Abstract
Objective:
To investigate subgroup responses to long-acting injectable (LAI) haloperidol decanoate (HD) and paliperidone palmitate (PP) in a randomized controlled trial that found no difference between the treatments on the primary outcome of efficacy failure.
Method:
A Comparison of Long-Acting Injectable Medications for Schizophrenia (ACLAIMS) enrolled 311 participants from March 2011 to July 2013 meeting DSM-VI-TR criteria for diagnoses of schizophrenia or schizoaffective disorder at risk of relapse due to medication non-adherence or substance abuse. Participants were randomly assigned to double-blinded treatment with HD or PP and followed for up to 2 years. A committee blinded to treatment assignment adjudicated efficacy failure based on meeting at least one of these criteria: psychiatric hospitalization, crisis stabilization, increased outpatient visits, could not discontinue oral antipsychotic, discontinued assigned LAI due to inadequate therapeutic benefit, or prolonged need for adjunctive oral antipsychotic medication. Survival analyses examined modification of treatment effects on efficacy failure by age, gender, race, substance abuse, baseline symptom severity, and baseline adherence. Mixed effect linear models and analysis of covariance examined this modification on safety outcomes.
Results:
An interaction between age and treatment (p=0.009) revealed younger participants assigned HD had longer time to efficacy failure than those assigned PP. Interactions were not significant between treatment group and gender, race, substance use disorder, baseline symptom severity, or baseline adherence. An interaction of treatment and age on akathisia (p=0.047) found an advantage for PP that was larger among younger persons. An advantage for HD on serum prolactin levels was larger among younger women (p=0.033).
Conclusion:
Among younger persons, HD was associated with lower rates of efficacy failure than PP. Age effects on adverse effects were mixed. Age-related heterogeneity of antipsychotic treatment effects warrants further investigation and consideration in clinical practice.
Clinical Trials Registration:
A Comparison of Long-acting Injectable Medications for Schizophrenia (ACLAIMS) https://clinicaltrials.gov/ct2/show/NCT01136772?term=ACLAIMS&rank=1, NCT01136772
Introduction
Long-acting injectable (LAI) antipsychotic medications are an important treatment option for individuals diagnosed with schizophrenia because they ensure medication delivery and allow for accurate assessment of medication adherence. This mode of medication delivery is widely believed to improve outcomes by improving medication adherence and thereby reducing symptoms and rates of relapse and rehospitalization.1 Treatment guidelines recommend LAI antipsychotics for patients who are at risk of nonadherence and for those who prefer bi-weekly or monthly injections to daily pills.2 There are increasingly frequent expert recommendations to use LAI antipsychotics among young people who are experiencing a first episode of schizophrenia because of high rates of non-adherence in this population and some evidence of improved outcomes with LAIs over oral antipsychotics.3–5
A Comparison of Long-acting Injectable Medications for Schizophrenia (ACLAIMS), a National Institute of Mental Health-sponsored randomized controlled trial that compared the effectiveness of paliperidone palmitate, a newer LAI, to haloperidol decanoate, which has been available for several decades.6 The study found no difference in rates of efficacy failure among study participants, all of whom had a history of relapse due to medication non-adherence or substance abuse.
In this investigation we explored whether different subgroups previously found to have differential responses to antipsychotics, defined by age, gender, race, the presence of a substance use disorder, Positive And Negative Syndrome Scale (PANSS)7 score at baseline, and baseline adherence responded differently to haloperidol decanoate and paliperidone palmitate. We investigated heterogeneity of effects in the primary outcome of efficacy failure, which was not different between the two medications in ACLAIMS, as well as secondary safety outcomes that were different in the overall analyses.
Methods
Participants
Analyses were conducted using data from ACLAIMS which took place from March 2011 to July 2013. Participants were eligible to join the study if they were between 18 and 65 years of age, met criteria from the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition, Text Revision; DSM-IV-TR) of diagnoses of schizophrenia or schizoaffective disorder,8 and had the capacity to provide informed consent.9 Of the 353 individuals assessed for eligibility, 311 were randomized to either the LAI paliperidone palmitate (PP) or LAI haloperidol decanoate (HD). The final intent-to-treat sample consisted of 294 participants (147 participants in the PP group and 147 participants in the HD group) who received at least one injection. The modified intent-to-treat sample consisted of 290 participants (145 in each group) with four participants removed who had no visit after their first injection. The study was conducted at 22 US clinical sites and each site obtained institutional review board approval to conduct the study. Further details on the design of ACLAIMS can be found in McEvoy et al.6
Outcome Measures
Primary outcome
The primary outcome of interest in this analysis was efficacy failure. This was defined as meeting at least one of the following criteria: a psychiatric hospitalization, a need for crisis stabilization, a clinically meaningful increase in the frequency of outpatient visits, clinicians’ decisions that oral antipsychotic medication could not be discontinued within eight weeks after starting the LAI, clinicians’ decisions to discontinue assigned LAI treatment due to inadequate therapeutic benefit, and ongoing or repeated need for adjunctive oral antipsychotic medication.6 A committee blinded to treatment assignment adjudicated efficacy failure based on these pre-determined criteria.
