Abstract
At the core of chromosome segregation is the centromere, which nucleates the assembly of a macromolecular kinetochore (centromere DNA and associated proteins) complex responsible for mediating spindle attachment. Recent advances in centromere research have led to identification of many kinetochore components, such as the centromeric-specific histone H3 variant, CenH3, and its interacting partner, Scm3. Both are essential for chromosome segregation and are evolutionarily conserved from yeast to humans. CenH3 is proposed to be the epigenetic mark that specifies centromeric identity. Molecular mechanisms that regulate the assembly of kinetochores at specific chromosomal sites to mediate chromosome segregation are not fully understood. In this review, we summarize the current literature and discuss results from our laboratory, which show that restricting the localization of budding yeast CenH3, Cse4, to centromeres and balanced stoichiometry between Scm3 and Cse4, contribute to faithful chromosome transmission. We highlight our findings that, similar to other eukaryotic centromeres, budding yeast centromeric histone H4 is hypoacetylated, and we discuss how altered histone acetylation affects chromosome segregation. This article is part of a Special Issue entitled: Chromatin in time and space.
Keywords: Cse4, Histone H4 acetylation, Scm3/HJURP, Kinetochore, CenH3, Chromosome segregation
1. Introduction
The accurate segregation of chromosomes relies on the correct temporal and spatial attachment of chromosomes to the spindle apparatus. The centromere (CEN) serves as the chromosomal location on which the kinetochore (CEN DNA and associated proteins) is assembled. The kinetochore is a mega-Dalton macromolecular complex that helps maintain cohesion between sister chromatids in the vicinity of CEN DNA, and mediates the attachment of chromosomes to the mitotic spindle and their subsequent movement to the spindle poles [1]. In addition to the kinetochores, telomeres, spindle pole bodies, microtubules, cohesins, and codensins are essential for proper chromosome segregation and maintenance of ploidy [2–5]. The spindle assembly checkpoint (SAC) monitors defects in spindle–kinetochore interactions. Errors in spindle–kinetochore attachment activate the SAC, which leads to an inhibition of the anaphase-promoting complex (APC), blocking entry into anaphase until all chromosomes are correctly aligned at the metaphase plate to prevent chromosome missegregation [6].
Centromeres are structurally categorized into three classes, referred to as point, regional, and holocentric [7]. Point centromeres found in budding yeast Saccharomyces cerevisiae are comprised of ~125 bp of DNA and are conserved among each of its 16 chromosomes [8–10]. In contrast, regional centromeres in metazoans, plants, and fission yeast span several kilobases to megabases with no obvious sequence conservation, and may contain repetitive alpha satellite sequences and flanking heterochromatin domains. The holocentric centromeres in Caenorhabditis elegans span the entire length of a chromosome without sequence specificity. Despite the centromeric DNA sequence heterogeneity, several kinetochore complexes are evolutionarily conserved between point and regional centromeres. A distinguishing feature that is common to all organisms studied to date is the presence of a centromeric histone H3 variant, CenH3 (Cse4 in budding yeast; Cnp1 in fission yeast; CID in fruit flies; and CENP-A in humans), which is essential for chromosome segregation [11]. In budding yeast, one to three Cse4 nucleosomes are found at each chromosome, and the centromeric nucleosomes are nearly perfectly positioned over a defined centromeric DNA region [12–16]. Centromeric assembly of CenH3 in budding and fission yeast, as well as in humans, requires the adaptor protein suppressor of chromosome missegregation (Scm3; holliday junction recognition protein [HJURP] in humans). Scm3/HJURP directly associates with CenH3 and is essential for assembly and maintenance of a functional kinetochore [17–24].
Over the past few years, at least six models for CenH3 nucleosomes have been proposed, each of which differs in composition and/or the conformational state of the centromeric DNA. The differences may be specific to the system being studied, biochemical assembly conditions, or the cell cycle stage. The simplest variation is a centromeric nucleosome wrapped with left-handed DNA around an octamer of histones H4, H2A, H2B, and CenH3 [25–32]. A second version, named a reversome, is identical to the first, except the DNA assumes a right-handed conformation [33]. A third model proposes that CenH3 nucleosomes are half the size of canonical nucleosomes, are comprised of a single molecule of each histone, H4, H2A, H2B, and CenH3, wrapped by right-handed DNA, and are referred to as a hemisome [34–36]. In a fourth model, only two molecules of CenH3 and two molecules of histone H4 assemble into a tetrasome, wrapped by left-handed DNA [22]. The last two models (hexasome and trisome) replace histones H2A and H2B with Scm3/HJURP. In the hexasome model, there are two molecules of CenH3, histone H4, and Scm3, wrapped by left-handed DNA [18,37]. A competing model, the trisome, proposes that there is only a single molecule of CenH3, histone H4, and Scm3 wrapped by right-handed DNA [35].
With respect to the first model, octameric structures containing CenH3 molecules have also been observed in vivo, but their chromosomal location is still a matter of debate [31,32,38]. It has been recently proposed that the sub-octameric complexes may be intermediate structures that occur during different cell cycle stages [39]. Future studies should resolve the differences in the models and provide a better understanding of the epigenetic mechanism that regulates CenH3 assembly (see [39–42] for detailed reviews on CenH3 nucleosome structure).
Although the precise structure of centromeric nucleosomes remains controversial [39], there is general agreement that CenH3 acts as the epigenetic mark that specifies centromeric identity [43,44]. Centromeric association of CenH3 in regional centromeres is largely independent of the underlying centromeric DNA sequence [7,42]. Also, several recent studies have shown the association of Cse4 to non-centromeric DNA, such as the yeast 2 μm plasmid and other non-centromeric chromosomal loci [31,45,46]. Taken together, these results raise three fundamental questions: (1) What factors control the assembly of CenH3 at centromeric chromatin? (2) How does the structure of centromeric chromatin contribute to the process of chromosome segregation? (3) What mechanisms are used to exclude CenH3 from non-centromeric regions?
Point centromeres in S. cerevisiae provide advantages for probing centromeric structure and function, because these centromeres have unique non-repetitive sequences (centromere DNA element [CDE] I-II-III) that can be easily and specifically assessed by chromatin immuno precipitation (ChIP) techniques [47]. Importantly, budding yeast Cse4 can complement RNAi-induced depletion of CENP-A in human cells, suggesting that, despite the differences in the two systems, the structural and functional properties of CenH3 are conserved [48]. Studies with budding yeast have also led to the identification of more than 100 kinetochore proteins, the cohesion and codensin complexes, and checkpoint signaling pathways [1]. As is the case for Cse4, many of the kinetochore and other proteins required for chromosome segregation are conserved from yeast to humans [49]. Research using S. cerevisiae to study chromosome segregation has benefited by the powerful genetic and biochemical tools and techniques recently developed in this system. These include ordered arrays of gene knockouts/mutants (~4000 non-essential and ~700 essential genes), libraries of genes cloned into low- and high-copy vectors, and synthetic genetic array (SGA) analysis for genome-wide studies [50]. In addition, the use of a yeast strain engineered with a reporter chromosome, in which the loss of the reporter gives rise to red sectors within white colonies, allows for facile visual detection and quantification of chromosome loss [51]. In this review, we summarize the current literature and focus on our work related to uncovering a role for hypoacetylation of centromeric histone H4 in kinetochore function [52]; chromosome loss due to mis-localization of Cse4 and identification of Cse4 domains and pathways that prevent ectopic localization of Cse4 to non-centromeric regions [53]; and how mis-regulation of a Cse4 interacting protein, Scm3, and its human homolog HJURP leads to chromosome segregation defects [54].
