Abstract
Background/Objective:
Midkine (MDK) and pleiotrophin (PTN) are two closely related heparin-binding growth factors which are overexpressed in a wide variety of human cancers. We hypothesized that these factors in washout of biopsy needles would be higher in breast and lung cancer than in benign lesions.
Methods:
Seventy subjects underwent pre-operative core needle biopsies of 78 breast masses (16 malignancies). In 11 subjects, fine needle aspiration was performed ex vivo on 7 non-small cell lung cancers and 11 normal lung specimens within surgically excised lung tissue. The biopsy needle was washed with buffer for immunoassay.
Results:
The MDK/DNA and the PTN/DNA ratio in most of the malignant breast masses were similar to the ratios in benign masses except one lobular carcinoma in situ (24-fold higher PTN/DNA ratio than the average benign mass). The MDK/DNA and PTN/DNA ratio were similar in most malignant and normal lung tissue except one squamous cell carcinoma (38-fold higher MDK/DNA ratio than the average of normal lung tissue).
Conclusions:
Both MDK and PTN are readily measurable in washout of needle biopsy samples from breast and lung masses and that levels may be highly elevated only in a specific subset of these malignancies.
Keywords: midkine, pleiotrophin, needle biopsy, breast mass, lung mass
Background
Midkine (MDK) and pleiotrophin (PTN) are two members of the heparin-binding growth factor family which are highly expressed in multiple embryonic tissues and in many malignant tissues. MDK is a basic cysteine-rich polypeptide with a molecular weight of 13 kDa. It is expressed in multiple tissues in the mouse embryo and decreases to undetectable levels by adulthood in many tissues, including breast [1–3]. High expression of MDK mRNA has been found in various human cancers such as breast, lung, stomach, colon, liver, ovary, urinary bladder, prostate, glioblastomas, neuroblastomas and Wilms’ tumor [4–7]. PTN is a related basic polypeptide with a molecular weight of 15 kDa, which is also expressed in multiple tissues of the mouse embryo and decreases with age in most tissues other than nervous tissue. PTN, like MDK, has been found to be overexpressed in various human cancers, including human breast, prostate, ovary, lung, pancreas, choriocarcinoma, melanoma, glioblastoma, and multiple myeloma [8, 9]. Both cytokines have been found to promote tumor growth, invasion and angiogenesis [10].
Both MDK and PTN act on several receptors, such as receptor-type protein-tyrosine phosphatase ζ (RPTP ζ), Anaplastic lymphoma kinase (ALK), Integrins, neuroglycan C (NGC), Low-density lipoprotein (LDL) receptor related protein (LRP), syndecan (SDC), and Notch [11]. Prior evidence suggests that MK and PTN act in part through the MAPK pathway which plays significant roles in cell proliferation and survival [12–14]. There is also evidence that expression of these growth factors can be induced by EGF and PDGF [15–16].
We recently reported that both MDK and PTN protein levels are increased in washouts of fine needle aspiration (FNA) biopsies of malignant thyroid nodules compared to benign thyroid nodules, suggesting that measurement of these two growth factors by ELISA in FNA biopsies might provide adjunctive diagnostic and/or prognostic information to supplement current cytological and molecular approaches [17–18]. Some prior studies suggest that MDK and PTN are overexpressed in breast and lung cancers [4–9].
Objectives
We therefore hypothesized that tissue concentrations measured by ELISA might also be elevated in needle biopsy samples of breast and lung cancer compared to benign breast masses and normal lung. As a pilot study to test this hypothesis, we obtained washout fluid from core needle biopsies of breast masses and from ex vivo FNA lung samples and measured MDK and PTN using high-sensitivity ELISAs. To adjust for tissue content, we normalized the resulting concentrations to DNA content.
Materials and Methods
Subjects and Sample Collection
For the study of breast cancer, subjects [n=70] were adult women who underwent core needle biopsies of breast masses at the Walter Reed National Military Medical Center (WRNMMC), protocol 385478, between August 2013 and June 2014. Core biopsies were performed for standard diagnostic indications under ultrasound (n=60 masses), mammography (n=17), or MRI guidance (n=1) using a 14-gauge core needle for ultrasound-guided or 9-gauge core needle device for mammography- or MRI-guided biopsies. After samples were collected from the needle lumen for conventional histology, the interior and exterior of the core needles were rinsed with 500 μL of phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA). Thus, the samples for immunoassays did not include the solid piece of tissue in core biopsy needle. The washout was aliquoted and stored immediately at −80 °C until the assays were performed. The cells in the washout were likely lysed because they were subjected to freezing for storage and because samples were diluted into buffer containing 0.5% Tween 20. Histological diagnoses were made by standard pathological examination.
Peripheral blood (2.7 mL) was collected in a plastic citrate tube at the time of biopsy from 39 subjects with breast masses who subsequently had a histological diagnosis. The blood was centrifuged at 4 °C for 15 minutes (3,000 g) within two hours of venipuncture. Plasma was aliquoted and stored at −80 °C until MDK assay.
