediated death apoptosis, and anti-inflammsatory pathways.
Conclusion
On the basis of the above results, we can conclude that the GLA exhibited a chemo-protective effect against DEN induced HCC that might be due to the altered hypoxic microenvironment, mitochondrial mediated death apoptosis, and anti-inflammatory pathway, respectively.
Keywords: gamma linolenic acid, apoptosis, hepatocellular carcinoma, diethynitrosamine, gene expression
Introduction
Deaths due to hepatic cancer are daily on the rise, with multiple reasons of mortality.1 Hepatocellular carcinoma (HCC) is the most prominent recognized danger to the hepatic tissue, and it is considered as the third most common reason for HCC death around the World.2,3 Cirrhotic liver leads to HCCs, followed by a long duration of liver damage brought on by alcohol, non-alcoholic steatohepatitis, environmental toxicants, and viral hepatitis.2,4 The available treatment for hepatic cancer can be counted in terms of chemotherapy, radiotherapy, removal of tissue part, and surgical medication, but the success of the treatment depends on the proper diagnosis and the stage of disease, due to more side-effects and the unfortunate outlook of HCC.5,6 If the above discussed treatments fail, the last option for the medicinal practitioner is liver transplantation, but the success rate of liver transplantation is limited and depends on the stage of the HCC.7,8 By this process, the unnatural openness of organs prohibits this selection for various people with HCC and increases the risk of tumor reappearance after liver transplantation, obscuring the efficacy of this treatment approach.7–10 Thus, it has become the need of the hour for researcher to focus on novel drug discovery with potential therapeutic targets that can be less toxic and can delay or reduce hepatic cancer incidence.
The environmental cancer agent diethyl nitrosamine (DEN), a nitroso compound generally present in nature and various food items such as mixed drinks, cheddar, tobacco, restorative and handled meats, is one of the best sources of a chemical carcinogen, particularly for the liver. It is sometimes found in home detergents and baby feeding nipples.11,12 DEN was found to be the one of the toxicanst to induce hepatic cancer (HCC). It is commonly used in various rodents to induce HCC.13–15
Polyunsaturated fatty acids (PUFAs) play an important role in the formation of the cell membrane and also play a significant role in the functioning of membrane fluidity and proteins. They exhibit various cellular, sub-cellular functions, processes, and gene expression.16,17 GLA is a member of the ω-6 family of PUFAs and is transfigured into arachidonic acid (AA) via a series of elongation and desaturation reactions. AA is further metabolized via cyclooxygenase (COX) enzymes into the prostaglandins or via 5-lipoxygenase into 5-hydroxy-eicosatetranoic acid and leukotrienes, which shows cellular inflammation. Due to the above facts, GLA is metabolized into the AA, and AA is further metabolized and acts as a pro-inflammatory agent. Due to this mechanism, GLA also acts as a pro-inflammatory in nature.18–21
GLA is mostly present in plants such as soy bean, grapes, sunflowers, and fish, and it should be present in the daily diet for a healthy lifestyle. Several researchers suggest that regular intake of GLA reduces the inflammatory reaction and inhibits the cancer and its associated conditions.18,21,22 Previous published literature in vitro and in vivo studies suggested the anticancer effect of GLA. GLA reduced tumor growth in a WBC256 rat model; it also inhibited the cell growth of various rat carcinogenesis cell lines and human neuroblastoma cell lines.19,20 GLA treatment down-regulated mammary gland carcinogenesis and tumor growth. It is also reduced the growth of various cultured human cell lines such as adenocarcinomic human alveolar basal epithelial cells (A549), human breast cancer cell lines (ZR-75-1), and human prostate cancer cell lines (PC-3). Various researchers suggest that GLA has the potential to have anticancer effects and, in our opinion, few researchers have scrutinized the anticancer effect of GLA against chemically induced HCC. In the current experimental study, we attempted to explore the anticancer effect of GLA against the DEN-induced HCC and tried to explore the possible mechanism of action.
Materials and methods
Drugs and reagents
GLA, diethylnitrosamine (DEN), Eagle balanced salt solution (EBSS), ponceau S, and RNase were procured from Sigma Aldrich (St Louis, MO, USA). Collagenase type 4, RNase, sodium cacodylate, hematoxylin, and eosin were purchased from Himedia. Bax and Bcl-2 were purchased from Biosynthesis Biotechnology (Beijing, China). The kits for aspartate aminotransferase (AST), alanine aminotransferase (ALT), alpha-fetoprotein (AFP), and alkaline phosphatase (ALP) were purchased from Beihuakangtai Biotechnology (Beijing, China). All other chemical and reagents used in the experimental study were acquired from the reputed vendor.
Experimental protocol
Swiss Wistar (both sexes; 120–150 gm, body weight) rats were used in the experimental investigation. All the animals were received from the institutional animal house and stored in a single cage (polyethylene) kept at standard experimental conditions (20°C±5°C, 12 hours light:dark cycle). The animals received standard food diet (China Animal Food, Beijing, China) and water ad libitum. The experimental study was conducted in accordance with the protocol of control and supervision of experiments on animals (CPCSEA), Government of India, and the study had prior approval from the Institutional animal ethics committee (IAEC) of Chandrasheker College of Pharmacy, India (CSP/18/02/018). They were acclimatized for 2 weeks before starting the experimental study. The animals were divided into the following groups, and each group contained 12 animals: Gp I, normal control received only carboxymethyl cellulose (CMC); Gp II, DEN control received a single oral dose of saline; Gp III–V, DEN control received GLA (0.125, 0.25, and 0.5 mL/kg, body weight) for the entire study period. All disease control group rats initilly received a single intraperitoneal dose of DEN (200 mg/kg) and every week thereafter received the pheobarbitol dose for induction of HCC.23–25 After 1 week of DEN administration, the alpha feto protein (ALP) level was estimated. During the HCC, the level of AFP was boosted more than 10-times as compared to normal.
The food, water, and body weight of all group animals were estimated at regular intervals. After completing the experimental investigation (180 days), the animals were sacrificed via dislocation of cervical and hepatic tissues were successfully removed for the further biochemical and histopathological investigation. The blood samples were collected via puncturing the retro orbital plexus.
Morphology and morphometry of hepatocyte nodules
Hepatic cancer was confirmed by morphological examination by the presence of hepatic nodules. Briefly, the rats were anesthetized by intramuscular injection of ketamine and xylzaine. Hepatic tissue were perfused through the portal vein using the heparinized saline solution, and the hepatic tissue was quickly removed from the group of rats, washed with PBS to remove the blood component from the tissue, and the tissue was blotted using a paper towel, the tissues were then weighed. All the animal tissues were macroscopically scrutinized by checking the color, which showed the development of nodules. The hepatic nodules easily identified, via color (white and gray), differences in size, and those covered with the non-nodular hepatic tissue (reddish brown in color). Further, tissues were evaluated via using the following scale (depending on the size of the hepatic nodules) viz., ≤1, 1–3 and ≥3.25,26
Biochemical parameter estimation
The hepatic parameters, such as serum AFP, ALT, ALP, and AST, non-hepatic parameter viz., total protein, albumin, total bilirubin, direct bilirubin, and blood urea nitrogen (BUN) were estimated from standard kits using the manufacturer instructions.
