Abstract
Objectives
Respiratory syncytial virus (RSV) infections are well recognized as a significant cause of morbidity and mortality in allogeneic haematopoietic stem cell transplant (allo-HSCT) recipients. We evaluated the spectrum of clinical manifestations, management (including ribavirin-based antiviral therapy) and outcomes of RSV infections and determined the risk factors associated with RSV lower respiratory tract infection (LRTI) and all-cause mortality.
Methods
In this retrospective study, we analysed clinical data from all laboratory-confirmed RSV infections in allo-HSCT recipients (n = 280) who presented at our institution from January 1996 to May 2009.
Results
Of the 280 patients, 80 (29%) developed LRTI within 20 days (median 1 day, range 0–19 days) and 44 (16%) died within 90 days (median 26 days, range 1–82 days) from RSV diagnosis. Multivariable logistic regression analyses identified several significant risk factors associated with RSV LRTI and all-cause mortality, including age, male sex, neutropenia, lymphocytopenia and lack of ribavirin-based antiviral therapy at the upper respiratory tract infection (URTI) stage. Aerosolized ribavirin-based therapy at the URTI stage was the single most significant factor in reducing the risk of RSV LRTI (83%), all-cause mortality (57%) and RSV-associated mortality (87%) in these patients (P < 0.05), irrespective of the year of RSV diagnosis.
Conclusions
Our results demonstrate that RSV infections are a significant cause of morbidity and mortality in high-risk allo-HSCT recipients and ribavirin-based antiviral therapy at the URTI stage had a positive impact on both outcomes in this vulnerable population with multiple risk factors.
Keywords: RSV, stem cell transplantation, immunocompromised, pneumonia
Introduction
Respiratory syncytial virus (RSV) infections are well recognized as a significant cause of morbidity and mortality in allogeneic haematopoietic stem cell transplant (allo-HSCT) recipients, with a reported incidence of 2%–17%.1–6 The reasons for this variation in incidence include changes in the levels of overall awareness, early detection of these infections in HSCT recipients over the past two decades, and the small sample sizes of published clinical studies.1 Allo-HSCT is a risk factor for RSV infection,6 which can proceed from the upper respiratory tract to the lower respiratory tract and even cause death. A high incidence (17%–84%) of RSV lower respiratory tract infection (LRTI) has been reported in HSCT recipients.2,7–12 Progression to LRTI increases the risk of RSV-associated mortality in these patients; the overall rate is 7%–83%.12–16
Recent studies of RSV infections in adult HSCT recipients identified age, myeloablative regimen, lymphocytopenia, mismatched or unrelated donor transplant, graft-versus-host disease (GVHD) and pre-engraftment (or early post-transplantation period) as factors that are significantly associated with progression to LRTI.2,5,6,8,11,17,18 Similarly, unrelated donors, cord blood transplantations and the need for mechanical ventilation were associated with RSV-associated mortality.13,19
In a recent systematic review of RSV infection management in HSCT recipients, ribavirin-based therapy may have prevented bad outcomes in high-risk HSCT recipients.1 Most of the clinical studies identified in our review1 were plagued by their small sample sizes, retrospective natures and inadequate power to determine the risk factors and outcomes of RSV infections and the associations among clinical factors (including antiviral therapy), confounders and RSV-associated morbidity and mortality.
For these reasons, we conducted a retrospective study of all RSV infections in the largest sample of allo-HSCT recipients over two decades. We sought to identify risk factors associated with RSV LRTI and all-cause mortality and the impact of antiviral therapy on these outcomes.
Patients and methods
All laboratory-confirmed RSV infections in allo-HSCT recipients who presented from January 1996 to May 2009 at our institution were included. Microbiology laboratory and infection control databases were used to identify infections in these patients. The MD Anderson institutional review board approved the study, and informed consent was waived.
Definitions
RSV cases were defined as allo-HSCT recipients who developed acute symptomatic respiratory illness and for whom a nasal wash, nasopharyngeal swab or bronchoalveolar lavage was positive for RSV by viral culture or direct immunofluorescence antigen detection testing. Cases were classified as community acquired if symptoms developed before hospitalization or within the first 5 days after admission; they were classified as nosocomial if symptoms developed any time after that during hospitalization. An upper respiratory tract infection (URTI) was defined as the onset of rhinorrhoea, nasal or sinus congestion, otitis media, pharyngitis or cough with no hypoxaemia or infiltrates on chest X-ray or CT scan of the chest in patients with positive RSV from a nasal wash specimen or nasopharyngeal swab. LRTI was defined as the onset of respiratory symptoms with new or changing pulmonary infiltrates, as seen on chest X-ray or CT, that were suggestive of viral aetiology (i.e. interstitial infiltrates or ground glass opacities) or RSV isolated from lower respiratory samples (e.g. endotracheal tube aspirate, sputum or bronchoalveolar lavage fluid).20 A resolution of all clinical manifestations of RSV infection, irrespective of viral shedding, was classified as a complete recovery. Neutropenia was defined as an absolute neutrophil count (ANC) <500/μL and lymphocytopenia as an absolute lymphocyte count (ALC) <200/μL. The isolation of another organism within 30 days prior to or after diagnosis of RSV infection was considered a coinfection. For identifying risk factors of RSV LRTI, coinfections diagnosed on the same day as the RSV diagnosis or within 2 weeks prior to RSV LRTI were included. Death from all causes was assessed within 90 days from RSV diagnosis and was attributed to RSV if a persistent or progressive RSV infection with respiratory failure was present at the time of death.
Data collection
Two investigators (J. N. S. and K. K. E. T.) collected all the demographic data, clinical characteristics and outcomes through chart reviews, each one with a list of different patients. Collected data were independently verified for accuracy and consistency by another investigator (D. P. S.). All outcome data and coinfections were further confirmed by a senior investigator (R. F. C.).