For the study of lung cancer, subjects (n=12) were adults who underwent surgical wedge resection of lung nodules at the WRNMMC between August 2013 and June 2014. Postoperative (ex vivo) FNA samples were performed. In the excised lung, the nodules of interest were identified by the surgeon and pathologist. The selected nodules with surrounding tissues were bisected for procurement, and then FNA was performed by passing a 25-gauge needle into the nodules under suction. The needle was then partially withdrawn and reinserted 15 to 20 times. For each nodule, 2 to 4 FNA samples were obtained. Two to 4 FNA samples of surrounding normal lung were also obtained. The needle, including tissue within the lumen, was washed with 500 μl PBS containing 1% BSA. The samples were aliquoted and stored immediately at −80 °C until assay. The cells in the washout were likely lysed because they were subjected to freezing for storage and because samples were diluted into buffer containing 0.5% Tween 20. Because of the very small amount of tissues in the washout samples, they were not centrifuged to remove the non-soluble components. FNA was performed on 7 nodules and 11 normal lung samples.
Histological diagnoses were made by standard pathological examination. None of our subjects had heart failure or chronic kidney disease, conditions which may increase circulating MDK concentrations [19–21]. None of our subjects had known metastases.
Study protocols were approved by the WRNMMC Institutional Review Board (protocol 385478), and all patients provided written informed consent to participate in the study. The investigators have adhered to the policies for protection of human subjects as prescribed in the 45 CFR, Part 46.
Midkine Sandwich ELISA Assay
MDK sandwich ELISA was performed using a commercial kit (Biovendor, Czech Republic) with modifications as previously described [11]. In summary, 50 μL of needle washout fluid from a breast core biopsy or lung FNA was diluted in 200 μL of TBSTA buffer (Tris-buffered saline: 50mM Tris-HCL, pH 8, 0.15 M NaCl, 0.5% Tween 20, 1% BSA). 100 μL of the diluted samples were pipetted into each of two 96-well plates, which had been coated by the manufacturer with capture antibody. The plate was incubated at 37°C for 2 hours without shaking, then washed 3 times with 0.32 ml per well of washing buffer provided in the kit, and then inverted and tapped to remove residual fluid. 10 μg/mL of poly-L-lysine (PLL, 150 – 300 kDa, Sigma, USA) in water was added to the biotin-labeled antibody solution provided with the kit, and then 100 μL of this solution was added to each well. The plate was incubated at room temperature for 1 hour, shaking at 300 rpm on an orbital microplate shaker. Then the wells were washed 5 times with 0.32 ml of the kit washing buffer per well. After vigorous tapping to remove residual fluid from the wells, 100 μL of streptavidin-HRP conjugate solution provided with the kit was added to each well. The plate was incubated at room temperature for 30 minutes, shaking at 300 rpm on an orbital microplate shaker. After washing 5 times with washing buffer and tapping, kit substrate solution was added to each well. The plate was covered with aluminum foil and incubated for 7 minutes at room temperature. Color development was stopped by adding 100 μl of kit stop solution. The absorbance of each well was measured using a microplate reader set to 450 nm. The standards for the assay consisted of recombinant MDK dissolved in TBSTA. The MDK assay showed good parallelism (Supplemental figure 1A). The intra-assay coefficient of variability was 5.2 % at 0.25 ng/mL and inter-assay coefficient of variability was 28 %.
Pleiotrophin Sandwich ELISA Assay
Mouse anti-pleiotropin monoclonal antibody (3B20, produced in the lab of Dr. Anton Wellstein) was diluted to 0.5 μg/mL in PBS and 100 μL/well was incubated in a 96-well plate at 4oC for 16 hrs. The wells were blocked with 250 μL per well of PBS containing 3% BSA and 0.2% Tween 20 for 2 hours at 4oC. Without washing, the plate was inverted and tapped to remove residual fluid. 100 μL of washout from a breast biopsy or lung FNA was diluted in 200 μL of PBSTA (PBS, 0.5% Tween 20, 1% BSA). 100 μL of the diluted samples were pipetted in duplicate into plate wells. The plate was incubated at room temperature for 2 hours, shaking at 300 rpm on an orbital microplate shaker. It was then washed 3 times with 0.25 mL per well of PBST (PBS, 0.5% Tween 20). After tapping the inverted plate to remove residual fluid, 100 μL/well of biotinylated anti-human pleiotrophin goat IgG (R & D Systems, USA) was added at a concentration of 500 ng/mL in 0.9% normal saline containing 5.7 meq/L calcium chloride and 0.5% BSA at pH 6. The plate was incubated shaking at 300 rpm at room temperature for 1 hour. Then the wells were washed 5 times with 250 μL of PBST per well. After vigorous tapping, 100 μL of streptavidin-HRP conjugate solution (Thermo Scientific, USA) was added at a concentration of 12.5 ng/mL in PBS to each well and the plate was incubated at room temperature for 30 minutes at 300 rpm on an orbital microplate shaker. After washing 5 times with PBST and tapping dry, 100 μL of TMB was added to each well. The plate was covered with aluminum foil and incubated for 7 minutes at room temperature. Color development was stopped by adding 100 μl of stop solution (0.16M sulfuric acid). The absorbance of each well was measured using a microplate reader set to 450 nm. The standards for the assay consisted of recombinant PTN dissolved in PBSTA. The PTN assay showed good parallelism (Supplemental figure 1B). The intra-assay coefficient of variability was 8.8% at 0.2 ng/mL and inter-assay coefficient of variability was 15 %.