Antioxidant parameters
The antioxidant parameters viz., superoxide dismutase (SOD), catalase (CAT), lipid peroxidation (LPO), glutathione (GSH) and glutathione peroxidise (GPx) were determined using the reported method of Kumar et al, with minor modification.25
Estimation of caspase level
Ninety-six well plates were used for the estimation of the level of caspase-3 and 8 in the different group of rats. Briefly, the serum samples of all group rats were taken into the cuvette followed by adding dithiotheritol (DTT), with a reaction mixture in equal quantity with final addition of DEVD-AFC (caspase 3), IETD-AFC (caspase 8), and it was incubated for 1 hour at room temperature. The free AFC level was estimated by fluorescence technique.
Western blotting
Briefly, radioimmunoprecipitation assay (RIPA) lysis buffer was used for homogenization of hepatic tissues as per Bradford et al.60 Then, Bardford reagent was used for extracting the protein sample via the precipitation method. The blot was incubated overnight with primary antibodies against Bax, Bcl-2, Bcl-xl, BAD, NFkB65, PHD2, UCHL-1, HIF-1α, VDAC, FASN, TNF-α, SREBP-1c, α-7nAChR, and HMGB-1 in 4°C and standard (β-actin).
qRT-PCR
For the estimation the qRT-PCR, the primer was designed from the primer tool. Briefly, Trizol reagents were used for the isolation the RNA from the hepatic tissue and the concentration was quantified by the previously reported method with minor modification. Hepatic tissue RNA (1 µg) was used in cDNA synthesis in a thermal cycler (96 well) and incubated for 15 minutes at 25°C, 100 minutes at 85°C, and 1,140 minutes at 37°C. cDNA (125 ng) was added in the reaction of qRT-PCR along with β-actin (standard reference). After that the program was again incubated for 2 seconds at 50°C and 10 seconds at 95°C and finally 20 seconds at 58°C, and the expression was calculated via the 2−ΔΔCT method with minor modification.
Statistical analysis
The result obtained in the current experimental studies was expressed as mean±SEM (n=12). One-way ANOVA was used to obtain the statistics, followed by least significant difference. P<0.05, P<0.01, and P<0.001 were considered as significant, more significant, and most significant, respectively.
Results
Effect of GLA on morphology and morphometry of hepatocyte nodules
The GLA treatment inhibits the morphology and morphometry of hepatocyte nodules of DEN-induced HCC rats (data not included in the manuscript). Normal control and GLA (0.5 mL/kg) did not exhibit the formation of any type of hepatic nodule formation. DEN-induced group rats showed the expansion of hepatic nodules which are white and grayish white in color. Table 1 exhibits the total number of hepatic nodules (252) in the DEN group, and GLA treatment significantly reduced the incidence of hepatic nodules (189, 102, and 35) at doses of 0.125, 0.25, and 0.5 mL/kg, respectively. The DEN group showed the hepatic nodules 115, 72, and 65 at a size of ≤1 mm, 1–3 mm, and ≥3 mm, respectively. The GLA treatment group inhibited the incidence of hepatic nodules 83 (≤1 mm), 70 (1–3 mm), and 36 (≥3 mm) at a dose of 0.125 mL; 46 (≤1 mm), 35 (1–3 mm), and 21 (≥3 mm) at a dose of 0.25 mL/kg; and 23 (≤1 mm), 8 (1–3 mm), and 4 (≥3 mm) at a dose of 0.5 mL/kg, respectively (Table 2).
Table 1.
Effect of GLA on the development of macroscopic hepatocytes nodules induced by DEN in rats
| S. No. | Groups | Number of rats/number of rats with nodules | Total number of nodules | Tumor incidence (%) |
|---|---|---|---|---|
| 1 | DEN control | 10/10 | 252 | 100 |
| 2 | DEN control+GLA (0.125 mL/kg) | 11/10 | 189 | 90.90 |
| 3 | DEN control+GLA (0.25 mL/kg) | 10/6 | 102 | 60 |
| 4 | DEN control+GLA (0.5 mg/kg) | 9/2 | 35 | 22.22 |
Note: Group I (normal control) and Group II (normal control+GLA 0.5 mg/kg) did not show any visible hepatocytes nodule.
Abbreviations: GLA, gamma linolenic acid; DEN, diethylnitrosamine.
Table 2.
Effect of GLA on the size distribution and growth of hepatocyte nodules induced by DEN in rats
| S. No. | Groups | Total number of nodules | Average number of nodules/nodules bearing rats | Relative size (% of number size) | ||
|---|---|---|---|---|---|---|
| ≤1 mm | 1–3 mm | ≥3 mm | ||||
| 1 | DEN control | 252 | 40.23±4.37 | 115 (45.64) | 72 (28.57) | 65 (25.69) |
| 2 | DEN control+GLA (0.125 mL/kg) | 189 | 28.34±3.05 | 83 (43.91) | 70 (37.07) | 36 (19.15) |
| 3 | DEN control+GLA (0.25 mL/kg) | 102 | 16.93±1.62 | 46 (45.09) | 35 (34.31) | 21 (20.58) |
| 4 | DEN control+GLA (0.5 mg/kg) | 35 | 6.04±2.93 | 23 (65.71) | 8 (22.86) | 4 (11.94) |
Note: Group I (normal control) and Group II (normal control+GLA 0.5 mg/kg) did not show any visible hepatocytes nodule.
Abbreviations: GLA, gamma linolenic acid; DEN, diethylnitrosamine.
Effect of GLA on the body weight
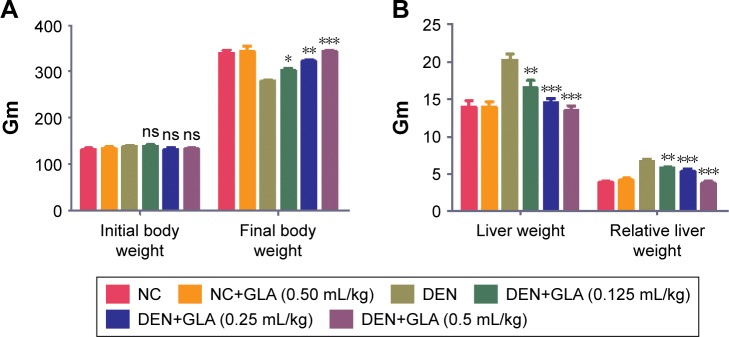
Body weight is a significant parameter for the estimation of disease effect. The same data was observed in our experimental study. DEN group rats showed increased body weight (279.83±5.34) as compared to initial body weight (138.74±4.81), but in comparison to the other group the body weight of DEN group rats was decreased. GLA treatment showed increased body weight (139±3.82) to (303.74±6.78) at a dose of 0.125 mL/kg; (131±4.04) to (322.38±5.93) at a dose of 0.25 mL/kg, and (132.74±4.23) to (375±8.93) at a dose of 0.5 mL/kg (Figure 1A).