Therapy and management
Ribavirin, alone or combined with intravenous immunoglobulins (IVIG) or palivizumab, was given to patients on the basis of the treating physicians' clinical judgement. Six grams a day of aerosolized ribavirin were administered on a continuous or split intermittent schedule. The continuous schedule consisted of 20 mg/mL for 18 h per day, delivered via a small-particle aerosol generator unit (SPAG-2) in a face mask while the patient remained inside a scavenging tent to prevent environmental contamination. In the split intermittent dosing schedule, 60 mg/mL was given over 2–3 h every 8 h. IVIG was administered at a dose of 500 mg/kg every other day for five to seven doses, whereas palivizumab was administered as a single intravenous infusion of 15 mg/kg.
Standard infection control practices were followed to reduce nosocomial transmission of respiratory viruses. All admitted patients occupied private rooms, and gowns, masks and gloves were used for contact isolation during any patient contact. Patients seen in outpatient clinics who had URTI symptoms were immediately placed in private rooms with masks. Furthermore, during the respiratory illness season, visitors and family members were screened by nursing staff for URTI symptoms before being allowed access to patients' rooms, and masks were provided to those who screened positive.
Statistical analyses
After examining the preliminary data, we determined the incidence of the two primary clinical endpoints: progression to RSV LRTI and all-cause mortality. Unadjusted ORs and 95% CIs with their P values for each clinical characteristic and RSV LRTI were calculated using logistic regression analysis. A final logistic regression model was constructed using a forward selection process by entering variables significant at P < 0.25 in the model, one at a time. Variables were retained in the model for their main effect if they were significant at the 0.05 level or their confounding effect if they induced a ≥15% change in the OR of another significant variable of primary interest.21 Previously eliminated variables (P > 0.25) were entered one at a time to ensure that all variables were given the opportunity to appear in the final model. In addition, a second model for RSV LRTI was built for a subgroup of patients who presented at the URTI stage. Unadjusted ORs and adjusted ORs (AORs) with 95% CIs were calculated for all-cause mortality. A Hosmer–Lemeshow goodness-of-fit test and the area under the receiver operating characteristic (ROC) curve were used to assess the fit of all the models. Kaplan–Meier failure curves were used to estimate the effect of early ribavirin-based treatment on progression to RSV LRTI in a subgroup of patients presenting at the URTI stage (stratified by year of RSV diagnosis) and on all-cause mortality, respectively. These survival probabilities were compared with a log-rank test of homogeneity. All statistical analyses were performed using STATA software version 10.1 (STATA Corporation, College Station, TX, USA).
Results
During the 14 year period, 280 laboratory-confirmed RSV infections occurred among 3822 allo-HSCT recipients, an incidence of 7%. The highest number of RSV infections was observed in the season 2000–01 (45 patients).
Of the 280 allo-HSCT recipients with RSV infections, 80 (29%) developed LRTI within 20 days and 44 (16%) died within 90 days after RSV diagnosis. These patients' clinical characteristics and outcomes are summarized in Table 1. The study population comprised patients of all ages (median 47 years; range 3–70 years) and both sexes [158 (56%) males], predominantly whites [181 (65%)]. The median duration from HSCT to RSV infection was 155 days (range 1–2931 days). Half the patients had received corticosteroids within 1 month before the onset of their RSV infection and 117 (42%) had undergone a myeloablative conditioning regimen prior to their infection. Most RSV infections were community acquired [250 (89%)], and most patients presented to our centre at the URTI stage [237 (85%)]. A substantial proportion of patients were hospitalized for RSV infections [197 (70%)] for a median duration of 8 days (range 2–65 days). Some patients required intensive care [24 (9%)] and mechanical ventilation [18 (6%)] as a result of serious RSV-related complications.
Table 1.
Clinical characteristics and outcomes of RSV infections in 280 allo-HSCT recipients
| Characteristics | Total (%) | Presented with URTI (n = 237) |
Presented with LRTI (n = 43) | |
|---|---|---|---|---|
| resolved at RSV URTI (n = 200) | progressed to RSV LRTI (n = 37) | |||
| Age (years), median (range) | 47 (3–70) | 46 (3–68) | 49 (4–67) | 49 (25–70) |
