Abstract
Medium-chain fatty acids (MCFAs) are key intermediates in the synthesis of medium-chain chemicals including α-olefins and dicarboxylic acids. In bacteria, microbial production of MCFAs is limited by the activity and product profile of acyl-ACP thioesterases. Here, we engineer a heterologous bacterial acyl-ACP thioesterase for improved MCFA production in Escherichia coli. Electrostatically matching the interface between the heterologous medium-chain Acinetobacter baylyi acyl-ACP thioesterase (AbTE) and the endogenous E. coli fatty acid ACP (E. coli AcpP) by replacing small nonpolar amino acids on the AbTE surface for positively charged ones increased secreted MCFA titers more than three-fold. Nuclear magnetic resonance titration of E. coli 15N-octanoyl-AcpP with a single AbTE point mutant and the best double mutant showed a progressive and significant increase in the number of interactions when compared to AbTE wildtype. The best AbTE mutant produced 131 mg/L of MCFAs, with MCFAs being 80% of all secreted fatty acid chain lengths. This work demonstrates that engineering the interface of heterologous enzymes to better couple with endogenous host proteins is a useful strategy to increase the titers of microbially-produced chemicals.
Introduction
Medium-chain fatty acids (MCFAs, C8–C12) are useful as antimicrobials and emulsifying agents, and their derivatization results in an array of chemicals including alkenes, α-olefins, esters, ω-hydroxy-carboxylic acids, α,ω-dicarboxylic acids, alcohols, and ketones1. Microbially, MCFAs are generated via hydrolysis of medium-chain fatty acyl-ACPs obtained from Type II fatty acid synthase (FAS)2, or medium-chain fatty acyl CoAs produced by Type I FAS3 or the reverse β-oxidation pathway4. Hydrolysis of fatty acyl intermediates is performed by medium-chain acyl-ACP thioesterases (TEs) from bacteria, higher plants (FatBs), and mammals, or medium-chain acyl-CoA TEs from bacteria. While bacterial acyl-ACP TEs tend to hydrolyze C8–C16 acyl-ACPs5, such as Acinetobacter baylyi TE6, FatBs have a narrower substrate profile, such as Cuphea palustris TE (C8:0 acyl-ACPs)7, and Umbellularia californica TE (C12:0 acyl-ACPs)8. The rat acyl-ACP TE has been shown to produce primarily C6–C8 fatty acids9. On the acyl-CoA front, E. coli ydiI hydrolyzes C6–C10 acyl-CoAs5.
Microbes do not naturally synthesize MCFAs extensively, and microbial MCFA production is hindered by the inefficient expression of heterologous plant TEs and the broad substrate profile of bacterial TEs. Long chain acyl-ACP TEs have been engineered for improved MCFA production via active site mutagenesis and computationally-guided approaches. For example, E. coli ‘TesA:L109P (signal peptide removed) preferentially hydrolyzes 12:0 and C14:0 acyl-ACPs, unlike the wild type’s preference for C16:0 acyl-ACPs10. Similarly, Pseudomonas aeruginosa TesA:D17S/L462R has improved activity on C12:0 acyl-CoAs11. More recently, using an iterative protein redesign and optimization algorithm ‘TesA sequences that preferentially bind C8–C12 substrates were selected leading to ‘TesA:S122K/Y145K/L146K and ‘TesA:M141L/Y145K/L146K, which had a 1.8-fold improvement in C12 mole fraction and a 10-fold improvement in C8 mole fraction over wild type, respectively12.
Using a natural medium-chain acyl-ACP TE as the engineering starting point, however, has the potential to result in a TE variant with an almost exclusive MCFA product profile, potentially leading to higher MCFA yields. Engineering medium-chain acyl-ACP TEs has a unique set of challenges. First, medium-chain acyl-ACP-TEs are heterologous to E. coli and may have problems interfacing with the host machinery. Second, active site engineering of medium-chain acyl-ACP TEs may not prove to be as fruitful, as mutations may need to be much more subtle, potentially in the second shell, and overall more difficult to identify. We hypothesized that engineering the interface of a heterologous medium chain TE to better complement the surface of E. coli fatty acid biosynthesis ACP (E. coli AcpP) may improve MCFA titers (Fig. 1). In Type II FAS, ACP is bound to the fatty acyl-chain and interacts with all the proteins in fatty acid biosynthesis. The fatty acyl chain is sequestered in the ACP hydrophobic core, and protein-protein interactions between the ACP and partner enzymes guide the maturing acyl chain from the ACP core and into each partner enzyme’s active site13. It is the selective binding of ACP to its enzyme partners, including the de novo FAS subunits, that enables efficient fatty acid biosynthesis14. For instance, crosslinking studies show that E. coli Type II FAS ACP binds more selectively to its cognate E. coli Type II FAS ketoacid synthase (KS) than Streptomyces maritimus Type II polyketide synthase KS14.
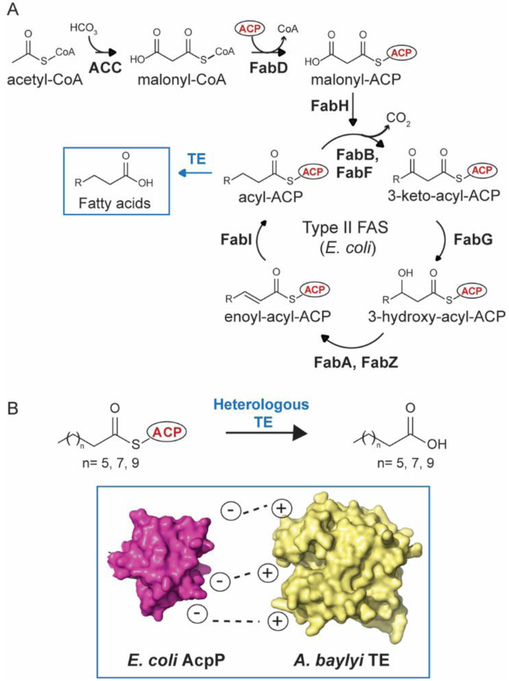
Figure 1.