DNA Assay
DNA concentration was measured using the Quant-it dsDNA HS assay kit (Life Technologies, Grand Island, NY). 20 μL of core needle or FNA washout was diluted in 200 μL of working solution and fluorescence was measured in 96 wells plates using a microplate reader (excitation/emission maxima ~485/520nm).
Plasma Midkine Sandwich ELISA Assay
125 μL of plasma was diluted in 125 μL of TBSTA. The rest of the procedure was identical to the procedure described above for assay of washout samples.
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics, version 19. For lung masses we assayed two FNA samples and averaged the values. Samples that showed DNA concentration less than 50 ng/mL were considered to have inadequate breast or lung tissue and were excluded from further analysis. The relationship between MDK vs. DNA and PTN vs. DNA concentrations were analyzed after log transformation by generalized linear model taking into account sampling from different nodules. Histological groups were compared using the Kruskal-Wallis and the Mann-Whitney U test with Holm correction for multiple comparisons. An α of 0.05 was considered the threshold for statistical significance. Data were expressed as mean ± SEM.
Results
Characteristics of Subjects and Masses
For MDK measurements, core needle biopsy samples were obtained from 78 breast masses in 70 subjects (mean age, 49.1 years; all females). Thirteen masses were excluded for low DNA content. For 15 masses (14 subjects), the core needle biopsy histology was read as malignant: invasive ductal carcinoma (IDC) in 5 masses, ductal carcinoma in situ (DCIS) in 9 masses and 1 invasive lobular carcinoma. Malignancy characteristics are summarized in Table 1. Three malignant breast samples were estrogen receptor negative (ER-; B23–1, B60–1, B63–1; Table 1). For 50 masses (45 subjects), the core needle biopsy histology was read as benign.
Table 1.
Malignant breast masses
| Sample ID | Histology | Pertinent Receptor status | Size of mass (cm) | MDK/DNA ratio (ng/μg) | PTN/DNA ratio (ng/μg) |
|---|---|---|---|---|---|
| B4–1 |
IDC | ER+, PR+, HER2- | 0.4 | 0.41 | 0 |
| B7–2 |
IDC | ER+, PR+, HER2- | 0.5 | 0.70 | 1.08 |
| B9–1 | DCIS | ER+, PR+ | 0.9 | 0.17 | 0 |
| B9–2 B23–1 |
DCIS LCIS |
ER+, PR+ ER-, PR- |
1.1 0.8 |
0.36 NA |
0 19.0 |
| B41–1 | IDC | ER+, PR+, HER2- | 0.5 | 0.10 | NA |
| B43–1 | IDC | ER+, PR+, HER2- | 0.5 | 0.13 | NA |
| B44–1 | DCIS | ER+, PR+ | 1.1 | 0.13 | NA |
| B44–2 | DCIS | ER+, PR+ | 0.5 | 0.35 | NA |
| B53–1 | DCIS | ER+, PR+ | 0.6 | 0.85 | 1.44 |
| B60–1 | DCIS | ER-, PR- | 0.4 | 0.24 | 0.86 |
| B62–1 | ILC | ER+, PR+, HER2 equivocal | 1.9 | 0.15 | 0 |
| B63–1 | DCIS | ER-, PR- | 0.5 | 0.41 | 0 |
| B73–1 | IDC | ER+, PR+, HER2- | 0.6 | 0.13 | 0 |
| B75–1 | DCIS | ER+, PR- | 2.0 | 0.29 | 1.48 |
| B81–1 | IDC | ER+, PR+, HER2- | 0.4 | 0.30 | 0 |
| B81–2 | IDC | ER+, PR+, HER2- | 0.5 | NA | 0 |
IDC, invasive ductal carcinoma; DCIS, ductal carcinoma in situ; ILC, invasive lobular carcinoma; LCIS, lobular carcinoma in situ; NA, not available
For PTN, we were only able to test 55 breast masses from 49 subjects (mean age, 51.1 years; all females) due to sample quantity. 4 masses were excluded for low DNA content. For 13 masses (10 subjects), the core needle biopsy histology was read as malignant: invasive ductal carcinoma (IDC) in 5 masses, ductal carcinoma in situ (DCIS) in 6 masses, invasive ductal carcinoa (IDC), and lobular carcinoma in situ (LCIS) in 1 mass. For 38 masses (34 subjects), the core needle biopsy histology was read as benign.
