Abstract
STUDY QUESTION
Does incisional endometriosis (IE) harbor somatic cancer-driver mutations?
SUMMARY ANSWER
We found that approximately one-quarter of IE cases harbor somatic-cancer mutations, which commonly affect components of the MAPK/RAS or PI3K-Akt-mTor signaling pathways.
WHAT IS KNOWN ALREADY
Despite the classification of endometriosis as a benign gynecological disease, it shares key features with cancers such as resistance to apoptosis and stimulation of angiogenesis and is well-established as the precursor of clear cell and endometrioid ovarian carcinomas. Our group has recently shown that deep infiltrating endometriosis (DE), a form of endometriosis that rarely undergoes malignant transformation, harbors recurrent somatic mutations.
STUDY DESIGN, SIZE, DURATION
In a retrospective study comparing iatrogenically induced and endogenously occurring forms of endometriosis unlikely to progress to cancer, we examined endometriosis specimens from 40 women with IE and 36 women with DE. Specimens were collected between 2004 and 2017 from five hospital sites in either Canada, Germany or the Netherlands. IE and DE cohorts were age-matched and all women presented with histologically typical endometriosis without known history of malignancy.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Archival tissue specimens containing endometriotic lesions were macrodissected and/or laser-capture microdissected to enrich endometriotic stroma and epithelium and a hypersensitive cancer hotspot sequencing panel was used to assess for presence of somatic mutations. Mutations were subsequently validated using droplet digital PCR. PTEN and ARID1A immunohistochemistry (IHC) were performed as surrogates for somatic events resulting in functional loss of respective proteins.
MAIN RESULTS AND THE ROLE OF CHANCE
Overall, we detected somatic cancer-driver events in 11 of 40 (27.5%) IE cases and 13 of 36 (36.1%) DE cases, including hotspot mutations in KRAS, ERBB2, PIK3CA and CTNNB1. Heterogeneous PTEN loss occurred at similar rates in IE and DE (7/40 vs 5/36, respectively), whereas ARID1A loss only occurred in a single case of DE. While rates of detectable somatic cancer-driver events between IE and DE are not statistically significant (P > 0.05), KRAS activating mutations were more prevalent in DE.
LIMITATIONS, REASONS FOR CAUTION
Detection of somatic cancer-driver events were limited to hotspots analyzed in our panel-based sequencing assay and loss of protein expression by IHC from archival tissue. Whole genome or exome sequencing, or epigenetic analysis may uncover additional somatic alterations. Moreover, because of the descriptive nature of this study, the functional roles of identified mutations within the context of endometriosis remain unclear and causality cannot be established.
WIDER IMPLICATIONS OF THE FINDINGS
The alterations we report may be important in driving the growth and survival of endometriosis in ectopic regions of the body. Given the frequency of mutation in surgically displaced endometrium (IE), examination of similar somatic events in eutopic endometrium, as well as clinically annotated cases of other forms of endometriosis, in particular endometriomas that are most commonly linked to malignancy, is warranted.
STUDY FUNDING/COMPETING INTEREST(S)
This study was funded by a Canadian Cancer Society Impact Grant [701603, PI Huntsman], Canadian Institutes of Health Research Transitional Open Operating Grant [MOP-142273, PI Yong], the Canadian Institutes of Health Research Foundation Grant [FDN-154290, PI Huntsman], the Canadian Institutes of Health Research Project Grant [PJT-156084, PIs Yong and Anglesio], and the Janet D. Cottrelle Foundation through the BC Cancer Foundation [PI Huntsman]. D.G. Huntsman is a co-founder and shareholder of Contextual Genomics Inc., a for profit company that provides clinical reporting to assist in cancer patient treatment. R. Aguirre-Hernandez, J. Khattra and L.M. Prentice have a patent MOLECULAR QUALITY ASSURANCE METHODS FOR USE IN SEQUENCING pending and are current (or former) employees of Contextual Genomics Inc. The remaining authors have no competing interests to declare.
TRIAL REGISTRATION NUMBER
Not applicable.
Keywords: endometriosis, somatic mutations, targeted sequencing, iatrogenic disease, cesarean section, incisional scar
Introduction
Endometriosis is a chronic, estrogen-dependent, inflammatory gynecological disease characterized by the growth and persistence of endometrial-like glands and stroma outside of the uterus (Giudice, 2010; Vercellini et al., 2014). Roughly 10% of reproductive-aged women and 50% of women experiencing chronic pelvic pain or infertility may be affected by endometriosis (Giudice, 2010). Clinical symptoms associated with endometriosis include chronic and/or cyclical pelvic pain, dyspareunia, dysmenorrhea and subfertility (Giudice, 2010; Vercellini et al., 2014). Not only does endometriosis greatly impair the health-related quality of life and work productivity of affected women (Nnoaham et al., 2011), but it poses a substantial economic burden on the healthcare system. The estimated cost of endometriosis (including both direct and indirect costs) to the USA in 2009 was roughly $69.4 billion USD (Simoens et al., 2012), thereby highlighting the importance of studying this disease beyond associated risk of certain cancers.
Despite the prevalence of endometriosis, its pathogenesis and mechanism of dissemination are poorly understood. Endometriosis primarily manifests itself in the pelvic region in three distinct forms: superficial peritoneal endometriosis, deep infiltrating endometriosis (DE) and ovarian endometriomas (cystic masses affecting the ovary) (Vercellini et al., 2014). It is important to note that endometriosis is not restricted to the pelvis (Giudice, 2010; Matalliotakis et al., 2017); past studies have reported on rare cases of extra-pelvic endometriosis in the lungs, liver, pericardium, surgical scars and even the central nervous system (Gunes et al., 2005; Ceccaroni et al., 2010; Bourgioti et al., 2017; Matalliotakis et al., 2017). Moreover, the specific origin of endometriosis is contentious and several theories on its etiology have been proposed including retrograde menstruation (the reflux of endometrial fragments through the fallopian tubes during menstruation), coelomic metaplasia, Müllerian remnants and lymphatic/vascular dissemination (Vercellini et al., 2014). While each theory is supported by at least circumstantial evidence, none appears sufficient to fully explain every incident case of endometriosis—it may be plausible that different mechanisms may give rise to distinct types of endometriosis.