Secondary outcomes
Tolerability failure was based on clinicians’ decisions and classified according to common antipsychotic adverse effects including weight gain, lipid changes, glucose changes, extrapyramidal symptoms (EPS), tardive dyskinesia (TD), akathisia, sexual dysfunction, gynecomastia/galactorrhea, and menstrual irregularities, as well as hypersensitivity during the oral antipsychotic trial.6
Other secondary outcomes were those examined by McEvoy et al,6 which are common adverse effects variably associated with different antipsychotics.10–14 These included weight change over the course of the study and incidence of gaining 15 pounds or more. Worst change from baseline of six laboratory measures were also examined. Worst change from baseline over the course of the study and incidence of clinically significant scores of three neurological effect measures were determined. These included the Abnormal Involuntary Movement Scale (AIMS)15 global severity score and incidence of scores ≥2, the Barnes Akathisia Rating Scale (BAS)16 global score and incidence of scores ≥3, and the Simpson-Angus Scale Abbreviated Form (SAS)17 mean score and incidence or scores ≥1. In addition, highest levels of prolactin and worst Arizona Sexual Side Effects (ASEX)18 score and incidence of scores ≥ 19 were determined grouped by gender.
Statistical Analyses
Primary Analysis
The primary analysis included the modified intent to treat population (N=290). Specific groups previously found to have heterogeneous responses to antipsychotic treatments were tested for modification of the effects of participants’ assigned treatments on efficacy failure.19–27 The Kaplan-Meier method28 was used for the survivor analysis to estimate survival probabilities of the population that did not experience efficacy failure in days from first injection. Modification of this association was then tested by age (continuous in years), gender (female or male), race (White or African American), substance use disorder (meeting criteria of at least 3 out of 5 on the Drug Use and/or Alcohol Use Scales)29, baseline PANSS score (median split), and baseline adherence (Brief Adherence Rating Scale (BARS)30 percentage taken in the last month (median split). Following the methods in McEvoy et al,6 participants were censored 90 days after their last injection. Analyses were adjusted for baseline PANSS score and study site.
Secondary Analyses
Exploratory analyses were conducted to determine whether there was significant modification by our groups of interest on the relationship between the assigned treatment and our outcome measures. Modification of the association between assigned treatment and tolerability failure was tested for each of the groups using a Wald χ2 test that adjusted for treatment site.
For the analysis of weight change over time, mixed-effect linear models with spatial power covariance structure were used to determine weight change (kg) in least squares means (LSMean) from baseline at four timepoints: six months from baseline, 12 months from baseline, 18 months from baseline and 24 months from baseline. Type III tests of fixed effects were used to determine the significance of the modification of the assigned treatment by our groups of interest, adjusting for treatment site and baseline weight.
A modified sample limited to those who had at least one laboratory assessment after their first injection (N=126 for HD, N=129 for PP) was used to determine the worst change from baseline of laboratory measures including HBA1C, blood glucose, total cholesterol, LDL cholesterol, triglycerides and HDL cholesterol. An Analysis of Covariance (ANCOVA) tested the significance of the interaction between our groups of interest and assigned treatment on the worst LSMean of these six laboratory assessments, using a Type III Sum of Squares (Type III SS) F-Test with a significance of α=0.05, adjusting for treatment site and baseline levels.
The same ANCOVA method was also used to determine the worst change from baseline in LSMean as the outcome for the AIMS, BAS, and SAS, and adjusted for treatment site and baseline scores. Modification of the relationship between these outcomes and assigned treatment by our groups of interest were tested for significance using the Type III SS F-Test with a significance of α=0.05. Finally, using the same ANCOVA method, we determined the highest levels of prolactin after baseline (LSMean), worst ASEX (LSMean) after baseline and incidence of an ASEX score >=19 grouped by gender. The interaction between the assigned treatment and our groups of interest were tested for significance using the Type III SS F-Test with a significance of α=0.05.
For all secondary analyses, data collected more than six weeks after each participant’s last injection were excluded. Individuals with baseline values equal to or greater than the clinically significant scores for the AIMS, BAS, SAS and ASEX were excluded from the individual analyses that examined incidence of the clinically significant score. SAS 9.431 was used to conduct these statistical analyses.
Results
The analytic sample was the modified intent-to-treat population, which included 290 participants who received at least one injection and returned for at least one follow-up visit. Table 1 shows the distribution of the subgroups among the treatments and the baseline characteristics of the study population. The survival analysis revealed a significant interaction between age and treatment assignment in the primary outcome of efficacy failure (p=0.009). There was no significant interaction between treatment assignment and gender, race, presence of a substance use disorder, baseline PANSS score, or baseline BARS score on efficacy failure. (Table 2)
Table 1.