| Male sex | 158 (56) | 108 (54) | 26 (70) | 24 (56) |
| Race | ||||
| non-Hispanic white | 181 (65) | 132 (66) | 24 (65) | 25 (58) |
| Hispanic | 51 (18) | 38 (19) | 4 (11) | 9 (21) |
| other | 48 (17) | 30 (15) | 9 (24) | 9 (21) |
| Type of cancer | ||||
| haematological | 274 (98) | 194 (97) | 37 (100) | 43 (100) |
| solid tumours | 6 (2) | 6 (3) | 0 | 0 |
| Corticosteroidsa | 141 (50) | 100 (50) | 17 (46) | 24 (55) |
| Myeloablative conditioning regimen | 117 (42) | 81 (41) | 17 (46) | 19 (44) |
| Time from HSCT (days), median (range) | 155 (1–2931) | 141 (1–2931) | 110 (1–1677) | 214 (1–1667) |
| Donor relationship | ||||
| matched related | 158 (56) | 104 (52) | 21 (57) | 33 (77) |
| matched unrelated | 112 (40) | 88 (44) | 15 (41) | 9 (21) |
| mismatched | 10 (4) | 8 (4) | 1 (3) | 1 (2) |
| Haematopoietic cell source | ||||
| marrow | 76 (27) | 56 (28) | 14 (38) | 6 (14) |
| peripheral | 190 (68) | 135 (68) | 20 (54) | 35 (81) |
| cord | 14 (5) | 9 (5) | 3 (8) | 2 (5) |
| GVHD at time of diagnosis of RSV infection | ||||
| acute | 76 (27) | 56 (28) | 9 (24) | 11 (26) |
| chronic | 85 (30) | 60 (30) | 8 (22) | 17 (40) |
| both | 19 (7) | 14 (7) | 2 (5) | 3 (7) |
| Neutropenia (ANC <500/μL)b | 15 (5) | 4 (2) | 7 (19) | 4 (9) |
| Lymphocytopenia (ALC <200/μL)b | 48 (17) | 24 (12) | 11 (30) | 13 (30) |
| Coinfectionsc | ||||
| pulmonary virald | 13 (5) | 8 (4) | 3 (8) | 2 (5) |
| pulmonary bacteriald | 14 (5) | 3 (2) | 3 (8) | 8 (19) |
| pulmonary fungald | 11 (4) | 4 (2) | 4 (11) | 3 (7) |
| non-pulmonary | 55 (20) | 27 (14) | 12 (32) | 16 (37) |
| Nosocomial | 30 (11) | 20 (10) | 10 (27) | 0 |
| Antiviral therapye | 216 (77) | 143 (72) | 32 (86) | 41 (95) |
| URTI stage | 157 (56) | 143 (72) | 14 (38) | — |
| LRTI stage | 59 (21) | — | 18 (49) | 41 (95) |
| Outcomes | ||||
| duration of symptoms (days), median (range) | 15 (1–72) | 13 (1–72) | 23 (4–68) | 21 (5–69) |
| hospitalized secondary to RSV infection | 197 (70) | 135 (68) | 31 (84) | 31 (72) |
| hospital stay (days), median (range) | 8 (2–65) | 7 (2–49) | 13 (2–56) | 11 (5–65) |
| admission to intensive care unit | 24 (9) | 5 (3) | 7 (19) | 12 (28) |
| mechanical ventilation | 18 (6) | 3 (2) | 7 (19) | 8 (19) |
| RSV-associated mortality within 90 days following RSV diagnosis | 22 (8) | 0 | 10 (27) | 12 (28) |
| all-cause mortality within 90 days following RSV diagnosis | 44 (16) | 12 (6) | 15 (41) | 17 (40) |
aWithin 1 month before RSV diagnosis.
bAt the time of first assessment of respiratory symptoms at the hospital.
cWithin 1 month prior to and after RSV diagnosis; 20 patients had both pulmonary and non-pulmonary coinfections.
dIn cases of LRTI, coinfections occurred on the same day as RSV diagnosis or within 2 weeks prior to RSV LRTI. Viral coinfections included influenza virus (7), parainfluenza virus (3), picornavirus (2) and adenovirus (1); bacterial coinfections included Klebsiella oxytoca (1), Pseudomonas aeruginosa and Pseudomonas fluorescens (4), methicillin-resistant Staphylococcus aureus (2), Stenotrophomonas maltophilia (6) and Escherichia coli (1); and fungal coinfections included 8 Aspergillus spp. and 3 Nocardia spp. infections.
eRibavirin alone or combined with IVIG or palivizumab.
RSV LRTI
Eighty patients (29%) experienced RSV LRTI during the course of their infection, including 37 who presented at the URTI stage. Risk factors associated with RSV LRTI (LRTI versus URTI) are displayed in Table 2. Compared with patients with URTI, patients with LRTI were older (AOR 1.4; 95% CI 1.1–1.8; P = 0.007) and were more likely to be neutropenic (AOR 5.24; 95% CI 1.23–22.36; P = 0.025) and lymphocytopenic (AOR 3.38; 95% CI, 1.38–8.25; P = 0.008) at the time of diagnosis. Interestingly, other factors, including sex, donor relationship, time between transplant and RSV infection, GVHD, corticosteroid use, intermittent versus continuous dosing schedule of aerosolized ribavirin, duration of therapy with aerosolized ribavirin and myeloablative conditioning regimen, were not significantly associated with RSV LRTI. On the other hand, one of the significant factors associated with RSV LRTI was lack of ribavirin-based antiviral therapy at the URTI stage (AOR 13.7; 95% CI 6.55–28.66; P < 0.001). Year of RSV diagnosis did not significantly affect progression to RSV LRTI; however, it was added to the multivariable risk model to demonstrate the independent effect of early antiviral therapy on decreasing the risk of progression to LRTI by specifically adjusting for the overall improvement in patient management over the years. The overall fit of the model was excellent with respect to the Hosmer–Lemeshow goodness-of-fit test (χ2 = 201.04; P = 0.2287) and area under the ROC curve (0.8597).
Table 2.
