Abstract
An estimated 3500 species of mosquitoes (family Culicidae) exist worldwide of which several are known vectors of pathogens that cause disease in humans and other vertebrates. Mosquitoes also host communities of microbes in their digestive tract that form a gut microbiota. Recent studies provide important insights on how mosquitoes acquire a gut microbiota and the community of microbes that are present. Results also indicate that the gut microbiota affects several aspects of mosquito biology. Altogether, these effects impact mosquito fitness with potential consequences for disease prevalence.
Introduction
All mosquitoes are aquatic during their juvenile stages and terrestrial as adults [1]. Larvae primarily consume organic detritus, unicellular organisms and small invertebrates while adults of both sexes commonly feed on extrafloral nectaries [2,3]. Adult females also usually blood feed on vertebrates, which provides nutrients for egg production but can result in transmission of pathogens between hosts [1]. Studies from the early 1900s noted that larval and adult stage mosquitoes harbor communities of extracellular microbes in their digestive tract that form a gut microbiota [4–6]. However, only in the last 10 years have these microbial communities and their roles in mosquito biology been more broadly studied. Results summarized in several recent reviews indicate that the gut microbiota of adult mosquitoes can both positively and negatively affect vector competency, which refers to the ability of females to acquire, maintain, and transmit vertebrate pathogens [7–10]. This short summary focuses on how mosquitoes acquire a gut microbiota, community composition, and known effects on larval development and adult physiology.
Acquisition of a gut microbiota by mosquito larvae
Some insects directly acquire their gut microbiota from parents or other individuals, while others primarily acquire their gut microbiota from the environment [11, 12]. Three lines of evidence indicate that mosquitoes predominantly acquire their gut microbiota anew each generation from the environment. First, experimental studies show that mosquito larvae hatch with no extracellular microbes in their gut [13]. Second, studies of gut community composition indicate that most microbes identified in larvae overlap with the community of microbes that are present in their aquatic habitat [13–18]. Third, mosquitoes host highly variable gut communities [13–18], which would not be expected if these communities were acquired directly from parents or congeners. Exceptions to environmental acquisition are results showing that several types of bacteria are present in the reproductive tracts of adult mosquitoes, and that some of these bacteria are on the surface of eggs females lay [13, 15, 19–25]. This can result in larvae directly acquiring these microbes by ingesting egg shell fragments at hatching or inoculation of the aquatic habitat where larvae feed. Several mosquito species host vertically transmitted intracellular bacteria in the genus Wolbachia and select other genera, which are thus present in eggs [7, 24, 26]. Certain viruses have also been shown to be vertically transmitted [27, 28]. However, these organisms are not part of the extracellular community of microbes that constitute the gut microbiota.
Culture-based studies initially suggested that mosquito larvae expel their gut microbiota in a meconium at metamorphosis, and that adults emerge from the pupal stage with few or no gut microbes [29]. These results further suggested adults reacquire a gut microbiota by imbibing water from the larval habitat and/or feeding on resources like extrafloral nectaries. However, controlled experiments coupled with analysis of gut community composition provide strong evidence that Aedes and Anopheles larvae transstadially transmit a portion of their gut microbiota to adults [13, 30, 31]. Thereafter though, the adult gut microbiota can change through consumption of microbe-containing water, nectar or other food sources [32–35]. Vertebrate blood usually contains few or no microbes, but several studies show that consumption of a blood meal persistently or transiently alters composition of the gut microbiota through alterations in redox status or metabolism [35–41]. Infection by different vector-borne pathogens can also affect the composition of the gut microbiota through unknown mechanisms [26, 41–44].
Composition of the gut microbiota
Insights into the composition of the mosquito gut microbiota primarily derive from metagenomic sequencing studies. Most identified microbes in larval and adult stage mosquitoes are gram-negative aerobes or facultative anaerobes that preferentially belong to four phyla (Proteobacteria, Firmicutes, Bacteroidetes and Actinobacteria) [13–18, 35, 45–48]. Bacteria in these phyla are also common community members in insects from several other orders that acquire their gut microbiota from the environment [49], which suggests prevalence in part reflects their abundance in habitats insects frequent. Several bacteria identified as gut community members in mosquitoes have also been isolated and successfully cultured [13, 18, 22, 31, 50–53]. Unicellular eukaryotes identified as gut community members in different species of mosquitoes include fungi, algae, and Apicomplexa but thus far none of these organisms have been isolated from mosquitoes and cultured independently [43, 48, 54–57]. Sequence-based surveys also identify a number of viruses in mosquitoes [58, 59]. Most belong to families with small RNA genomes such as the Flaviviridae which includes species that are mosquito-vectored vertebrate pathogens [59]. In contrast, the absence of bacteriophages in published studies suggest either viruses infecting bacteria in the gut are underrepresented or few bacteriophages infect bacteria present in the mosquito gut.