Baseline Characteristics of the modified Intent-To-Treat sample.
| Full Sample | Haloperidol | Paliperidone | |
|---|---|---|---|
| N (%) | 290 | 145 (50.00) | 145 (50.00) |
| Age (M, SD) | 44 (12.5) | 45 (12.3) | 43 (12.6) |
| 18–45 years (N, %) | 141 (48.62) | 66 (45.52) | 75 (51.72) |
| 46–65 years (N, %) | 149 (51.38) | 79 (54.48) | 70 (48.28) |
| Female (N, %) | 74 (25.61) | 35 (24.1) | 39 (27.1) |
| Race (N, %) | |||
| African American | 173 (60.49) | 85 (60.28) | 88 (60.69) |
| White | 107 (37.41) | 52 (36.88) | 55 (37.93) |
| Other* | 6 (2.10) | 4 (2.84) | 2 (1.38) |
| Hispanic (N, %) | 14 (4.83) | 8 (5.52) | 6 (4.14) |
| Substance Use Disorder (N, %) | 72 (24.83) | 36 (24.83) | 36 (24.83) |
| Clinical | |||
| Weight kg (M, SD) | 89.94 (22.06) | 90 (22.5) | 90 (21.7) |
| HBA1C (M, SD) | 5.77 (1.00) | 5.6 (0.6) | 5.9 (1.3) |
| Blood Glucose mg/dL (M, SD) | 99.29 (25.16) | 94.80 (17.61) | 103.85 (30.39) |
| Total Cholesterol mg/dL (M, SD) | 180.55 (40.29) | 181.14 (42.11) | 179.96 (38.51) |
| LDL Cholesterol mg/dL (M, SD) | 106.25 (34.15) | 108.11 (33.18) | 104.38 (35.12) |
| Triglycerides mg/dL (M, SD) | 122.29 (83.45) | 120.16 (80.65) | 124.43 (86.41) |
| HDL Cholesterol mg/dL (M, SD) | 49.66 (14.66) | 48.73 (13.02) | 50.61 (16.14) |
| Prolactin μg/L (M, SD) | |||
| Female | 33.12 (36.81) | 32.40 (38.98) | 33.77 (35.25) |
| Male | 17.80 (17.62) | 17.83 (13.30) | 17.76 (21.30) |
| PANSS Total Score (Med, Range) | 71.00 (36.00–116.00) | 70.00 (36.00–116.00) | 72.00 (37.00–116.00) |
| BARS Proportion Taken (Med, Range) | 93.00 (0–100.00) | 93.50 (0–100.00) | 90.00 (0–100.00) |
| Time to Efficacy Failure (days) | |||
| Age 18–45 years (M, SD) | 336.03 (209.63) | 313.71 (216.31) | |
| Age 46–65 years (M, SD) | 287.33 (217.14) | 304.03 (186.22) | |
| AIMS Global Severity Score (Med, Range) | 0 (0–3.00) | 0 (0–3.00) | 0 (0–2.00) |
| BAS Global Score (Med, Range) | 0 (0–3.00) | 0 (0–3.00) | 0 (0–3.00) |
| SAS Mean Score (Med, Range) | 0 (0–1.50) | 0.17 (0–1.50) | 0 (0–1.50) |
| ASEX Score (Med, Range) | |||
| Female | 19.00 (7.00–30.00) | 19.00 (7.00–30.00) | 18.00 (10.00–30.00) |
| Male | 14.00 (5.00–30.00) | 14.00 (5.00–30.00) | 14.00 (5.00–30.00) |
Other includes: American Indian, Alaska Native, Asian, Native Hawaiian, Pacific Islander
AIMS: Abnormal Involuntary Movement Scale (higher is worse); ASEX: Arizona Sexual Side Effects (higher is worse); BARS: Brief Adherence Rating Scale (higher is better); BAS: Barnes Akathisia Rating Scale (higher is worse); PANSS: Positive and Negative Syndrome Scale (higher is worse); SAS: Simpson-Angus Extrapyramidal Side Effects Scale Abbreviated Form (higher is worse)
Table 2.
Modification of assigned treatment by groups of interest on efficacy failure. Modified Intent-To-Treat sample (N=290).