Risk factor analysis for RSV LRTI in allo-HSCT recipients
| Characteristic | No. of patients (%) |
Overall URTI versus LRTI (n = 280) |
Restricted to patients presenting with URTI (n = 237) |
|||||
|---|---|---|---|---|---|---|---|---|
| LRTI (n = 80) | URTI (n = 200) | COR (95% CI) | P value | AOR (95% CI) | P value | AOR (95% CI) | P value | |
| Age (years), median (range)a | 49 (4–70) | 46 (3–68) | 1.21 (0.99, 1.47) | 0.053 | 1.4 (1.1, 1.8) | 0.007 | 1.27 (0.94, 1.7) | 0.117b |
| Male sex | 50 (63) | 108 (54) | 1.42 (0.83, 2.42) | 0.196 | — | — | — | — |
| Non-white race | 49 (61) | 132 (66) | 1.23 (0.72, 2.1) | 0.453 | — | — | — | — |
| Corticosteroidsc | 41 (51) | 100 (50) | 1.05 (0.63, 1.77) | 0.85 | — | — | — | — |
| Myeloablative conditioning regimen | 36 (45) | 81 (41) | 1.2 (0.71, 2.03) | 0.491 | — | — | — | — |
| Time from HSCT (days), median (range)a | 186 (1–1677) | 141 (1–2931) | 1.00 (0.99, 1.02) | 0.465 | — | — | — | — |
| Donor relationship | — | — | — | — | ||||
| matched related | 54 (68) | 104 (52) | 1.0 | |||||
| matched unrelated | 24 (30) | 88 (44) | 0.53 (0.3, 0.92) | 0.024 | ||||
| mismatched | 2 (3) | 8 (4) | 0.48 (0.1, 2.35) | 0.366 | ||||
| Haematopoietic cell source | — | — | — | — | ||||
| marrow | 20 (25) | 56 (28) | 1.0 | |||||
| peripheral | 55 (69) | 135 (68) | 1.14 (0.47, 5.2) | 0.667 | ||||
| cord | 5 (6) | 9 (5) | 1.56 (0.63, 2.08) | 0.473 | ||||
| GVHD at the time of diagnosis of RSV infection | 50 (63) | 130 (65) | 0.89 (0.52, 1.54) | 0.693 | — | — | — | — |
| Neutropenia (ANC <500/μL)d | 11 (14) | 4 (2) | 7.81 (2.41, 25.34) | 0.001 | 5.24 (1.23, 22.36) | 0.025 | 6.78 (1.48, 31.05) | 0.014 |
| Lymphocytopenia (ALC <200/μL)d | 24 (30) | 24 (12) | 3.14 (1.66, 5.96) | <0.001 | 3.38 (1.38, 8.25) | 0.008 | 2.85 (0.99, 8.23) | 0.053b |
| Pulmonary coinfectionse | ||||||||
| viral | 5 (6) | 8 (4) | 2.03 (0.64, 6.45) | 0.23 | 2.24 (0.52, 9.68) | 0.281 | 2.55 (0.55, 11.86) | 0.233 |
| bacterial | 11 (14) | 3 (2) | 11.9 (3.2, 44.13) | <0.001 | 5.2 (1.02, 26.5) | 0.047 | 3.23 (0.41, 25.16) | 0.263 |
| fungal | 7 (9) | 4 (2) | 5.68 (1.6, 20.1) | 0.007 | 9.25 (1.85, 46.36) | 0.007 | 9.53 (1.83, 49.73) | 0.007 |
| Nosocomial infection | 10 (13) | 20 (10) | 1.29 (0.57, 2.88) | 0.542 | — | — | — | — |
| Lack of antiviral therapy at the URTI stagef | 66 (83) | 57 (29) | 11.83 (6.15, 22.73) | <0.001 | 13.7 (6.55, 28.66) | <0.001 | 4.45 (1.95, 10.17) | <0.001 |
| Year of RSV diagnosis (2001 and later) | 58 (73) | 159 (80) | 0.68 (0.37, 1.24) | 0.207 | 0.5 (0.24, 1.05) | 0.068b | 0.48 (0.19, 1.19) | 0.112b |
COR, crude OR.
aMeasure of effect for age and time from HSCT expressed per 10 year and 30 day interval, respectively.
bAge and lymphocytopenia were retained in the model due to confounding effects and year of RSV diagnosis was included in the model to adjust for the overall advances in the clinical management of patients during recent years.
cWithin 1 month before RSV diagnosis.
dAt the time of first assessment of respiratory symptoms.
eWithin 1 month prior to and after RSV diagnosis. In cases of LRTI, coinfections occurred on the same day as RSV diagnosis or within 2 weeks prior to RSV LRTI. Viral coinfections included influenza virus (7), parainfluenza virus (3), picornavirus (2) and adenovirus (1); bacterial coinfections included Klebsiella oxytoca (1), Pseudomonas aeruginosa and Pseudomonas fluorescens (4), methicillin-resistant Staphylococcus aureus (2), Stenotrophomonas maltophilia (6) and Escherichia coli (1); and fungal coinfections included 8 Aspergillus spp. and 3 Nocardia spp. infections.
fRibavirin alone or combined with IVIG or palivizumab.
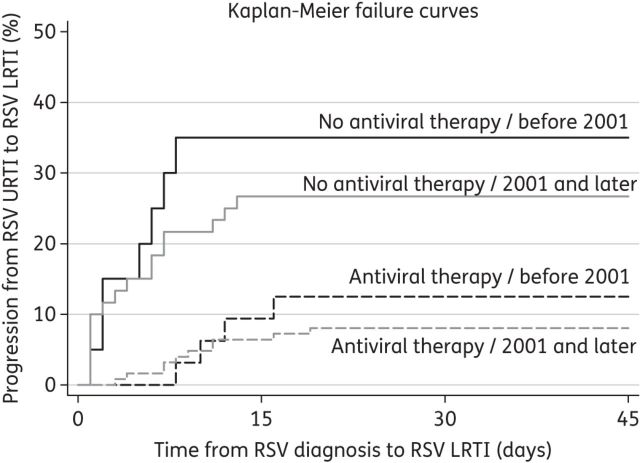
In a subgroup analysis of 237 patients who presented with URTI, neutropenia (P = 0.014), fungal coinfections (P = 0.007) and lack of ribavirin-based antiviral therapy (P < 0.001) were independently associated with progression to LRTI. Although age and lymphocytopenia were not statistically significant, they were retained in the model because of their confounding effects. The overall fit of this secondary risk model was also good with respect to the Hosmer–Lemeshow goodness-of-fit test (χ2 = 168.83; P = 0.1805) and area under the ROC curve (0.7778). Kaplan–Meier curves for patients presenting at the URTI stage (Figure 1) demonstrate the effect of early ribavirin-based antiviral therapy in reducing the overall cumulative probability of progression to RSV LRTI (P < 0.001 for log-rank test), after stratifying for year of RSV diagnosis.
Figure 1.