Figure 1.
Effect of GLA on body weight and tissue weight.
Notes: (A) The initial body weight and final body weight of all group rats, and (B) the hepatic tissue and relative tissue weight method as described in the Materials and methods section. All values are presented as mean±SEM. Statistical analysis by one-way ANOVA followed by Dunnett’s multiple comparison. *P<0.05, **P<0.01 and ***P<0.001.
Abbreviations: ns, non-significant; DEN, diethylnitrosamine; GLA, gamma linolenic acid; NC, normal control.
The liver weight and relative liver weight of normal control and GLA (0.5 mL/kg) showed an almost similar trend. DEN group rats showed increased liver tissue weight due to expansion of hepatic nodules, and the increase in weight of hepatic tissue in this group showed an increase in relative liver weight. Concentration-dependent treatment of GLA exhibited reduced liver tissue weight and relative tissue weight as companion to DEN group rats (Figure 1B).
Effect of GLA on hepatic parameters
Several researchers suggest that the AFP is the gold parameter and consider it as the indicator for the hepatic cancer. During the HCC condition, hepatic parameters boosted a significant level into the serum due to leakage into blood. DEN rats exhibited the upregulation of AFP (305.84±0.394), AST (211.7±3.82), ALT (135.84±4.95), and ALP (195.4±3.74). Concentration-dependent treatment of GLA significantly reduced the level of hepatic parameters near tot the normal control (Figure 2).
Figure 2.
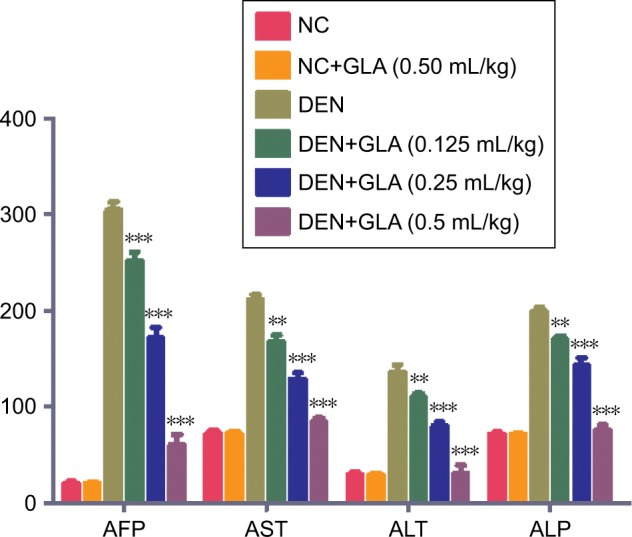
The effect of GLA on hepatic parameters of all group rats, for AFP, AST, ALT, and ALP, as described in the Materials and methods section.
Notes: All values are presented as mean±SEM. Statistical analysis by one-way ANOVA followed by Dunnett’s multiple comparison. *P<0.05, **P<0.01, and ***P<0.001.
Abbreviations: AFP, Alpha feto protein; ALP, Alkaline phosphatase; ALT, Alanine Aminotransferase; AST, Aspartate Aminotransferase; DEN, diethylnitrosamine; GLA, gamma linolenic acid.
Effect of GLA on non-hepatic parameters
A similar trend (hepatic parameters) was found in the non-hepatic parameters. DEN-induced group rats demonstrated the level of non-hepatic parameters like albumin (0.85±0.03), total protein (2.4±0.12), BUN (40±3.91), total bilirubin (77.5±4.83), and direct bilirubin (21.02±1.92). GLA treatment altered the level of non-hepatic parameters like albumin (3.92±0.23), total protein (7.5±0.92), BUN (13.25±2.83), total bilirubin (14.5±3.55), and direct bilirubin (7.74±1.83) (Figure 3).
Figure 3.
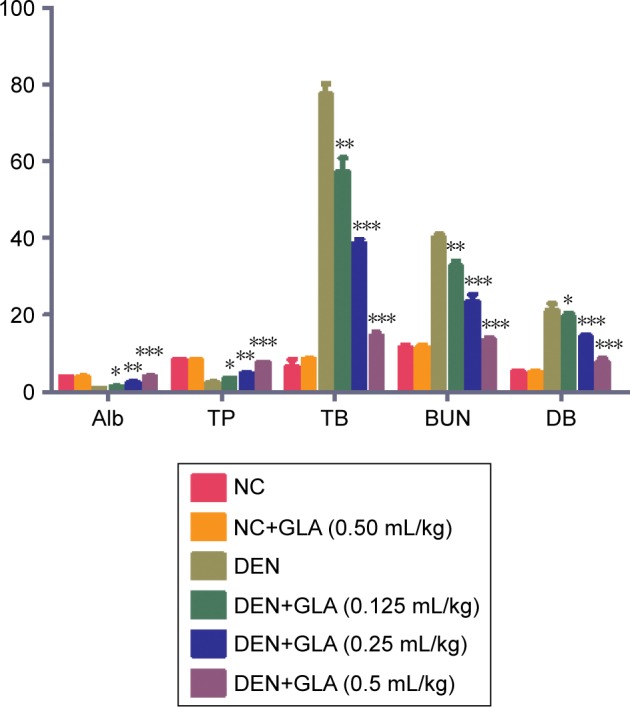
The effect GLA on non-hepatic parameters of all group rats, for Alb, TP, TB, BUN, and DB, as described in the Materials and methods section.
Notes: All values are presented as mean±SEM. Statistical analysis by one-way ANOVA followed by Dunnett’s multiple comparison. *P<0.05, **P<0.01 and ***P<0.001.
Abbreviations: Alb, Albumin; BUN, Blood urea nitrogen; DB, Direct bilirubin; DEN, diethylnitrosamine; GLA, gamma linolenic acid; NC, normal control; TB, Total bilirubin; TP, Total protein.
Effect of GLA on antioxidant parameters
Figure 4 demonstrates the antioxidant effect of GLA on all groups of rats. A concentration-dependent treatment of GLA restored the antioxidant level near to the normal control level. GLA successfully down-regulated the level of LPO (8.1±1.45) and upregulated the level of CAT (0.65±0.004), SOD (1.36±0.08), GST (0.31±0.002), and GPx (5.8±0.18), as compared to DEN-treated rats.
Figure 4.