Although endometriosis is estimated to progress to cancer in only 1% of cases (Vercellini et al., 2014), studies have established endometriosis as the precursor to clear cell ovarian carcinomas (CCOCs) and endometrioid ovarian carcinomas (ENOCs) (Anglesio et al., 2015; Anglesio and Yong, 2017). Women suffering from endometriosis have a 2–4-fold greater risk of developing these cancers (Pearce et al., 2012), moreover, mutational studies demonstrate a clonal relationship between endometriosis-associated ovarian carcinomas and endometriotic lesions. For instance, CCOCs or ENOCs and concurrent endometriotic lesions from the same cases harbored identical somatic mutations in ARID1A or PIK3CA (Wiegand et al., 2010; Anglesio et al., 2015)—consequently, these alterations contribute to the mutational burden in ovarian endometriosis and have widely been considered early events in the malignant transformation of such lesions.
Our group has recently shown that DE harbors recurrent somatic cancer-driver mutations (Anglesio et al., 2017). Malignant transformation of this particular form of endometriosis is exceedingly rare. Nonetheless, we identified somatic cancer-driver hotspot mutations in PIK3CA, KRAS and PPP2R1A, and loss of function mutations in ARID1A, all together affecting 7/27 (25.9%) cases of DE subjected to broad genomic analysis. KRAS activating mutations were confirmed in 3/12 additional cases with focused analysis (Anglesio et al., 2017). The function of these mutations in endometriosis is unclear despite the presence of these mutations being clearly non-random. While endometriosis is not considered a malignancy, it shares many notable pathophysiological features with cancers, such as resistance to apoptosis and stimulation of angiogenesis (Taylor et al., 2002; Bulun, 2009). Endometriosis is also capable of invading local tissue, such as in DE (Kavallaris et al., 2003). Therefore, such somatic cancer-driver mutations may be advantageous for the overall survival and growth of endometrial tissue outside of a native uterine microenvironment. Considering the relative rarity of malignant transformation of endometriosis overall, and particularly DE, it is unlikely that these mutations act solely as early events in malignant transformation. Nevertheless, to understand the importance of these cancer-driver mutations in a non-malignant etiology, it is crucial to determine their prevalence across various forms of the disease.
In the current study, we sought to investigate the prevalence of somatic cancer-driver mutations in endometriosis by comparing DE to another tissue-infiltrating form of endometriosis that is unlikely to undergo malignant transformation. Specifically, we examined incisional endometriosis (IE), an iatrogenic form of endometriosis that occurs in the resulting surgical scars of obstetric or gynecological procedures (Leng et al., 2006). Unlike other forms of endometriosis, the uterine origin of cells is well accepted for IE: endometrial cells, both stroma and epithelium, are mechanically transferred to the abdominal fascia or subcutaneous tissue around sites of incision following procedures such as cesarean sections, hysterectomies, myomectomies appendectomies, tubal ligations and episiotomies (Kaloo et al., 2002; Gunes et al., 2005; Nominato et al., 2010). We compared somatic driver mutation profiles between DE and IE to determine whether there were differences in mutation profile between these two types of endometriosis with unique differences in their etiologies (endogenously occurring vs iatrogenically induced, respectively).
Materials and Methods
Patient identification and tissue collection
We obtained formalin-fixed and paraffin-embedded (FFPE) tissue specimens from four independent cohorts of women with IE. The Vancouver General Hospital in Vancouver, BC, Canada contributed endometriotic tissue samples from 12 IE patients. The Referral Centre for Gynecopathology in Mannheim, Germany contributed tissue samples from 10 IE patients. The University Hospital Tuebingen in Tuebingen, Germany contributed tissue samples from 15 IE patients. Lastly, the VU University Medical Center (VUMC) in Amsterdam, The Netherlands contributed tissue samples from three IE patients. Inclusion criteria for the IE cohort were diagnosis with incisional, umbilical or post C-sectional endometriosis lesions containing both epithelial and stromal components by extensive pathology review, the absence of cancer or dysplasia, and a lesion size sufficient for tissue coring, macrodissection, and/or laser-capture microdissection (LCM). Details of prior surgery and the time interval between suspected inciting surgery and subsequent diagnosis with IE were available for most, but not all, patients (Supplementary Table SI). Note that a few cases included in our IE cohort lacked details of surgical history, or only had a history of surgical abortion, and therefore may represent spontaneous cases of abdominal wall or subcutaneous endometriosis (rather than iatrogenic disease). Adjacent tissue blocks of endometriosis (same anatomical site) were available for sampling for some IE patients (Supplementary Table SI).
In addition, we obtained FFPE or molecular-fixed (Sakura Finetek, USA) and paraffin-embedded (MFPE) tissue specimens from two independent cohorts of women with DE. Endometriotic tissue samples from 23 DE patients were retrieved from local pathology archives and the prospective tissue bank at the BC Women’s Centre for Pelvic Pain and Endometriosis in Vancouver, BC, Canada. Ten cases (Patients 41–50) overlap with our previous study (Supplementary Table SII) wherein they were analyzed by droplet digital PCR for KRAS mutations alone (Anglesio et al., 2017). Here we include them with a broader genomic analysis as noted below. The VUMC contributed tissue samples from an additional 13 DE patients. Inclusion criteria for the DE cohort were local invasion >5 mm, pathologist-confirmed endometriosis, the absence of cancer or dysplasia, and a lesion size sufficient for tissue coring, macrodissection and/or LCM. Blocks of tissue representing DE at distant/anatomically distinct sites were available for several cases (Supplementary Table SII).
Ethics approval
Institutional review boards at each respective hospital approved tissue collection and collection of clinical data. See the Supplemental methods for further details.
Sample processing and DNA extraction
Except as noted below, specimens were sectioned at 8 μm onto glass slides, deparaffinized with xylene and stained with 10% diluted hematoxylin and eosin (H&E; Supplementary Fig. S1). Using a standard H&E slide as a guide, we manually macrodissected the stained specimens under a stereo microscope using the tip of a 20-guage needle. All tissues for patients 38–40 and 64–76 were enriched by laser-captured microdissection (LCM) by sectioning at 5 μm onto PEN membrane slides (Leica Microsystems Inc., Switzerland), staining with Toluidine blue, and dissecting stromal and epithelial components of endometriosis together using the Leica Laser MicroDissection (LMD) 7 system (Leica Microsystems Inc., Switzerland).
DNA from all enriched specimen was extracted using the ARCTURUS® PicoPure® DNA Extraction Kit (ThermoFisher Scientific, USA) and quantitated using the Qubit 2.0 Fluorometer (ThermoFisher Scientific, USA).