Kaplan–Meier failure curves for progression to RSV LRTI in 237 patients presenting with URTI, stratified by ribavirin-based antiviral therapy at the URTI stage and year of RSV diagnosis. Antiviral therapy indicates ribavirin-based antiviral therapy at the URTI stage. Progression to RSV LRTI was significantly lower in patients receiving ribavirin-based antiviral therapy at the URTI stage compared with those who did not receive it (P < 0.001 for log-rank test). There was no significant difference in outcomes based on the year of RSV diagnosis (based on log-rank test).
Overall, patients with LRTI experienced a significantly longer duration of symptoms (median 23 versus 13 days), higher hospital admission rates, a longer median hospital stay, more ICU stays and more need for mechanical ventilation than patients with only URTI.
All-cause mortality
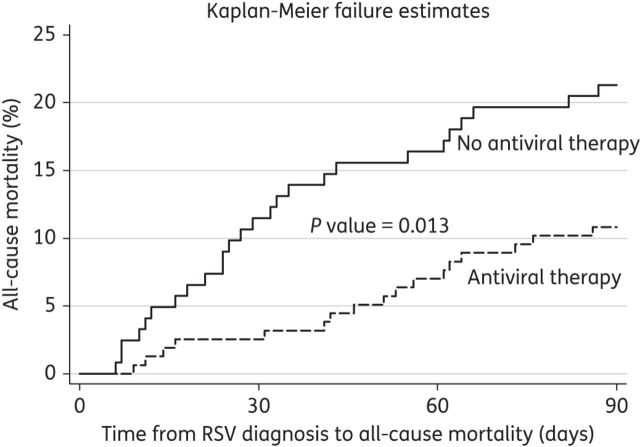
Overall, 44 patients died within 90 days from RSV diagnosis, yielding an all-cause mortality rate of 16%. This rate was even higher for those who experienced progression to RSV LRTI (44/80, 55%). Risk factors for all-cause mortality are shown in Table 3. After adjusting for year of RSV diagnosis, multiple risk factors for mortality were identified in these patients, such as increasing age (for every 10 year interval: AOR 1.45; 95% CI 1.1–1.9; P = 0.008), male sex (AOR 2.76; 95% CI 1.25–6.09; P = 0.012), time from HSCT to RSV diagnosis (for every 30 day interval: AOR 0.94; 95% CI, 0.90–0.99; P = 0.012), neutropenia (AOR 3.21; 95% CI 0.91–11.33; P = 0.069), lymphocytopenia (AOR 3.03; 95% CI 1.33–6.91; P = 0.008) and lack of ribavirin-based therapy at the URTI stage (AOR 2.43; 95% CI 1.16–5.11; P = 0.019). The overall fit of this mortality risk model was excellent with respect to the Hosmer–Lemeshow goodness-of-fit test (χ2 = 2.98; P = 0.936) and area under the ROC curve (0.7914). Kaplan–Meier curves (Figure 2) demonstrate a significantly higher probability of all-cause mortality in patients who were not treated compared with those who were treated with ribavirin-based therapy at the URTI stage (P < 0.05 for log-rank test).
Table 3.
Risk factor analysis for all-cause mortality in allo-HSCT recipients
| Characteristic | All-cause mortality (n = 44) | Survival (n = 236) | COR (95% CI) | P value | AOR (95% CI) | P value |
|---|---|---|---|---|---|---|
| Age (years), median (range)a | 50 (14–70) | 47 (3–68) | 1.29 (1.0, 1.66) | 0.046 | 1.45 (1.1, 1.9) | 0.008 |
| Male sex | 33 (75) | 125 (53) | 2.66 (1.29, 5.52) | 0.008 | 2.76 (1.25, 6.09) | 0.012 |
| Non-white race | 16 (36) | 83 (35) | 1.05 (0.54, 2.06) | 0.879 | — | — |
| Corticosteroidsb | 28 (64) | 113 (48) | 1.9 (0.98, 3.7) | 0.058 | — | — |
| Myeloablative conditioning regimen | 18 (41) | 99 (42) | 0.96 (0.49, 1.84) | 0.898 | — | — |
| Time from HSCT (days), median (range)a | 91 (1–1426) | 173 (1–2167) | 0.94 (0.9, 0.98) | 0.008 | 0.94 (0.9, 0.99) | 0.012 |
| Donor relationship | — | — | ||||
| matched related | 27 (61) | 131 (56) | 1.0 | |||
| matched unrelated | 14 (32) | 98 (42) | 0.69 (0.35, 1.39) | 0.302 | ||
| mismatched | 3 (7) | 7 (3) | 2.08 (0.51, 8.56) | 0.310 | ||
| Haematopoietic cell source | — | — | ||||
| marrow | 12 (27) | 64 (27) | 1.0 | |||
| peripheral | 31 (71) | 159 (67) | 1.04 (0.5, 2.15) | 0.916 | ||
| cord | 1 (2) | 13 (6) | 0.41 (0.05, 3.44) | 0.411 | ||
| GVHD at the time of diagnosis of RSV infection | 27 (61) | 153 (65) | 0.86 (0.44, 1.67) | 0.66 | — | — |
| Neutropenia (ANC <500/μL)c | 8 (18) | 7 (3) | 7.27 (2.48, 21.27) | <0.001 | 3.21 (0.91, 11.33) | 0.069d |
| Lymphocytopenia (ALC <200/μL)c | 17 (39) | 31 (13) | 4.16 (2.04, 8.51) | <0.001 | 3.03 (1.33, 6.91) | 0.008 |
| Pulmonary coinfectionse | ||||||
| viral | 3 (7) | 10 (4) | 2.12 (0.55, 8.14) | 0.274 | — | — |
| bacterial | 8 (18) | 6 (3) | 9.42 (3.06, 29.03) | <0.001 | — | — |
| fungal | 3 (7) | 8 (3) | 2.65 (0.67, 10.54) | 0.167 | — | — |
| Nosocomial infection | 7 (16) | 23 (10) | 1.75 (0.7, 4.38) | 0.230 | — | — |
| LRTI at presentation | 17 (39) | 26 (11) | 5.09 (2.45, 10.56) | <0.001 | — | — |
| Lack of antiviral therapy at the URTI stagef | 27 (61) | 96 (41) | 2.32 (1.2, 4.48) | 0.013 | 2.43 (1.16, 5.11) | 0.019 |
| Year of RSV diagnosis (2001 and later) | 35 (80) | 182 (77) | 1.15 (0.52, 2.55) | 0.724 | 1.33 (0.55, 3.24) | 0.532d |
COR, crude OR.