For MDK measurements, FNA samples were obtained from 7 lung nodules and 11 normal lung specimens in 12 subjects (mean age, 66.5 years; 9 males, 3 females). For all 7 nodules, the histology was read as malignant: adenocarcinoma in 4 nodules and squamous cell carcinoma in 2 nodules and mucinous adenocarcinoma in 1 nodule.
For PTN, we were only able to test 6 lung nodules in 10 subjects (mean age, 66.4 years; 7 males, 3 females) due to sample quantity. For 6 nodules, the histology was read as malignant: adenocarcinoma in 4 nodules and squamous cell carcinoma in 2 nodules. Malignancy characteristics were summarized in Table 2.
Table 2.
Malignancies in lung masses
| Sample ID | Histology | Size of masses (cm) | MDK/DNA ratio (ng/μg) |
PTN/DNA ratio (ng/μg) |
|---|---|---|---|---|
| L1 | Squamous Cell Carcinoma | 1.3 | 6.79 | 0.58 |
| L7 | Mucinous Adenocarcinoma |
4.71 | 0.061 | NA |
| L9 | Adenocarcinoma | 1.6 | 0.12 | 0 |
| L10 | Adenocarcinoma | 1.5 | 0.47 | 0.3 |
| L11 | Squamous Cell Carcinoma | 4.5 | 0.11 | 0.04 |
| L14 | Adenocarcinoma | 3.8 | 0.20 | 0.08 |
| L15 | Adenocarcinoma | 0.5 | 0.10 | 0.35 |
Midkine, Pleiotrophin and DNA Concentrations in Needle Biopsy Washout of Breast Masses
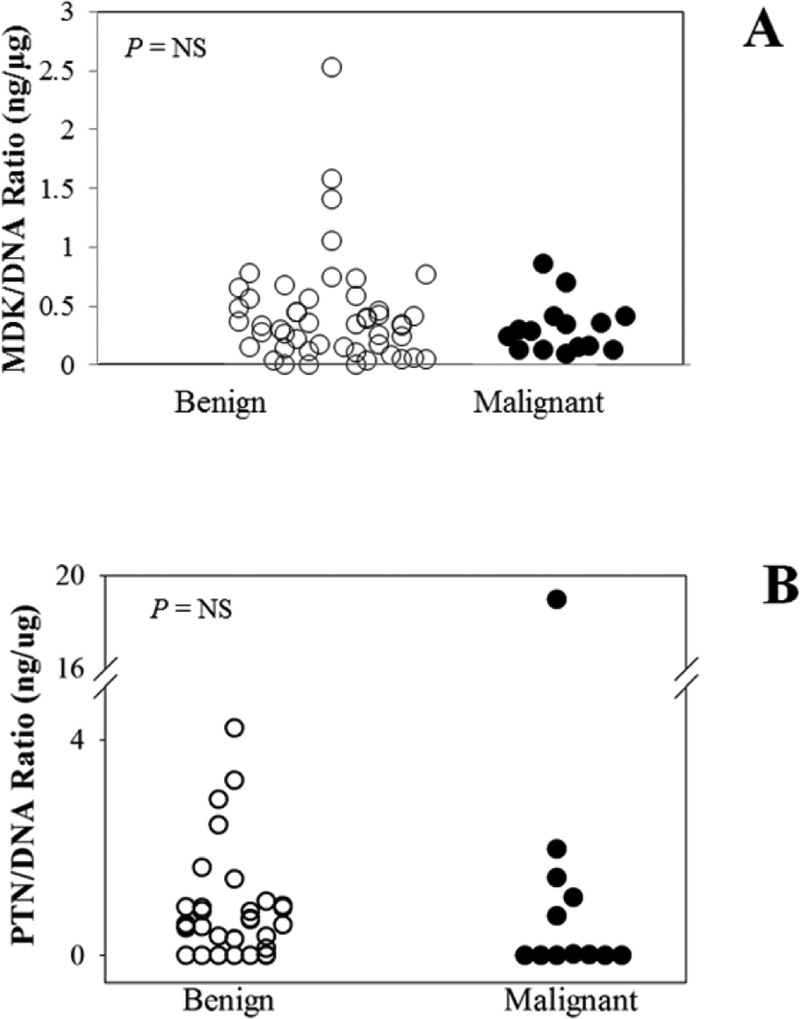
The concentrations of MDK in washout of core needle biopsies were similar in malignant breast masses and benign breast masses (0.17 ± 0.03 ng/mL vs 0.14 ± 0.02 ng/mL, mean ± SEM, P = NS, figure 1A). Among malignant masses, MDK concentration did not differ significantly in IDC and DCIS (0.13 ± 0.04 ng/mL vs 0.21 ± 0.04 ng/mL, P = NS). Of 78 core needle washout samples, 13 samples had DNA concentration less than 50 ng/mL and were therefore considered to contain inadequate breast tissue and were excluded from further analysis. DNA concentrations were similar in malignant and in benign masses (0.32 ± 0.41 vs 0.55 ± 0.39, μg/mL, P = NS).
Figure 1.