Additionally, a subset of samples initially macrodissected and where somatic mutations were observed, were subject to LCM of distinct stromal and epithelial compartments of endometriosis so as to ascertain which cell populations were affected. DNA was extracted as noted above for other LCM samples.
Targeted sequencing
A proprietary hypersensitive cancer hotspot assay, FIND ITTM version 3.4 (Contextual Genomics, Canada), was used to sequence macrodissected or laser captured endometriotic specimens. Hotspot regions from 33 genes were analyzed (Supplementary Table SI). Libraries were constructed using 45–75 ng of total DNA input. Quality assurance methods based on DNA sequence barcodes were incorporated into the assay and bioinformatics pipeline to increase sensitivity of called mutations. Candidate variants for orthogonal validation were selected as those with probability scores ≥0.8 and variant allele frequency (VAF) ≥0.8% for macrodissected samples or VAF ≥ 5.0% for laser-captured samples, as well as having been previous reported in the Catalogue of Somatic Mutations in Cancer (COSMIC) (Forbes et al., 2017).
Droplet digital PCR assays
We used droplet digital polymerase-chain-reaction (ddPCR) assays to orthogonally validate hotspot mutations identified by targeted sequencing. Moreover, independent of the targeted sequencing results, one sample from each patient was also analyzed for all common KRAS activating G12 mutations (G12A, G12C, G12D, G12V, G12R, G12S, a subset of samples also included G13D) by ddPCR assays to rule out false positive and false negative next-generation sequencing-based errors related to KRAS (see Supplemental methods for primer/probes sequence). DNA from macrodissected specimens was pre-amplified for 10 cycles before subsequent droplet generation using the QX200 Droplet Generator (Bio-Rad Laboratories, USA). After thermal cycling, the QX200 Droplet Reader (Bio-Rad Laboratories, USA) was used to quantify droplets.
ddPCR was also used to detect select mutations from distinct LCM-enriched stromal and epithelial compartments from a subset of endometriosis lesions (see text and Supplemental methods).
ARID1A and PTEN immunohistochemistry
Loss of nuclear ARID1A immunoreactivity was used as a surrogate for ARID1A loss-of-function mutations (Khalique et al., 2018; Kobel et al., 2018). Specimens were stained either on the Dako Omnis automated immunostainer (Agilent Technologies, USA) using a 1:150 dilution of an ARID1A rabbit polyclonal antibody, HPA005456 (Sigma-Aldrich), or on the BenchMark Ultra autostainer (Ventana Medical Systems, USA) using a 1:100 dilution of the same ARID1A rabbit polyclonal antibody (HPA005456). Similarly, loss of PTEN immunoreactivity was used as a surrogate for PTEN loss-of-function mutations. Specimens were stained either on the Ventana Discovery Ultra (Ventana Medical Systems, USA) immunostainer using a 1:25 dilution of rabbit monoclonal antibody, 138G6 (Cell Signaling, USA), or on the BenchMark Ultra autostainer (Ventana Medical Systems, USA) using a 1:100 dilution of rabbit monoclonal antibody, SP218 (Spring Bioscience, USA). T.M.N., B.T.C. or H.M.H. scored all ARID1A and PTEN immunostained slides. Specific detail on assays used for individual specimens can be found in the Supplemental methods.
Statistical analyses
The Student’s t-test was used to compare mean age of IE and DE patients included in this study. Since somatic cancer-driver events are not mutually exclusive of one another, we conducted the Fisher’s exact test on each individual pairwise comparison of driver event rates in IE and DE to assess whether they were significantly different. All tests were two-sided and a P-value <0.05 was considered to be statistically significant.
Results
Sample description
We examined somatic mutations in common cancer hotspots in 40 women with IE (total of 59 specimens studied), and in 36 women with DE (total of 43 specimens studied). The mean age of women with IE (Patients 1–40) was 36.5 years (28–49 years) (Supplementary Table SII and Supplementary Fig. S2). Between one and four tissue blocks from each patient were collected and analyzed. In four patients (Patients 5, 10, 12 and 28) eutopic endometrium samples were available for sequencing—we did not detect somatic cancer-driver mutations in the available eutopic endometrium specimens. Of IE cases with obtainable surgical history, the original surgical procedure performed was most often cesarean section and the interval between the most recent gynecological or obstetric surgery and subsequent diagnosis with IE ranged from 1 month to 11 years. The mean age of women with DE (Patients 41–76) was 33.9 years (22–50 years) (Supplementary Table SIII and Supplementary Fig. S2). Although most women were affected with DE at a single anatomical site, several women had multiple DE lesions at distinct anatomical sites, these additional lesions were included in analysis when available. The mean age of women in the IE and DE cohorts were not significantly different (P = 0.0765, Student’s t-test) (Supplementary Fig. S2).
Sequencing findings
Of 40 patients with IE, four patients (10.0%) harbored somatic COSMIC hotspot mutations in either KRAS (2), PIK3CA (1) or ERBB2 (1) (Table I). Of 36 patients with DE, eight patients (22.2%) harbored somatic cancer-driver mutations in either KRAS (7) or CTNNB1 (1) (Table I and Supplementary Table SIV). No association between detection of mutations and institutional cohort or processing was observed.
Table I.
Somatic cancer-driver mutations detected in endometriosis (EMS) specimens from women with incisional endometriosis (IE) and deep infiltrating endometriosis (DE). The variant allele frequency (VAF) of macrodissected or laser-capture microdissected (LCM) specimens as determined by means of targeted panel sequencing and corresponding droplet digital PCR (ddPCR) assays are presented below. ‘Adjacent’ refers to tissue specimens obtained from a different archival tissue block yet the same anatomical site as the index block. ‘Separate’ refers to specimens obtained from an anatomically distinct site from the index block.