Medium-chain fatty acid biosynthesis in Escherichia coli. A. E. coli Type II fatty acid synthase (FAS) extends and reduces an acyl chain bound to acyl-carrier protein (ACP). All enzymes in FAS interact with ACP. Thioesterases (TEs) hydrolyze acyl-ACPs to free fatty acids of different chain lengths according to their substrate specificity. B. Matching the surface interface between the native E. coli AcpP and heterologous medium-chain Acinetobacter baylyi TE, improves medium-chain fatty acid production in E. coli.
Here, we engineer the medium-chain acyl-ACP TE from Acinetobacter baylyi (AbTE) to better interface with E. coli AcpP to improve MCFA production. First, we docked E. coli AcpP with the endogenous E. coli TE ‘TesA to identify potential contact residues involved in stabilizing the AcpP-’TesA interaction. Next, we mutated the equivalent positions in AbTE to the amino acids found in E. coli ‘TesA and measured its fatty acid profile. We find that mutation of just two residues on the AbTE surface, G17 and A165 to arginines, improves MCFA titers more than 3-fold when compared to expression of AbTE wild type in E. coli. We proved that the AbTE mutations lead to new selective protein-protein interactions with E. coli ACP by performing NMR titrations between 15N-octanoyl-AcpP and the AbTEs. This work demonstrates that engineering the interface of heterologous enzymes to better couple with endogenous host enzymes is a useful strategy to improve the microbial production of chemicals that require the expression of heterologous enzymes. Improving the microbial production of MCFAs is significant because MCFAs are key intermediates in the biosynthesis of medium-chain chemicals, including α-olefins, dicarboxylic acids and hydroxyacids, which are important targets in the polymer industry.
Results
Screening medium-chain acyl-ACP TEs and E. coli hosts for MCFA production.
To identify the acyl-ACP TE that results in the highest secreted MCFA titers, we expressed the bacterial TE from Acinetobacter baylyi6, and the plant TEs from Cocos nucifera7, Cuphea palustris7, and Umbellularia californica15 in E. coli MG1655 (Fig. 2A). The percent sequence identity of these TEs to one another ranges from 15–17%; A. baylyi TE has the highest percent identity with E. coli ‘TesA at 38% (Fig. SI1). We measured secreted fatty acids as they could be continuously extracted from the culture broth, overcoming issues with cell lysis, and potentially reducing the overall cost for MCFA production. AbTE results in the highest secreted MCFA titers at 29 mg/L, consisting of octanoic, decanoic and dodecanoic acid. C nucifera TE produced only dodecanoic acid at 5 mg/L, while C. palustris TE produced mostly octanoic acid at 8 mg/L. U. californica TE produced a mixture of decanoic and dodecanoic acid at 3 mg/L and 12 mg/L respectively. Given that AbTE resulted in the highest MCFA titers, we decided to engineer this enzyme.
Figure 2.
Medium-chain fatty acid production and calculated E. coli AcpP interphase with TEs. A. Secreted fatty acid titers of E. coli (MG1655) expressing four heterologous TEs. B. Secreted fatty acid titers by different E. coli strains expressing A. baylyi TE (AbTE:WT). C. Secreted and total (secreted plus intracellular and membrane bound) fatty acid titers produced by E. coli expressing AbTE:WT and inactive AbTE (AbTE:S11A). The experiments were done in triplicate and the error bars represent the standard deviation from the mean. D. Docking of E.coli AcpP (magenta, PDB ID: 1FAE) and E. coli ‘TesA (cyan, PDB ID 1U8U) identifies potential residues on the ‘TesA surface that are important for interactions between ‘TesA and AcpP. E. Homology model of AbTE with surface residues equivalent to ‘TesA labelled.
The E. coli genomic background has been shown to affect chemical production16. We expressed AbTE in five different E. coli hosts: DH5α, BL21, DH10B, MG1655, and BW25113 ΔfadE, and measured secreted MCFA titers. We included the fadE deletion as it has been shown to improve fatty acid production in E. coli17. Surprisingly, E. coli hosts BL21 and MG1655 resulted in the highest MCFA productions at 26 mg/L and BW25113 ΔfadE produced only 12 mg/L (Fig. 2B). Based on these results, we moved forward with AbTE expressed in E. coli MG1655.
Engineering AbTE for improved MCFA titers.
Expression of the non-functional AbTE:S11A in E. coli produces only saturated long-chain (C14-C18) fatty acids (LCFA) due to the presence of endogenous long chain TEs. Expression of AbTE wild type (AbTE:WT) in E. coli produced ~29 mg/L of secreted MCFAs or ~52% of all secreted fatty acid chain lengths. When total fatty acids were measured, i.e. secreted fatty acids plus intracellular and membrane bound fatty acids, AbTE:WT expressed in E. coli produced ~48 mg/L of MCFAs, which is ~22% of total fatty acid chain lengths. While MCFA levels increased by 65% when taking into account intracellular and membrane bound fatty acids, LCFA levels increase more than 6-fold. Specifically, AbTE:WT produced octanoic, decanoic, and dodecanoic acid at 9 mg/L, 6, mg/L and 14 mg/L, respectively (Fig. 2A). In addition to saturated fatty acids, AbTE:WT also produced small levels of unsaturated C12-C16 fatty acids.