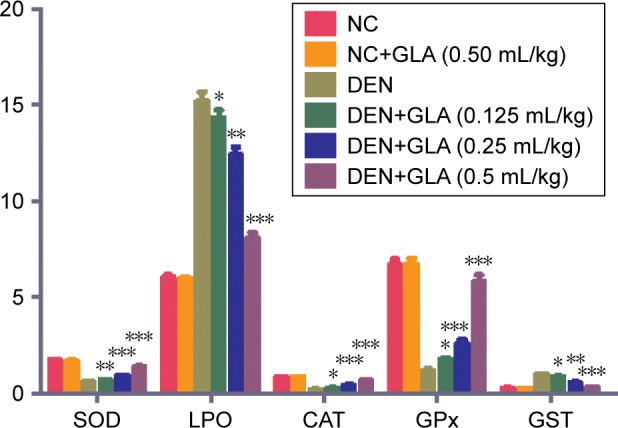
The effect of GLA on antioxidant parameters of all group rats, for SOD, LPO, CAT, GPx, and GST, as described in Materials and methods section.
Notes: All values are presented as mean±SEM. Statistical analysis by one-way ANOVA followed by Dunnett’s multiple comparison. *P<0.05, **P<0.01 and ***P<0.001.
Abbreviations: CAT, Catalase; DEN, diethylnitrosamine; GLA, gamma linolenic acid; GPx, Glutathione peroxidase; GST, Glutathione S-transferase; LPO, Lipid peroxidation; NC, normal control; SOD, Superoxide dismutase.
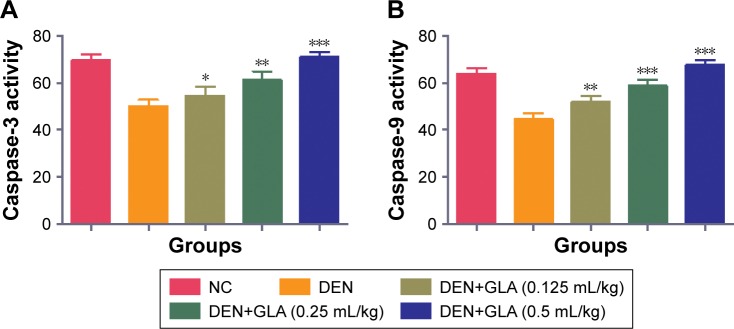
GLA effect on caspase
During the HCC disease, the level of caspase considerably decreased due to an increase in the inflammatory reactions. GLA treatment significantly (P<0.001) increased the level of caspase-3 and -7 in a dose-dependent manner (Figure 5).
Figure 5.
The effect of GLA on caspase.
Notes: (A) caspase-3 and (B) caspase-8, as described in the Materials and methods section. All values are presented as mean±SEM. Statistical analysis by one-way ANOVA followed by Dunnett’s multiple comparison. *P<0.05, **P<0.01, and ***P<0.001.
Abbreviations: DEN, diethylnitrosamine; GLA, gamma linolenic acid; NC, normal control.
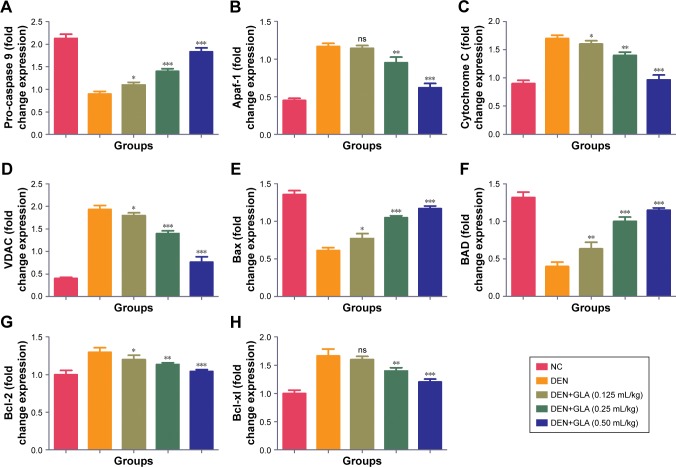
GLA boosted apoptosis in hepatic tissue
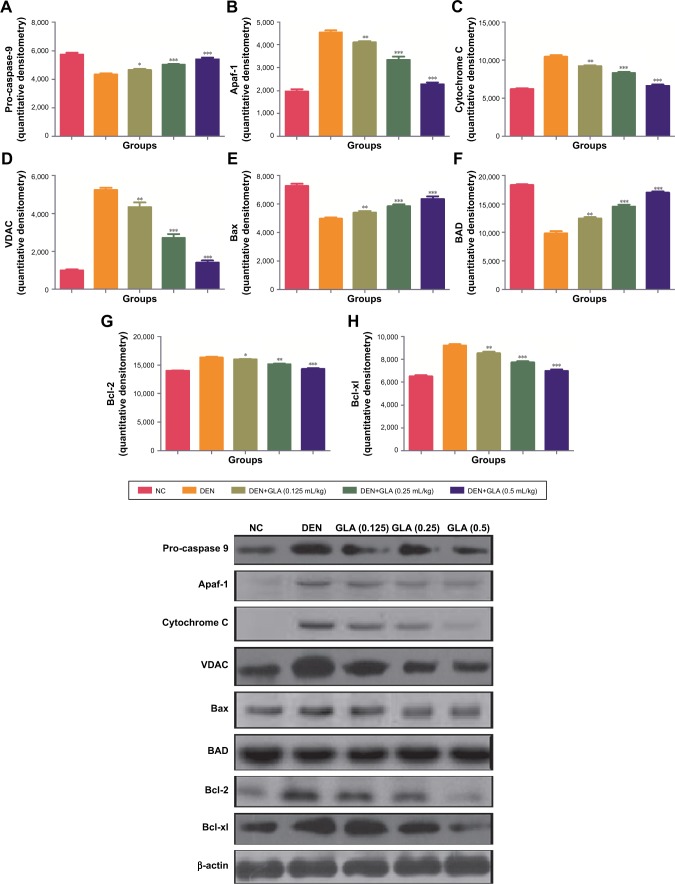
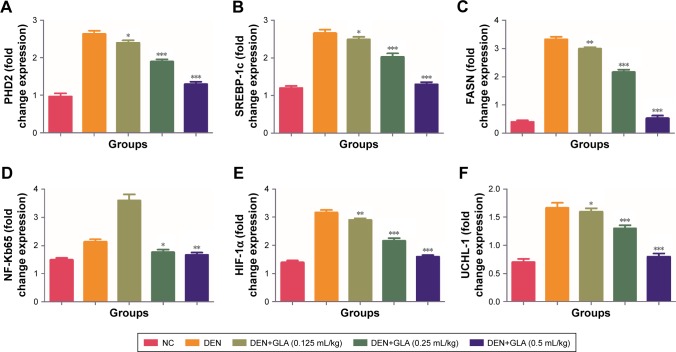
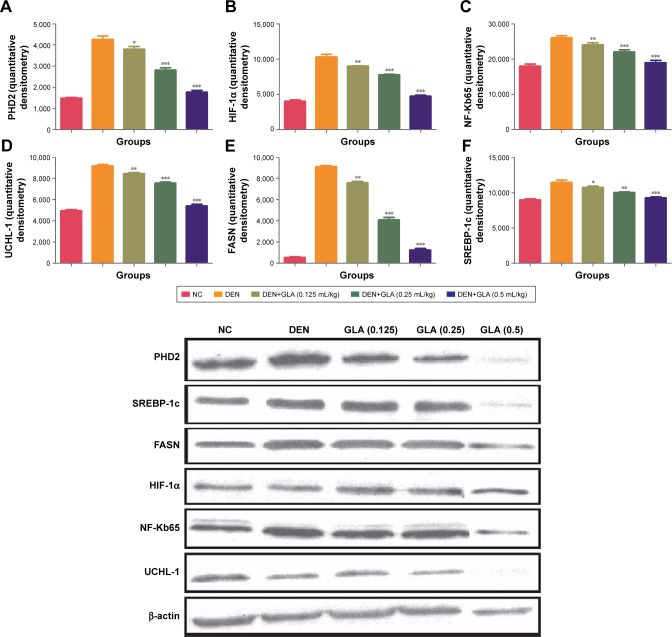
Figure 6 represents the quantitative real time PCR results explaining the critical boosting level of mRNA expression of antiapoptotic proteins and proapototic markers. DEN treatment boosted the mitochondria mediated apoptosis (cytochrome c, VADC, pro-caspase 9, and Apaf-1), along with the upregulation of cytochorme c expression (Figure 6). A similar trend was found in the Western blot (Figure 7). GLA treatment significantly protects the cell from apoptosis. DEN treatment also afforded commendable hypoxia, which was perceived via boosting the HIF-1α, FASN, UCHL-1, SREBP-1c, and NFκBp65, along with reducing PHD2 expression (Figures 8 and 9).