| EMS type | Patient and block | Descriptor | Driver mutation identified | Collection method and component | VAF (%)—targeted sequencing | VAF (%)— ddPCR |
|---|---|---|---|---|---|---|
| IE | 8A | Index | KRAS G12V | Macrodissection: mixed | 3.04 | 2.53 |
| KRAS G12V | LCM: mixed | 28.7 | ||||
| 8B | Adjacent | KRAS G12V | Macrodissection: mixed | Not detected | 3.29 | |
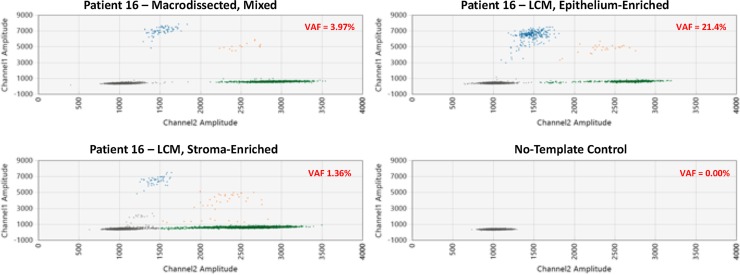
| IE | 16A | Index | ERBB2 S310F | Macrodissection: mixed | 3.336 | 3.97 |
| ERBB2 S310F | LCM: mixed | 18.2 | ||||
| ERBB2 S310F | LCM: epithelium | 21.4 | ||||
| ERBB2 S310F | LCM: stroma | 1.36 | ||||
| IE | 19A | Index | KRAS G12C | Macrodissection: mixed | 4.833 | 3.32 |
| KRAS G12C | LCM: mixed | 29.5 | ||||
| IE | 25A | Index | PIK3CA H1047R | Macrodissection: mixed | 5.359 | 5.79 |
| PIK3CA H1047R | LCM: epithelium | 24.9 | ||||
| PIK3CA H1047R | LCM: stroma | 0.567 | ||||
| DE | 41A | Index | CTNNB1 G34V | Macrodissection: mixed | 3.933 | 3.88 |
| CTNNB1 G34V | LCM: epithelium | 19.5 | ||||
| CTNNB1 G34V | LCM: stroma | 0.664 | ||||
| DE | 42A | Index | KRAS G12D | Macrodissection: mixed | 2.807 | 2.14 |
| KRAS G12D | LCM: epithelium | 38.125 | ||||
| KRAS G12D | LCM: stroma | 0.002 | ||||
| DE | 45A | Index | KRAS G12D | Macrodissection: mixed | 0.932 | n/a |
| KRAS G12D | LCM: mixed | 2.065 | ||||
| DE | 50A | Index | KRAS G12V | Macrodissection: mixed | Not detected | 0.941 |
| KRAS G12V | LCM: mixed | 3.589 | ||||
| DE | 51A | Index | KRAS G12D | Macrodissection: mixed | 1.108 | 1.03 |
| DE | 54A | Index | KRAS G12C | Macrodissection: mixed | 1.05 | 1.19 |
| DE | 61B | Separate | KRAS G12V | Macrodissection: mixed | 2.627 | 2.81 |
| DE | 72A | Index | KRAS G12A | LCM: mixed | 10.749 | 10.41 |
Analyzing laser-captured microdissected epithelial and stromal cell fractions using ddPCR, we previously determined KRAS G12 mutations to be restricted to the glandular epithelial component of endometriotic lesions (Anglesio et al., 2017). In this study, using the same method, we were able to confirm that similar to KRAS observations: CTNNB1, PIK3CA and ERBB2 hotspot mutations were also enriched only in the glandular-epithelial compartment of endometriotic lesions (Table I and Fig. 1; Supplementary Fig. S3). Hotspot KRAS mutations remained the most common somatic cancer-driver mutations detected in both IE and DE: there were KRAS mutations in 2 of 40 patients with IE (5%) compared to 7 of 36 patients with DE (19.4%), however, given our limited sample sizes, this difference is not significant (P = 0.076, Fisher’s exact test).
Figure 1.
Droplet digital PCR (ddPCR) validation of ERBB2 c.929C>T (p.S310F) mutation in the epithelial component of endometriosis in Patient 16. Patient 16 harbors an ERRB2 S310F mutation as detected by ddPCR in a macrodissected endometriosis sample. The variant allelic frequency (VAF) in the epithelium-enriched, laser-captured microdissected (LCM) sample (from the same tissue block) is notably higher than in the stroma-enriched LCM sample (21.4 vs 1.4%, respectively), consistent with this somatic alteration being epithelial-restricted.
Immunohistochemistry
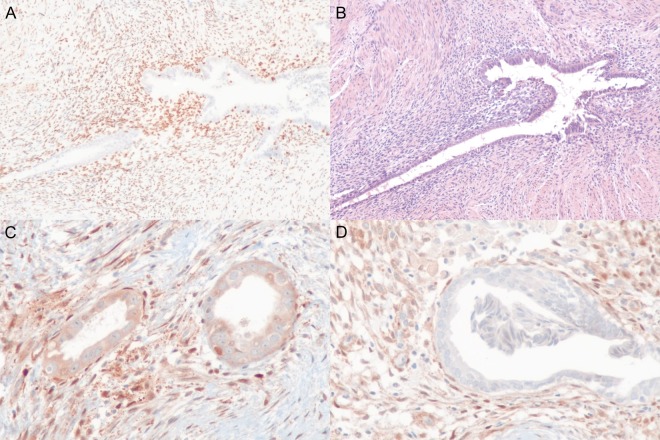
Immunohistochemical (IHC) staining revealed loss of ARID1A protein to be a rare event with only a single case of ARID1A-loss in a DE case (1/36; 3%) and no detectable loss in IE cases (Fig. 2A and B; Supplementary Tables SV and SVI). Conversely, 7 of 40 patients with IE (18%), and 5 of 36 patients with DE, (14%) exhibited loss of PTEN. Whole slide sections revealed a heterogeneous pattern of PTEN loss in endometriotic lesions, wherein only some glands demonstrate loss of PTEN immunoreactivity (Fig. 2C and D; Supplementary Fig. S4; Supplementary Tables SV and SVI). While in some cases PTEN-null glands tended to be clustered (Supplementary Fig. S4), in others the number of endometriosis glands were limited and thus difficult to infer spatial distribution. Consistent with laser-capture analysis of cancer-driver mutations, we observed ARID1A-loss and PTEN-loss only in the epithelial compartment of endometriotic lesions.
Figure 2.
Immunohistochemistry of deep and incisional endometriosis samples showing (A) loss of ARID1A in epithelial endometriosis cells and (B) matching haematoxylin and eosin staining from deep endometriosis patient 47. Similarly, we observed both deep and incisional endometriosis with heterogeneous expression of PTEN. For example, incisional endometriosis patient 17 (C) shows intermediate PTEN expression in some regions of glandular epithelium, while (D) loss of PTEN expression was apparent in other regions. In both the context of ARID1A and PTEN immunohistochemistry, the endometriosis stromal compartment (and surrounding normal tissues) provides a clear, block-specific, positive control with strong expression visible for both proteins, respectively.