To identify the interface between E. coli ‘TesA and E. coli AcpP we used ClusPro18, which takes into account only the ‘TesA-AcpP protein interactions to dock E. coli AcpP (PDB ID: 2FAE) and E. coli ‘TesA bound to octanoic acid (PDB ID: 1U8U) (Fig. 2D). Using the model, we identified eight positions on ‘TesA that are potentially part of the AcpP-’TesA interface: Y15, R16, R77, N112, R115, R116, D153, and R160. Structural alignment of ‘TesA with a AbTE homology19 model revealed that all positions except for R16 (AbTE: G17), R115 (AbTE: T120), R116 (AbTE: A121), D153 (AbTE: N158) and R160 (AbTE: A165) had the same amino acids in these two proteins (Fig. 2E, Fig. SI2). Interestingly, four of the five amino acids that are different between ‘TesA and AbTE are positively charged arginines, which could help stabilize the ‘TesA-AcpP interaction as E. coli AcpP has a highly negative surface. We hypothesized that we could replicate these interactions between AbTE and E. coli AcpP to improve MCFA production.
We mutated positions 17, 120, 121, and 165 on AbTE to arginines to generate AbTE:G17R, AbTE:T120R, AbTE: A121R and AbTEA165R, and measured their secreted fatty acid titers (Fig. 3A, Fig. SI3). Expression of AbTE:G17R in E. coli resulted in ~76mg/L of secreted MCFAs, more than double the secreted MCFA titers from AbTE:WT. The MCFAs produced by AbTE:G17R accounted for ~74% of secreted fatty acids of all chain lengths. In particular, octanoic, decanoic and dodecanoic acid were produced at 30 mg/L, 18 mg/L and 28 mg/L, respectively. Expression of AbTE:A165R in E. coli resulted in slightly lower secreted MCFA titers than AbTE:WT. To determine if the effects of these mutations on MCFA titers were additive, we constructed all double mutants using AbTE:G17R as the scaffold. Expression of AbTE:G17R/A165R in E. coli resulted in ~98 mg/L of secreted MCFAs. This is a 29% increase in secreted MCFA titers when compared to expression of AbTE:G17R and a more than 3-fold in secreted MCFA titers when compared to AbTE:WT. Although AbTE:G17R/A165R secreted MCFA titers improved, the MCFA percentage was only 55% of secreted fatty acids of all chain lengths. The major constituent in the AbTE:G17R/A165R secreted MCFA profile was dodecanoic acid at 45 mg/L. The increase in secreted MCFA titer achieved by AbTE:G17R/A165R was unexpected as AbTE:A165R resulted in the lowest MCFA titers from all single mutants. Finally, we generated the triple mutants using AbTE:G17R/A165R as the scaffold. At this stage, we also mutated the fifth position on AbTE that varies from ‘TesA, AbTE:N158D. This fifth position does not change the amino acid to a positively charged arginine, but to a negatively charged aspartate. Nevertheless, the aspartate could still form part of a stabilizing interaction. As Figure 3C shows, none of the triple mutants resulted in improved secreted MCFA titers. Of note, positions A121 and T120 are located on the other side of the AbTE binding pocket than G17 and A165. For completion, we generated the remaining double mutants AbTE:T120R/A121R, AbTE:T120R/A121R, and AbTE:A121R/A165R and measured their secreted fatty acid titers (Fig. SI4). While two of these double mutants produced comparable secreted MCFA titers to AbTE:WT, AbTE:T120R/A121R produced ~45 mg/L of secreted MCFAs. Taken together, AbTE:G17R/A165R results in the highest secreted MCFA titers (98 mg/L), yet AbTE:G17R has the highest percentage of secreted MCFAs (74%), a trend that holds true whether analyzing secreted or total fatty acids (Table 1).
Figure 3.
Secreted fatty acid and protein levels of E. coli expressing A. baylyi TE (AbTE) and AbTE mutants. A. Secreted fatty acid levels of E .coli expressing AbTE single, double, and triple arginine mutants, as well as AbTE single and double glutamate mutants. All experiments were done in triplicate and the error bars represent the standard deviation from the mean. B. Time course of saturated fatty acid titers produced by E. coli expressing AbTE, AbTE:G17R, and AbTE:G17R/A165R. C. Coomassie stained SDS-PAGE gel of Serial dilution of induced E. coli cultures expressing AbTE:WT, AbTE:G17R and AbTE:G17R/A165R.
Table 1.
: Saturated fatty acid percent composition produced by AbTE:WT and variants
| Thioesterase | E. coli strain | Media | C8:0 (%) | C10:0 (%) | C12:0 (%) | C14:0 (%) | C16:0 (%) | C18:0 (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Supernatant | AbTE:WT | MG1655 | M9 | 16.5 | 10.5 | 25.2 | 11.5 | 17.1 | 19.2 |
| AbTE:G17R | MG1655 | M9 | 29.3 | 17.5 | 27.3 | 4.7 | 9.1 | 12.1 | |
| AbTE:G17R/A165R | MG1655 | M9 | 18.5 | 11.7 | 25.4 | 21.2 | 14.2 | 9 | |
| Total | AbTE:WT | MG1655 | M9 | 4.7 | 3.3 | 14.4 | 41.7 | 30.4 | 5.5 |
| AbTE:G17R | MG1655 | M9 | 16.1 | 9.9 | 25.0 | 29.4 | 13.9 | 5.7 | |
| AbTE:G17R/A165R | MG1655 | M9 | 9.4 | 6.0 | 18.8 | 38.7 | 18.6 | 8.5 |
Extending cultivation time to increase MCFA titers.