Figure 6.
The effect of GLA on activation of mitochondrial mediated pathway.
Notes: (A) Pro-caspase 9, (B) Apaf-1, (C) Cytochrome C, (D) VDAC, (E) Bax, (F) BAD, (G) Bcl-2, and (H) Bcl-xl, as described in the Materials and methods section. All values are presented as mean±SEM. Statistical analysis by one-way ANOVA followed by Dunnett’s multiple comparison. *P<0.05, **P<0.01, and ***P<0.001.
Abbreviations: DEN, diethylnitrosamine; GLA, gamma linolenic acid; NC, normal control; ns, non-significant.
Figure 7.
The effect of GLA on activation of mitochondrial mediated pathway via immunoblotting assay.
Notes: (A) Pro-caspase 9, (B) Apaf-1, (C) Cytochrome C, (D) VDAC, (E) Bax, (F) BAD, (G) Bcl-2, and (H) Bcl-xl, as described in the Materials and methods section. All values are presented as mean±SEM. Statistical analysis by one-way ANOVA followed by Dunnett’s multiple comparison. *P<0.05, **P<0.01 and ***P<0.001.
Abbreviations: DEN, diethylnitrosamine; GLA, gamma linolenic acid; NC, normal control.
Figure 8.
The effect of GLA on activation of hypoxic cancer cells metabolic pathway.
Notes: (A) PHD2, (B) SREBP-1c, (C) FASN, (D) NF-Kb65, (E) HIF-1α, and (F) UCHL-1, as described in the Materials and methods section. All values are presented as mean±SEM. Statistical analysis by one-way ANOVA followed by Dunnett’s multiple comparison. *P<0.05, **P<0.01 and ***P<0.001.
Abbreviations: DEN, diethylnitrosamine; GLA, gamma linolenic acid; NC, normal control.
Figure 9.
The effect of GLA on activation of hypoxic cancer cells metabolic pathway via immunoblotting assay.
Notes: (A) PHD2, (B) HIF-1α, (C) NF-Kb65, (D) UCHL-1, (E) FASN and (F) SERBC-1c, as described in the Materials and methods section. All values are presented as mean±SEM. Statistical analysis by one-way ANOVA followed by Dunnett’s multiple comparison. *P<0.05, **P<0.01 and ***P<0.001.
Abbreviations: DEN, diethylnitrosamine; GLA, gamma linolenic acid; NC, normal control.
Discussion
HCC is the most common malignancy in among all types of malignancies. The developed, as well as undeveloped countries are affected by this disease. It is considered as the most common hepatic cancer disease due to the late signs of diagnosis. Various factors, such as high consumption of ethanol, hepatitis (B and C), and aflatoxin, are various pathogens involved in the progression of disease. Generally HCC is induced via fungal poison, chemical poison, and food contamination.2,3 DEN (nitroso compound) is most commonly used to induce hepatic cancer, which induces the HCC in rodents similarly to in humans. In the current investigation, we used the DEN and phenobarbital to induce HCC in Wistar rats and we observed that DEN group rats showed the generation of hepatic nodules and dose-dependent treatment of GLA significantly (P<0.001) down-regulated the formation of these hepatic nodules.27,28 The available literature suggests that the formation of pre-cancerous hepatic nodules is the precursor of hepatic cancer. The dose-dependent treatment of GLA significantly reduced the formation of pre-cancerous hepatic nodules and suggests the chemoprotective effect of GLA on HCC.
Body weight, tissue weight, and relative tissue weight are significant parameters to estimate the expansion of the cancer disease. Several researchers suggest that, during the progression of disease, body weight reduced, and a similar result was found in our experimental study.29 DEN group rats showed that reduced body weight, as compared to the other group rats, and dose-dependent treatment of GLA significantly (P<0.001) increased the body weight in a dose-dependent manner. Other parameters, such as relative liver weight, significantly increased in the DEN group rats due to expansion of hepatic nodules, and GLA treatment significantly (P<0.001) decreased the relative hepatic weight. On the basis of this result, we can say that GLA increases the body weight and relative tissue weight. The current hypothesis was supported by a reduction of the tumor formation in GLA treatment group rats.
Biochemical markers plays an important role in screening specific conditions of malignancy and help with treatment start, hypothesis advancement observing, and finally evaluation of reactions for treatment.30,31 These catalysts are extraordinary, and changes in their level/concentration directly showed the effect on the cell multiplication with expansion of potential and their metabolic turnover.32,33 The alterations of the activity of these enzymes have been showed to relate well with the quantities changed in the cells during the malignancy condition. During the malignancy condition, there is a disorder of transport function, which is performed via cell organelles of hepatocytes. During the cancer, the enzymes start to secrete into the circulation and modulate the content in the serum due to alteration of plasma membrane permeability.34,35 The increase in enzymes into the circulation due to damage of the cell structure and its integrity alters the enzymatic concentration. Serum enzymes like ALP and transaminases are used as an indicator in liver damage. DEN group rats showed an increased level of ALT, AST, and ALP and suggest the dysfunction of hepatic tissue. Transaminases enzymes viz., AST and ALT are both directly co-related to conversion of amino acid to keto acids and play a significant role in the expansion of HCC.36 GLA treatment shows the inhibitory effect of DEN-induced boosted enzymatic activity, which is avowed via GLA and protects the liver from harm.