Excluding rare sequence reads, most studies indicate that bacterial diversity in the mosquito gut is low (≤200 species) when compared to vertebrates [12]. However, diversity estimates in mosquitoes are comparable to other holometabolous insects that acquire their gut microbiota from the environment [11, 49]. Differences in bacterial species diversity have been reported between larval stadia of Culex quinquefasciatus [60]. Studies of Aedes aegypti further show that substantial numbers of bacteria in the gut die at the end of each instar prior to molting, which provides opportunity for altering gut community composition in the succeeding instar [61]. Other patterns of note include that bacterial species diversity is consistently higher in mosquito larvae than adults [13, 15, 18, 47], and that diversity is lower in laboratory reared versus field collected mosquitoes of the same species [13, 14, 35].
A large number of studies indicate that bacterial diversity differs substantially both between and within mosquito species as a function of collection location and other factors [14–18, 30, 42–46, 62, 63]. On a local scale, factors implicated in affecting community composition include habitat preferences of different mosquito species and larval feeding behavior [18, 30, 62]. Studies that have analyzed mosquito larvae from multiple sites that are in close proximity to one another report greater similarities in gut community composition among individuals of the same or different species from the same site and collection date than among individuals from different sites and collection dates [14, 15, 47]. This strongly suggests the community of microbes present in aquatic habitats can vary considerably even when in close proximity to one another. It also means that larvae from a given site can harbor similar communities of gut microbes but adults collected from a given local area can exhibit high levels of variation due to heterogeneity between larval sites [14–16, 35, 45]. In addition, while bacteria in particular phyla or classes are commonly found in mosquitoes, no evidence currently indicates that particular species of mosquitoes across their geographic range host a ‘core’ microbiota that always includes particular microbes [10, 12]. Instead, current results indicate that environment dominates over genetics in shaping the gut microbiota of mosquitoes, which is a similar conclusion to studies of other insects and vertebrates that acquire their gut microbiota from the environment [49, 65].
Functional roles of the gut microbiota in mosquito larvae
Several early studies reported that mosquito larvae exhibit higher mortality and/or delayed growth to the pupal stage when the abundance of microbes in the aquatic habitat is reduced [4, 5, 19]. More recently, increased mortality and delayed growth have also been reported when larvae are treated with antibiotics [66, 67], whereas inoculating larvae with certain bacteria or yeast has been reported to promote growth [68, 69]. These findings collectively suggest a positive role for the gut microbiota in development of mosquito larvae although the approaches used provide no insights into the mechanisms involved. Antibiotic treatment also does not fully eliminate the gut microbiota due to high levels of resistance in several bacterial community members and insensitivity of eukaryotic community members [47].
Alternatively, surface sterilizing eggs produces axenic larvae with no gut microbiota, which can then be fed a sterile diet and/or inoculated with known microbes to produce gnotobiotic larvae [13]. This approach showed that axenic Ae. aegypti first instars consume a standard rearing diet like conventional (non-sterile) larvae but do not grow which results in larvae dying after several days as first instars [13]. In contrast, Escherichia coli and several other species of bacteria identified as gut community members in different populations of Ae. aegypti [13, 48] colonize the midgut of axenic larvae and rescue growth, whereas dead bacteria do not [13]. Axenic larvae from field collected populations of Ae. aegypti as well as several other species of mosquitoes further exhibit the same responses [13, 18, 47], which altogether suggests several mosquitoes require living bacteria in their gut as larvae for growth and molting.
In considering how bacteria might promote growth, recent studies show that Ae. aegypti larvae inoculated with a mixed community of bacteria or wild-type E. coli exhibit markedly lower gut oxygen levels in the midgut (hypoxia) than axenic larvae or gnotobiotic larvae with bacteria defective for aerobic respiration [61]. This suggested that bacteria in the midgut induce a gut hypoxia response which could function as a growth signal. Functional assays supporting this hypothesis include the finding that gut hypoxia activates hypoxia-induced transcription factors (HIFs) in Ae. aegypti larvae which leads to activation of the insulin/insulin growth factor pathway, select mitogen activated kinases and other processes with essential growth functions [61, 70] (Figure 1). Transcriptome analysis also identifies several genes with functions in digestion and nutrient acquisition as additional targets that are potentially regulated by the gut microbiota or microbiota-induced hypoxia [71].
Figure 1.