2. Acetylation pattern of centromeric histone H4 affects chromosome segregation
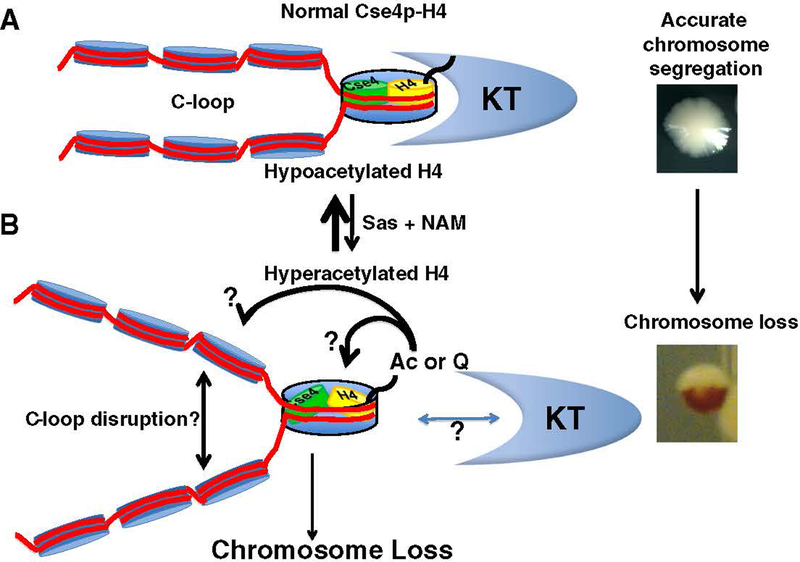
Post-translational modifications (PTMs) of histones modulate chromatin structure and regulate cellular processes such as chromosome segregation [55]. Studies of regional centromeres have revealed a unique pattern of centromeric and pericentromeric histone H3 and H4 modifications. Centromeric histone H3 is methylated at lysine 4 (K4), which is typically associated with active transcription, and the pericentromeric histone H3 is dimethylated at lysine 9 (K9), which is usually associated with heterochromatic regions [56]. In humans, fruit flies, and fission yeast, histones H3 and H4 are hypoacetylated in both the centromeric and pericentromeric regions [57,58]. The proper assembly of heterochromatin in the pericentromeric region is required for centromere establishment and maintenance [59–61]. Even though histone H4 acetylation is well established in mediating changes in chromatin structure [62,63], the precise molecular role for these modifications in chromosome segregation is not clearly defined. Using point centromeres of budding yeast, we have investigated the PTMs of histone H4 and CenH3 to understand the roles of these modifications in genome stability [52]. We characterized the acetylation of histone H4 at the centromeric and pericentromeric regions using ChIP assays with antibodies to tetra-acetylated histone H4 (acetylated at lysines K5, K8, K12, and K16) or to acetylated histone H4 at lysine 16 (K16) only. We observed low levels of both forms of acetylated H4 at centromeres. We were particularly intrigued by the very low levels of H4K16 acetylation (H4K16Ac), as this PTM is critical for chromatin compaction and is sufficient to prevent higher-order folding of nucleosome arrays [64]. We also found hypoacetylated H4K16 at non-centromeric regions containing Cse4, suggesting that H4, when associated with Cse4, tends to favor H4K16 that is hypoacetylated. The functional significance of H4K16 hypoacetylation was demonstrated by showing that increasing histone acetyltransferase (HAT) activity or inhibiting histone deacetylase complexes (HDACs) leads to increased levels of chromosome loss, growth inhibition in CSE4 and histone H4 mutants, and increased centromeric H4K16Ac. Specifically, we showed that overexpression of the something about silencing (SAS) complex, an H4K16 HAT, deletion of Sir2, an H4K16 deacetylase, treatment with nicotinamide (NAM), an HDAC inhibitor, or expression of the H4K16Q acetyl-mimic mutant all lead to increased chromosome loss (Fig. 1) [52]. We propose that the Cse4–H4 interactions mediated by the histone fold domain of Cse4 may act in protecting cells from high levels of histone H4 acetylation. When these interactions are weakened, a hypoacetylated N-terminal H4 becomes essential to maintain kinetochore integrity (Fig. 1). Our results suggest that hypoacetylated H4 at the centromere is a conserved feature between yeast and metazoans, despite having highly divergent centromeric sequences. Furthermore, ectopic expression of histone demethylases and acetyltransferases causes chromosome loss and confers lethality in kinetochore mutants (unpublished data). Taken together, these data suggest that there is likely an interplay between histone modifications for a functional kinetochore that may be conserved from yeast to humans.
Fig. 1.
Acetylation pattern of centromeric histone H4 affects chromosome segregation. (A) Centromeric histone H4 N-terminal tails are hypoacetylated, which is associated with proper C-loop (light blue nucleosomes to left of Cse4/H4 nucleosome) and kinetochore (KT) assembly to mediate accurate chromosome segregation. Note that the Cse4 nucleosome illustration does not indicate the actual composition of the centromeric nucleosome (refer to the introduction for various models of centromeric nucleosomes). (B) Phenotypes resulting from hyperacetylated centromeric histone H4 at K16. Increased dosage of histone H4K16 acetylation (Ac) by overexpression of Sas2 histone acetyltransferase activity (Sas), growth on nicotinamide (NAM), an inhibitor of nicotinamide adenine dinucleotide (NAD)-dependent deacetylases that act on H4K16, or use of an H4K16Q acetyl-mimic mutant (Q – glutamine) results in chromosome loss that may be caused by disruption of the C-loop and/or improper kinetochore assembly at the centromere (?). (Far right). We use a visual assay to monitor chromosome loss in a strain engineered with a reporter chromosome. Loss of the reporter chromosome results in red sectors in an otherwise white colony. Chromosome loss within the first cell division results in half-sectored red colonies. Refer to Section 2 for details.
Based on our studies of H4 PTMs and the interactions of Cse4 with H4, we explored the PTMs of Cse4 and their role in chromosome segregation. Phosphorylation of CENP-A in humans was described almost a decade ago, and there has been one report so far on the role of this modification in cytokinesis [65–67]. To identify Cse4 PTMs, we used biochemical methods that allowed us to purify Cse4 while preserving potential PTMs for mass spectrometric analysis. We created CSE4 mutants that could not be modified or mimicked a modified state and found that these mutants displayed genetic interactions with kinetochore and spindle biorientation mutants (unpublished data). Our work on the PTM of Cse4 will provide new insights into kinetochore function that may be evolutionarily conserved.