aMeasure of effect for age and time from HSCT expressed per 10 year and 30 day interval, respectively.
bWithin 1 month before RSV diagnosis.
cAt the time of first assessment of respiratory symptoms at the hospital.
dNeutropenia was retained in the model due to confounding effects and year of RSV diagnosis was included in the model to adjust for the overall advances in the clinical management of patients during recent years.
eWithin 1 month prior to and after RSV diagnosis. In cases of LRTI, coinfections occurred on the same day as RSV diagnosis or within 2 weeks prior to RSV LRTI. Viral coinfections included influenza virus (7), parainfluenza virus (3), picornavirus (2) and adenovirus (1); bacterial coinfections included Klebsiella oxytoca (1), Pseudomonas aeruginosa and Pseudomonas fluorescens (4), methicillin-resistant Staphylococcus aureus (2), Stenotrophomonas maltophilia (6) and Escherichia coli (1); and fungal coinfections included 8 Aspergillus spp. and 3 Nocardia spp. infections.
fRibavirin alone or combined with IVIG or palivizumab.
Figure 2.
Kaplan–Meier failure curves for all-cause mortality, stratified by ribavirin-based therapy at the URTI stage. Antiviral therapy indicates ribavirin-based antiviral therapy at the URTI stage. All-cause mortality was significantly lower in patients receiving ribavirin-based therapy at the URTI stage compared with those who did not receive it (P < 0.05 for log-rank test). There was no significant difference in outcomes based on the year of RSV diagnosis (based on log-rank test); stratification by this variable is not shown in the graph.
Treatment
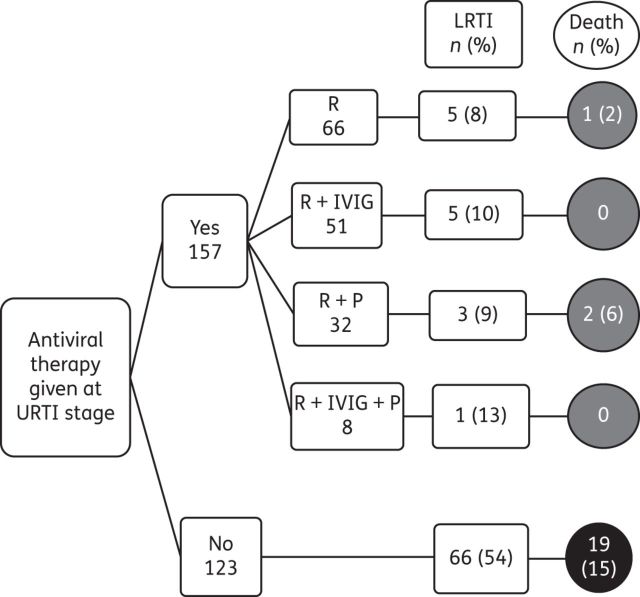
Only four patients received IVIG alone; one developed LRTI and died with an RSV infection. A substantial proportion of allo-HSCT recipients received ribavirin-based therapy [216 (77%)] as monotherapy [77 (28%)], in combination with IVIG [71 (25%)] or palivizumab [49 (18%)] or all three combined [19 (7%)]. However, only about half of the patients [157 (56%)] had received the ribavirin-based antiviral therapy at the URTI stage and their outcomes are illustrated in Figure 3. The incidence of RSV LRTI and RSV-associated mortality was much higher in patients who were not treated with ribavirin-based antiviral therapy [66 (54%) and 19 (15%), respectively] than in patients who were treated at the URTI stage [14 (9%) and 3 (2%), respectively] (Figure 3). Ribavirin-based therapy at the URTI stage reduced the risk of progression to LRTI by 83%, RSV-associated mortality by 87% and all-cause mortality by 57%.
Figure 3.
Outcomes of all RSV infections in allo-HSCT recipients, stratified by the type of antiviral therapy at the URTI stage. R, ribavirin; P, palivizumab; Death, RSV-associated mortality.
Discussion
In this largest study of RSV infections in allo-HSCT recipients to date, we found that aerosolized ribavirin-based therapy at the URTI stage reduced significantly the risk of progression to LRTI and all-cause mortality in these patients.
No patient demographic characteristics, except age and male sex, were significantly associated with progression to LRTI or mortality in this study. Age was also found to be a significant risk factor for progression in other studies of respiratory infections, including RSV, in HSCT patients.4,5,17 Like Nichols et al.,17 we found an association between mortality and male sex; however, unlike that study, we did not find that donor type was a significant factor in determining outcome.