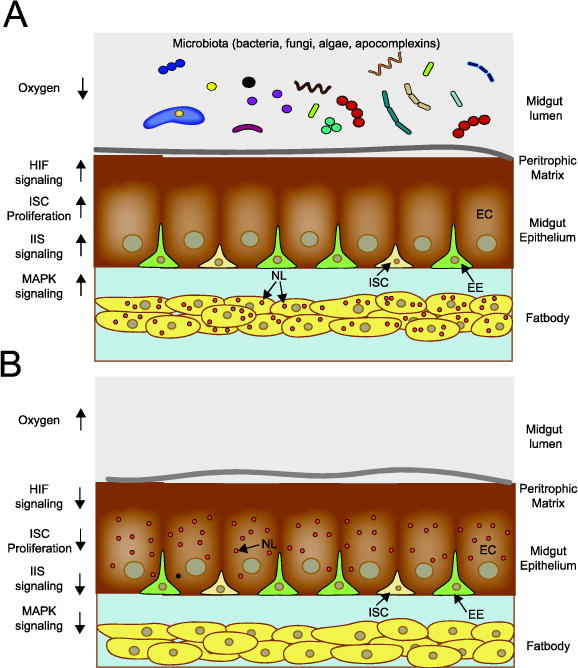
Schematic illustrating the known effects of the gut microbiota on larval growth and nutrient acquisition. A. When living microbes are present in the midgut lumen, gut oxygen levels fall below 5%, HIF-α is stabilized with resulting HIF signaling stimulating: 1) proliferation and differentiation of intestinal stem cells (ISCs) into endocrine cells (EE) and enterocytes, 2) insulin- insulin growth factor signaling (IIS), 3) mitogen activated kinase (MAPK) signaling, and 4) biosynthesis and transport of neutral lipids (NL) by midgut enterocytes (ECs) to the fat body. B. When living microbes are absent from the midgut lumen, gut oxygen levels remain above 5%, which is associated with no stabilization of HIF-α and: 1) an absence of ISC proliferation, 2) no activation of IIS, 3) no activation of MAPK signaling, and 4) biosynthesis of neutral lipids in ECs but an absence of transport to the fat body.
Axenic Ae. aegypti larvae were recently reported to grow into adults when fed a liver powder: yeast extract diet supplemented with heat-killed bacteria, which suggests other diets could obviate a requirement by mosquito larvae for living microbes under certain conditions [72]. In contrast, another study that bioassayed the same diets found no evidence axenic Ae. aegypti or An. gambiae larvae grow beyond the first instar but did find that several eukaryotic gut community members induce gut hypoxia, HIF signaling and larval growth if viable [73]. However, the preceding study and others also show that the proportion of larvae that develop into adults as well as the size, longevity, and fecundity of surviving adults varies among different bacteria and unicellular eukaryotes [18, 42,73–75]. Thus, microbes from diverse taxa can induce a gut hypoxia response and larval growth, whereas in the absence of micriobiota larvae do not grow or molt. In this respect the presence of a microbiota is more critical than its composition when compared to mosquitoes with no gut microbiota. However, community composition does affect several measures of mosquito fitness, which indicates the individual species of microbes or particular combinations of microbes benefit mosquito fitness more than others. Whether these differences reflect variation nutrients microbes provide in conjunction with the diet larvae are fed, the ability of certain microbes to facilitate digestion, or the production of products that promote gut function or other activities is currently unknown.
Effects of the gut microbiota on midgut function in adult mosquitoes
In addition to affecting vector competence and fitness metrics like size and fecundity, a few studies indicate the gut microbiota also affects midgut function in adult mosquitoes. These include evidence based on oral antibiotic treatment of adult An. gambiae that the gut microbiota affects immune gene expression in the midgut [50]. Antibiotic treatment of adult An. coluzzi further suggest the gut microbiota is required for peritrophic matrix formation around the blood bolus after blood feeding [41]. In turn, disabled peritrophic matrix formation allows bacteria to contact midgut cells, which could play a role in altered expression of immune genes and increased risk of systemic infection by gut microbes [41]. Other results implicate the gut microbiota in blood meal digestion either directly through the hemolytic activity of some community members or indirectly by affecting midgut function [76].
Conclusions
In addition to affecting vector competency, current results indicate the gut microbiota affects larval growth, adult fitness and select other traits that likely affect vector populations and disease prevalence. Results published in the last ten years substantially advance understanding of how mosquitoes acquire a gut microbiota and community composition but with a bias toward studies of bacteria. Going forward additional information is needed about the identity and diversity of unicellular eukaryotes in the mosquito gut as well as studies that begin to examine how particular networks of microbes interact, the stability of different communities and their effects on mosquito fitness. How mosquito diet, habitat and other factors interact to shape the composition of the gut microbiota is also largely unknown. While several recent papers indicate that the gut microbiota positively affects mosquito physiology, understanding of the underlying mechanisms remain understudied. To address this issue, more experimental data are needed that formally examine how the gut microbiota affects growth and other physiological processes, and the function of particular community members in these processes.