AFP is an oncofetal protein, considered as an indicator for hepatic cancer. During the disease, the content of AFP considerably boosts, and this is also used as a specific tool for the estimation of HCC.37,38 AFP is clinically utilized for diagnosis of tumor markers. The introduction of specific cancer inducing agents, eg, DEN, has been found to increase the concentration of AFP to a significant level.39,40 The concentration of AFP was lower during the birth but, during the HCC, the concentration of AFP considerably enhanced in HCC patients. A similar momentum was observed in our experimental study, DEN-induced group rats exhibited an increased level of AFP, which was significantly (P<0.001) down-regulated by the GLA treatment dose-dependently.
Another way to treat the disease is to scavenge the free radical. During the HCC, the free radical is induced by DNA and expands the toxic reactions. DEN induces the alteration of DNA structure, especially the expansion of alkyl DNA, and causes chromosomal abnormalities, additionally it induces the micronuclei in hepatic tissue.41,42 Most of the drug metabolizes into the liver, and DEN also digests into the hepatic tissue; during the DEN digestion it releases a lot of free radical or reactive oxygen species, which are involved in the different stages of carcinogenesis via initiation, expansion, and progression.43 It alters the endogenous antioxidant redox system and induces a disturbance in the endogenous redox systems.44 It also starts the deposition of protein and lipid into the hepatic tissue and decreases the membrane micro-viscosity of hepatic cells. Various cellular components, such as DNA, lipids, carbohydrates, low molecular weight compound, and thiol, attract to the ROS and start the oxidation of macromolecules and finally expand the pathogenesis of disease. MDA, an indicator of LPO, is a prominent marker of oxidative stress and increasing the concentration of MDA suggests an increase in oxidative stress via DEN, which was activated.45 An increase in MDA level starts damaging the cells, initiates cell death, induces oxidative stress, generates ROS, and alters cellular function finally a carcinogenesis. During the induction of disease, LPO starts the production of peroxy and alkoxy radicals, which results in dysfunction of the antioxidant system. The role of the endogenous antioxidant system is to scavenge the free radical and toxic effect of free radicals.46 SOD and CAT are both first line endogenous antioxidant enzymes, which play a significant role in protecting the cell from the free radical via scavenging the superoxide into hydrogen peroxide and detoxification of the hydrogen peroxide.47 During the disease, the levels of SOD and CAT both decline, and dose-dependent treatment of GLA significantly (P<0.001) increases the MDA level as compared to DEN control and suggests the antioxidant effect of GLA.
Angiogenesis and cell proliferation play significant roles in cancer progression.48,49 Both mechanisms have a substantial role in the tissue architecture and the same is found in the morphological evaluation.50 The apoptosis can be defined via two pathways, such as mitochondrial intrinsic and death receptor mediated apoptotic pathways. Bcl-2 family protein regulates the mitochondrial pathway via involvement of pro- and anti-apoptotic protein members. During the apoptosis process, BAD and BAX (proapoptotic) protein translocate into the mitochondria outer membrane and boost the secretion of cytochrome c.51,52 On the contrary, Bcl-2 and BCL-XL (antiapoptotic) proteins down-regulate the secretion of cytochrome C.50 In the current experimental study, we observed that the GLA treatment enhanced the expression of Bcl-2 and BCL-XL (antiapoptotic) and decreased the expression of BAD and BAX (proapoptotic). The same momentum was observed in the mRNA expression estimated via q-RT-PCR. Cytochrome c secretion triggers the congregation of cytochrome apopto-some and apoptosome is consider as the complex form of cytochrome-c, procaspase 9, and Apaf-1. The formation of apoptosome reduces the cytosolic level of pro-caspase-9 and Apaf-1. A similar result was observed in our experimental study, and the expression of VDAC and cytochrome c is reduced during progress of the disease. We found cleavage of procaspase 9 with the generation of apoptosome and lead the caspase 9 formation, which further activated the caspase 3 and 8.53 The activation of caspase starts the activation of a downstream caspase cascade leading to apoptosis. On the basis of the result, we can say that GLA treatment reduced the proliferative and angiogenic effect of DEN via activation of a mitochondrial mediated apoptosis pathway.
It is well documented that tumor cells want energy from the glycolysis due to the hypoxic condition of cells.54 Previous published literature suggests that enhanced glycolytic activity in tumor cells boost the synthesis of fatty acid, which is required via de novo fatty acid synthesis.55,56 Hypoxia regulates via HIF-1α and also regulates via PHD2 (iron dependent hydroxylases enzyme) and 2-oxoglutarate.57 The HIF-1α expression significantly reduces and confirms down-regulation of UCHL-1 and NF-kBp65 expression.58,59 Concentration-dependent treatment of GLA inhibits the expression of SREBP-1 and FASN (marker of de nova fatty acid synthesis). We can say that GLA down-regulated the DEN induced hypoxia and protected rodent HCC.
Conclusion
Henceforth, On the basis of this result, we can conclude that GLA exhibited a chemoprotective effect against the DEN induced HCC via a regulated hypoxia induced cell signaling pathway, anti-inflammatory pathway, and mitochondria mediated death apoptosis.