| Predicting | Efficacy Failure | |
|---|---|---|
| χ2 | p-valuea | |
| Age (Continuous Years) | 7.2597 | 0.009 |
| Gender | 0.1287 | 0.7198 |
| Substance Use Disorder | 0.1387 | 0.7096 |
| Race | 2.2666 | 0.1322 |
| PANSS (Median split) | 0.8281 | 0.3628 |
| BARS (Median split) | 0.5102 | 0.4750 |
=Adjusted for Baseline PANSS Score and Site
BARS: Brief Adherence Rating Scale; PANSS: Positive and Negative Syndrome Scale
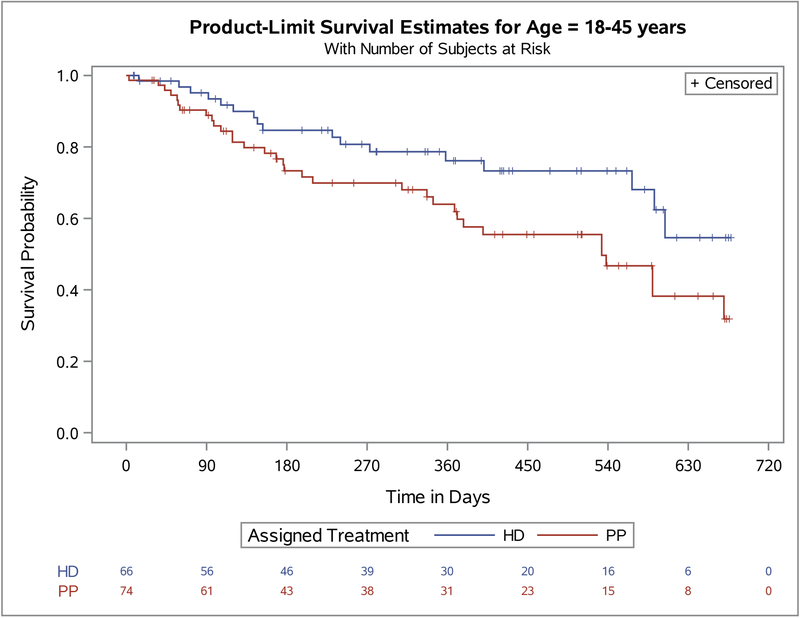
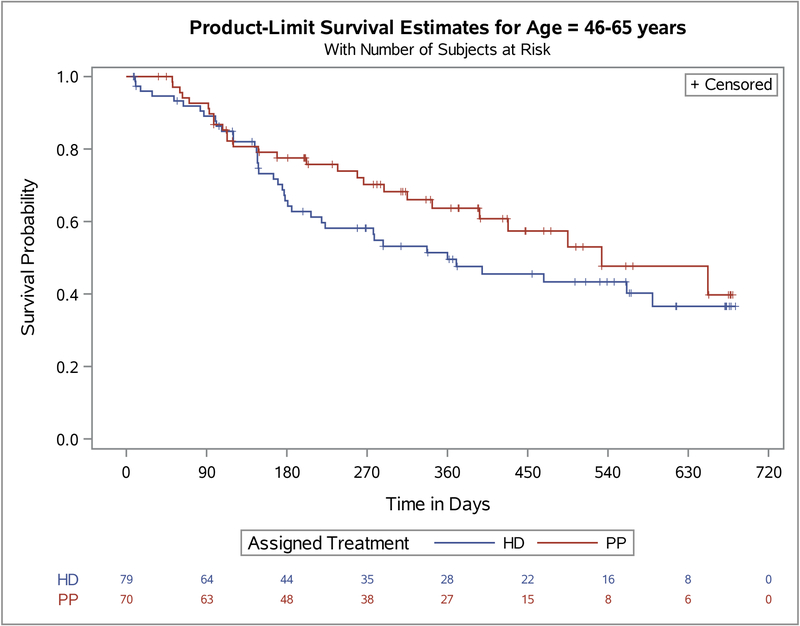
To further investigate the modification of assigned treatment efficacy failure by age we split the population at the median age as seen in Figure 1. We found that among the younger group (age 18–45), HD was associated with a significantly longer time to efficacy failure (p=0.029) than PP. Among the older group (age 46–65), there was a trend for longer time to efficacy failure among the PP group (p=0.196). In addition, among the criteria for efficacy failure (Table 3), the need for psychiatric hospitalization and the need for crisis stabilization were significantly different between the assigned treatments in the younger age group (i.e., p=0.016 and p=0.025 respectively). All the other, rarer criteria defining efficacy failure trended in the same direction, with participants assigned to PP having more events than those assigned HD. No differences between assigned treatment and efficacy failure criteria were seen in the older age group.
Figure 1.
Primary outcome. Survival analysis of Paliperidone Palmitate (PP) versus Haloperidol Decanoate (HD) stratified by age groups.
Age 18–45 years Log-rank P=.03; Age 46–65 years Log-rank P=.21
Table 3.
Criteria for Efficacy Failure within age groups by medication. Modified Intent-To-Treat sample (N=290).*
| 18–45 Years | 46–65 Years | |||||
|---|---|---|---|---|---|---|
| HD | PP | p-value | HD | PP | p-value | |
| N (%) | 66 (46.81) | 75 (53.19) | 79 (53.02) | 70 (46.98) | ||
| Efficacy Failure (N, %) | 17 (25.76) | 34 (45.33) | 0.0158 | 39 (49.37) | 27 (38.57) | 0.1855 |
| Criteria for Efficacy Failure (N, %) | ||||||
| Need for psychiatric hospitalization | 9 (13.64) | 23 (30.67) | 0.0160 | 29 (36.71) | 23 (32.86) | 0.6225 |
| Need for crisis stabilization | 4 (6.06) | 14 (18.67) | 0.0252 | 17 (21.52) | 7 (10.00) | 0.0563 |
| Increased frequency outpatient visits | 0 | 2 (2.67) | 0.1815 | 4 (5.06) | 3 (4.29) | 0.8229 |
| Oral antipsychotic medication could not be discontinued | 1 (1.52) | 5 (6.67) | 0.1305 | 10 (12.66) | 6 (8.57) | 0.4213 |
| Discontinue the assigned LAI inadequate benefit | 6 (9.09) | 12 (16.00) | 0.2199 | 20 (25.32) | 16 (22.86) | 0.7263 |
| Need for adjunctive oral antipsychotic medication | 3 (4.55) | 7 (9.33) | 0.2691 | 11 (13.92) | 9 (12.86) | 0.8488 |
Not mutually exclusive categories
Other assigned treatment outcomes modified by age included akathisia measured by the BAS and increases in serum prolactin levels.(Table 4) The greater mean increase in the BAS score associated with HD was larger in the 18–45 age group (p=0.047). All prolactin analyses were conducted by gender; the greater increase associated with PP was of larger magnitude among younger women (p=0.033).