Acknowledgments
Writing of this summary and some of the results reported were supported by a grant from the National Institutes of Health (R01AI106892) and National Science Foundation (IOS 1656236).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Clements AN. The Biology of Mosquitoes, Vol. 1. Development, Nutrition, and Reproduction. Chapman & Hall; New York: 1992. [Google Scholar]
- 2.Merritt RW, Dadd RH, Walder ED. Feeding behavior, natural food, and nutritional relationships of larval mosquitoes. Annu Rev Entomol. 1992;37:349–376. doi: 10.1146/annurev.en.37.010192.002025. [DOI] [PubMed] [Google Scholar]
- 3.Foster WA. Mosquito sugar feeding and reproductive energetics. Annu Rev Entomol. 40:443–474. doi: 10.1146/annurev.en.40.010195.002303. [DOI] [PubMed] [Google Scholar]
- 4.Hinman EH. A study of the food of mosquito larvae. Am J Hygiene. 1930;12:238–270. [Google Scholar]
- 5•.Rozeboom LE. The relation of bacteria and bacterial filtrates to the development of mosquito larvae. Am J Hygiene. 1935;21:167–179. This paper presents the first evidence implicating living microbes in the gut as a requirement for growth of mosquito larvae. [Google Scholar]
- 6.Chao J, Wistreich GA. Microbial isolations from the mid-gut of Culex tarsalis Coquillet. J Insect Pathol. 1959;1:311–318. [Google Scholar]
- 7.Hegde S, Rasgon JL, Hughes GL. The microbiome modulates arbovirus transmission in mosquitoes. Curr Op Virol. 2015;15:97–102. doi: 10.1016/j.coviro.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Tol, Dimopoulos G. Influences of the mosquito microbiota on vector competence. Adv Insect Physiol. 2016;51:243–291. [Google Scholar]
- 9.Gendrin M. A swiss army knife to cut malaria transmission. Cell Host Microbe. 2017;22:577–579. doi: 10.1016/j.chom.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Guegan M, Zouache K, Demichel C, Minard G, van Tran V, Potier P, Mavingui P, Moro CV. The mosquito holobiont: fresh insight into mosquito-microbiota interactions. Microbiome. 2018;6:49. doi: 10.1186/s40168-018-0435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engel P, Moran NA. The gut microbiota of insects-diversity in structure and function. FEMS Microbiol Revs. 2013;37:699–735. doi: 10.1111/1574-6976.12025. [DOI] [PubMed] [Google Scholar]
- 12.Strand MR. The gut microbiota of mosquitoes: diversity and function. In: Wikel S, Aksoy S, Dimopoulos G, editors. Arthropod Vector: Controller of Disease Transmission. Vol. 1. Elsevier; 2017. pp. 185–199. [Google Scholar]
- 13••.Coon KL, Vogel KJ, Brown MR, Strand MR. Mosquitoes rely on their gut microbiota for development. Mol Ecol. 2014;23:2727–2739. doi: 10.1111/mec.12771. This article outlines methods for producing axenic mosquito larvae, experimental evidence that larvae transstadially transmit a portion of the gut microbiota to adults, and data indicating that living microbes are required for growth of multiple mosquito species. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boissiere A, Tchioffo MT, Bachar D, Abate L, Marie A, Nsango SE, Shahbazkia HR, Awono-Ambene PH, Levashina EA, Christen R, Mollais I. Midgut microbiota of the malaria mosquito vector Anopheles gambiae and interactions with Plasmodium falciparum infection. PLoS Pathog. 2012;8:e1002742. doi: 10.1371/journal.ppat.1002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gimonneau G, Tchioffo MT, Abate L, Boissiere A, Awono-Ambene PH, Nsango SE, Christen R, Moriais I. Composition of Anopheles coluzzii and Anopheles gambiae microbiota from larval to adult stages. Infect Genet Evol. 2014;28:715–24. doi: 10.1016/j.meegid.2014.09.029. [DOI] [PubMed] [Google Scholar]
- 16.Muturi EJ, Kim CH, Bara J, Bach EM, Siddappaji MH. Culex pipiens and Culex restuans mosquitoes harbor distinct microbiota dominated by few bacterial taxa. Para Vector. 2016;9:18. doi: 10.1186/s13071-016-1299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buck M, Nilsson LKJ, Brunius C, Dabire RK, Hopkins R, Terenius O. Bacterial associations reveal spatial population dynamics in Anopheles gambiae mosquitoes. Sci Rep. 2016;6:22806. doi: 10.1038/srep22806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18••.Dickson LB, Jiolle D, Minard G, Moltini-Conclois I, Volant S, Ghozlane A, Bouchier C, Ayala D, Paupy Carryover effects of larval exposure to different environmental bacteria drive adult trait variation in a mosquito vector. Sci Adv. 2017;3:e1700585. doi: 10.1126/sciadv.1700585. This study provides strong evidence that variation in the gut microbiota in larval stage mosquitoes impacts multiple fitness traits in adults. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chao J, Wistreich GA, Moore J. Failure to isolate microorganisms from within mosquito eggs. Microbial isolations from the mid-gut of Culex tarsalis Coquillet. Ann Entomol Soc Am. 1963;56:559–561. [Google Scholar]