After adjusting for ribavirin-based therapy at the URTI stage and the year of RSV diagnosis, we identified multiple clinical factors associated with RSV LRTI, including increasing age, lymphocytopenia, neutropenia and associated bacterial and fungal coinfections. All of these factors, except for coinfections, increased the risk of RSV-associated mortality significantly. One retrospective study reported an association between myeloablative regimens and LRTI incidence during the first 100 days after HSCT in patients with respiratory infections, including RSV.6 However, in our study the use of a myeloablative conditioning regimen was not associated with an increased risk of RSV LRTI or mortality, even after stratifying incidence by time after HSCT. Interestingly, most of the significant predictors of LRTI and RSV mortality in our study population can be surrogate indicators of an underlying immunodeficiency or of a severe infection at presentation. Older patients with lymphocytopenia and neutropenia at presentation were more likely to experience progression to LRTI and mortality. Lymphocytopenia and neutropenia have also been reported to be associated with unfavourable RSV outcomes in HSCT recipients in other studies.5,8,11,22
Both multivariable risk models demonstrated that lack of ribavirin-based therapy at the URTI stage significantly increased the risk of LRTI and mortality in these patients. Thus, timely initiation of ribavirin-based therapy may decrease mortality in these patients. As noted earlier, various studies have reported varying levels of success with antiviral therapy in preventing RSV LRTI or RSV-associated mortality.3,8,15,23–26 The diversity in their results can be attributed to the small sample size of these studies and the fact that many of them did not focus on studying the effect of antiviral therapy on RSV infections in allo-HSCT recipients. In a recent systematic review of all published data on ribavirin as treatment for RSV infections in HSCT recipients,1 we found that the rate of progression to LRTI was much lower in HSCT recipients treated with any form of ribavirin at the URTI stage and regardless of the duration of therapy or the addition of an immunomodulator, with subsequent lower mortality rate. In the current study, which had the largest sample size to date, our findings were in agreement with those of the published review.
One of the major limitations of our study is that the data were collected retrospectively. This may lead to recall bias for some patient history records; however, this was not a major concern because RSV is an acute infection and most data collected were laboratory, pharmacological and radiological findings. We may also have overestimated RSV-associated morbidity rates, particularly when some patients may not have sought medical attention for minimal respiratory symptoms. More importantly, there are no standardized definitions for RSV LRTI or even mortality in this complex patient population, who may have different types of infections concomitantly. It is very difficult to attribute death to an infectious cause in a complex population such as ours; hence we used all-cause mortality as the primary endpoint in our study. Another limitation is that no definite criteria existed for prescribing antiviral therapy; therapy was initiated at the discretion of the treating physician. However, in 2003 we developed an algorithm for the management of RSV infections in HSCT recipients that specifically included aerosolized ribavirin use only in patients at risk; this algorithm is part of the standard of care in the Department of Stem Cell Transplantation and Cellular Therapy at our institution.27 Finally, an immortal time bias may exist in our study by assuming that the treated patients lived for the duration of treatment or until a decision to treat was reached. However, such a bias is very minimal in cases of acute infections, such as RSV infections, where the time to initiation of therapy was 1–3 days in most of our patients and the average duration of treatment was 7 days. Furthermore, outcomes were defined such that they could have occurred at any time during the exposure period, including before initiation of treatment, and hence all outcomes were included in the analysis.
In summary, we found that allo-HSCT recipients with RSV infections who experience progression to LRTI have the highest risk of mortality. In the absence of a placebo-controlled randomized trial to assess the effectiveness of aerosolized ribavirin or any form of ribavirin in treating RSV infections in HSCT recipients (a placebo arm would be unethical) and on the basis of our findings, we recommend prompt identification of high-risk allo-HSCT recipients with RSV infections (such as older patients with lymphocytopenia and neutropenia) and early initiation of ribavirin-based therapy to prevent progression to LRTI and subsequent mortality.
Funding
R. F. C. received a research grant from ADMA Biologics and Valeant Pharmaceuticals.
Transparency declarations
All authors have indicated that they have neither financial relationships nor commercial associations that would pose a conflict of interest.
Author contributions
All authors made substantial contributions to conception and design, acquisition of data and critical review, and have checked the final version of the manuscript.
Acknowledgements
We thank Ms Ann Sutton (Department of Scientific Publications, The University of Texas MD Anderson Cancer Center) for her editorial support.
References
- 1.Shah JN, Chemaly RF. Management of RSV infections in adult recipients of hematopoietic stem cell transplantation. Blood. 2011;117:2755–63. doi: 10.1182/blood-2010-08-263400. [DOI] [PubMed] [Google Scholar]
- 2.Whimbey E, Champlin RE, Couch RB, et al. Community respiratory virus infections among hospitalized adult bone marrow transplant recipients. Clin Infect Dis. 1996;22:778–82. doi: 10.1093/clinids/22.5.778. [DOI] [PubMed] [Google Scholar]
- 3.Khanna N, Widmer AF, Decker M, et al. Respiratory syncytial virus infection in patients with hematological diseases: single-center study and review of the literature. Clin Infect Dis. 2008;46:402–12. doi: 10.1086/525263. [DOI] [PubMed] [Google Scholar]
- 4.Raboni SM, Nogueira MB, Tsuchiya LRV, et al. Respiratory tract viral infections in bone marrow transplant patients. Transplantation. 2003;76:142–6. doi: 10.1097/01.TP.0000072012.26176.58. [DOI] [PubMed] [Google Scholar]