Table 4.
Worst change from baseline and incidence of clinically significant scores for neurological effects and prolactin levels among age groups
| 18–45 years | 46–65 years | ||||
|---|---|---|---|---|---|
| HD | PP | HD | PP | p-valuea | |
| AIMS Global Severity Score | |||||
| Incidence of AIMS>=2 (N, %) | 8 (11.94) | 9 (11.84) | 24 (30.00) | 22 (30.99) | 0.98 |
| Worst change from baseline (M, 95% CI) | 0.38 (0.20–0.57) | 0.26 (0.09–0.43) | 0.71 (0.54–0.87) | 0.62 (0.45–0.79) | 0.82 |
| BAS Global Score | |||||
| Incidence of BAS>=3 (N, %) | 10 (14.93) | 4 (5.26) | 4 (5.00) | 2 (2.82) | 0.22 |
| Worst Change from Baseline (M, 95% CI) | 0.88 (0.65–1.11) | 0.37 (0.16–0.58) | 0.49 (0.29–0.70) | 0.41 (0.20–0.62) | 0.047 |
| SAS Mean Score | |||||
| Incidence of SAS>=1 (N, %) | 11 (16.42) | 8 (10.53) | 15 (18.75) | 9 (12.68) | 0.75 |
| Worst Change from Baseline (M, 95% CI) | 0.21 (0.13–0.30) | 0.20 (0.12–0.28) | 0.34 (0.26–0.42) | 0.28 (0.20–0.36) | 0.57 |
| Serum Prolactin Levels | |||||
| Among Women Only | |||||
| Highest Level After Baseline (M, 95% CI) | 21.92 (0.79–43.05) | 89.06 (69.37–108.74) | 26.60 (6.50–46.71) | 55.56 (38.97–72.16) | 0.033 |
| Worst ASEX After Baseline (M, 95% CI) | 21.89 (17.37–26.40) | 22.39 (18.19–26.60) | 24.78 (20.49–29.07) | 25.07 (21.52–28.61) | 0.95 |
| ASEX Score >=19 (N, %) | 2 (12.50) | 6 (30.00) | 2 (10.00) | 3 (15.79) | 0.47 |
| Among Men Only | |||||
| Highest Level After Baseline (M, 95% CI) | 14.79 (7.55–22.03) | 35.34 (28.37–42.31) | 15.93 (9.24–22.61) | 33.24 (26.18–40.30) | 0.64 |
| Worst ASEX After Baseline (M, 95% CI) | 18.70 (16.74–20.65) | 16.32 (14.41–18.22) | 17.30 (15.49–19.10) | 19.41 (17.53–21.29) | 0.018 |
| ASEX Score >=19 (N, %) | 7 (13.73) | 7 (12.73) | 7 (11.67) | 12 (23.08) | 0.15 |
= Significance of interaction (modification) of Treatment by Age Group
AIMS: Abnormal Involuntary Movement Scale (higher is worse); BAS: Barnes Akathisia Rating Scale (higher is worse); HD: Haloperidol Decanoate; PP: Paliperidone Palmitate; SAS: Simpson-Angus Extrapyramidal Side Effects Scale Abbreviated Form (higher is worse); ASEX: Arizona Sexual Side Effects Scale (higher is worse)
PP was associated with more weight gain than HD; there was no modification by age (weight change by assigned treatment modified by age group, at 6 months p=0.82; at 12 months p=0.28; at 18 months p=0.34; at 24 months p=0.95; ever gained 15 pounds or more by assigned treatment modified by age group, p=0.44). No effects on laboratory measures were modified by age (HBA1C p=0.39; blood glucose p=0.90; total cholesterol p=0.12; LDL p=0.29; triglycerides p=0.76; HDL p=0.69). Thirty people in each treatment group discontinued the study medication because of poor tolerability; we found no significant interaction between age (p=0.29), gender (p=0.41), race (p=0.41), presence of a substance use disorder (p=0.81), baseline PANSS score (p=0.60), or baseline BARS score (p=0.66) and treatment assignment on this outcome.