- 20.Favia G, Ricci I, Damiani C, Raddadi N, Crotti E, Marzorati M, Rizzi A, Urso R, Brusetti L, Borin S, Mora D, Scuppa P, Pasqualini L, Clementi E, Genchi M, Conona S, Negri I, Grandi G, Alma A, Kramer L, Esposito F, Bandi C, Sacchi L, Daffonchio D. Bacteria of the genus Asaia stably associate with Anopheles stephensi, an Asian malarial mosquito vector. Proc Natl Acad Sci USA. 2007;104:9047–9051. doi: 10.1073/pnas.0610451104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zouache K, Voronin D, Tran-Van V, Mousson L, Failloux A, Mavingui P. Persistent Wolbachia and cultivable bacteria infection in the reproductive and somatic tissues of the mosquito vector Aedes albopictus. PLoS One. 2009;4:e6388. doi: 10.1371/journal.pone.0006388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Damiani C, Ricci I, Crotti E, Rossi P, Rizzi A, Scuppa P, Capone A, Ulissi, Ulissi U, Epis S, Genchi M, Sagnon N, Faye I, Kang A, Chouaia B, Whitehorn C, Moussa GW, Mandrioli M, Esposito F, Sacchi L, Bandi C, Daffonchio D, Favia G. Mosquito-bacteria symbiosis: the case of Anopheles gambiae and Asaia. Microb Ecol. 2010;60:644–654. doi: 10.1007/s00248-010-9704-8. [DOI] [PubMed] [Google Scholar]
- 23.Sharma P, Sharma S, Maurya RK, De TD, Thomas T, Lata S, Singh N, Pandey KC, Valecha N, Dixit R. Salivary glands harbor more diverse microbial communities than gut in Anopheles culicifacies. Para Vectors. 2014;7:235. doi: 10.1186/1756-3305-7-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xi Z, Khoo CC, Dobson SL. Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science. 2005;310(6):326–328. doi: 10.1126/science.1117607. [DOI] [PubMed] [Google Scholar]
- 25.Segata N, Baldini F, Pompon J, Garrett WS, Trouong DT, Dabire RK, Diabate A, Levashina EA, Catteruccia F. The reproductive tracts of two malaria vectors are populated by a core microbiome and by gender- and swarm-enriched microbial biomarkers. Sci Rep. 2016;6:24207. doi: 10.1038/srep24207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zouache K, Michelland RJ, Failloux AB, Grundmann GL, Mavingui P. Chiungunya virus impacts diversity of symbiotic bacteria in mosquito vector. Mol Ecol. 2012;21:2297–2309. doi: 10.1111/j.1365-294X.2012.05526.x. [DOI] [PubMed] [Google Scholar]
- 27.Saiyasombat R, Bolling BG, Brault AC, Bartholomay LC, Blitvich BJ. Evidence of efficient transovarial transmission of Culex flavivirus by Culex pipiens (Diptera: Culicidae) J Med Entomol. 2011;48:1031–1038. doi: 10.1603/me11043. [DOI] [PubMed] [Google Scholar]
- 28.Haddow AD, Guzman H, Popov VL, Wood TG, Widen SG, Haddow AD, Tesh RB, Weaver SC. First isolation of Aedes flavivirus in the western hemisphere and evidence of vertical transmission in the mosquito Aedes (Stegomyia) albopictus (Diptera: Culicidae) Virology. 2013;440:134–139. doi: 10.1016/j.virol.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Moll RM, Romoser WS, Modrzakowski MC, Moncayo AC, Lerdthusnee K. Meconial peritrophic membranes and the fate of midgut bacteria during mosquito (Diptera: Culicidae) metamorphosis. J Med Ent. 2001;38:29–32. doi: 10.1603/0022-2585-38.1.29. [DOI] [PubMed] [Google Scholar]
- 30.Duguma D, Hall MW, Rugman-Jones P, Stouthamer R, Terenius O, Neufeld JD, Walton WE. Developmental succession of the microbiome of Culex mosquitoes. BMC Microbiology. 2013;15:140. doi: 10.1186/s12866-015-0475-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chavshin AR, Oshaghi MA, Vatandoost H, Yakhchali B, Zarenejad F, Terenius O. Malphighian tubules are important determinants of Pseudomans transstadial transmission and longtime persistence in Anopheles stephensi. Para Vectors. 2015;21:8. doi: 10.1186/s13071-015-0635-6. :36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Briones AM, Shililu J, Githure J, Novak R, Raskin L. Thorsellia anophelis is the dominant bacterium in a Kenyan population of adult Anopheles gambiae mosquitoes. ISME J. 2008;2:74–82. doi: 10.1038/ismej.2007.95. [DOI] [PubMed] [Google Scholar]