Our data for the effect of altered H4 acetylation and the role of Cse4 modifications on chromosome segregation provide many avenues for future research. These include determining the mechanisms that regulate the PTMs of centromeric histone H4 and Cse4, and identifying how these modifications affect the architecture of centromeric and pericentromeric regions, which, in turn, affects kinetochore function. Future studies may also determine whether the PTMs of histone H4 and Cse4 are a prerequisite for assembly into centromeric chromatin or the modification occurs after centromeric incorporation, and identify how these events may be coupled to kinetochore assembly.
Recent studies have shown that the centromeric chromatin structure forms a 50 kb intramolecular chromatin loop (C-loop) with the Cse4 nucleosome at the apex, a structure that is critical for correct biorientation [68–70]. We propose that the presence and/or absence of specific PTMs modulate the conformation of the chromatin that comprises the C-loop to maximize the probability of correct biorientation. We have performed experiments to examine the C-loop structure in histone H4 mutants and found that hyperacetylation may antagonize assembly of the C-loop (Fig. 1 and unpublished data). Future studies should help identify proteins that contribute to a functional C-loop and determine how PTMs of Cse4 and H4 may modulate its structure. These studies will provide new insight(s) into the mechanism of how PTMs of kinetochore proteins and centromeric histones mediate faithful chromosome segregation not only in S. cerevisiae but also in higher eukaryotes.
3. Mechanisms that prevent non-centromeric localization of Cse4 to maintain genome stability
Histones and histone variants confer specific properties to chromatin and affect processes such as DNA replication, transcription, and chromosome segregation [11]. Hence, it is conceivable that mislocalization of CenH3 to loci where canonical histone H3 resides may have a profound effect on cellular physiology. Although assembly of CenH3 at centromeres has been studied extensively, reports of the physiological consequences and mechanisms that prevent mis-localization of CenH3 are very limited. For example, in fruit flies, overexpression of CenH3 leads to ectopic kinetochore formation and severe chromosome loss [71,72]. In humans, CENP-A is over-expressed and mis-localized in primary colorectal cells that are aneuploid [73]. Higher levels of CENP-A expression are reported for patients with hepatocellular carcinoma [74], and CENP-A overexpression was one of the core markers in neoplastic intratubular germ cells, suggesting that it may be a new biological marker for human disease[75]. It has recently been shown that CENP-A overexpression promotes genome instability in pRb-depleted cells [76]. These observations suggest that chromosome transmission fidelity may depend on the recruitment of CenH3 to centromeres and its exclusion from non-centromeric regions.
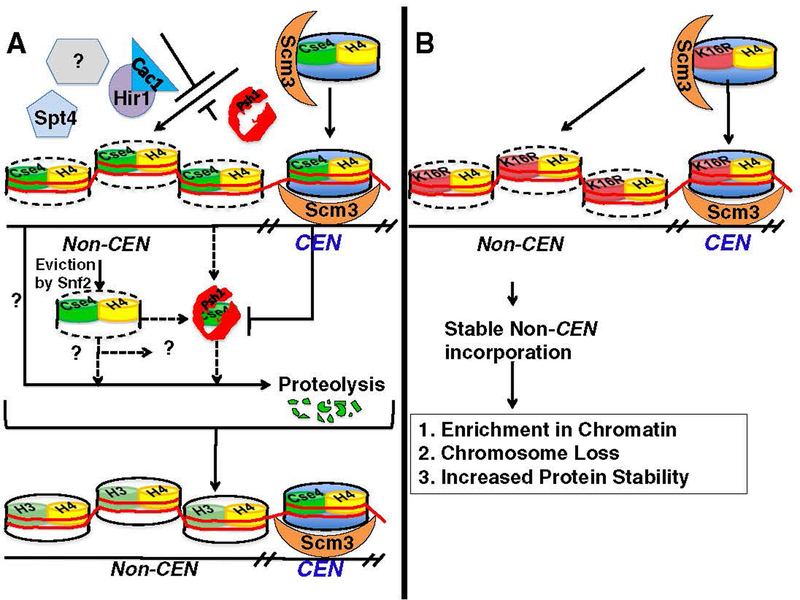
Several recent studies in S. cerevisiae have indicated that Cse4 has the potential to localize to both centromeric and non-centromeric regions (Fig. 2A) [31,38,46]. Deletions of S. cerevisiae transcription factor Spt4 or histone chaperones Cac1 and Hir1 exhibit mis-localization of Cse4 and defects in chromosome segregation [77,78]. Recent studies reported eviction of non-centromeric Cse4 by Snf2, a member of the Swi/Snf ATP-dependent chromatin remodeling complex. Deletion of SNF2 results in mis-localization of Cse4 to non-centromeric regions and defects in chromosome segregation [79]. Taken together, these studies suggest that the chromosome loss phenotype of spt4, cac1 hir1, and snf2 strains may be due to mis-localization of Cse4. SPT4, CAC1, HIR1, and SNF2 are non-essential for haploid growth, indicating that additional or redundant pathways may regulate Cse4 localization. Studies with budding yeast and fruit flies have shown that ubiquitin-dependent proteolysis of Cse4 and CID, respectively, contributes to their exclusion from non-centromeric regions [80–82]. Recently, Psh1 was identified as an E3 ligase that mediates the ubiquitination and proteolysis of Cse4 [83,84]. These studies have shown that deletion of PSH1 results in mis-localization of Cse4 to non-centromeric regions, and overexpression of CSE4 leads to lethality in PSH1 deletion strains. The Psh1–Cse4 interaction is dependent on the unique centromeric targeting domain (CATD) in the C-terminus of Cse4p, which is not present in histone H3. Psh1 localizes to centromeric DNA, and it is proposed that the interaction of Cse4 with Scm3 at the centromere protects Cse4 from degradation [84]. Despite its role in directing Cse4 proteolysis, PSH1 is not essential, and PSH1 deletion strains do not have high rates of chromosome loss [84]. Results from these studies have led to the proposal that additional domains within Cse4 and mechanisms independent of Psh1 also mediate the exclusion of Cse4 from non-centromeric regions.
Fig. 2.
Mechanisms that prevent non-centromeric localization of Cse4 to maintain genome stability. (A) Cse4 can localize to non-centromeric regions; however, mis-localization is suppressed by Psh1, Snf2, Cac1-Hir1, Spt4, and other proteins (?). Psh1 is an E3 ubiquitin ligase that mediates proteolytic degradation of Cse4 and excludes its incorporation into non-centromeric DNA. Centromeric incorporation of Cse4 and its interaction with Scm3 is thought to protect Cse4 from proteolysis by Psh1. Snf2 is proposed to remove Cse4 from non-centromeric regions. Cse4 eviction by Snf2 may or may not be mutually exclusive from known (Psh1) and unknown (?) proteolytic pathways. The centromeric nucleosome is depicted in blue, and the non-centromeric regions of Cse4 are denoted by the cylinders with a dotted outline. Note that the Cse4 nucleosome illustration does not indicate the actual composition of the centromeric nucleosome (refer to the introduction for various models of centromeric nucleosomes). (B) Overexpression of cse4K16R leads to its mis-localization to non-centromeric regions. Scm3-mediated centromeric localization of cse4K16R is inferred from complementation of a CSE4 deletion strain by cse4K16R. Stable non-centromeric incorporation of cse4K16R leads to its enrichment in chromatin, chromosome loss, and increased protein stability. Refer to Section 3 for details.