Total mutation rates
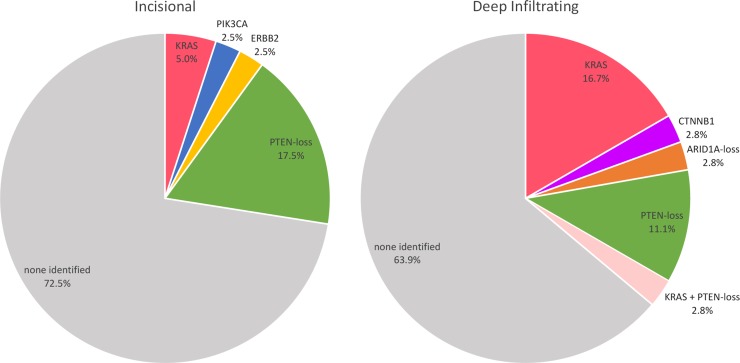
Accounting for both ddPCR-validated somatic COSMIC hotspot mutations and the IHC findings, the overall rate of somatic cancer-driver events in IE and DE was 27.5% (proportion) and 36.1% (proportion), respectively. The pattern of somatic mutations compared between the IE and DE cases is illustrated in Fig. 3 (see also Supplemental methods and Supplementary Table SVII).
Figure 3.
Overview of somatic cancer-driver events in incisional endometriosis (left) and deep infiltrating endometriosis (right). The reported proportion of cases is based on an observed event from any specimen(s) from each case, this is important in the context of deep endometriosis especially as multiple blocks and anatomical sites were assayed. Our results suggest that somatic alterations were sub-clonal in all informative cases, therefore, it is possible that additional re-sampling may increase the proportion of patients in both deep or incisional categories with one or more observable somatic alteration in one or more lesion(s).
Discussion
Beyond the association of endometriosis and ovarian cancer, endometriosis is an understudied disease as its origin remains contentious and pathophysiology poorly understood. An estimated 176 million women are affected by endometriosis worldwide (Adamson et al., 2010) and thus the disease has a profound impact on the healthcare costs and wellbeing of women in many different countries. Current standard treatment for endometriosis consists of adjunctive medical therapy or surgical resection of endometriotic lesions (Giudice, 2010), however, rates of endometriosis recurrence may be as high as 43–68% (Tandoi et al., 2011). Furthermore, surgical staging of endometriosis, largely based on anatomical presentation, does not appear to correlate well with pain symptoms (Vercellini et al., 2007) and lacks prognostic value for clinical endpoints such as recurrence or risk of malignant transformation (Johnson et al., 2017). Expanding recent finding of somatic molecular alterations across endometriosis types stands to benefit endometriosis classification and may lead to a novel and more biologically informative system of classification. Widespread knowledge on the prevalence of mutations may highlight common pathway dysfunction. Even with difficulties in targeting RAS-pathway (Samatar and Poulikakos, 2014) and potential toxicities related to PI3K-Akt pathway inhibitors (Engelman, 2009), molecular characterization may justify the use of targeted therapies in select circumstances and will undoubtedly drive innovation for novel intervention strategies.
Our previous study revealed the presence of recurrent somatic cancer-driver mutations (particularly KRAS) in DE (Anglesio et al., 2017). In the current study, we analyzed the prevalence of somatic cancer-driver events in IE, another form of endometriosis with little malignant potential, using a hypersensitive cancer hotspot assay combined with orthogonal validation by ddPCR or IHC staining. We found that the overall rates of somatic cancer-driver events to be similar for IE and DE, moreover, the spectrum of affected pathways was similar. The similarity in the rates of mutation and mutational profile of IE and DE is consistent with endometriotic cells in both forms of endometriosis originating from a similar etiology. Because IE is accepted to originate from endometrial cells in the uterus via iatrogenic transplantation, this may further the uterine origin of DE as well (e.g. secondary to retrograde menstruation).
Although our sample sizes were insufficiently large to conclude differences in either overall rates of somatic events or enrichment of particular alterations when comparing IE and DE, it is apparent that alterations resulting in upregulation of the MAPK/RAS or PI3K-Akt-mTOR signaling pathways are present in a substantial fraction of endometriosis cases. In endometriosis, somatic alterations in these pathways may confer a survival advantage to cells. For example via production of high levels of VEGF and BCL-2 or BCL-xL and therefore the stimulation of angiogenesis and resistance to apoptosis respectively (Taylor et al., 2002; Beliard et al., 2004; Braun et al., 2007; Pylayeva-Gupta et al., 2011). In fact, studies have demonstrated the upregulation of VEGF and other pro-angiogenic factors in oncogenic KRAS-transformed epithelial cells (Matsuo et al., 2009; Wang et al., 2015), though not yet in the context of endometriosis. Additionally, the expression of oncogenic Ras results in the upregulation of BCL-xL in colon cancer cells and the upregulation of both BCL-2 and BCL-xL in hematopoietic cells in vitro (Kinoshita et al., 1995; Okamoto et al., 2015). In support of our hypothesis, Cheng et al. (2011) were able to develop a mouse model of endometriosis by transplanting endometrium from KRASG12V/+ donor mice into subcutaneous, abdominal pockets of immunocompetent recipient mice. In this model, oncogenic KRAS promoted the formation of endometriosis and enabled the prolonged survival of endometriotic lesions but does not result in malignant transformation (Cheng et al., 2011). In a more recent study, KRAS activation in (mouse) eutopic endometrial tissues was suggested to regulate progesterone receptor transcriptional function via SIRT1 and lead to progesterone resistance (Yoo et al., 2017). SIRT1 has also been shown to be required in maintaining stemness/enabling transformation in a Ras-driven model of glial tumors and increases activation of p44/42 (ERK) seen in combination with reduced cellular senescence in lung fibroblasts (Huang et al., 2008; Lee et al., 2015; O’Callaghan and Vassilopoulos, 2017). However, it should be noted that neither of these models are estrogen/progesterone dependent. While SIRT1 activity linked to KRAS activation may indeed be important in endometriosis pathogenesis, any link to progesterone receptor activity remains tenuous, in particular since the Yoo et al. (2007) model may be confounded due to its use of the Pgr-CRE promotor to induce KRASG12D expression. Nonetheless, before accepting any potential mechanism related to our observed cancer-driver mutations, model systems incorporating somatic mutations into non-malignant endometriosis must be improved and interrogated.