- 33.Lindh JM, Borg-Karlson A-K, Faye I. Transstadial and horizontal transfer of bacteria within a colony of Anopheles gambiae (Diptera: Culicidae) and oviposition response to bacteria-containing water. Acta Tropica. 2008;107:2421–250. doi: 10.1016/j.actatropica.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 34.Crotti E, Rizzi A, Chouaia B, Ricci I, Favia G, Alma A, Sacchi L, Bourtzis K, Mandrioli M, Cherif A, Bandi C, Daffonchio D. Acetic acid bacteria, newly emerging symbionts of insects. Appl Env Microbiol. 2010;76:6963–6970. doi: 10.1128/AEM.01336-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35•.Wang Y, Gilbreath TM, Kukutla P, Yan GY, Xu JN. Dynamic gut microbiome across life history of the malaria mosquito Anopheles gambiae in Kenya. PLoS One. 2011;6:e24767. doi: 10.1371/journal.pone.0024767. This study was among the first to use metagenomics to characterize bacterial community members of the gut microbiota across different life stages of mosquitoes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar S, Molina-Cruz A, Gupta L, Rodrigues J, Barillas-Mury C. A peroxidase/ dual oxidase system modulates midgut epithelial immunity in Anopheles gambiae. Science. 2010;327:1644–1648. doi: 10.1126/science.1184008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaio AD, Gusmão DS, Santos AV, Berbert-Molina MA, Pimenta PFP, Lemos FJA. Contribution of midgut bacteria to blood digestion and egg production in Aedes aegypti (Diptera: Culicidae) (L.) Para Vectors. 2011;4:105. doi: 10.1186/1756-3305-4-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38•.Oliveira JHM, Goncalves RLS, Lara FA, Dias FA, Gandara AC, Menna, Barreto RF, Edwards MC, Laurindo FR, Silva-Neto MA, Sorgine MH, Oliveira PL. Blood meal-derived heme decreases ROS levels in the midgut of Aedes aegypti and allows proliferation of intestinal microbiota. PLoS Pathogens. 2011;7:1001320. doi: 10.1371/journal.ppat.1001320. This study presents strong evidence that blood feeding affects gut bacteria in adult mosquitoes and results that implicate particular mechanisms in proliferation of some gut community members. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Short SM, Mongodin EF, MacLeod HJ, Talyuli OAC, Dimopoulos G. Amino acid metabolic signaling influences Aedes aegypti midgut microbiome variability. PLoS Negl Trop Dis. 2017;11:e0005677. doi: 10.1371/journal.pntd.0005677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodgers FH, Gendrin M, Wyer CAS, Christophides GK. Microbiota-induced peritrophic matrix regulates midgut homeostasis and prevents systemic infection of malaria vector mosquitoes. PLoS Pathogens. 2017;13:e1006391. doi: 10.1371/journal.ppat.1006391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramirez JL, Souza-Neto J, Torres Cosme R, Rovira J, Ortiz A, Pascale JM, Dimopoulos G. Reciprocal tripartite interactions between the Aedes aegypti midgut microbiota, innate immune system and dengue virus influences vector competence. PLoS Negl Trop Dis. 2012;6:e1561. doi: 10.1371/journal.pntd.0001561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muturi EJ, Bara JJ, Rooney AP, Hansen AK. Midgut fungal and bacterial microbiota of Aedes triseriatus and Aedes japonicus shift in response to La Crosse virus infection. Mol Ecol. 2016;25:4075–4090. doi: 10.1111/mec.13741. [DOI] [PubMed] [Google Scholar]
- 44.Villegas LEM, Campolina TB, Barnabe NR, Orfano AS, Chaves BA, Norris DE, Pimenta PFP, Secundino NFC. Zika virus infection modulates the bacterial diversity associated with Aedes aegypti as revealed by metagenomics analysis. PLoS One. 2018;13:e0190352. doi: 10.1371/journal.pone.0190352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45•.Osei-Poku J, Mbogo CM, Palmer WJ, Jiggins FM. Deep sequencing reveals extensive variation in the gut microbiota of wild mosquitoes from Kenya. Mol Ecol. 2012;21:5138–5150. doi: 10.1111/j.1365-294X.2012.05759.x. This study presents compelling evidence for high level interspecific variation in gut microbiota diversity in adult stage mosquitoes. [DOI] [PubMed] [Google Scholar]