To elucidate the mechanisms and consequence(s) of CenH3 mis-localization, we are investigating whether mis-localization of Cse4 contributes to chromosome loss, identifying domains within Cse4 required to prevent mis-localization, and characterizing pathways that mediate the degradation of Cse4, thereby preventing its localization to non-centromeric regions. In order to determine if mis-localization of Cse4 leads to chromosome segregation defects, we examined phenotypes of mis-localized Cse4 in wild-type yeast strains. We used a strain in which all lysines of Cse4 were replaced with arginines (cse4K16R), tagged with 13-Myc epitopes at the amino terminus, and controlled by a galactose-inducible promoter (cse4K16R) [53,80]. Cse4K16R is relatively stable and highly enriched in chromatin, and exhibits a diffuse nuclear localization pattern that is different from the discrete Cse4 foci characteristic of kinetochore localization [53,80]. We observed that strains overexpressing cse4K16R exhibited a chromosome loss phenotype, suggesting that mis-localization of cse4K16R contributes to genome stability (Fig. 2) [53]. Since cse4K16R phenotypes could be suppressed by constitutive expression of slightly higher levels of histone H3, we propose that this is due to displacement of cse4K16R from non-centromeric regions. A C-terminal mutant form of H3 that cannot form tetramers with H4 could not suppress the cse4K16R-mediated defects, and co-overexpression of histone H4 and cse4K16R led to even higher rates of chromosome loss. These results strongly suggest that Cse4-H4 complexes are incorporated into non-centromeric loci.
To identify functional domains within Cse4 that mediate its exclusion from ectopic sites, we created mutant alleles of CSE4 lacking the N-terminus or lysines in the N-terminal or the C-terminal domain mutated to arginine. We determined that mutations or truncation of the Cse4 N-terminus resulted in stabilization, chromatin enrichment, localization to centromeric and non-centromeric regions, and defects in chromosome segregation (unpublished data). Our data suggest that proteolysis of Cse4 requires more than just the CATD of Cse4. This work provides important insights into the role of the N-terminal domain of Cse4p in Psh1-dependent and -independent pathways for Cse4 proteolysis. To identify additional proteins that facilitate Cse4 proteolysis, we are using genome-wide approaches (e.g., SGA) to examine genetic interactions with different CSE4 mutant alleles in loss-of-function mutations in virtually every yeast gene [50]. As proof of principle, our screens led to the identification of PSH1, whose deletion resulted in a severe decrease in fitness when CSE4 was overexpressed. These studies are very exciting, as they will enable us to identify domains within Cse4 that interact with other proteins to prevent incorporation of Cse4 into non-centromeric regions. We anticipate that screens with cse4K16R will enable us to identify mechanisms that may be ubiquitin- and/or Psh1-independent.
Taken together, our findings demonstrate that cells have multiple regulatory mechanisms to prevent Cse4 mis-localization and maintain genome stability. Cse4 is able to associate with both centromeric and non-centromeric DNA. However, several factors, such as domains within Cse4, its interaction with other proteins, and the structure of the non-centromeric chromatin, prevent the stable incorporation of Cse4 at ectopic sites. Future studies will help us better understand the role of each of these factors and how the nature of non-centromeric chromatin allows promiscuous incorporation of mutant Cse4 proteins. We are interested in determining whether co-localization of Scm3 to regions of Cse4 mis-localization contributes to their stable incorporation into chromatin, similar to that observed for centromeric Cse4 [83]. We would also like to investigate whether the transcriptional role of SPT4 and its interacting partners, SPT5 and SPT6, contribute to the exclusion of Cse4 from non-centromeric regions and to the suppression of chromosome instability (CIN). Precedence for this hypothesis is based on a recent study suggesting that the exclusion of Cse4 from promoters may be due to rapid turnover of histones in this region by histone chaperones such as Cac1 and Hir1, whereas exclusion of Cse4 from coding regions may be due to transcriptional activity [78]. Future work will provide a better understanding of how cells safeguard against the stable incorporation of Cse4 at ectopic sites to maintain genome stability.
4. Mis-regulation of Scm3/HJURP leads to chromosome loss in yeast and human cells
The studies described above provide evidence that budding yeast and other eukaryotes restrict Cse4 assembly to the kinetochore in order to maintain faithful chromosome transmission. In this section, we summarize recent work on the Cse4 adapter protein, Scm3, which is essential for centromeric localization of Cse4 [17,18,20,21]. The mechanism of Scm3-dependent Cse4 assembly is likely to be highly conserved, as Scm3 orthologs are present in other organisms ranging from fission yeast (SpScm3) to Xenopus (xHJURP), mice (mmHJURP), and humans (HJURP) [19,22,24,85,86]. Scm3 and its homologs share an evolutionarily conserved domain that specifically interacts with CenH3 to facilitate its assembly at the centromere [37,54].
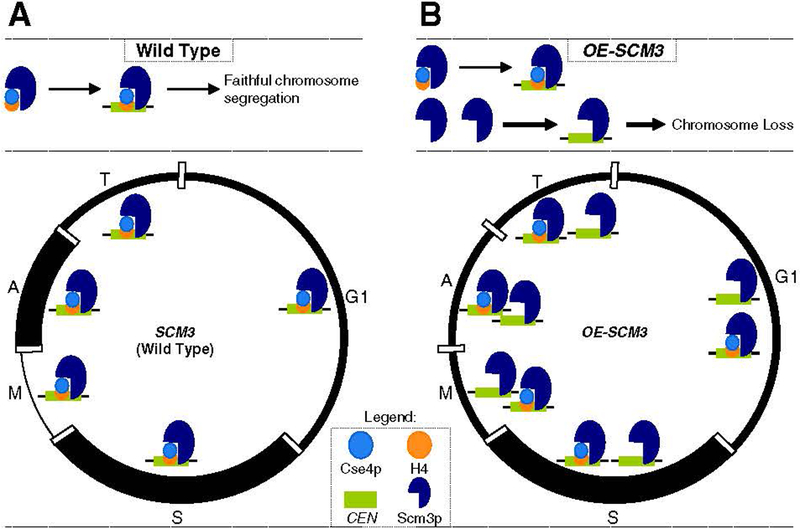
Understanding the molecular role of Scm3 in kinetochore assembly has been an area of active investigation in recent years. Scm3 in budding yeast was first identified in a genetic screen for suppressors of temperature-sensitive CSE4 mutants [20], and recent studies have shown the interdependence among Scm3, Ndc10, and Cse4 for centromeric localization [17]. Our studies of budding yeast have shown that the centromeric association of Scm3 is cell cycle-regulated and that Scm3 is enriched at centromeric DNA in S phase and anaphase, and depleted in early mitotic cells (Fig. 3) [54]. The incorporation of Cse4 at centromeres occurs in S phase and is coupled with centromeric DNA replication [87]. Notably, Scm3 and Cse4 are both enriched at centromeres in S phase cells, consistent with a role for Scm3 as a Cse4 assembly factor [54]. Centromeric enrichment of Scm3 in anaphase has also been observed recently by Luconi et al. [88], who suggested that such an enrichment was required for the maintenance of Cse4 at the kinetochore under tension caused by spindle elongation. Scm3 homologs in other eukaryotes also exhibit a cell cycle-dependent centromeric association pattern [19,22–24]. For example, centromeric association of HJURP is coincident with CenH3 localization in G1 [23,24]. The CenH3 homolog in Drosophila, Cid, is recruited to centromeric DNA in metaphase, which is dependent upon CENP-C and a newly identified protein CAL1 [89]. Although CAL1 exhibits no obvious sequence homology to Scm3/HJURP proteins, these proteins have conserved function as a chaperone for the centromeric assembly of CenH3. Collectively, these studies show that Scm3 and its homologs are required for the incorporation of CenH3 and that centromeric association of these proteins is cell cycle-regulated. Future studies will help us understand how cell cycle-dependent centromeric association of Scm3/HJURP is regulated and whether this association is required for accurate chromosome segregation.