Abbreviations
- HCC
hepatocellular carcinoma
- GLA
gamma linolenic acid
- DEN
diethylnitrosamine
- RT-PCR
real-time polymerase chain reaction
- PUFAs
polyunsaturated fatty acids
- AA
arachidonic acid
- COX
cyclooxygenase
- EBSS
eagle balanced salt solution
- AST
aspartate ami-notransferase
- ALT
alanine aminotransferase
- AFP
alpha-fetoprotein
- ALP
alkaline phosphatise
- CMC
carboxymethyl cellulose
- SOD
superoxide dismutase
- CAT
catalase
- LPO
lipid peroxidation
- GSH
glutathione
- GPx
glutathione peroxidase
- RIPA
radioimmunoprecipitation assay
- A549
adenocarcinomic human alveolar basal epithelial cells
- ZR-75-1
human breast cancer cell line
- PC-3
human prostate cancer
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Cummings LC, Payes JD, Cooper GS. Survival after hepatic resection in metastatic colorectal cancer: a population-based study. Cancer. 2007;109(4):718–726. doi: 10.1002/cncr.22448. [DOI] [PubMed] [Google Scholar]
- 2.Balogh J, Victor D, Asham EH, et al. Hepatocellular carcinoma: a review. J Hepatocell Carcinoma. 2016;3:41–53. doi: 10.2147/JHC.S61146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 4.Benedict M, Zhang X. Non-alcoholic fatty liver disease: An expanded review. World J Hepatol. 2017;9(16):715–¨C732. doi: 10.4254/wjh.v9.i16.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lafaro KJ, Demirjian AN, Pawlik TM. Epidemiology of hepatocellular carcinoma. Surg Oncol Clin N Am. 2015;24(1):1–17. doi: 10.1016/j.soc.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 6.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 7.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379(9822):1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 8.Farazi PA, Depinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6(9):674–687. doi: 10.1038/nrc1934. [DOI] [PubMed] [Google Scholar]
- 9.Benedict M, Zhang X. Non-alcoholic fatty liver disease: An expanded review. World J Hepatol. 2017;9(16):715–C732. doi: 10.4254/wjh.v9.i16.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264–1273. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verma A, Ahmed B, Anwar F, et al. Novel glycoside from Wedelia calendulacea inhibits diethyl nitrosamine-induced renal cancer via downregulating the COX-2 and PEG2 through nuclear factor-κB pathway. Inflammopharmacology. 2017;25(1):159–175. doi: 10.1007/s10787-017-0310-y. [DOI] [PubMed] [Google Scholar]
- 12.Kumar V, Bhatt PC, Rahman M, et al. Fabrication, optimization, and characterization of umbelliferone β-D-galactopyranoside-loaded PLGA nanoparticles in treatment of hepatocellular carcinoma: in vitro and in vivo studies. Int J Nanomedicine. 2017;12:6747–6758. doi: 10.2147/IJN.S136629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kadasa NM, Abdallah H, Afifi M, Gowayed S. Hepatoprotective effects of curcumin against diethyl nitrosamine induced hepatotoxicity in albino rats. Asian Pac J Cancer Prev. 2015;16(1):103–108. doi: 10.7314/apjcp.2015.16.1.103. [DOI] [PubMed] [Google Scholar]
- 14.Afzal M, Kazmi I, Gupta G, Rahman M, Kimothi V, Anwar F. Preventive effect of Metformin against N-nitrosodiethylamine-initiated hepatocellular carcinoma in rats. Saudi Pharm J. 2012;20(4):365–370. doi: 10.1016/j.jsps.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mandal AK, Ghosh D, Sarkar S, Ghosh A, Swarnakar S, Das N. Nano-capsulated quercetin downregulates rat hepatic MMP-13 and controls diethylnitrosamine-induced carcinoma. Nanomedicine. 2014;9(15):2323–2337. doi: 10.2217/nnm.14.11. [DOI] [PubMed] [Google Scholar]
- 16.Calder PC. Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot Essent Fat Acids. 2006;75(3):197–202. doi: 10.1016/j.plefa.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Chowdhury R, Warnakula S, Kunutsor S, et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med. 2014;160(6):398–406. doi: 10.7326/M13-1788. [DOI] [PubMed] [Google Scholar]
- 18.Roy S, Rawat AK, Sammi SR, et al. Alpha-linolenic acid stabilizes HIF-1 α and downregulates FASN to promote mitochondrial apoptosis for mammary gland chemoprevention. Oncotarget. 2017;8(41):70049–70071. doi: 10.18632/oncotarget.19551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saha B, Patro BS, Koli M, Pai G, Ray J, Bandyopadhyay SK, Chattopadhyay S. trans-4,4′-Dihydroxystilbene (DHS) inhibits human neuroblastoma tumor growth and induces mitochondrial and lysosomal damages in neuroblastoma cell lines. Oncotarget. 2017;8(43):73905–73924. doi: 10.18632/oncotarget.17879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yadav RK, Singh M, Roy S, Ansari MN, Saeedan AS, Kaithwas G. Modulation of oxidative stress response by flaxseed oil: Role of lipid peroxidation and underlying mechanisms. Prostaglandins Other Lipid Mediat. 2018;135:21–26. doi: 10.1016/j.prostaglandins.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Rani A, Roy S, Singh M, et al. α-Chymotrypsin regulates free fatty acids and UCHL-1 to ameliorate N-methyl nitrosourea induced mammary gland carcinoma in albino wistar rats. Inflammopharmacology. 2016;24(5):277–286. doi: 10.1007/s10787-016-0280-5. [DOI] [PubMed] [Google Scholar]
- 22.Balakumar P, Taneja G. Fish oil and vascular endothelial protection: bench to bedside. Free Radic Biol Med. 2012;53(2):271–279. doi: 10.1016/j.freeradbiomed.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Khan R, Kazmi I, Afzal M, et al. Fixed dose combination therapy loperamide and niacin ameliorates diethylnitrosamine-induced liver carcinogenesis in albino Wistar rats. RSC Adv. 2015;5(83):67996–68002. [Google Scholar]
- 24.Anwar F, Mushtaq G, Kazmi I, et al. Anticancer effect of rosiglitazone in rats treated with N-nitrosodiethylamine via inhibition of DNA synthesis: an implication for hepatocellular carcinoma. RSC Adv. 2015;5(84):68385–68391. [Google Scholar]
- 25.Kumar V, Bhatt PC, Rahman M, Al-Abbasi FA, Anwar F, Verma A. Umbelliferon-α-d-glucopyranosyl-(2 I → 1 II)-α-Dglucopyranoside ameliorates Diethylnitrosamine induced precancerous lesion development in liver via regulation of inflammation, hyperproliferation and antioxidant at pre-clinical stage. Biomed Pharmacother. 2017;94:834–842. doi: 10.1016/j.biopha.2017.07.047. [DOI] [PubMed] [Google Scholar]
- 26.Verma A, Singh D, Anwar F, Bhatt PC, Al-Abbasi F, Kumar V. Triterpenoids principle of Wedelia calendulacea attenuated diethynitrosamine-induced hepatocellular carcinoma via down-regulating oxidative stress, inflammation and pathology via NF-kB pathway. Inflammopharmacology. 2018;26(1):133–146. doi: 10.1007/s10787-017-0350-3. [DOI] [PubMed] [Google Scholar]