MDK/DNA ratios (A), and PTN/DNA ratios (B) in core needle biopsy washout from histologically confirmed benign (open circles) and malignant (closed circles) breast masses.
MDK and PTN were measured by highly sensitive ELISAs. DNA concentration was included as a measure of tissue content in each sample. MDK, PTN, and DNA concentrations and MDK/DNA and PTN/DNA ratios were similar in most benign and malignant samples, except for a single high PTN/DNA ratio in the one lobular carcinoma in situ studied. MDK and DNA concentrations were significantly associated (R2 = 0.124, P = 0.033). MDK concentrations were normalized to DNA content to adjust for the amount of tissue sampled. The MDK/DNA ratio did not differ significantly between malignant breast masses and benign breast masses (0.34 ± 0.06 ng/μg vs 0.57± 0.09 ng/μg, P = NS)(figure 1B) or between IDC and DCIS (0.28 ± 0.08 ng/μg vs 0.4 ± 0.09 ng/μg, P = NS). The concentrations of PTN in washout of core needle biopsies were similar in malignant breast masses and benign breast masses (0.25 ± 0.12 ng/mL vs. 0.26 ± 0.06 ng/mL, P = NS)(figure 1C). Among malignant masses, PTN concentration did not differ significantly in IDC, DCIS and LCIS (0.09 ± 0.08 ng/mL vs 0.2 ± 0.35 ng/mL vs. 1.50 ng/mL, P = NS). DNA concentration was also measured in the core needle washout samples. Of 55 core needle washout samples, 3 samples had DNA concentration less than 50 ng/mL and were therefore considered to contain inadequate breast tissue and were excluded from further analysis. DNA concentrations were similar in malignant and in benign masses (0.51 ±0.13 μg/mL vs. 0.56 ± 0.05 μg/mL, P = NS)(figure 1C). MDK and DNA concentrations were significantly associated (R2 = 0.18, P = 0.025). To correct for the amount of breast tissue in each sample, we normalized PTN concentrations to DNA content by calculating the ratio of PTN concentration to DNA concentration (PTN/DNA, ng/μg). Samples from malignant masses had similar PTN/DNA ratios compared to benign masses (1.38 ± 0.4 vs 0.78 ± 0.18 ng/μg, P = NS)(figure 1D). PTN/DNA was not significantly different between IDC and DCIS (0.18 ± 0.16 vs 0.7 ± 0.35 ng/μg, P = NS). The single sample of LCIS had a high PTN/DNA ratio (19.0 ng/μg, figure 1D) compared to other malignant masses (0.44 ± 0.2 ng/μg) or benign masses.
Midkine, Pleiotrophin and DNA Concentrations in Needle Biopsy Washout of Lung Masses
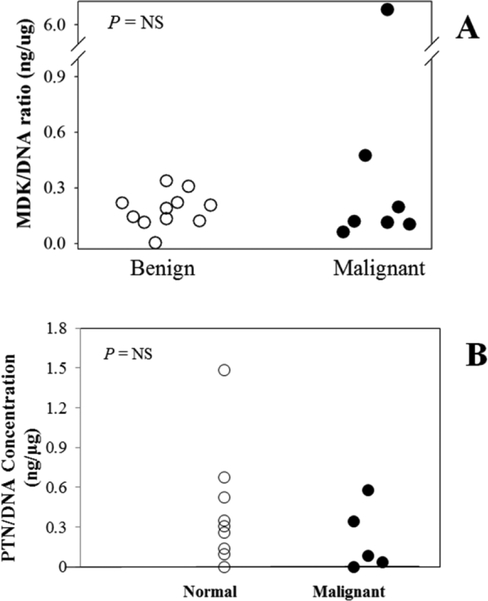
The concentrations of MDK in washout of FNA was significantly different in malignant lung nodules and normal lung tissue (0.62 ± 0.48 vs. 0.09 ± 0.03, P = 0.033)(figure 2A). DNA concentrations were significantly elevated in malignant nodules compared to normal lung tissue (0.99 ± 0.15 μg/mL vs. 0.47 ± 0.1 μg/mL, P <0.001). MDK and DNA concentrations were positively associated (R2 = 0.298, P < 0.002). MDK concentrations were normalized to DNA content to adjust for the amount of tissue sampled. The MDK/DNA ratio did not differ significantly between malignant lung nodules and normal lung tissue (1.23 ± 1.11 ng/μg vs 0.18 ± 0.02 ng/μg, P = NS)(figure 2B). However, one squamous cell carcinoma did have an elevated MDK/DNA ratio (6.79 ng/μg, figure 2B). MDK and PTN were measured by highly sensitive ELISAs. DNA concentration was included as a measure of tissue content in each sample. MDK, PTN, and DNA concentrations and MDK/DNA and PTN/DNA ratios were similar in most normal and malignant samples, except for a high MDK/DNA ratio in one squamous cell carcinoma studied.
Figure 2.
MDK/DNA ratios (A), and PTN/DNA ratios (B) in washout fluid from fine needle aspiration samples of normal lung tissue (open circles) and malignant lung masses (closed circles).