Fig. 3.
Mis-regulation of Scm3 results in increased chromosome loss in budding yeast. Scm3 forms a stoichiometric complex with Cse4/H4 and mediates the assembly of Cse4 at the centromeric DNA. Depicted is the cell cycle of budding yeast with G1, S, mitotic (M), anaphase (A), and telophase (T) cells. (A). Wild-type cells with a balanced stoichiometry of Scm3 with Cse4/H4 contribute to faithful chromosome segregation. The schematic figure depicts the cell cycle following ChIP experiments using epitope-tagged Scm3 expressed from its endogenous promoter. Scm3 is enriched in S phase and anaphase (A) and shows depletion in early mitotic (M) phase cells. The enrichment and depletion are denoted by the thickness of the lines in each phase. (B) Strains overexpressing SCM3 (OE-SCM3) have an excess of Scm3 compared to the available pools of Cse4 and H4. We propose that centromeric association of Scm3 devoid of Cse4/H4 leads to chromosome loss in strains overexpressing SCM3. Schematic of cell cycle as in (A) shows results of ChIP experiments using epitope-tagged overexpressed SCM3, which fails to show the depletion of centromeric Scm3 in early mitotic cells and constitutively associates with centromeres in all phases of the cell cycle. The thickness of the lines in each phase represents the levels of centromeric Scm3. Figure is adapted from our publication [54]. Refer to Section 4 for details.
Recent studies have shown that HJURP-mediated deposition of CENP-A is sufficient to recruit centromeric proteins to an ectopic site [86]. Notably, HJURP overexpression is observed in lung and breast cancer cell lines that exhibit CIN and cell immortality [90,91]. These characteristics are associated with poor prognosis in cancer patients [91]. To study the effects of overexpressed SCM3 and HJURP, we used strains in which SCM3 and HJURP expression were controlled by a galactose-inducible promoter. Unlike Scm3 expressed from its own promoter, the centromeric association of galactose-induced SCM3 is not cell cycle-regulated (Fig. 3)[54]. Overexpression of SCM3 leads to increased chromosome loss, reduced viability in kinetochore mutants, premature separation of sister chromatids, and reduced levels of Cse4 and histone H4 at centromeres (Fig. 3). In turn, overexpression of CSE4 or histone H4 suppressed the chromosome loss and restored levels of Cse4 at centromeres in strains overexpressing SCM3 [54]. It is possible that the altered cell cycle pattern of centromeric association of Scm3, when overexpressed, may contribute to chromosome loss. We explored the possibility that HJURP overexpression might contribute to the CIN by testing its effect in yeast and human cells. These studies indicated that overexpressed HJURP in S. cerevisiae and human cells led to chromosome loss and mitotic defects, respectively, suggesting that increased HJURP levels may directly cause CIN in human cells [54]. Notably, it has been shown previously that histone H3 overexpression also causes chromosome loss and reduction of centromeric Cse4, similar to the effects of SCM3 overexpression [53,92]. Taken together, these studies show that balanced stoichiometry of histone H3, CenH3, and Scm3/HJURP is critical for chromosome transmission fidelity.
The observation that SCM3 overexpression causes reduction in centromere-bound Cse4 suggests that the chromosome loss phenotype may be due to centromeric association of Scm3 devoid of Cse4 (Fig. 3). Support for this hypothesis was derived from the use of mutant alleles of SCM3, which failed to bind to Cse4 but were proficient in binding to centromeric DNA. Overexpression of this scm3 allele led to high rates of chromosome loss that was not suppressed by overexpression of CSE4 [54]. Furthermore, we determined that the centromeric association of Scm3 is mediated through its N-terminal domain [54], and these results are consistent with recent data from in vitro studies [37]. It is not known whether centromeric DNA binding properties of Scm3 in budding yeast represent a unique feature of “point centromeres” or are evolutionarily conserved properties. Our computational analysis predicts a putative DNA binding motif within the C-terminus of fission yeast Scm3 (unpublished data), but no specific centromeric DNA binding domain within HJURP was detected. Future studies should help us understand the molecular mechanisms that maintain regulation of Scm3/HJURP expression to suppress chromo-some instability, and help us identify and develop therapeutic targets for cancers with mis-regulated HJURP expression.
In summary, our studies and those of others have identified PTMs of histone and kinetochore proteins and the potential role of these modifications in kinetochore function. The findings that hypoacetylation of centromeric histone H4 contributes to genome stability raises a fundamental question of how enzymatic activities of HATs and HDACs distinguish centromeres from other regions. Notably, cancer therapies based on anti-mitotic drugs are being actively investigated [93]. Our findings suggest that a combination of HDAC inhibitors with drugs that inhibit kinetochore function may provide an efficacious approach to treat cancers, with minimal effect on normal cells. Studies addressing how mis-localization of Cse4 or deregulation of Scm3/HJURP leads to chromosome loss in budding yeast may provide insights into how perturbations in similar pathways contribute to mis-localized CENP-A and overex-pressed HJURP in human disease. Taken together, studies with budding yeast kinetochore proteins and centromeric histones promise to provide new insights into the mechanisms of centromeric chromatin assembly and regulation.
Acknowledgements
We are grateful to Lars Boeckmann for comments, and Anthony Dawson for comments and illustrations in Figs. 1 and 2. Research in the authors’ laboratory is supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Footnotes
This article is part of a Special Issue entitled: Chromatin in time and space.