- 46.Yadav KK, Bora A, Datta S, Chandel K, Gogoi HK, Prasad GB, Veer V. Molecular characterization of midgut microbiota of Aedes albopictus and Aedes aegypti from Arunachal Pradesh, India. Para Vector. 2015;8:641. doi: 10.1186/s13071-015-1252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47•.Coon KL, Brown MR, Strand MR. Mosquitoes host communities of bacteria that are essential for development but vary greatly between local habitats. Mol Ecol. 2016;25:5806–5826. doi: 10.1111/mec.13877. This study presents evidence for high level intraspecific variation in gut community diversity in larval stage mosquitoes from different local breeding sites, and strong evidence for the existence of high level resistance to broad spectrum antibiotics in certain bacterial community members of the gut microbiota. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thongsripong P, Chadler JA, Green B, Kittayapong P, Wilcox BA, Kapan DD, Bennett SN. Mosquito vector-associated microbiota: metabarcoding bacteria and eukaryotic symbionts across habitat types in Thailand endemic for dengue and other arthropod- borne diseases. Ecol Evol. 2017;8:1352–1368. doi: 10.1002/ece3.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yun J-H, Roh SW, Whon TW, Jung M-J, Kim M-S, Park D-S, Yoon C, Nam Y-D, Kim Y-J, Choi J-H, Kim J-Y, Shin N-S, Kim S-H, Lee W-J, Bae J-W. Insect gut bacterial diversity determined by environmental habitat, diet, developmental stage, and phylogeny of host. Appl Env Microbiol. 2014;80:5254–5264. doi: 10.1128/AEM.01226-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dong YM, Manfredini F, Dimopoulos G. Implication of the mosquito midgut microbiota in defense against malaria parasites. PLoS Pathogens. 2009;5:1000423. doi: 10.1371/journal.ppat.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gusmão DS, Santos AV, Marini DC, Bacci M, Berbert-Molina MA, Lemos FJA. Culture- dependent and culture-independent characterization of microorganisms associated with Aedes aegypti (Diptera: Culicidae) (L.) and dynamics of bacterial colonization in the midgut. Acta Tropica. 2010;115:275–281. doi: 10.1016/j.actatropica.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 52.Djadid ND, Jazayeri H, Raz A, Favia G, Ricci I, Zakeri S. Identification of the midgut microbiota of An. stephensi and An. maculipennis for their application as a paratransgenic tool against malaria. PLoS One. 2011;6:e28484. doi: 10.1371/journal.pone.0028484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Terenius O, Lindh JM, Eriksson-Gonzales K, Bussiere L, Laugen AT, Bergguist H, Titanji K, Faye I. Midgut bacterial dynamics in Aedes aegypti. FEMs Microbial Ecology. 2012;80:556–565. doi: 10.1111/j.1574-6941.2012.01317.x. [DOI] [PubMed] [Google Scholar]
- 54.Chandler JA, Liu RM, Bennett SN. RNA shotgun metagenomics sequencing of northern California (USA) mosquitoes uncovers viruses, bacteria and fungi. Front Microbiol. 2015;6:185. doi: 10.3389/fmicb.2015.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Belda E, Coulibaly B, Fofana A, Beavogui AH, Traore SF, Gohl DM, Vernick KD, Riehle MM. Preferential suppression of Anopheles gambiae host sequences allows detection of the mosquito eukaryotic microbiome. Sci Rep. 2017;12:3241. doi: 10.1038/s41598-017-03487-1. This study presents approaches for preferentially characterizing eukaryotic members of the mosquito gut microbiota. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steyn A, Roets F, Botha A. Yeasts associated with Culex pipiens and Culex theileri mosquito larvae and the effect of selected yeast strains on the ontogeny of Culex pipiens. Microb Ecol. 2016;71:747–760. doi: 10.1007/s00248-015-0709-1. [DOI] [PubMed] [Google Scholar]
- 57.Bozic J, Capone A, Pediconi D, Mensah P, Cappelli A, Valzano M, Mancici MV, Scuppa P, Martin E, Epis S, Rossi P, Favia G, Ricci I. Mosquitoes can harbour yeasts of clinical significance and contribute to their environmental dissemination. Environ Microbiol Rep. 2017;9:642–648. doi: 10.1111/1758-2229.12569. [DOI] [PubMed] [Google Scholar]
- 58.Bolling BG, Weaver SC, Tesh RB, Vasilakis N. Insect-specific virus discovery: significance for the arbovirus community. Viruses. 2015;7:4911–28. doi: 10.3390/v7092851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roundy CM, Azar SR, Rossi SL, Weaver SC, Vasilakis N. Insect-specific viruses: a historical overview and recent developments. Adv Virus Res. 2017;98:119–46. doi: 10.1016/bs.aivir.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 60.Pennington MJ, Prager SM, Walton WE, Trumble JT. Culex quinquefasciatus larval microbiomes vary with instar and exposure to common wastewater contaminants. Sci Rep. 2016;6:21969. doi: 10.1038/srep21969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61•.Coon KL, Valzania L, McKinney DA, Vogel KJ, Brown MR, Strand MR. Bacteria-mediated hypoxia functions as a signal for mosquito development. Proc Natl Acad Sci USA. 2017;114:E5362–E5369. doi: 10.1073/pnas.1702983114. This study presents evidence that bacteria-induced hypoxia functions as a growth signal for development of mosquito larvae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yee DA, Allgood D, Kneitel JM, Kuehn KA. Constitutive differences between natural and artificial container mosquito habitats: vector communities, resources, microorganisms, and habitat parameters. J Med Entomol. 2012;49:482–491. doi: 10.1603/me11227. [DOI] [PubMed] [Google Scholar]