Expecting to accumulate higher MCFA titers during a longer cultivation period, we increased the cultivation time from 24hrs to 72hrs (Fig. 3B). When expressing AbTE:WT in E. coli, secreted MCFA titers almost tripled between 24hrs and 72hrs from ~29 mg/L to ~79 mg/L, jumping from being 52% to 69% of all secreted fatty acid chain lengths. We speculate that LCFAs are more likely to be ligated to CoA by endogenous FadD and enter the β-oxidation pathway20, thus leading to a reduction of LCMA levels over time. Interestingly, cultivating E. coli expressing AbTE:G17R for 72hrs did not result in any changes in MCFA titers. When expressing AbTE:G17R/A165R in E. coli, secreted MCFAs increased from 98 mg/L to 131 mg/L when extending the cultivation form 24hrs to 72 hrs. This increasing in MCFA titers increased the percentage of secreted MCFAs from 55% to 80%. It is possible that the low increase in MCFA titers observed with the AbTE mutants between 24hrs and 72hrs is due to lost in activity after 24hrs. It has been shown that adding solubility tags to heterologous proteins increases their viability inside the cell21. Attaching maltose binding protein (MBP) to the N-terminus of the AbTE mutants, the terminus at the opposite end of the AcpP-AbTE interface, results only in LCFAs and no MCFAs were detected (Fig. SI5).
AbTE:G17R and AbTE:G17R/A165R mode of action.
It is possible that the improved MCFA profile of AbTE:G17R and AbTE:G17R/A165R when compared to AbTE:WT is the result of the mutants’ superior expression in E. coli. Positions 17 and 165 are located on the AbTE surface and mutating small hydrophobic amino acids to positively charged arginines may improve solubility. A SDS-PAGE gel of E. coli expressing AbTE:WT, AbTE:G17R or AbTE:G17R/A165R showed comparable soluble expression of the three enzymes over 3 dilutions of cell lysate (Fig. 3C, Fig SI6). In E. coli, arginines on the surface of FAS enzymes are essential for interaction with AcpP, which has a highly negatively charged surface22. Mutation of these arginines in FAS enzymes to negatively charged amino acids results in decreased interactions with ACP (FabA), decreased specific activity with ACP substrates (FabH, FabG), and decrease Kcat and increased KM with ACP substrates (FabI)23–26. If the newly introduced arginines on AbTE help stabilize AbTE-AcpP interactions, mutating AbTE positions 17 and 165 to a negatively charged amino acids, such as glutamate, should disrupt E. coli AcpP interactions resulting in lower MCFA titers. E. coli expression of both the single AbTE:G17E and AbTE:G17E/A165E result in overall lower secreted fatty acid titers than AbTE:WT (Fig. 3A). AbTE:G17E and AbTE:G17E/A165E results in ~21mg/L and 14 mg/L of secreted MCFAs, respectively, which is ~69% of secreted fatty acids of all chain lengths. Interestingly, AbTE:G17R/A165E results in ~57 mg/L of secreted MCFAs, which is ~79% of secreted fatty acids of all chain lengths. This favorable effect in secreted MCFAs or secreted fatty acids of all chain lengths is not seen in AbTE:G17E/A165R, making position 17 key in stabilizing AbTE-AcpP interactions. Taken together, the arginines on the AbTE surface do not affect the protein expression in E. coli, and enhance AbTE’s interaction with E. coli AcpP.