Our data also broadly question the defacto-labeling of ‘cancer driver’ genes as single mutational hits do not appear adequate to trigger malignant progression of endometriosis. To date, the generation of (mouse) models resembling the subtypes of ovarian cancer associated with endometriosis (CCOCs or ENOCs) require at minimum two somatic alterations (Dinulescu et al., 2005; Wu et al., 2007; Chandler et al., 2015), and none develop a validated physiological analogue of endometriosis. Furthermore, mutations in oncogenes such as KRAS and tumor suppressor genes such as PTEN are well described to trigger cellular senescence in benign lesions after an initial period of proliferation (Courtois-Cox et al., 2008). It remains to be seen if such a paradigm of negative feedback could apply to endometriosis carrying driver gene alterations, e.g. could such alterations provide sufficient proliferative advantage for lesion establishment yet ultimately abrogate progression? However, endometriotic lesions grow, or persist, in 78% of cases and rarely subside spontaneously (Abbott et al., 2004) and endometriosis is reported to exhibit aberrant response to anti-proliferative signals.
Similar to our previous report, ddPCR assays and IHC staining revealed that all somatic cancer-driver events observed (hotspot mutations in KRAS, CTNNB1 and PIK3CA, loss of PTEN, or loss of ARID1A) affected only the epithelial compartment of endometriotic lesions. Moreover, visualization of lesions with PTEN-loss or ARID1A-loss revealed that only some, generally clustered, glands were affected by these somatic events whereas other surrounding glands had normal expression—consistent with clonal expansion of an affected epithelial cell. While our methods do not allow us to make similar observation for the hotspot mutations, we would expect this to be the case. Curiously, we observed PTEN-loss in several women with IE or DE yet, based on our analysis, it is unclear by which mechanism loss of PTEN occurs in endometriosis. Targeted panel sequencing did not reveal loss-of-function hotspot point mutations in PTEN, despite partial coverage over this gene. However, it is possible that PTEN is lost through large-scale deletion or epigenetic mechanisms/methylation.
Finally, it is unclear whether mutations independently arise in implanted endometrial cells or whether they are already present in the endometrium prior to implantation/seeding. In other words, are such cancer-driver events naturally present at low levels in the eutopic endometrium? It is conceivable that accumulation of somatic alterations, including driver mutations in selective/permissive microenvironments, reflects the aging of tissues (Risques and Kennedy, 2018). A recent study analyzing uterine lavage fluid reported cancer-associated mutations, including mutations in KRAS and PIK3CA, in roughly half of women analyzed (51 of 95) that lacked histopathological evidence of (endometrial) cancer (Nair et al., 2016). Likewise, peritoneal washing revealed TP53 mutations in 19 of 20 control women (women unaffected by cancer or reported benign pathology), albeit at ultra-low allelic frequencies (<0.1%), with an apparent increase in mutational burden correlating with age (Krimmel et al., 2016). As the mutations we have identified in women with IE and DE are common in CCOCs and ENOCs (Committee on the State of the Science in Ovarian Cancer Research, 2016; Wang et al., 2017), establishing the prevalence of these mutation across types of endometriosis is warranted, in particular for endometriomas where relative risk of malignant transformations is considerably higher (Saavalainen et al., 2018).
By taking into account the diffuse cellular make-up of endometriosis specimens and challenges of ultra-low input sequencing from FFPE tissue, we have confirmed the presence of somatic cancer-driver events in women with IE as well as DE. These two forms of endometriosis are associated with very low malignant potential, however, nearly one-third (31.6%) of all endometriosis cases analyzed harbored cancer-driver events, most commonly activating mutations of KRAS and loss of PTEN expression. Our screen for mutations is not exhaustive, and it is possible that whole genome/exome sequencing, or epigenetic analysis, may uncover additional somatic ‘driver’ events. Nevertheless, it is evident that these somatic events, particularly those involving the RAS/MAPK pathway or PI3K-Akt-mTOR pathway, are inherent features of endometriosis outside of the context of cancer and represent potential mechanisms that contribute to endometriosis pathology. Further exploration on the prevalence and function of these alterations is greatly needed to enhance our understanding and management of this vastly understudied disease.
Supplementary Material
Acknowledgements
We thank the women who have allowed us to use their tissue samples in our research study. We would also like to thank the staff at the Genetic Pathology Evaluation Centre (GPEC) and the Department of Anatomical Pathology at the Vancouver General Hospital in Vancouver for their assistance with specimen collection, immunohistochemical staining and optimization, and tissue sectioning. Lastly, we would like to acknowledge the NKI-AVL Core Facility Molecular Pathology & Biobanking (CFMPB) for supplying NKI-AVL Biobank material and /or lab support.
Author’s Roles
V.L., H.M.H., P.J.Y., D.G.H. and M.S.A. designed the study. V.L., L.V., T.M.N., B.T.C., T.P., N.L.O., H.N., A.L., J.K., L.M.P., D.C., M.K., V.M., A.F.L., J.P., M.C.B., B.K., S.Y.B., F.K. and S.K. collected specimens and data. T.M.N., B.T.C. and H.M.H. scored immunostained slides. V.L. and R.A.H. performed data analysis. V.L. and L.V. drafted the article. All authors revised the manuscript and approved submission of the final version.
Funding
Canadian Cancer Society Impact Grant [701603, PI Huntsman], Canadian Institutes of Health Research Transitional Open Operating Grant [MOP-142273, PI Yong], the Canadian Institutes of Health Research Foundation Grant [FDN-154290, PI Huntsman], the Canadian Institutes of Health Research Project Grant [PJT-156084, PIs Yong and Anglesio], and the Janet D. Cottrelle Foundation through the BC Cancer Foundation [PI Huntsman]. The BC Women’s Hospital and Health Centre Foundation and Women’s Health Research Institute (Nelly Auersperg Grant) provided support to the BC Women’s Centre for Pelvic Pain and Endometriosis. The BC Cancer Foundation and the VGH and UBC Hospital Foundation provided funding to OVCARE: BC’s Ovarian Cancer Research Team (including V.L., T.M.N., B.C.C., T.P., A.L., D.C., P.J.Y., D.G.H. and M.S.A.). A Frederick Banting and Charles Best Canada Graduate Scholarship from the Canadian Institutes of Health Research provided support to V.L. The Dutch Cancer Society translational research fellowship [KWF 2013-5869] provided support to H.M.H. The Michael Smith Foundation for Health Research Health Professional Investigator Award provided support to P.J. Yong. The Janet D. Cottrelle Foundation Scholars fund provided support to M.S. Anglesio. The Dr Chew Wei Memorial Professorship in Gynecologic Oncology and the Canada Research Chairs program (Research Chair in Molecular and Genomic Pathology) provided support to D.G. Huntsman.