We conducted several post-hoc analyses to further investigate the modification of assigned treatment on efficacy failure by age. To examine the possibility that differential adherence between the two medications might explain the significant findings, we determined whether there was an association between non-adherence to assigned LAI among those assigned to that LAI and whether that association was modified by age. The association was not significant (χ2=0.45, p=0.50). There was no difference between the assigned treatments and duration and dose equivalents 32 of the oral supplementation phase, with the oral supplementation discontinued by week 8 for the vast majority of participants. Because antipsychotics may not be as effective at treating affective symptoms as psychosis, we tested whether there was an interaction between assigned treatment and age on the likelihood that ending treatment was due to affective symptomatology. Age did not modify this association (χ2=1.79, p=0.18). The effect of anticholinergic load on memory and cognition was tested by first identifying participants who started anticholinergic medications after entering the study to determine if the initiation of anticholinergics by assigned treatment was modified by age. Second, we calculated whether the association between mean change from baseline of the Verbal Memory Response (measured from the Brief Assessment of Cognition, BAC 33) and assigned LAI was modified by age. Age did not modify either of these associations (i.e., χ2=0.49, p=0.48 for anticholinergic naïve participants, and χ2=1.4, p=0.24 for the BAC).
Discussion
This randomized trial compared HD to PP in participants considered likely to benefit from LAI medications and did not find an advantage for PP on the main outcome of efficacy failure. This is consistent with a long history of research that finds that standard antipsychotics (i.e., other than clozapine) are generally similarly effective and are most distinct in their side effect profiles.34,35
The desire to personalize treatments has led to numerous calls to investigate heterogeneity of treatment effects in patient subgroups. A prior investigation of antipsychotics found ethnic differences in metabolic complications from antipsychotic therapy.22 Sernyak et al11 found an increased risk of diabetes in younger adults (<40 years of age) taking atypical antipsychotics. A reduction in the risk of hospitalization was associated with older age in individuals with schizophrenia who were compliant with their medication regimens.36 Sex differences have been found in the incidence and progression of schizophrenia.37 Another investigation found that clusters of individuals with differing levels of cognitive impairment had differential responses to treatments.38 Those with schizophrenia who are less adherent taking their medications as prescribed are more likely to experience relapse than those who are highly adherent.39
The analyses presented here evaluated whether some subgroups of clinical interest responded differently to HD and PP. The effect of age was strongly significant, but there was no modification by gender, race, presence of a substance use disorder, baseline symptoms (PANSS score), or baseline adherence (BARS score). Age also modified the effects of the treatments on akathisia and serum prolactin levels. In both cases, younger participants had an exaggerated adverse effect compared to older participants. To illustrate the possible clinical significance of our preliminary findings we found that the NNT for those aged 18–45 taking HD versus PP was 5.26, while the NNT for PP versus HD for those aged 46–65 was 10. An NNT of 5 or lower is considered effective, while higher values indicate less effectiveness. 40
Limitations
Limitations of our study are that our analyses do not explain why HD is associated with lower rates of efficacy failure than PP in younger participants. The expectation that PP might be better tolerated among young persons who are more sensitive to side effects was not confirmed.41 One possible explanation is use of different dosages of medications in younger or older patients.42 However, analysis of the maximum dosage prescribed after baseline of assigned treatments found no difference by age (p=0.56 for the HD group and p=0.69 for the PP group).
In addition, subgroup analyses may affect the balance achieved through the initial randomization. We tested for differences between assigned LAIs for each of our subgroups and none were significant (see Table 5). However, unmeasured differences between assigned LAIs for our subgroups may still exist, which could affect our results.
Table 5.
Sensitivity analysis of differences between assigned treatments within each subgroup.
| Subgroup | Haloperidol | Paliperidone | p-value* |
|---|---|---|---|
| Age (M, SD) | 45.01 (12.34) | 42.61 (12.57) | 0.21 |
| Female (N, %) | 35 (24.14) | 39 (26.90) | 0.85 |
| Race (N, %) | 0.93 | ||
| African American | 85 (62.04) | 88 (61.54) | |
| White | 52 (37.96) | 55 (38.46) | |
| Substance Use Disorder (N, %) | 36 (24.83) | 36 (24.83) | 1 |
| PANSS Median Split (N, %) | 0.73 | ||
| Lower | 78 (53.79) | 65 (44.83) | |
| Upper | 67 (46.21) | 80 (55.17) | |
| BARS Median Split (N, %) | 0.87 | ||
| Lower | 92 (63.45) | 95 (65.52) | |
| Upper | 53 (36.55) | 50 (34.48) |
Differences between prevalence of assigned LAI for each subgroup.
We investigated six possible treatment modifiers, which increases the chance that the significant finding is due to chance. If we were to control for multiple comparisons using a Bonferroni correction (0.05/6=.008), then the interaction of age with assigned treatment would still closely approximate the usual standard for statistical significance.43
Our post hoc analyses of treatment heterogeneity must be considered preliminary. Further efforts to examine heterogeneity of treatment response of antipsychotic medications by age are needed. If differential effects of medications by age are confirmed, this may lead to improved selection of treatments, shorter time to treatment response, and better outcomes.
CLINICAL POINTS.
Identification of heterogeneous treatment response in clinical subgroups may lead to improved medication selection.