The concentrations of PTN in washout of FNA were similar in malignant lung nodules and normal lung tissue (0.14 ± 0.06 ng/mL vs. 0.16 ± 0.05 ng/mL, P = NS)(figure 2C). DNA concentrations were elevated in malignant nodules compared to normal lung tissue (1.06 ± 0.22 μg/mL vs. 0.48 ± 0.11 μg/mL, P = 0.001)(figure 2C). PTN and DNA concentrations were not significantly associated (R2 = 0.09, P = 0.1). The PTN/DNA ratio did not differ significantly between malignant lung nodules and normal lung tissue (0.21 ± 0.11 ng/μg vs. 0.43 ± 0.15 ng/μg, P = NS)(figure 2D).
Plasma Midkine Concentrations
Plasma MDK concentrations did not differ between subjects with malignant breast masses and those with benign breast masses (0.28 ± 0.07 ng/mL vs. 0.23 ± 0.01 ng/mL, P = NS).
Discussion
We previously reported that the concentrations of two heparin-binding growth factors, MDK and PTN were higher in needle biopsy washouts of malignant thyroid nodules than in benign nodules [17–18]. Because MDK and PTN are both reportedly overexpressed in breast cancer cells, we used highly sensitive ELISAs to measure MDK and PTN concentrations in the washouts of core needle biopsies from breast masses to determine whether levels differed in malignant and benign lesions. In order to correct for the amount of breast tissue present, we normalized the MDK and PTN concentration to the DNA concentration. We found that, in general, neither MDK nor PTN protein levels in core needle biopsy washout samples were significantly higher in malignant than in benign breast masses. However, one lobular carcinoma in situ showed a PTN/DNA ratio 24- fold higher than the average benign mass. Because PTN was only measured in one LCIS, it is unclear whether PTN overexpression is a general property of LCIS. We also measured MDK concentration in plasma and did not find significant differences in plasma MDK concentrations between subjects with malignant and benign masses.
Several previous studies have suggested that MDK and PTN are overexpressed in some breast cancers. In an early study, using non-quantitative RT-PCR, Garver et al. detected MDK mRNA expression in more malignant samples than in normal samples [4]. Similarly, Yu et al. found higher MDK mRNA expression in breast cancer samples than in normal breast tissue using semi-quantitative northern analysis [22], and Qin et al reported that 86% of IDC samples showed immunohistochemical staining for MDK expression [23]. In contrast to the above studies, Miyashiro et al., using RT-PCR and northern analysis, identified MDK mRNA similarly in malignant and normal breast tissue samples, but found differential expression of a truncated form of MDK, which was identified in 6 of 26 malignant samples but not in normal breast tissue [24]. MDK expression seems to be unrelated to the level of estrogen and progesterone receptors [4].
For PTN expression in breast cancer, there are conflicting results. Fang et al. reported PTN mRNA expression in 62% of breast cancer samples but not in normal breast tissue using an RNAse protection assay [25]. In that study, the status of ER or PR was not correlated with PTN mRNA expression. However, Garver et al. found that most breast cancers and most normal breast tissue samples expressed PTN mRNA as assessed by RT-PCR. More recent microarray analysis by Turashvili et al. and by Casey et al. actually found decreased PTN mRNA expression in malignant compared to normal breast tissue [26–27].
Because MDK and PTN are both reportedly overexpressed in lung cancer cells, we also used highly sensitive ELISAs to measure MDK and PTN concentrations in the washouts of FNA needle biopsies from ex vivo lung nodules to determine whether levels differed in malignant lesions and normal lung. We normalized the MDK and PTN concentration to the DNA concentration to correct for the amount of lung tissue present. Neither MDK nor PTN protein levels in FNA needle biopsy washout samples were significantly higher in malignant lung nodules than in normal lung tissue. However, one squamous cell carcinoma showed MDK/DNA ratio 38-fold higher than the average sample of normal lung tissue. MDK was only measured in two squamous cell carcinoma nodules, and therefore the frequency of MDK overexpression in squamous cell carcinomas is unclear.
Prior analyses have suggested that MDK and PTN are overexpressed in some lung cancers. Ostroff et al. identified PTN as a serum marker that helps identify individuals with non-small cell lung cancer [28]. Similarly, Du et al. reported significant increases in serum PTN levels of patients with lung cancer compared to controls, and, in two surgical specimens, found elevation of PTN mRNA and protein in and around non-small cell lung cancer lesions compared to normal lung tissue [29]. However, using non-quantitative RT-PCR, Garver et al. reported decreased expression of PTN in malignant lung samples compared to resected normal lung but increased expression of MDK [5]. Serum midkine levels are also reportedly elevated in patients with lung cancer [30]. In addition, there is in vitro evidence that MDK promotes lung cancer epithelial-mesenchymal transition [31] and cell migration [32], raising the possibility that those lung cancers that overexpression MDK might be treated by targeting this pathway.