References
- [1].Westermann S, Drubin DG, Barnes G, Structures and functions of yeast kineto-chore complexes, Annu. Rev. Biochem 76 (2007) 563–591. [DOI] [PubMed] [Google Scholar]
- [2].Jaspersen SL, Winey M, The budding yeast spindle pole body: structure, duplication, and function, Annu. Rev. Cell Dev. Biol 20 (2004) 1–28. [DOI] [PubMed] [Google Scholar]
- [3].Cheung AL, Deng W, Telomere dysfunction, genome instability and cancer, Front. Biosci 13 (2008) 2075–2090. [DOI] [PubMed] [Google Scholar]
- [4].Nasmyth K, Segregating sister genomes: the molecular biology of chromosome separation, Science 297 (2002) 559–565. [DOI] [PubMed] [Google Scholar]
- [5].Clarke DJ, Bachant J, Kinetochore structure and spindle assembly checkpoint signaling in the budding yeast, Saccharomyces cerevisiae, Front. Biosci 13 (2008) 6787–6819. [DOI] [PubMed] [Google Scholar]
- [6].Cheeseman IM, Desai A, Molecular architecture of the kinetochore–microtubule interface, Nat. Rev. Mol. Cell Biol 9 (2008) 33–46. [DOI] [PubMed] [Google Scholar]
- [7].Ekwall K, Epigenetic control of centromere behavior, Annu. Rev. Genet 41 (2007) 63–81. [DOI] [PubMed] [Google Scholar]
- [8].Hieter P, Pridmore D, Hegemann JH, Thomas M, Davis RW, Philippsen P, Functional selection and analysis of yeast centromeric DNA, Cell 42 (1985) 913–921. [DOI] [PubMed] [Google Scholar]
- [9].Fitzgerald-Hayes M, Clarke L, Carbon J, Nucleotide sequence comparisons and functional analysis of yeast centromere DNAs, Cell 29 (1982) 235–244. [DOI] [PubMed] [Google Scholar]
- [10].Clarke L, Carbon J, Isolation of a yeast centromere and construction of functional small circular chromosomes, Nature 287 (1980) 504–509. [DOI] [PubMed] [Google Scholar]
- [11].Smith MM, Centromeres and variant histones: what, where, when and why? Curr. Opin. Cell Biol 14 (2002) 279–285. [DOI] [PubMed] [Google Scholar]
- [12].Cole HA, Howard BH, Clark DJ, The centromeric nucleosome of budding yeast is perfectly positioned and covers the entire centromere, Proc. Natl. Acad. Sci. U. S. A 108 (2011) 12687–12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Furuyama S, Biggins S, Centromere identity is specified by a single centromeric nucleosome in budding yeast, Proc. Natl. Acad. Sci. U. S. A 104 (2007) 14706–14711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lawrimore J, Bloom KS, Salmon ED, Point centromeres contain more than a single centromere-specific Cse4 (CENP-A) nucleosome, J. Cell Biol 195 (2011) 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Henikoff S, Henikoff JG, ‘Point’ centromeres of Saccharomyces harbor single CenH3 nucleosomes, Genetics (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Coffman VC, Wu P, Parthun MR, Wu JQ, CENP-A exceeds microtubule attachment sites in centromere clusters of both budding and fission yeast, J. Cell Biol 195 (2011) 563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Camahort R, Li B, Florens L, Swanson SK, Washburn MP, Gerton JL, Scm3 is essential to recruit the histone H3 variant Cse4 to centromeres and to maintain a functional kinetochore, Mol. Cell 26 (2007) 853–865. [DOI] [PubMed] [Google Scholar]
- [18].Mizuguchi G, Xiao H, Wisniewski J, Smith MM, Wu C, Nonhistone Scm3 and histones CenH3-H4 assemble the core of centromere-specific nucleosomes, Cell 129 (2007) 1153–1164. [DOI] [PubMed] [Google Scholar]
- [19].Pidoux AL, Choi ES, Abbott JK, Liu X, Kagansky A, Castillo AG, Hamilton GL,Richardson W, Rappsilber J, He X, Allshire RC, Fission yeast Scm3: A CENP-A receptor required for integrity of subkinetochore chromatin, Mol. Cell 33 (2009) 299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Stoler S, Rogers K, Weitze S, Morey L, Fitzgerald-Hayes M, Baker RE, Scm3, an essential Saccharomyces cerevisiae centromere protein required for G2/M progression and Cse4 localization, Proc. Natl. Acad. Sci. U. S. A 104 (2007) 10571–10576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shivaraju M, Camahort R, Mattingly M, Gerton JL, Scm3 is a centromeric nucleosome assembly factor, J. Biol. Chem 286 (2011) 12016–12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Williams JS, Hayashi T, Yanagida M, Russell P, Fission yeast Scm3 mediates stable assembly of Cnp1/CENP-A into centromeric chromatin, Mol. Cell 33 (2009) 287–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dunleavy EM, Roche D, Tagami H, Lacoste N, Ray-Gallet D, Nakamura Y, Daigo Y, Nakatani Y, Almouzni-Pettinotti G, HJURP is a cell-cycle-dependent maintenance and deposition factor of CENP-A at centromeres, Cell 137 (2009) 485–497. [DOI] [PubMed] [Google Scholar]
- [24].Foltz DR, Jansen LE, Bailey AO, Yates III JR, Bassett EA, Wood S, Black BE, Cleveland DW, Centromere-specific assembly of CENP-A nucleosomes is mediated by HJURP, Cell 137 (2009) 472–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shelby RD, Monier K, Sullivan KF, Chromatin assembly at kinetochores is uncoupled from DNA replication, J. Cell Biol 151 (2000) 1113–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sekulic N, Bassett EA, Rogers DJ, Black BE, The structure of (CENP-A-H4)(2) reveals physical features that mark centromeres, Nature 467 (2010) 347–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Palmer DK, Margolis RL, Kinetochore components recognized by human autoantibodies are present on mononucleosomes, Mol. Cell. Biol 5 (1985) 173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Palmer DK, O’Day K, Wener MH, Andrews BS, Margolis RL, A 17-kD centromere protein (CENP-A) copurifies with nucleosome core particles and with histones, J. Cell Biol 104 (1987) 805–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Foltz DR, Jansen LE, Black BE, Bailey AO, Yates III JR, Cleveland DW, The human CENP-A centromeric nucleosome-associated complex, Nat. Cell Biol 8 (2006) 458–469. [DOI] [PubMed] [Google Scholar]
- [30].Conde e Silva N, Black BE, Sivolob A, Filipski J, Cleveland DW, Prunell A, CENP-A-containing nucleosomes: easier disassembly versus exclusive centromeric localization, J. Mol. Biol 370 (2007) 555–573. [DOI] [PubMed] [Google Scholar]
- [31].Camahort R, Shivaraju M, Mattingly M, Li B, Nakanishi S, Zhu D, Shilatifard A, Workman JL, Gerton JL, Cse4 is part of an octameric nucleosome in budding yeast, Mol. Cell 35 (2009) 794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhang W, Colmenares SU, Karpen GH, Assembly of Drosophila centromeric nucleosomes requires CID dimerization, Mol. Cell 45 (2011) 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lavelle C, Recouvreux P, Wong H, Bancaud A, Viovy JL, Prunell A, Victor JM, Right-handed nucleosome: myth or reality? Cell 139 (2009) 1216–1217 (author reply 1217–1218). [DOI] [PubMed] [Google Scholar]