Long-acting injectable (LAI) antipsychotic medications are important options ensuring medication delivery in schizophrenia.
Although a large NIMH-sponsored study found no difference in effectiveness between paliperidone palmitate and haloperidol decanoate (HD), this analysis found HD more effective among younger patients, warranting further research into age effects.
Acknowledgments
Funding/Support: This study was funded by R01MH081107, and this funder did not have a role in the conduct and publication of this study. Dr. Bareis was funded by 5T32MH020004-18, and this funder did not have a role in the conduct and publication of the study.
Footnotes
Potential Conflicts of Interest: Dr. Stroup received a research grant from Auspex Pharmaceuticals. Dr. McEvoy has received consulting fees, honoraria, and/or grants from Alkermes Inc, Avanir, Boehringer Ingelheim, Neurocrine, Teva, and Otsuka. Drs. Bareis, Swartz, and Rosenheck have no conflicts of interest to disclose.
REFERENCES
- 1.Tiihonen J, Mittendorfer-Rutz E, Majak M, et al. Real-World Effectiveness of Antipsychotic Treatments in a Nationwide Cohort of 29823 Patients With Schizophrenia. JAMA Psychiatry. 2017;74(7):686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchanan RW, Kreyenbuhl J, Kelly DL, et al. The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subotnik KL, Casaus LR, Ventura J, et al. Long-Acting Injectable Risperidone for Relapse Prevention and Control of Breakthrough Symptoms After a Recent First Episode of Schizophrenia. A Randomized Clinical Trial. JAMA Psychiatry. 2015;72(8):822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Correll CU, Citrome L, Haddad PM, et al. The Use of Long-Acting Injectable Antipsychotics in Schizophrenia: Evaluating the Evidence. J Clin Psychiatry. 2016;77(suppl 3):1–24. [DOI] [PubMed] [Google Scholar]
- 5.Biagi E, Capuzzi E, Colmegna F, et al. Long-Acting Injectable Antipsychotics in Schizophrenia: Literature Review and Practical Perspective, with a Focus on Aripiprazole Once-Monthly. Adv Ther. 2017;34(5):1036–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McEvoy JP, Byerly M, Hamer RM, et al. Effectiveness of paliperidone palmitate vs haloperidol decanoate for maintenance treatment of schizophrenia: a randomized clinical trial. JAMA. 2014;311(19):1978–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kay SR, Opler LA, Spitzer RL, Williams JB, Fiszbein A, Gorelick A. SCID-PANSS: two-tier diagnostic system for psychotic disorders. Compr Psychiatry. 1991;32(4):355–361. [DOI] [PubMed] [Google Scholar]
- 8.Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 9.Jeste DV, Palmer BW, Appelbaum PS, et al. A new brief instrument for assessing decisional capacity for clinical research. Arch Gen Psychiatry. 2007;64(8):966–974. [DOI] [PubMed] [Google Scholar]
- 10.Gao K, Fang F, Wang Z, Calabrese JR. Subjective Versus Objective Weight Gain During Acute Treatment With Second-Generation Antipsychotics in Schizophrenia and Bipolar Disorder. J Clin Psychopharmacol. 2016;36(6):637–642. [DOI] [PubMed] [Google Scholar]
- 11.Sernyak MJ, Leslie DL, Alarcon RD, Losonczy MF, Rosenheck R. Association of diabetes mellitus with use of atypical neuroleptics in the treatment of schizophrenia. Am J Psychiatry. 2002;159(4):561–566. [DOI] [PubMed] [Google Scholar]
- 12.Bakker PR, de Groot IW, van Os J, van Harten PN. Long-stay psychiatric patients: a prospective study revealing persistent antipsychotic-induced movement disorder. PLoS One. 2011;6(10):e25588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henderson DC, Doraiswamy PM. Prolactin-related and metabolic adverse effects of atypical antipsychotic agents. J Clin Psychiatry. 2008;69 Suppl 1:32–44. [PubMed] [Google Scholar]
- 14.Baggaley M Sexual dysfunction in schizophrenia: focus on recent evidence. Hum Psychopharmacol. 2008;23(3):201–209. [DOI] [PubMed] [Google Scholar]
- 15.Guy W Abnormal Involuntary Movement Scale (AIMS) In: ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: U.S. Dept. of Health, Education, and Welfare; 1976:534 – 537. [Google Scholar]
- 16.Barnes TR. The Barnes Akathisia Rating Scale--revisited. J Psychopharmacol. 2003;17(4):365–370. [DOI] [PubMed] [Google Scholar]
- 17.Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–19. [DOI] [PubMed] [Google Scholar]
- 18.McGahuey CA, Gelenberg AJ, Laukes CA, et al. The Arizona Sexual Experience Scale (ASEX): reliability and validity. J Sex Marital Ther. 2000;26(1):25–40. [DOI] [PubMed] [Google Scholar]