- 27.Wang Q, Yu WN, Chen X, et al. Spontaneous Hepatocellular Carcinoma after the Combined Deletion of Akt Isoforms. Cancer Cell. 2016;29(4):523–535. doi: 10.1016/j.ccell.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghosh D, Choudhury ST, Ghosh S, et al. Nanocapsulated curcumin: oral chemopreventive formulation against diethylnitrosamine induced hepatocellular carcinoma in rat. Chem Biol Interact. 2012;195(3):206–214. doi: 10.1016/j.cbi.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Damodaran A, Kabali B. Autonomic dysfunction in central obesity. World J Med Sci. 2013;8(2):118–122. [Google Scholar]
- 30.Henao-Mejia J, Elinav E, Jin C, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482(7384):179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dapito DH, Mencin A, Gwak GY, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21(4):504–516. doi: 10.1016/j.ccr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iida G, Asano K, Seki M, et al. Intraoperative identification of canine hepatocellular carcinoma with indocyanine green fluorescent imaging. J Small Anim Pract. 2013;54(11):594–600. doi: 10.1111/jsap.12148. [DOI] [PubMed] [Google Scholar]
- 33.Sawan AS. Prevalence of Obstruction Meibomian Gland Disease among Ophthalmology Patients. J King Abdulaziz Univ. 2009;16(2):69–76. [Google Scholar]
- 34.Park EJ, Lee JH, Yu GY, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140(2):197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lv P, Lin XZ, Li J, Li W, Chen K. Differentiation of small hepatic hemangioma from small hepatocellular carcinoma: recently introduced spectral CT method. Radiology. 2011;259(3):720–729. doi: 10.1148/radiol.11101425. [DOI] [PubMed] [Google Scholar]
- 36.Abbasi A, Bhutto AR, Butt N, Munir SM. Corelation of serum alpha fetoprotein and tumor size in hepatocellular carcinoma. J Pak Med Assoc. 2012;62(1):33–36. [PubMed] [Google Scholar]
- 37.Mizejewski GJ. Levels of alpha-fetoprotein during pregnancy and early infancy in normal and disease states. Obstet Gynecol Surv. 2003;58(12):804–826. doi: 10.1097/01.OGX.0000099770.97668.18. [DOI] [PubMed] [Google Scholar]
- 38.Mizejewski GJ. Biological roles of alpha-fetoprotein during pregnancy and perinatal development. Exp Biol Med. 2004;229(6):439–463. doi: 10.1177/153537020422900602. [DOI] [PubMed] [Google Scholar]
- 39.Mizejewski GJ. Review of the putative cell-surface receptors for alpha-fetoprotein: identification of a candidate receptor protein family. Tumour Biol. 2011;32(2):241–258. doi: 10.1007/s13277-010-0134-5. [DOI] [PubMed] [Google Scholar]
- 40.Hishinuma M, Ohashi KI, Yamauchi N, et al. Hepatocellular oncofetal protein, glypican 3 is a sensitive marker for alpha-fetoprotein-producing gastric carcinoma. Histopathology. 2006;49(5):479–486. doi: 10.1111/j.1365-2559.2006.02522.x. [DOI] [PubMed] [Google Scholar]
- 41.Shanab SM, Mostafa SS, Shalaby EA, Mahmoud GI. Aqueous extracts of microalgae exhibit antioxidant and anticancer activities. Asian Pac J Trop Biomed. 2012;2(8):608–615. doi: 10.1016/S2221-1691(12)60106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luedde T, Beraza N, Kotsikoris V, et al. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell. 2007;11(2):119–132. doi: 10.1016/j.ccr.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 43.Prasad KN, Xie H, Hao J, et al. Antioxidant and anticancer activities of 8-hydroxypsoralen isolated from wampee [Clausena lansium (Lour.) Skeels] peel. Food Chem. 2010;118(1):62–66. [Google Scholar]
- 44.Ip BC, Wang XD. Non-alcoholic steatohepatitis and hepatocellular carcinoma: implications for lycopene intervention. Nutrients. 2013;6(1):124–162. doi: 10.3390/nu6010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sutton A, Nahon P, Pessayre D, et al. Genetic polymorphisms in antioxidant enzymes modulate hepatic iron accumulation and hepatocellular carcinoma development in patients with alcohol-induced cirrhosis. Cancer Res. 2006;66(5):2844–2852. doi: 10.1158/0008-5472.CAN-05-2566. [DOI] [PubMed] [Google Scholar]
- 46.Federico A, Dallio M, Loguercio C. Silymarin/Silybin and chronic liver disease: A marriage of many years. Molecules. 2017;22(2):191. doi: 10.3390/molecules22020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimizu M, Shirakami Y, Sakai H, et al. Chemopreventive potential of green tea catechins in hepatocellular carcinoma. Int J Mol Sci. 2015;16(3):6124–6139. doi: 10.3390/ijms16036124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ouyang L, Shi Z, Zhao S, et al. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012;45(6):487–498. doi: 10.1111/j.1365-2184.2012.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lunt SY, vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 50.Michaylira CZ, Nakagawa H. Hypoxic microenvironment as a cradle for melanoma development and progression. Cancer Biol Ther. 2006;5(5):476–479. doi: 10.4161/cbt.5.5.2749. [DOI] [PubMed] [Google Scholar]
- 51.Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015;15(9):540–555. doi: 10.1038/nrc3982. [DOI] [PubMed] [Google Scholar]
- 52.Sarvaiya PJ, Guo D, Ulasov I, Gabikian P, Lesniak MS. Chemokines in tumor progression and metastasis. Oncotarget. 2013;4(12):2171–2185. doi: 10.18632/oncotarget.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park DH, Shin JW, Park SK, et al. Diethylnitrosamine (DEN) induces irreversible hepatocellular carcinogenesis through overexpression of G1/S-phase regulatory proteins in rat. Toxicol Lett. 2009;191(2–3):321–326. doi: 10.1016/j.toxlet.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 54.Guillaumond F, Leca J, Olivares O, et al. Strengthened glycolysis under hypoxia supports tumor symbiosis and hexosamine biosynthesis in pancreatic adenocarcinoma. Proc Natl Acad Sci U S A. 2013;110(10):3919–3924. doi: 10.1073/pnas.1219555110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schornack PA, Gillies RJ. Contributions of cell metabolism and H+ diffusion to the acidic pH of tumors. Neoplasia. 2003;5(2):135–145. doi: 10.1016/s1476-5586(03)80005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu RSS. Hypoxia: From molecular responses to ecosystem responses. Mar Pollut Bull. 2002;45(1–12):35–45. doi: 10.1016/s0025-326x(02)00061-9. [DOI] [PubMed] [Google Scholar]
- 57.Aragonés J, Schneider M, van Geyte K, et al. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet. 2008;40(2):170–180. doi: 10.1038/ng.2007.62. [DOI] [PubMed] [Google Scholar]
- 58.Siddiq A, Ayoub IA, Chavez JC, et al. Hypoxia-inducible factor prolyl 4-hydroxylase inhibition. A target for neuroprotection in the central nervous system. J Biol Chem. 2005;280(50):41732–41743. doi: 10.1074/jbc.M504963200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woo KJ, Lee TJ, Park JW, Kwon TK. Desferrioxamine, an iron chelator, enhances HIF-1α accumulation via cyclooxygenase-2 signaling pathway. Biochem Biophys Res Commun. 2006;343(1):8–14. doi: 10.1016/j.bbrc.2006.02.116. [DOI] [PubMed] [Google Scholar]
- 60.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]