- [34].Dalal Y, Wang H, Lindsay S, Henikoff S, Tetrameric structure of centromeric nucleosomes in interphase Drosophila cells, PLoS Biol 5 (2007) e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Furuyama T, Henikoff S, Centromeric nucleosomes induce positive DNA super-coils, Cell 138 (2009) 104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Dimitriadis EK, Weber C, Gill RK, Diekmann S, Dalal Y, Tetrameric organization of vertebrate centromeric nucleosomes, Proc. Natl. Acad. Sci. U. S. A 107 (2010) 20317–20322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Xiao H, Mizuguchi G, Wisniewski J, Huang Y, Wei D, Wu C, Nonhistone Scm3 binds to AT-rich DNA to organize atypical centromeric nucleosome of budding yeast, Mol. Cell 43 (2011) 369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Krassovsky K, Henikoff JG, Henikoff S, Tripartite organization of centromeric chromatin in budding yeast, Proc. Natl. Acad. Sci. U. S. A 109 (2012) 243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Black BE, Cleveland DW, Epigenetic centromere propagation and the nature of CENP-A nucleosomes, Cell 144 (2011) 471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Dalal Y, Bui M, Down the rabbit hole of centromere assembly and dynamics, Curr. Opin. Cell Biol 22 (2010) 392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Henikoff S, Furuyama T, Epigenetic inheritance of centromeres, Cold Spring Harb. Symp. Quant. Biol 75 (2010) 51–60. [DOI] [PubMed] [Google Scholar]
- [42].Black BE, Jansen LE, Foltz DR, Cleveland DW, Centromere identity, function, and epigenetic propagation across cell divisions, Cold Spring Harb. Symp. Quant. Biol 75 (2010) 403–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Black BE, Bassett EA, The histone variant CENP-A and centromere specification, Curr. Opin. Cell Biol 20 (2008) 91–100. [DOI] [PubMed] [Google Scholar]
- [44].Choo KH, Centromerization, Trends Cell Biol 10 (2000) 182–188. [DOI] [PubMed] [Google Scholar]
- [45].Huang CC, Hajra S, Ghosh SK, Jayaram M, Cse4 (CenH3) association with the Saccharomyces cerevisiae plasmid partitioning locus in its native and chromosomally integrated states: implications in centromere evolution, Mol. Cell. Biol 31 (2011) 1030–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lefrancois P, Euskirchen GM, Auerbach RK, Rozowsky J, Gibson T, Yellman CM, Gerstein M, Snyder M, Efficient yeast ChIP-Seq using multiplex short-read DNA sequencing, BMC Genomics 10 (2009) 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Meluh PB, Yang P, Glowczewski L, Koshland D, Smith MM, Cse4p is a component of the core centromere of Saccharomyces cerevisiae, Cell 94 (1998) 607–613. [DOI] [PubMed] [Google Scholar]
- [48].Wieland G, Orthaus S, Ohndorf S, Diekmann S, Hemmerich P, Functional complementation of human centromere protein A (CENP-A) by Cse4p from Saccharomyces cerevisiae, Mol. Cell. Biol 24 (2004) 6620–6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kitagawa K, Hieter P, Evolutionary conservation between budding yeast and human kinetochores, Nat. Rev. Mol. Cell Biol 2 (2001) 678–687. [DOI] [PubMed] [Google Scholar]
- [50].Tong AH, Evangelista M, Parsons AB, Xu H, Bader GD, Page N, Robinson M, Raghibizadeh S, Hogue CW, Bussey H, Andrews B, Tyers M, Boone C, Systematic genetic analysis with ordered arrays of yeast deletion mutants, Science 294 (2001) 2364–2368. [DOI] [PubMed] [Google Scholar]
- [51].Spencer F, Gerring SL, Connelly C, Hieter P, Mitotic chromosome transmission fidelity mutants in Saccharomyces cerevisiae, Genetics 124 (1990) 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Choy JS, Acuna R, Au WC, Basrai MA, A role for histone H4K16 hypoacetylation in Saccharomyces cerevisiae kinetochore function, Genetics 189 (2011) 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Au WC, Crisp MJ, DeLuca SZ, Rando OJ, Basrai MA, Altered dosage and mislocalization of histone H3 and Cse4p lead to chromosome loss in Saccharomyces cerevisiae, Genetics 179 (2008) 263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Mishra PK, Au WC, Choy JS, Kuich PH, Baker RE, Foltz DR, Basrai MA, Mis-regulation of Scm3p/HJURP causes chromosome instability in Saccharomyces cerevisiae and human cells, PLoS Genet 7 (2011) e1002303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Allshire RC, Karpen GH, Epigenetic regulation of centromeric chromatin: old dogs, new tricks? Nat. Rev. Genet 9 (2008) 923–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sullivan BA, Karpen GH, Centromeric chromatin exhibits a histone modification pattern that is distinct from both euchromatin and heterochromatin, Nat. Struct. Mol. Biol 11 (2004) 1076–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ekwall K, Olsson T, Turner BM, Cranston G, Allshire RC, Transient inhibition of histone deacetylation alters the structural and functional imprint at fission yeast centromeres, Cell 91 (1997) 1021–1032. [DOI] [PubMed] [Google Scholar]
- [58].Hayashi T, Fujita Y, Iwasaki O, Adachi Y, Takahashi K, Yanagida M, Mis16 and Mis18 are required for CENP-A loading and histone deacetylation at centromeres, Cell 118 (2004) 715–729. [DOI] [PubMed] [Google Scholar]
- [59].Allshire RC, RNA interference, heterochromatin, and centromere function, Cold Spring Harb. Symp. Quant. Biol 69 (2004) 389–395. [DOI] [PubMed] [Google Scholar]
- [60].Folco HD, Pidoux AL, Urano T, Allshire RC, Heterochromatin and RNAi are required to establish CENP-A chromatin at centromeres, Science 319 (2008) 94–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kagansky A, Folco HD, Almeida R, Pidoux AL, Boukaba A, Simmer F, Urano T, Hamilton GL, Allshire RC, Synthetic heterochromatin bypasses RNAi and centromeric repeats to establish functional centromeres, Science 324 (2009) 1716–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Kan PY, Caterino TL, Hayes JJ, The H4 tail domain participates in intra- and internucleosome interactions with protein and DNA during folding and oligomerization of nucleosome arrays, Mol. Cell. Biol 29 (2009) 538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wang X, Hayes JJ, Acetylation mimics within individual core histone tail domains indicate distinct roles in regulating the stability of higher-order chromatin structure, Mol. Cell. Biol 28 (2008) 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Shogren-Knaak M, Ishii H, Sun JM, Pazin MJ, Davie JR, Peterson CL, Histone H4-K16 acetylation controls chromatin structure and protein interactions, Science 311 (2006) 844–847. [DOI] [PubMed] [Google Scholar]
- [65].Kunitoku N, Sasayama T, Marumoto T, Zhang D, Honda S, Kobayashi O, Hatakeyama K, Ushio Y, Saya H, Hirota T, CENP-A phosphorylation by Aurora-A in prophase is required for enrichment of Aurora-B at inner centromeres and for kinetochore function, Dev. Cell 5 (2003) 853–864. [DOI] [PubMed] [Google Scholar]