Conflict of interest
D.G.H. is a co-founder and shareholder of Contextual Genomics Inc., a for profit company that provides clinical reporting to assist in cancer patient treatment. R.A.-H., J.K. and L.M.P. have a patent MOLECULAR QUALITY ASSURANCE METHODS FOR USE IN SEQUENCING pending and are current (or former) employees of Contextual Genomics Inc. The remaining authors have no competing interests to declare.
References
- Abbott J, Hawe J, Hunter D, Holmes M, Finn P, Garry R. Laparoscopic excision of endometriosis: a randomized, placebo-controlled trial. Fertil Steril 2004;82:878–884. [DOI] [PubMed] [Google Scholar]
- Adamson GD, Kennedy SH, Hummelshoj L. Creating solutions in endometriosis: global collaboration through the World Endometriosis Research Foundation. J Endometr 2010;2:3–6. [Google Scholar]
- Anglesio MS, Bashashati A, Wang YK, Senz J, Ha G, Yang W, Aniba MR, Prentice LM, Farahani H, Li Chang H et al. Multifocal endometriotic lesions associated with cancer are clonal and carry a high mutation burden. J Pathol 2015;236:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglesio MS, Papadopoulos N, Ayhan A, Nazeran TM, Noe M, Horlings HM, Lum A, Jones S, Senz J, Seckin T et al. Cancer-associated mutations in endometriosis without cancer. N Engl J Med 2017;376:1835–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglesio MS, Yong PJ. Endometriosis-associated ovarian cancers. Clin Obstet Gynecol 2017;60:711–727. [DOI] [PubMed] [Google Scholar]
- Beliard A, Noel A, Foidart JM. Reduction of apoptosis and proliferation in endometriosis. Fertil Steril 2004;82:80–85. [DOI] [PubMed] [Google Scholar]
- Bourgioti C, Preza O, Panourgias E, Chatoupis K, Antoniou A, Nikolaidou ME, Moulopoulos LA. MR imaging of endometriosis: spectrum of disease. Diagn Interv Imaging 2017;98:751–767. [DOI] [PubMed] [Google Scholar]
- Braun DP, Ding J, Shaheen F, Willey JC, Rana N, Dmowski WP. Quantitative expression of apoptosis-regulating genes in endometrium from women with and without endometriosis. Fertil Steril 2007;87:263–268. [DOI] [PubMed] [Google Scholar]
- Bulun SE. Endometriosis. N Engl J Med 2009;360:268–279. [DOI] [PubMed] [Google Scholar]
- Ceccaroni M, Clarizia R, Placci A. Pericardial, pleural, and diaphragmatic endometriosis. J Thorac Cardiovasc Surg 2010;140:1189–1190. [DOI] [PubMed] [Google Scholar]
- Chandler RL, Damrauer JS, Raab JR, Schisler JC, Wilkerson MD, Didion JP, Starmer J, Serber D, Yee D, Xiong J et al. Coexistent ARID1A-PIK3CA mutations promote ovarian clear-cell tumorigenesis through pro-tumorigenic inflammatory cytokine signalling. Nat Commun 2015;6:6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CW, Licence D, Cook E, Luo F, Arends MJ, Smith SK, Print CG, Charnock-Jones DS. Activation of mutated K-ras in donor endometrial epithelium and stroma promotes lesion growth in an intact immunocompetent murine model of endometriosis. J Pathol 2011;224:261–269. [DOI] [PubMed] [Google Scholar]
- Committee on the State of the Science in Ovarian Cancer Research; Board on Health Care Services; Institute of Medicine; National Academies of Sciences, Engineering, and Medicine. Ovarian cancers: evolving paradigms in research and care. Washington, DC: National Academies Press (US), 2016. [PubMed]
- Courtois-Cox S, Jones SL, Cichowski K. Many roads lead to oncogene-induced senescence. Oncogene 2008;27:2801–2809. [DOI] [PubMed] [Google Scholar]
- Dinulescu DM, Ince TA, Quade BJ, Shafer SA, Crowley D, Jacks T. Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat Med 2005;11:63–70. [DOI] [PubMed] [Google Scholar]
- Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer 2009;9:550–562. [DOI] [PubMed] [Google Scholar]
- Forbes SA, Beare D, Boutselakis H, Bamford S, Bindal N, Tate J, Cole CG, Ward S, Dawson E, Ponting L et al. COSMIC: somatic cancer genetics at high-resolution. Nucleic Acids Res 2017;45:D777–D783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudice LC. Clinical practice. Endometriosis. N Engl J Med 2010;362:2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunes M, Kayikcioglu F, Ozturkoglu E, Haberal A. Incisional endometriosis after cesarean section, episiotomy and other gynecologic procedures. J Obstet Gynaecol Res 2005;31:471–475. [DOI] [PubMed] [Google Scholar]
- Huang J, Gan Q, Han L, Li J, Zhang H, Sun Y, Zhang Z, Tong T. SIRT1 overexpression antagonizes cellular senescence with activated ERK/S6k1 signaling in human diploid fibroblasts. PLoS One 2008;3:e1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NP, Hummelshoj L, Adamson GD, Keckstein J, Taylor HS, Abrao MS, Bush D, Kiesel L, Tamimi R, Sharpe-Timms KL et al. World Endometriosis Society consensus on the classification of endometriosis. Hum Reprod 2017;32:315–324. [DOI] [PubMed] [Google Scholar]
- Kaloo P, Reid G, Wong F. Caesarean section scar endometriosis: two cases of recurrent disease and a literature review. Aust N Z J Obstet Gynaecol 2002;42:218–220. [DOI] [PubMed] [Google Scholar]
- Kavallaris A, Kohler C, Kuhne-Heid R, Schneider A. Histopathological extent of rectal invasion by rectovaginal endometriosis. Hum Reprod 2003;18:1323–1327. [DOI] [PubMed] [Google Scholar]
- Khalique S, Naidoo K, Attygalle AD, Kriplani D, Daley F, Lowe A, Campbell J, Jones T, Hubank M, Fenwick K et al. Optimised ARID1A immunohistochemistry is an accurate predictor of ARID1A mutational status in gynaecological cancers. J Pathol Clin Res 2018;4:154–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Yokota T, Arai K, Miyajima A. Regulation of Bcl-2 expression by oncogenic Ras protein in hematopoietic cells. Oncogene 1995;10:2207–2212. [PubMed] [Google Scholar]