Our study of heparin-binding growth factor levels in breast and lung cancer has several strengths compared to prior studies. First, unlike many previous studies, we assessed expression at the protein, rather than the mRNA level, which has greater relevance for pathogenesis. Second, unlike many older studies, we used a highly quantitative method, ELISA. The assays for both MDK and PTN are highly sensitive [17], readily allowing the detection of both MDK and PTN in the samples studied. Third, we assessed protein levels not only in serum, but directly in malignant tissues, thus potentially increasing the ability to detect increased expression.
However, the current study also has several important limitations. It is a pilot study with a small sample size of patients with breast and lung malignancies. Although we identified specific malignancies with elevated levels of heparin-binding growth factors, additional larger studies would be required to determine the frequency of this overexpression in various subtypes of breast and lung cancer, and to assess whether or not this overexpression has prognostic significance. A second limitation is that we did not have any benign lung masses available for study, and thus the only comparison was to normal lung. A third limitation is that we did not study subjects with advanced, metastatic breast or lung cancer; such subjects might have higher MDK or PTN levels, including elevated circulating MDK, as previously reported [30].
Conclusions
In conclusion, this pilot study indicates that neither MDK nor PTN concentrations in needle biopsy washout samples are higher in most malignant breast or lung masses compared to benign breast masses or normal lung, respectively. However, one lobular carcinoma in situ of the breast showed PTN/DNA ratio 24-fold higher than the average benign mass and one squamous cell carcinoma of the lung showed MDK/DNA ratio 38-fold higher than the average sample of normal lung tissue, suggesting that a subset of breast and lung cancers may strongly overexpress heparin-binding growth factors. Additional studies would be required to determine the frequency of this overexpression, whether or not it has prognostic significance, and whether these overexpressing malignancies could be treated by targeting the MDK/PTN pathways, for example with monoclonal antibodies.
Supplementary Material
Acknowledgments
Funding
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The views expressed are those of the authors and do not reflect the official policy of the Department of the Army, the Department of Defense or the U.S. Government.
Footnotes
Conflict of interest
Y.H.J and J.B. are co-inventors on a patent application entitled, “Assay to measure midkine and pleiotrophin level for diagnosing a growth” (U.S. Patent Application No. 14/646,078 – Filed: May 20, 2015), filed by the National Institutes of Health that covers the measurement of MDK and PTN in FNA samples. This does not alter our adherence to PLOS ONE policies on sharing data and materials. The authors declare that there is no conflict of interest, employment, consultancy, or products in development or modified products that could be perceived as prejudicing the impartiality of the research reported.
References
- [1].Matsubara S et al. , Structure of a retinoic acid responsive gene, MK, which is transiently activated during the differentiation of embryonal carcinoma cells and the mid-gestation period of mouse embryogenesis, in: The Journal of Biological Chemistry 265(16) (1990), 9441–9443. [PubMed] [Google Scholar]
- [2].Kadomatsu K et al. , A retinoic acid responsive gene MK found in the teratocarcinoma system is expressed in spatially and temporally controlled manner during mouse embryogenesis, in: The Journal of Cell Biology 110(3) (1990), 607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mitsiadis T et al. , Expression of the heparin-binding cytokines, midkine (MK) and HB-GAM (pleiotrophin) is associated with epithelial-mesenchymal interaction during fetal development and organogenesis, in: Development 121(1) (1995), 37–51. [DOI] [PubMed] [Google Scholar]
- [4].Garver R et al. , Midkine and pleiotrophin expression in normal and malignant breast tissue, in: Cancer 74(5) (1994), 1584–1590. [DOI] [PubMed] [Google Scholar]
- [5].Garver R et al. , Reciprocal expression of pleiotrophin and midkine in normal versus malignant lung tissues, in: American Journal of Respiratory Cell and Molecular Biology 9(5) (1993), 463–466. [DOI] [PubMed] [Google Scholar]
- [6].Tsutsui J et al. , A new family of heparin binding growth/differentiation factors. Increased midkine expression in Wilm’s tumor and other human carcinomas, in: Cancer Research 53(6) (1993), 1281–1285. [PubMed] [Google Scholar]
- [7].Kato S et al. , Monoclonal antibody to human midkine reveals increased midkine expression in human brain tumors, in: Journal Neuropathology and Experimental Neurology 58(5) (1999), 430–441. [DOI] [PubMed] [Google Scholar]
- [8].Rosenfield S et al. , Pleiotrophin (PTN) expression and function and in the mouse mammary gland and mammary epithelial cells, in: Public Library of Science One 7(10) (2012), e47876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Papadimitriou E et al. , Roles of pleiotrophin in tumor growth and angiogenesis, in: European Cytokine Network 20(4) (2009), 180–190. [DOI] [PubMed] [Google Scholar]