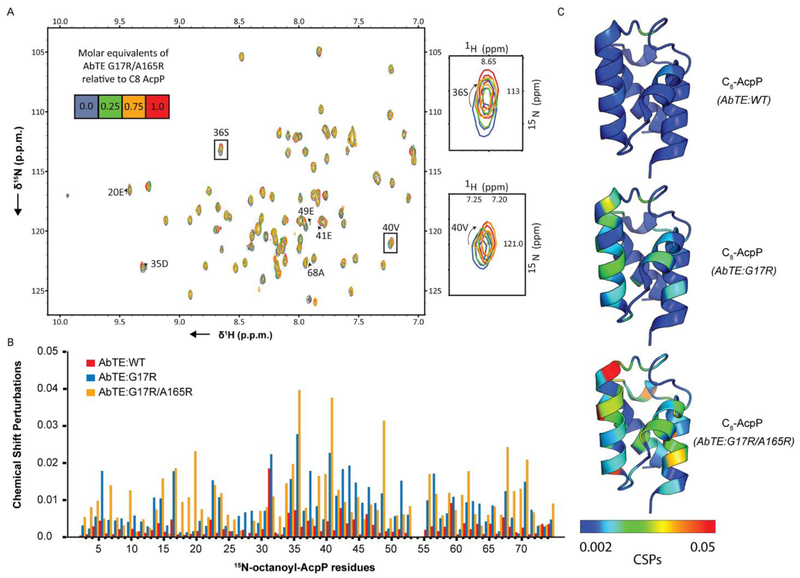
To directly test whether there are increased interactions between E. coli AcpP and AbTE:G17R or AbTE:G17R/A165R when compared to AbTE:WT, we performed a two dimensional 1H/15N heteronuclear single quantum coherence (HSQC) titration experiment. Specifically, 15N-octanoyl-AcpP was titrated with an increasing molar ratio of AbTE:WT, AbTE:G17R, or AbTE:G17R/A165R, and movement of AcpP residues on the HSQC spectra were analyzed27. The degree of movement of each residue’s peak on the HSQC spectra is quantified as a chemical shift perturbation (CSP), which combines the 1H and 15N dimension movement with a scaling factor to yield a simple value28. This method yields a residue-by-residue quantification of the rapid binding events that normally result in catalysis. In the presence of AbTE:WT, E. coli AcpP CPSs are very low, indicating only a few sites of weak interaction between these two enzymes (Fig. 4B, Fig. SI7). In the presence of AbTE:G17R, E. coli AcpP showed significantly more interactions (Fig. SI8). In particular, there are increased perturbations with E. coli AcpP surface residues 20E, 36S, 38D, 41E, 49E, 68A, and 71Y. In the presence of AbTE:G17R/A165R, E. coli AcpP showed global increases in CSPs across all residues, indicating significant increase in protein-protein interactions (Fig. SI9). Of note, there are large CSPs at E. coli AcpP residue 36S and 41E, and many of the CSPs occur at negative residues demonstrating the need to match the positive residues of the partner to AcpP’s negative face. Furthermore, 36S, the residue which is functionalized with phosphopantetheine, is greatly perturbed most likely due to AbTEG17R/A165R interacting with the acyl chain. Taken together, the titration experiments show that AbTE:G17R/A165R has significantly increased molecular contacts with E. coli AcpP compared to AbTE:WT. Although AbTE:G17R/A165R induced E. coli AcpP CSP values that are two orders of magnitude smaller than the ones triggered by E. coli native enzymes27, the CSP values triggered by AbTE:G17R/A165R are ten times larger than the ones induced by AbTE:WT. The data herein demonstrates that the previously overlooked protein-protein interactions between the interfaces are critical for the development of novel functional interactions.
Figure 4.
Solution NMR analysis of molecular interactions between A. baylyi thioesterase (AbTE) and E. coli AcpP. A. HSQC titration of AbTE:G17R/A165R with E. coli 15N-octanoyl-AcpP. Right: Zoom of two selected cross-peaks 36S and 40V. B. CSPs were measured for each 5N-octanoyl-AcpP residue in the presence of AbTE:WT (red), AbTE:G17R (blue), or AbTE:G17R/A165R (orange) and plotted by residue number. C. CSPs are plotted onto the structure of AcpP (PDB ID = 2FAD) in the presence of AbTE:WT, AbTE:G17R, or AbTE:G17R/A165R. Shifts in 1H and 15N dimensions of 15N-octanoyl-AcpP upon titration with AbTE:WT, AbTE:G17R and AbTE:G17R/A165R used to calculate chemical shift perturbations can be found in Fig.SI 9.
Discussion
We engineered the surface of a heterologous enzyme (AbTE) to better couple to an endogenous E. coli enzyme (AcpP) to increase chemical titers (MCFAs). Replacement of two small nonpolar residues on the AbTE surface predicted to contact E. coli AcpP, which has a highly negatively charged surface, with positively charged arginines, the amino acid found at the equivalent positions in E. coli ‘TesA, resulted in more than 3-fold improvement in secreted MCFA titers. Titration NMR experiments demonstrate that the mutations indeed affect the AbTE-AcpP binding interface, demonstrating the power of protein interface engineering between heterologous proteins and interacting host proteins to increase the titers of microbially-produced chemicals.
In the future, engineering the interface of heterologous proteins to better match the interface of host enzymes could be applied to more distantly related proteins, such as plant TEs. Such an approach may prove even more beneficial for more distantly related enzymes that may only marginally interact with endogenous host proteins. A key limitation will be the low sequence homology between heterologous and endogenous proteins, making it difficult to identify key residues for mutagenesis (SI Fig. 1). The recently determined structure of U. californica TE should assist with the identification of plant TE-AcpP interactions29. In addition to MCFA production, the matching interface strategy could also be applied to medium-chain methyl ketone production by better coupling E. coli AcpPs to heterologous β-ketoacyl-ACP TEs, such one from Solanum habrochaites (ShMKS2)30.
Methods
Plasmid construction.
Non-codon optimized Acinetobacter baylyi TE (AbTE), S. cerevisiae codon-optimized Cuphea palustris TE (CpTE), E. coli codon-optimized Umbellularia californica TE (UcTE) were commercially synthesized and cloned under PTRC in pMB1-PTRC-AgGPPS-(GSG)2-AgPS (pSS185) between NcoI/XmaI to generate pMB1-PTRC-AbTE (pSS192), pMB1-PTRC-CpTE (pSS183), and pMB1-PTRC-UcTE (pSS193). S. cerevisiae codon-optimized CnTE was amplified from pESC-LEU2-PTEF1-PHXT7-CnTE (pSS81) with primers SS455/SS456 and cloned under PTRC in pSS185 between NcoI/XmaI to generate pMB1-PTRC-CnTE (pSS174). AbTE mutants were generated using QuikChange protocol with some modifications (SI). Details on templates and primers used are in Table SI4. For protein expression, a C-terminal His6-tag was introduced into AbTE:WT, AbTE:G17R and AbTE:G17R/A165R using primers TB1/TB2 and cloned into pET-28b (amplified using primers TB3/TB4) to generate pET-28b -AbTE:WT, pET-28b -AbTE:G17R, and pET-28b -AbTE:G17R/A165R.