- Kobel M, Anglesio MS, Brenton JD. You won’t believe this old test… that does cheap single-cell mutation detection. J Pathol Clin Res 2018;4:149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimmel JD, Schmitt MW, Harrell MI, Agnew KJ, Kennedy SR, Emond MJ, Loeb LA, Swisher EM, Risques RA. Ultra-deep sequencing detects ovarian cancer cells in peritoneal fluid and reveals somatic TP53 mutations in noncancerous tissues. Proc Natl Acad Sci USA 2016;113:6005–6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Park JR, Kwon OS, Lee TH, Nakano I, Miyoshi H, Chun KH, Park MJ, Lee HJ, Kim SU et al. SIRT1 is required for oncogenic transformation of neural stem cells and for the survival of ‘cancer cells with neural stemness’ in a p53-dependent manner. Neuro Oncol 2015;17:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng J, Lang J, Guo L, Li H, Liu Z. Carcinosarcoma arising from atypical endometriosis in a cesarean section scar. Int J Gynecol Cancer 2006;16:432–435. [DOI] [PubMed] [Google Scholar]
- Matalliotakis M, Goulielmos GN, Kalogiannidis I, Koumantakis G, Matalliotakis I, Arici A. Extra pelvic endometriosis: retrospective analysis on 200 cases in two different countries. Eur J Obstet Gynecol Reprod Biol 2017;217:34–37. [DOI] [PubMed] [Google Scholar]
- Matsuo Y, Campbell PM, Brekken RA, Sung B, Ouellette MM, Fleming JB, Aggarwal BB, Der CJ, Guha S. K-Ras promotes angiogenesis mediated by immortalized human pancreatic epithelial cells through mitogen-activated protein kinase signaling pathways. Mol Cancer Res 2009;7:799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair N, Camacho-Vanegas O, Rykunov D, Dashkoff M, Camacho SC, Schumacher CA, Irish JC, Harkins TT, Freeman E, Garcia I et al. Genomic analysis of uterine lavage fluid detects early endometrial cancers and reveals a prevalent landscape of driver mutations in women without histopathologic evidence of cancer: a prospective cross-sectional study. PLoS Med 2016;13:e1002206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nnoaham KE, Hummelshoj L, Webster P, d’Hooghe T, de Cicco Nardone F, de Cicco Nardone C, Jenkinson C, Kennedy SH, Zondervan KT, World Endometriosis Research Foundation Global Study of Women’s Health consortium . Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril 2011;96:366–373.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nominato NS, Prates LF, Lauar I, Morais J, Maia L, Geber S. Caesarean section greatly increases risk of scar endometriosis. Eur J Obstet Gynecol Reprod Biol 2010;152:83–85. [DOI] [PubMed] [Google Scholar]
- O’Callaghan C, Vassilopoulos A. Sirtuins at the crossroads of stemness, aging, and cancer. Aging Cell 2017;16:1208–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K, Zaanan A, Kawakami H, Huang S, Sinicrope FA. Reversal of mutant KRAS-mediated apoptosis resistance by concurrent Noxa/Bik induction and Bcl-2/Bcl-xL antagonism in colon cancer cells. Mol Cancer Res 2015;13:659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, Webb PM, Nagle CM, Doherty JA, Cushing-Haugen KL, Wicklund KG et al. Assocation between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncol 2012;13:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D. RAS oncogenes: weaving a tumorigenic web. Nat Rev Cancer 2011;11:761–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risques RA, Kennedy SR. Aging and the rise of somatic cancer-associated mutations in normal tissues. PLoS Genet 2018;14:e1007108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavalainen L, Lassus H, But A, Tiitinen A, Harkki P, Gissler M, Pukkala E, Heikinheimo O. Risk of gynecologic cancer according to the type of endometriosis. Obstet Gynecol 2018;131:1095–1102. [DOI] [PubMed] [Google Scholar]
- Samatar AA, Poulikakos PI. Targeting RAS-ERK signalling in cancer: promises and challenges. Nat Rev Drug Discov 2014;13:928–942. [DOI] [PubMed] [Google Scholar]
- Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, Brodszky V, Canis M, Colombo GL, DeLeire T et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod 2012;27:1292–1299. [DOI] [PubMed] [Google Scholar]
- Tandoi I, Somigliana E, Riparini J, Ronzoni S, Vigano’ P, Candiani M. High rate of endometriosis recurrence in young women. J Pediatr Adolesc Gynecol 2011;24:376–379. [DOI] [PubMed] [Google Scholar]
- Taylor RN, Lebovic DI, Mueller MD. Angiogenic factors in endometriosis. Ann N Y Acad Sci 2002;955:89–100. discussion 118, 396–406. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod 2007;22:266–271. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Vigano P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol 2014;10:261–275. [DOI] [PubMed] [Google Scholar]
- Wang YK, Bashashati A, Anglesio MS, Cochrane DR, Grewal DS, Ha G, McPherson A, Horlings HM, Senz J, Prentice LM et al. Genomic consequences of aberrant DNA repair mechanisms stratify ovarian cancer histotypes. Nat Genet 2017;49:856–865. [DOI] [PubMed] [Google Scholar]
- Wang ZD, Wei SQ, Wang QY. Targeting oncogenic KRAS in non-small cell lung cancer cells by phenformin inhibits growth and angiogenesis. Am J Cancer Res 2015;5:3339–3349. [PMC free article] [PubMed] [Google Scholar]
- Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, Senz J, McConechy MK, Anglesio MS, Kalloger SE et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med 2010;363:1532–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R, Hendrix-Lucas N, Kuick R, Zhai Y, Schwartz DR, Akyol A, Hanash S, Misek DE, Katabuchi H, Williams BO et al. Mouse model of human ovarian endometrioid adenocarcinoma based on somatic defects in the Wnt/beta-catenin and PI3K/Pten signaling pathways. Cancer Cell 2007;11:321–333. [DOI] [PubMed] [Google Scholar]
- Yoo JY, Kim TH, Fazleabas AT, Palomino WA, Ahn SH, Tayade C, Schammel DP, Young SL, Jeong JW, Lessey BA. KRAS Activation and over-expression of SIRT1/BCL6 Contributes to the Pathogenesis of Endometriosis and Progesterone Resistance. Sci Rep 2017;7:6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.