- 63.Kim C-H, Lampman RL, Muturi EJ. Bacterial communities and midgut microbiota associated with mosquito populations from waste tires in East-Central Illinois. J Med Entomol. 2015;52:63–75. doi: 10.1093/jme/tju011. [DOI] [PubMed] [Google Scholar]
- 64.Dada N, Jumas-Bilak E, Manguin S, Seidu R, Stenström T-A, Overgaard HJ. Comparative assessment of the bacterial communities associated with Aedes aegypti larvae and water from domestic water storage containers. Para Vectors. 2014;7:391. doi: 10.1186/1756-3305-7-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, Costea PI, Godneva A, Kalka IN, Bar N, Shilo S, Lador D, Vila AV, Zmora N, Pevsner-Fischer M, Israeli D, Kosower N, Malka G, Wolf BC, Avnit-Sagi T, Lotan-Pompan M, Weinberger A, Halpern Z, Carmi S, Fu J, Wijmenga C, Zhernakova A, Elinav E, Segal E. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555:210–215. doi: 10.1038/nature25973. [DOI] [PubMed] [Google Scholar]
- 66.Wotton RS, Chaloner DT, Yardley CA, Merritt RW. Growth of Anopheles mosquito larvae on dietary microbiota in aquatic surface microlayers. Med Vet Entomol. 11:65–70. doi: 10.1111/j.1365-2915.1997.tb00291.x. [DOI] [PubMed] [Google Scholar]
- 67.Chouaia B, Rossi P, Epis S, Mosca M, Ricci I, Damiani C, Ulissi U, Crotti E, Daffonchio D, Bandi C, Favia G. Delayed larval development in Anopheles mosquitoes deprived of Asaia bacterial symbionts. BMC Microbiology. 2012;12:S2. doi: 10.1186/1471-2180-12-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mitraka E, Stathopoulos S, siden-Kiamos I, Christophides GK, Louis C. Asaia accelerates development of Anopheles gambiae. Pathog Glob Health. 2013;107:305–311. doi: 10.1179/2047773213Y.0000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Diaz-Nieto LM, Alessio CD, Perotti MA, Beron CM. Culex pipiens development is greatly influenced by native bacteria and exogenous yeast. PLoS One. 2016;11:e0153133. doi: 10.1371/journal.pone.0153133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70••.Valzania L, Coon KL, Vogel KJ, Brown MR, Strand MR. Hypoxia-induced transcription factor signaling is essential for larval growth of the mosquito. Aedes aegypti Proc Natl Acad Sci USA. 2018;115:457–465. doi: 10.1073/pnas.1719063115. This study presents evidence that microbiota-induced gu hypoxia activates multiple processes required for growth of mosquitoes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vogel KJ, Valzania L, Coon KL, Brown MR, Strand MR. Transcriptome sequencing reveals large-scale changes in axenic Aedes aegypti larvae. PLoS Negl Trop Dis. 2017;11:e0005273. doi: 10.1371/journal.pntd.0005273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Correa MA, Brackney DE, Steven B. Axenic Aedes aegypti develop without live bacteria, but exhibit delayed development and reduced oviposition. bioRxiv. doi:org/10.1101/264978. [Google Scholar]
- 73.Valzania L, Martinson VG, Harrison RE, Boyd BM, Coon KL, Brown MR, Strand MR. Both living bacteria and eukaryotes in the mosquito gut promote growth of larvae. PLoS Negl Trop Dis. 2018 doi: 10.1371/journal.pntd.0006638. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gendrin M, Rodgers FH, Yerbanga RS, Ouédraogo JB, Basáñez M-G, Cohuet A, Christophides GK. Antibiotics in ingested human blood affect the mosquito microbiota and capacity to transmit malaria. Nat Commun. 2015;6:5921. doi: 10.1038/ncomms6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Coon KL, Brown MR, Strand MR. Gut bacteria differentially affect egg production in the anautogenous mosquito Aedes aegypti and facultatively autogenous mosquito Aedes atropalpus (Diptera: Culicidae) Parasit Vectors. 2016;30:375. doi: 10.1186/s13071-016-1660-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gaio AD, Gusmão DS, Santos AV, Berbert-Molina MA, Pimenta PFP, Lemos FJA. Contribution of midgut bacteria to blood digestion and egg production in Aedes aegypti (Diptera: Culicidae) (L.) Para Vectors. 2011;4:105. doi: 10.1186/1756-3305-4-105. [DOI] [PMC free article] [PubMed] [Google Scholar]