- 5.Martino R, Porras RP, Rabella N, et al. Prospective study of the incidence, clinical features, and outcome of symptomatic upper and lower respiratory tract infections by respiratory viruses in adult recipients of hematopoietic stem cell transplants for hematologic malignancies. Biol Blood Marrow Transplant. 2005;11:781–96. doi: 10.1016/j.bbmt.2005.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiffer JT, Kirby K, Sandmaier B, et al. Timing and severity of community acquired respiratory virus infections after myeloablative versus non-myeloablative hematopoietic stem cell transplantation. Haematologica. 2009;94:1101–8. doi: 10.3324/haematol.2008.003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Englund JA, Sullivan CJ, Jordan MC, et al. Respiratory syncytial virus infection in immunocompromised adults. Ann Intern Med. 1988;109:203–8. doi: 10.7326/0003-4819-109-3-203. [DOI] [PubMed] [Google Scholar]
- 8.Ljungman P. Respiratory virus infections in stem cell transplant patients: the European experience. Biol Blood Marrow Transplant. 2001;7(Suppl):5S–7S. doi: 10.1053/bbmt.2001.v7.pm11777102. [DOI] [PubMed] [Google Scholar]
- 9.Boeckh M, Berrey MM, Bowden RA, et al. Phase 1 evaluation of the respiratory syncytial virus-specific monoclonal antibody palivizumab in recipients of hematopoietic stem cell transplants. J Infect Dis. 2001;184:350–4. doi: 10.1086/322043. [DOI] [PubMed] [Google Scholar]
- 10.Boeckh M, Englund J, Li Y, et al. Randomized controlled multicenter trial of aerosolized ribavirin for respiratory syncytial virus upper respiratory tract infection in hematopoietic cell transplant recipients. Clin Infect Dis. 2007;44:245–9. doi: 10.1086/509930. [DOI] [PubMed] [Google Scholar]
- 11.Chemaly RF, Ghosh S, Bodey GP, et al. Respiratory viral infections in adults with hematologic malignancies and human stem cell transplantation recipients: a retrospective study at a major cancer center. Medicine (Baltimore) 2006;85:278–87. doi: 10.1097/01.md.0000232560.22098.4e. [DOI] [PubMed] [Google Scholar]
- 12.Avetisyan G, Mattsson J, Sparrelid E, et al. Respiratory syncytial virus infection in recipients of allogeneic stem-cell transplantation: a retrospective study of the incidence, clinical features, and outcome. Transplantation. 2009;88:1222–6. doi: 10.1097/TP.0b013e3181bb477e. [DOI] [PubMed] [Google Scholar]
- 13.McCarthy AJ, Kingman HM, Kelly C, et al. The outcome of 26 patients with respiratory syncytial virus infection following allogeneic stem cell transplantation. Bone Marrow Transplant. 1999;24:1315–22. doi: 10.1038/sj.bmt.1702078. [DOI] [PubMed] [Google Scholar]
- 14.Harrington RD, Hooton TM, Hackman RC, et al. An outbreak of respiratory syncytial virus in a bone marrow transplant center. J Infect Dis. 1992;165:987–93. doi: 10.1093/infdis/165.6.987. [DOI] [PubMed] [Google Scholar]
- 15.Lewinsohn DM, Bowden RA, Mattson D, et al. Phase I study of intravenous ribavirin treatment of respiratory syncytial virus pneumonia after marrow transplantation. Antimicrob Agents Chemother. 1996;40:2555–7. doi: 10.1128/aac.40.11.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Machado CM, Boas LS, Mendes AV, et al. Low mortality rates related to respiratory virus infections after bone marrow transplantation. Bone Marrow Transplant. 2003;31:695–700. doi: 10.1038/sj.bmt.1703900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nichols WG, Gooley T, Boeckh M. Community-acquired respiratory syncytial virus and parainfluenza virus infections after hematopoietic stem cell transplantation: the Fred Hutchinson Cancer Research Center experience. Biol Blood Marrow Transplant. 2001;7(Suppl):11S–5S. doi: 10.1053/bbmt.2001.v7.pm11777098. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh S, Champlin RE, Englund J, et al. Respiratory syncytial virus upper respiratory tract illnesses in adult blood and marrow transplant recipients: combination therapy with aerosolized ribavirin and intravenous immunoglobulin. Bone Marrow Transplant. 2000;25:751–5. doi: 10.1038/sj.bmt.1702228. [DOI] [PubMed] [Google Scholar]
- 19.de Fontbrune FS, Robin M, Porcher R, et al. Palivizumab treatment of respiratory syncytial virus infection after allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2007;45:1019–24. doi: 10.1086/521912. [DOI] [PubMed] [Google Scholar]
- 20.Torres HA, Aguilera EA, Mattiuzzi GN, et al. Characteristics and outcome of respiratory syncytial virus infection in patients with leukemia. Haematologica. 2007;92:1216–23. doi: 10.3324/haematol.11300. [DOI] [PubMed] [Google Scholar]
- 21.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129:125–37. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 22.Boeckh MJ, Gooley T, Englund J, et al. Respiratory syncytial virus (RSV) infection in hematopoietic stem cell transplant (HCT) recipients: risk factors for acquisition and lower respiratory tract disease, and impact on mortality. ASH Annual Meeting Abstracts. 2004;104:187. [Google Scholar]
- 23.Sparrelid E, Ljungman P, Ekelof-Andstrom E, et al. Ribavirin therapy in bone marrow transplant recipients with viral respiratory tract infections. Bone Marrow Transplant. 1997;19:905–8. doi: 10.1038/sj.bmt.1700752. [DOI] [PubMed] [Google Scholar]
- 24.Schleuning M, Buxbaum-Conradi H, Jager G, et al. Intravenous ribavirin for eradication of respiratory syncytial virus (RSV) and adenovirus isolates from the respiratory and/or gastrointestinal tract in recipients of allogeneic hematopoietic stem cell transplants. Hematol J. 2004;5:135–44. doi: 10.1038/sj.thj.6200358. [DOI] [PubMed] [Google Scholar]
- 25.Hall CB, McBride JT, Walsh EE, et al. Aerosolized ribavirin treatment of infants with respiratory syncytial viral infection. A randomized double-blind study. N Engl J Med. 1983;308:1443–7. doi: 10.1056/NEJM198306163082403. [DOI] [PubMed] [Google Scholar]
- 26.Hertz MI, Englund JA, Snover D, et al. Respiratory syncytial virus-induced acute lung injury in adult patients with bone marrow transplants: a clinical approach and review of the literature. Medicine (Baltimore) 1989;68:269–81. doi: 10.1097/00005792-198909000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Granwehr BP, Chemaly RF, Kontoyiannis DP. Fungal and viral infections in cancer patients. In: Kantarjian HM, Wolff RA, Koller CA, editors. MD Anderson Manual of Medical Oncology. Houston, TX: The McGraw-Hill Companies; 2011. pp. 1205–34. [Google Scholar]