AbTE and ACP expression and purification.
pET-AbTE:WT, pET-AbTE:G17R, and pET-AbTE:G17R/A165R were transformed in E. coli BL21 (DE3) and grown in the presence of 50 mg/L of kanamycin. Cells were induced with 1mM IPTG at OD600=0.8 and grown for 16 to 18 hours at 16° C. Cells pellets were resuspended in 50 mM Hepes (pH 7.4), 250 mM NaCl, and 10% glycerol before lysis by sonication and clarification at 22,000 rcf. Clarified lysate was allowed to batch bind the Ni-NTA resin for 20 minutes followed by washing with buffer containing 25 mM imidazole. Final elution was performed with buffer containing 250mM imidazole, followed by dialysis into 50 mM Tris buffer (pH 7.4), 150 mM NaCl and 10% glycerol. After concentration to ~2mL, the enzymes were purified on a GE Superdex 200 gel filtration column and the fractions containing the desired protein were checked by UV trace and SDS PAGE before concentration. The same procedure was followed for the 15N-AcpP, however, AcpP was grown in 1g 15N ammonium chloride, 4g of 12C glucose, 1L of M9 media, and 50mg of kanamycin.
15N-octanoyl-AcpP synthesis.
15N-AcpP was incubated overnight with Pseudomonas aeruginosa ACP hydrolase to generate pure apo 15N-AcpP as confirmed by a urea PAGE31. C8-pantethenamide32 was loaded onto apo 15N-AcpP to generate 15N-octanoyl-AcpP using 12.5mM MgCl2, 10mM ATP, 0.1μM E. coli CoaA, 0.1μM E. coli CoaD, 0.1μM E. coli CoaE, 0.2μM Bacillus subtilis surfactin phosphopantetheinyl transferase, 0.02% Triton X, 0.01% azide, and 0.1% tris(2-carboxyethyl)-phosphine (TCEP).
NMR titration experiments.
AbTE:WT, AbTE:G17R, AbTE:G17R/A165R and 15N-octanoyl-AcpP were purified separately on a Superdex 75 gel filtration column into 50 mM pH 7.4 potassium phosphate with 0.01% azide and 0.5 mM TCEP. Samples were prepared to a volume of 500μL with 50 μL of D2O. The AbTE:WT, AbTE:G17R, and AbTE:G17R/A165R experiments were performed with 0.75 mM, 0.101 mM, and 0.74 mM 15N-octanoyl-AcpP. The AcpPs were titrated to a final concentration of 1.5, 2.0, and 1 molar equivalents of AbTE: WT, AbTE :G17R, and AbTE G17R/A165R, respectively. Experiments were performed on a Bruker Avance 800MHz spectrometer, in 50 mM potassium phosphate pH 7.4 with 0.5mM TCEP and 0.1% sodium azide. Chemical shift perturbations were calculated using the equation where α=0.2.
MCFA production, derivatization and quantification.
MCFA Production: Overnight cultures of E.coli MG1655 expressing AbTE:WT or AbTE variants were diluted 1:50 in 5 mL of M9 media (0.5% glucose, amp100) and grown at 37°C, 250 r.p.m. until reaching an OD600 = 0.3–0.4. The cells were then induced with 500 μM of IPTG (500 mM stock) and grown at 30°C, 250 r.p.m. for 24 or 72 hrs. Fatty acid analysis: For secreted fatty acids, E. coli cultures were vortexed for 3 sec, 600 μL of culture removed and centrifuged for 10 min at 7354g. Next, 400 μL of the supernatant was removed for derivatization. For total fatty acids, 400 μL of culture was used for derivatization. Fatty acid derivatization: Fatty acids were derivatized to fatty acid methyl esters and analyzed via GC/MS as described in Torella et. al., 201333 with some modifications. To the 400 μL of sample, 50 μL of 10% (wt/vol) NaCl, 50 μL of glacial acetic acid, 20 μL of 90.5 mg/L nonanoic acid (internal standard), and 200 μL of ethyl acetate were added and the mixture was vortexed for 5 sec. The mixture was then centrifuged at 12,098g for 10 min. Methyl esters were generated by mixing 100 μL of the ethyl acetate layer with 900 μL of a 30:1 mixture of methanol and 37% (vol/vol) HCl in a 2 mL microcentrifuge tube, vortexed for 5 sec, and incubated at 50°C for 1 hr. After cooling to room temperature, 500 μL of water and 500 μL of hexanes were added. The mixture was vortexed for 5 sec, 100μL of the hexane layer was taken and mixed with 400 μL of ethyl acetate for analysis via GC-MS. FAME quantification: The samples were analyzed using Agilent 7890A/Agilent 5975 MS detector using a DB-5MS column. The inlet temperature was set to 300°C, flow at 1 mL/min, the oven at 70°C for 1 min, ramp at 30°C/min to 290°C, and held for 1 min at 290°C. Standard curves of C8–C18 fully saturated FAMEs (Alfa Aesar/TCI) were used for sample quantification.
Supplementary Material
Table SI1: Table of Strains.
Table SI2: Table of plasmids.
Table SI3: Table of primers.
Table SI4: Site-directed mutagenesis primers and templates.
Figure SI1. Percent sequence identity between TEs used in this work.
Figure SI2. Amino acid sequence alignment of AbTE and E. coli 'TesA.