- [66].Zeitlin SG, Barber CM, Allis CD, Sullivan KF, Differential regulation of CENP-A and histone H3 phosphorylation in G2/M, J. Cell Sci 114 (2001) 653–661. [DOI] [PubMed] [Google Scholar]
- [67].Zeitlin SG, Shelby RD, Sullivan KF, CENP-A is phosphorylated by Aurora B kinase and plays an unexpected role in completion of cytokinesis, J. Cell Biol 155 (2001) 1147–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Anderson M, Haase J, Yeh E, Bloom K, Function and assembly of DNA looping, clustering, and microtubule attachment complexes within a eukaryotic kinetochore, Mol. Biol. Cell 20 (2009) 4131–4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Stephens AD, Haase J, Vicci L, Taylor II RM, Bloom K, Cohesin, condensin, and the intramolecular centromere loop together generate the mitotic chromatin spring, J. Cell Biol 193 (2011) 1167–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Yeh E, Haase J, Paliulis LV, Joglekar A, Bond L, Bouck D, Salmon ED, Bloom KS, Pericentric chromatin is organized into an intramolecular loop in mitosis, Curr. Biol 18 (2008) 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Heun P, Erhardt S, Blower MD, Weiss S, Skora AD, Karpen GH, Mislocalization of the Drosophila centromere-specific histone CID promotes formation of functional ectopic kinetochores, Dev. Cell 10 (2006) 303–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Olszak AM, van Essen D, Pereira AJ, Diehl S, Manke T, Maiato H, Saccani S, Heun P, Heterochromatin boundaries are hotspots for de novo kinetochore formation, Nat. Cell Biol 13 (2011) 799–808. [DOI] [PubMed] [Google Scholar]
- [73].Tomonaga T, Matsushita K, Yamaguchi S, Oohashi T, Shimada H, Ochiai T, Yoda K, Nomura F, Overexpression and mistargeting of centromere protein-A in human primary colorectal cancer, Cancer Res 63 (2003) 3511–3516. [PubMed] [Google Scholar]
- [74].Liu L, Li Y, Zhang S, Yu D, Zhu M, Hepatitis B virus X protein mutant upregulates CENP-A expression in hepatoma cells, Oncol. Rep 27 (2012) 168–173. [DOI] [PubMed] [Google Scholar]
- [75].Li Y, Zhu Z, Zhang S, Yu D, Yu H, Liu L, Cao X, Wang L, Gao H, Zhu M, ShRNA-targeted centromere protein A inhibits hepatocellular carcinoma growth, PLoS One 6 (2011) e17794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Amato A, Schillaci T, Lentini L, Di Leonardo A, CENPA overexpression promotes genome instability in pRb-depleted human cells, Mol. Cancer 8 (2009) 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Crotti LB, Basrai MA, Functional roles for evolutionarily conserved Spt4p at centromeres and heterochromatin in Saccharomyces cerevisiae, EMBO J 23 (2004) 1804–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Lopes da Rosa J, Holik J, Green EM, Rando OJ, Kaufman PD, Overlapping regulation of CenH3 localization and histone H3 turnover by CAF-1 and HIR proteins in Saccharomyces cerevisiae, Genetics 187 (2011) 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Gkikopoulos T, Singh V, Tsui K, Awad S, Renshaw MJ, Scholfield P, Barton GJ,Nislow C, Tanaka TU, Owen-Hughes T, The SWI/SNF complex acts to constrain distribution of the centromeric histone variant Cse4, EMBO J 30 (2011) 1919–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Collins KA, Furuyama S, Biggins S, Proteolysis contributes to the exclusive centromere localization of the yeast Cse4/CENP-A histone H3 variant, Curr. Biol 14 (2004) 1968–1972. [DOI] [PubMed] [Google Scholar]
- [81].Moreno-Moreno O, Torras-Llort M, Azorin F, Proteolysis restricts localization of CID, the centromere-specific histone H3 variant of Drosophila, to centromeres, Nucleic Acids Res 34 (2006) 6247–6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Moreno-Moreno O, Medina-Giro S, Torras-Llort M, Azorin F, The F box protein partner of paired regulates stability of Drosophila centromeric histone H3, CenH3(CID), Curr. Biol 21 (2011) 1488–1493. [DOI] [PubMed] [Google Scholar]
- [83].Hewawasam G, Shivaraju M, Mattingly M, Venkatesh S, Martin-Brown S, Florens L, Workman JL, Gerton JL, Psh1 is an E3 ubiquitin ligase that targets the centromeric histone variant Cse4, Mol. Cell 40 (2010) 444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Ranjitkar P, Press MO, Yi X, Baker R, MacCoss MJ, Biggins S, An E3 ubiquitin ligase prevents ectopic localization of the centromeric histone H3 variant via the centromere targeting domain, Mol. Cell 40 (2010) 455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Bernad R, Sanchez P, Rivera T, Rodriguez-Corsino M, Boyarchuk E, Vassias I,Ray-Gallet D, Arnaoutov A, Dasso M, Almouzni G, Losada A, Xenopus HJURP and condensin II are required for CENP-A assembly, J. Cell Biol 192 (2011) 569–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Barnhart MC, Kuich PH, Stellfox ME, Ward JA, Bassett EA, Black BE, Foltz DR, HJURP is a CENP-A chromatin assembly factor sufficient to form a functional de novo kinetochore, J. Cell Biol 194 (2011) 229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Pearson CG, Yeh E, Gardner M, Odde D, Salmon ED, Bloom K, Stable kinetochore-microtubule attachment constrains centromere positioning in metaphase, Curr. Biol 14 (2004) 1962–1967. [DOI] [PubMed] [Google Scholar]
- [88].Luconi L, Araki Y, Erlemann S, Schiebel E, The CENP-A chaperone Scm3 becomes enriched at kinetochores in anaphase independently of CENP-A incorporation, Cell Cycle 10 (2011) 3369–3378. [DOI] [PubMed] [Google Scholar]
- [89].Mellone BG, Grive KJ, Shteyn V, Bowers SR, Oderberg I, Karpen GH, Assembly of Drosophila centromeric chromatin proteins during mitosis, PLoS Genet 7 (2011) e1002068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Kato T, Sato N, Hayama S, Yamabuki T, Ito T, Miyamoto M, Kondo S, Nakamura Y, Daigo Y, Activation of Holliday junction recognizing protein involved in the chromosomal stability and immortality of cancer cells, Cancer Res 67 (2007) 8544–8553. [DOI] [PubMed] [Google Scholar]
- [91].Hu Z, Huang G, Sadanandam A, Gu S, Lenburg ME, Pai M, Bayani N, Blakely EA, Gray JW, Mao JH, The expression level of HJURP has an independent prognostic impact and predicts the sensitivity to radiotherapy in breast cancer, Breast Cancer Res 12 (2010) R18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Castillo AG, Mellone BG, Partridge JF, Richardson W, Hamilton GL, Allshire RC, Pidoux AL, Plasticity of fission yeast CENP-A chromatin driven by relative levels of histone H3 and H4, PLoS Genet 3 (2007) e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Jackson JR, Patrick DR, Dar MM, Huang PS, Targeted anti-mitotic therapies: can we improve on tubulin agents? Nat. Rev. Cancer 7 (2007) 107–117. [DOI] [PubMed] [Google Scholar]