- [10].Muramatsu T and Muramatsu H, Glycosaminoglycan-binding cytokines as tumor markers, in: Proteomics 8(16) (2008), 3350–3359. [DOI] [PubMed] [Google Scholar]
- [11].Xu C et al. , Functional receptors and intracellular signal pathways of midkine (MK) and pleiotrophin (PTN), in: Biolological and Pharmaceutical Bulletin 37(4) (2014), 511–520. [DOI] [PubMed] [Google Scholar]
- [12].Owada K et al. , Midkine inhibits caspase-dependent apoptosis via the activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase in cultured neurons, in: Journal of Neurochemistry 73(5) (1999), 2084–2092. [PubMed] [Google Scholar]
- [13].Muramatsu T, Structure and function of midkine as the basis of its pharmalogical effects, in: British Journal of Pharmacology 171(4) (2014), 814–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sethi G et al. , PTN signaling: Components and mechanistic insights in human ovarian cancer, in: Molecular Carcinogenesis 54(12) (2015), 1772–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rawnaq T et al. , The Multifunctional growth factor midkine promotes proliferation and migration in pancreatic cancer, in: Molecular Cancer Research 12(5) (2014), 670–680. [DOI] [PubMed] [Google Scholar]
- [16].Li YS, Gurrieri M, and Deuel TF, Pleiotrophin gene expression is highly restricted and is regulated by platelet-derived growth factor, in: Biochemical and Biophysical Research Communications 184(1) (1992), 427–432. [DOI] [PubMed] [Google Scholar]
- [17].Jee YH et al. , Midkine Concentration in Fine-needle Aspiration of Benign and Malignant Thyroid Nodules, in: Clinical Endocrinology 83(6) (2015), 977–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].H Jee Y et al. , Increased Pleiotrophin Concentrations in Papillary Thyroid Cancer, in: Public Library of Science One 11(2) (2016), e0149383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kitahara T et al. , Serum midkine as a predictor of cardiac events in patients with chronic heart failure, in: Journal of Cardiac Failure 16(4) (2010), 308–313. [DOI] [PubMed] [Google Scholar]
- [20].Andreucci M et al. , The ischemic/nephrotoxic acute kidney injury and the use of renal biomarkers in clinical practice, in: European Journal of Internal Medicine 39 (2017), 1–8. [DOI] [PubMed] [Google Scholar]
- [21].Krzystek-Korpacka M et al. , Midkine, a multifunctional cytokine, in patients with severe sepsis and septic shock: a pilot study, in: Shock 35(5) (2011), 471–477. [DOI] [PubMed] [Google Scholar]
- [22].Yu L et al. , Midkine Promoter Can Mediate Transcriptional Activation of a Fused Suicide Gene in a Broader Range of Human Breast Cancer Compared with c-erbB-2 Promoter, in: Oncology 66(2) (2004), 143–149. [DOI] [PubMed] [Google Scholar]
- [23].L Qin L et al. , Expression of Midkine and Endoglin in Breast Carcinomas with Different Immunohistochemical Profiles, in: APMIS: Acta Pathologica, Microbiologica, et Immunologica Scandinavica 119(2) (2011), 103–110. [DOI] [PubMed] [Google Scholar]
- [24].Miyashiro I et al. , Midkine Expression in Human Breast Cancers: Expression of Truncated Form, in: Breast Cancer Research and Treatment 43(1) (1997) 1–6. [DOI] [PubMed] [Google Scholar]
- [25].Fang W et al. , Pleiotrophin Stimulates Fibroblasts and Endothelial and Epithelial Cells and Is Expressed in Human Cancer, in; Journal of Biological Chemistry 267(36) (1992), 25889–25897. [PubMed] [Google Scholar]
- [26].Turashvili G et al. , Novel Markers for Differentiation of Lobular and Ductal Invasive Breast Carcinomas by laser Microdissection and Microarray Analysis, in: Biomed Central Cancer 7 (2007), 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Casey T et al. , Molecular Signature Suggest a Major Role for Stromal Cells in Development of Invasive Breast Cancer, in: Breast Cancer Research and Treatment 114(1) (2009), 47–62. [DOI] [PubMed] [Google Scholar]
- [28].Ostroff RM et al. , Unlocking Biomarker Discovery: Large Scale Application of Aptamer Proteomic Technology for Early Detection of Lung Cancer, in: Public Library of Science One 5(12) (2010), e15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Du ZY et al. , Serum Pleiotrophin Could Be an Early Indicator for Diagnosis and Prognosis of Non-Small Cell Lung Cancer, in: Asian Pacific Journal of Cancer Prevention 16(4) (2015), 1421–1425. [DOI] [PubMed] [Google Scholar]
- [30].Ikematsu S et al. , Serum Midkine Levels are Increased in Patients with Various Types of Carcinomas, in: British Journal of Cancer 83(6) (2000), 701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhao G et al. , ERβ-Mediated Estradiol Enhances Epithelial Mesenchymal Transition of Lung Adenocarcinoma through Increasing Transcription of Midkine, in: Molecular Endocrinology 26(8) (2012), 1304–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Feng ZJ et al. , Lung Cancer Cell Migration is Regulated Via Repressing, Growth Factor PTN/RPTP β/ζ Signaling by Menin, in: Oncogene 29(39) (2010), 5416–5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.