- 19.Jeste DV, Maglione JE. Atypical antipsychotics for older adults: are they safe and effective as we once thought? J Comp Eff Res. 2013;2(4):355–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rado J, Janicak PG. Pharmacological and clinical profile of recently approved second-generation antipsychotics: implications for treatment of schizophrenia in older patients. Drugs Aging. 2012;29(10):783–791. [DOI] [PubMed] [Google Scholar]
- 21.Grossman LS, Harrow M, Rosen C, Faull R, Strauss GP. Sex differences in schizophrenia and other psychotic disorders: a 20-year longitudinal study of psychosis and recovery. Compr Psychiatry. 2008;49(6):523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ader M, Garvey WT, Phillips LS, et al. Ethnic heterogeneity in glucoregulatory function during treatment with atypical antipsychotics in patients with schizophrenia. J Psychiatr Res. 2008;42(13):1076–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swartz MS, Wagner HR, Swanson JW, et al. The effectiveness of antipsychotic medications in patients who use or avoid illicit substances: results from the CATIE study. Schizophr Res. 2008;100(1–3):39–52. [DOI] [PubMed] [Google Scholar]
- 24.Leatherman SM, Liang MH, Krystal JH, et al. Differences in treatment effect among clinical subgroups in a randomized clinical trial of long-acting injectable risperidone and oral antipsychotics in unstable chronic schizophrenia. J Nerv Ment Dis. 2014;202(1):13–17. [DOI] [PubMed] [Google Scholar]
- 25.Barkhof E, Meijer CJ, de Sonneville LM, Linszen DH, de Haan L. Interventions to improve adherence to antipsychotic medication in patients with schizophrenia--a review of the past decade. Eur Psychiatry. 2012;27(1):9–18. [DOI] [PubMed] [Google Scholar]
- 26.Szymanski S, Lieberman J, Pollack S, et al. Gender differences in neuroleptic nonresponsive clozapine-treated schizophrenics. Biol Psychiatry. 1996;39(4):249–254. [DOI] [PubMed] [Google Scholar]
- 27.Case M, Stauffer VL, Ascher-Svanum H, et al. The heterogeneity of antipsychotic response in the treatment of schizophrenia. Psychol Med. 2011;41(6):1291–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stel VS, Dekker FW, Tripepi G, Zoccali C, Jager KJ. Survival analysis I: the Kaplan-Meier method. Nephron Clin Pract. 2011;119(1):c83–88. [DOI] [PubMed] [Google Scholar]
- 29.Drake RE, Osher FC, Noordsy DL, Hurlbut SC, Teague GB, Beaudett MS. Diagnosis of alcohol use disorders in schizophrenia. Schizophr Bull. 1990;16(1):57–67. [DOI] [PubMed] [Google Scholar]
- 30.Byerly MJ, Nakonezny PA, Rush AJ. The Brief Adherence Rating Scale (BARS) validated against electronic monitoring in assessing the antipsychotic medication adherence of outpatients with schizophrenia and schizoaffective disorder. Schizophr Res. 2008;100(1–3):60–69. [DOI] [PubMed] [Google Scholar]
- 31.SAS 9.4 [computer program]. SAS Institute, Inc.
- 32.Leucht S, Samara M, Heres S, Davis JM. Dose Equivalents for Antipsychotic Drugs: The DDD Method. Schizophr Bull. 2016;42 Suppl 1:S90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68(2–3):283–297. [DOI] [PubMed] [Google Scholar]
- 34.Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951–962. [DOI] [PubMed] [Google Scholar]
- 35.Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–1223. [DOI] [PubMed] [Google Scholar]
- 36.Kozma CM, Weiden PJ. Partial compliance with antipsychotics increases mental health hospitalizations in schizophrenic patients: analysis of a national managed care database. Am Health Drug Benefits. 2009;2(1):31–38. [PMC free article] [PubMed] [Google Scholar]
- 37.Aleman A, Kahn RS, Selten JP. Sex differences in the risk of schizophrenia: evidence from meta-analysis. Arch Gen Psychiatry. 2003;60(6):565–571. [DOI] [PubMed] [Google Scholar]
- 38.Gilbert E, Merette C, Jomphe V, et al. Cluster analysis of cognitive deficits may mark heterogeneity in schizophrenia in terms of outcome and response to treatment. Eur Arch Psychiatry Clin Neurosci. 2014;264(4):333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marder SR. Overview of partial compliance. J Clin Psychiatry. 2003;64 Suppl 16:3–9. [PubMed] [Google Scholar]
- 40.Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ. 1995;310(6977):452–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel NC, Crismon ML, Hoagwood K, et al. Trends in the use of typical and atypical antipsychotics in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2005;44(6):548–556. [DOI] [PubMed] [Google Scholar]
- 42.dosReis S, Zito JM, Buchanan RW, Lehman AF. Antipsychotic dosing and concurrent psychotropic treatments for Medicaid-insured individuals with schizophrenia. Schizophr Bull. 2002;28(4):607–617. [DOI] [PubMed] [Google Scholar]
- 43.Bender R, Lange S. Adjusting for multiple testing--when and how? J Clin Epidemiol. 2001;54(4):343–349. [DOI] [PubMed] [Google Scholar]