Figure SI3. Gas chromatograms of secreted fatty acids produced by E. coli expressing AbTE and AbTE variants.
Figure SI4. Secreted fatty acid production of E. coli expressing AbTE:WT, AbTE:T120R/A121R, AbTE:T120R/A165R, AbTE:121R/A165R.
Figure SI5. Effect of fusing maltose binding protein to AbTE mutants.
Figure SI6. Protein levels of Acitenobacter baylyi TE expressed in E. coli.
Figure SI7. HSQC titration of wild type AbTE with E. coli 15N-octanoyl-AcpP.
Figure SI8. HSQC titration of AbTE:G17R with E. coli 15N-octanoyl-AcpP.
Figure SI9. HSQC titration of AbTE:G17R/A165R with E. coli 15N-octanoyl-AcpP perturbations separated by resonance in 1H and 15N dimension.
Figure SI10. The HSQC spectrum of 15N-octanoyl-AcpP with peak labels. TE sequences.
Acknowledgements
Work in the P.P-Y laboratory was supported by Georgia Institute of Technology Start-Up funds, a DARPA Young Investigator Award, and a DuPont Young Professor Award. Work at M.D.B laboratory was supported by NIH GM095970. We thank Dr. X. Huang for NMR assistance.
Footnotes
Supplementary information.
Materials, homology model and docking, AbTE mutant generation, SDS-PAGE gel of abTE:WT and AbTE variants.
References
- 1.Sarria S, Kruyer NS & Peralta-Yahya P (2017) Microbial synthesis of medium-chain chemicals from renewables. Nat. Biotechnol 35, 1158–1166. [DOI] [PubMed] [Google Scholar]
- 2.Pfleger BF, Gossing M, and Nielsen J (2015) Metabolic engineering strategies for microbial synthesis of oleochemicals. Metabol. Eng 29, 1–11. [DOI] [PubMed] [Google Scholar]
- 3.Schweizer E, and Hofmann J (2004) Microbial type I fatty acid synthases (FAS): major players in a network of cellular FAS systems, Microbiol. Mol. Biol. Rev 68, 501–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dellomonaco C, Clomburg JM, Miller EN, and Gonzalez R (2011) Engineered reversal of the beta-oxidation cycle for the synthesis of fuels and chemicals. Nature 476, 355–359. [DOI] [PubMed] [Google Scholar]
- 5.McMahon MD, and Prather KL (2014) Functional screening and in vitro analysis reveal thioesterases with enhanced substrate specificity profiles that improve short-chain fatty acid production in Escherichia coli. Appl. Environ. Microbiol 80, 1042–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng Y, Li L, Liu Q, Qin W, Yang J, Cao Y, Jiang X, Zhao G, and Xian M (2012) Boosting the free fatty acid synthesis of Escherichia coli by expression of a cytosolic Acinetobacter baylyi thioesterase. Biotechnol. Biofuels 5, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jing F, Cantu DC, Tvaruzkova J, Chipman JP, Nikolau BJ, Yandeau-Nelson MD, and Reilly PJ (2011) Phylogenetic and experimental characterization of an acyl-ACP thioesterase family reveals significant diversity in enzymatic specificity and activity. BMC Biochem. 12, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollard MR, Anderson L, Fan C, Hawkins DJ, and Davies HM (1991) A specific acyl-ACP thioesterase implicated in medium-chain fatty acid production in immature cotyledons of Umbellularia californica. Arch. Biochem. Biophys 284, 306–312. [DOI] [PubMed] [Google Scholar]
- 9.Leber C, and Da Silva NA (2014) Engineering of Saccharomyces cerevisiae for the synthesis of short chain fatty acids. Biotechnol. Bioeng 111, 347–358. [DOI] [PubMed] [Google Scholar]
- 10.Choi YJ, Lee SY, (2013) Microbial production of short-chain alkanes. Nature 502, 571–574. [DOI] [PubMed] [Google Scholar]
- 11.Kovacic F, Granzin J, Wilhelm S, Kojic-Prodic B, Batra-Safferling R, and Jaeger KE (2013) Structural and functional characterisation of TesA - a novel lysophospholipase A from Pseudomonas aeruginosa. PloS one 8, e69125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grisewood MJ, Hernández-Lozada NJ, Thoden JB, Gifford NP, Mendez-Perez D, Schoenberger HA, Allan MF, Floy ME, Lai R-Y, Holden HM, Pfleger BF, and Maranas CD (2017) Computational Redesign of Acyl-ACP Thioesterase with Improved Selectivity toward Medium-Chain-Length Fatty Acids. ACS Catal. 7, 3837–3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zornetzer GA, Tanem J, Fox BG and Markley JL (2010) The Length of the Bound Fatty Acid Influences the Dynamics of the Acyl Carrier Protein and the Stability of the Thioester Bond. Biochemistry 49, 470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Worthington AS, Hur GH and Burkart MD (2011) Activity-guided engineering of natural product carrier proteins. Mol. Biosyst 7, 365–370. [DOI] [PubMed] [Google Scholar]
- 15.Voelker TA and Davies HM (1994) Alteration of the specificity and regulation of fatty acid synthesis of Escherichia coli by expression of a plant medium-chain acyl-acyl carrier protein thioesterase. J. Bacteriol 176, 7320–7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bond-Watts BB, Bellerose RJ & Chang MCY (2011) Enzyme mechanism as a kinetic control element for designing synthetic biofuel pathways. Nat. Chem. Biol 7, 222–227. [DOI] [PubMed] [Google Scholar]
- 17.Steen EJ, Kang Y, Bokinsky G, Hu Z, Schirmer A, McClure A, Del Cardayre SB, and Keasling JD (2010) Microbial production of fatty-acid-derived fuels and chemicals from plant biomass. Nature 463, 559–562. [DOI] [PubMed] [Google Scholar]
- 18.Kozakov D, Beglov D, Bohnuud T, Mottarella SE, Xia B, Hall DR, and Vajda S (2013) How good is automated protein docking?. Proteins: Struc., Func., Bioinf 81, 2159–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelley LA, Mezulis S, Yates CM, Wass MN & Sternberg MJE (2015) The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc 10, 845–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ford TJ, and Way JC (2015) Enhancement of E. coli acyl-CoA synthetase FadD activity on medium chain fatty acids. PeerJ 3, e1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiue E, and Prather KL (2014) Improving D-glucaric acid production from myo-inositol in E. coli by increasing MIOX stability and myo-inositol transport. Metab. Eng 22, 22–31. [DOI] [PubMed] [Google Scholar]
- 22.Chan DI, and Vogel HJ (2010) Current understanding of fatty acid biosynthesis and the acyl carrier protein, Biochem. J 430, 1–19. [DOI] [PubMed] [Google Scholar]
- 23.Zhang YM, Wu B, Zheng J, and Rock CO (2003) Key residues responsible for acyl carrier protein and beta-ketoacyl-acyl carrier protein reductase (FabG) interaction. J. Biol. Chem 278, 52935–52943. [DOI] [PubMed] [Google Scholar]
- 24.Zhang YM, Rao MS, Heath RJ, Price AC, Olson AJ, Rock CO, and White SW (2001) Identification and analysis of the acyl carrier protein (ACP) docking site on beta-ketoacyl-ACP synthase III, J. Biol. Chem 276, 8231–8238. [DOI] [PubMed] [Google Scholar]
- 25.Rafi S, Novichenok P, Kolappan S, Stratton CF, Rawat R, Kisker C, Simmerling C, and Tonge PJ (2006) Structure of acyl carrier protein bound to FabI, the FASII enoyl reductase from Escherichia coli. J. Biol. Chem 281, 39285–39293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finzel K, Nguyen C, Jackson DR, Gupta A, Tsai SC, and Burkart MD (2015) Probing the Substrate Specificity and Protein-Protein Interactions of the E. coli Fatty Acid Dehydratase. FabA, Chem. Biol 22, 1453–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen C, Haushalter RW, Lee DJ, Markwick PR, Bruegger J, Caldara-Festin G, Finzel K, Jackson DR, Ishikawa F, O’Dowd B, McCammon JA, Opella SJ, Tsai SC and Burkart MD (2014) Trapping the dynamic acyl carrier protein in fatty acid biosynthesis. Nature 505, 427–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williamson MP (2013) Using chemical shift perturbation to characterize ligand binding. Prog Nucl Mag Res Sp 73, 1–16. [DOI] [PubMed] [Google Scholar]
- 29.Feng YB, Wang YY, Liu J, Liu YH, Cao XP & Xue S (2017) Structural Insight into Acyl-ACP Thioesterase toward Substrate Specificity Design. ACS Chem Biol 12, 2830–2836. [DOI] [PubMed] [Google Scholar]
- 30.Yu G, Nguyen TT, Guo Y, Schauvinhold I, Auldridge ME, Bhuiyan N, Ben-Israel I, Iijima Y, Fridman E, Noel JP, and Pichersky E (2010) Enzymatic functions of wild tomato methylketone synthases 1 and 2, Plant Physiol. 154, 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kosa NM, Haushalter RW, Smith AR & Burkart MD (2012) Reversible labeling of native and fusion-protein motifs. Nat Methods 9, 981–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haushalter RW, Worthington AS, Hur GH & Burkart MD (2008) An orthogonal purification strategy for isolating crosslinked domains of modular synthases. Bioorg Med Chem Lett 18, 3039–3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torella JP, Ford TJ, Kim SN, Chen AM, Way JC, and Silver PA (2013) Tailored fatty acid synthesis via dynamic control of fatty acid elongation. PNAS 110, 11290–11295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI1: Table of Strains.
Table SI2: Table of plasmids.
Table SI3: Table of primers.
Table SI4: Site-directed mutagenesis primers and templates.
Figure SI1. Percent sequence identity between TEs used in this work.
Figure SI2. Amino acid sequence alignment of AbTE and E. coli 'TesA.
Figure SI3. Gas chromatograms of secreted fatty acids produced by E. coli expressing AbTE and AbTE variants.
Figure SI4. Secreted fatty acid production of E. coli expressing AbTE:WT, AbTE:T120R/A121R, AbTE:T120R/A165R, AbTE:121R/A165R.
Figure SI5. Effect of fusing maltose binding protein to AbTE mutants.
Figure SI6. Protein levels of Acitenobacter baylyi TE expressed in E. coli.
Figure SI7. HSQC titration of wild type AbTE with E. coli 15N-octanoyl-AcpP.
Figure SI8. HSQC titration of AbTE:G17R with E. coli 15N-octanoyl-AcpP.
Figure SI9. HSQC titration of AbTE:G17R/A165R with E. coli 15N-octanoyl-AcpP perturbations separated by resonance in 1H and 15N dimension.
Figure SI10. The HSQC spectrum of 15N-octanoyl-AcpP with peak labels. TE sequences.