Abstract
Background
As a nuclear protein and a secreted protein, HMGB1 is involved in many cellular processes such as proliferation, transcription, and inflammation. The overexpression of HMGB1 in various types of cancers is reported, but its clinical significance and prognostic value in glioblastoma multiforme (GBM) has not been well defined.
Material/Methods
The expression of HMGB1 in 116 patients with GBM was investigated with immunohistochemistry, and was detected with qRT-PCR in 12 pairs of tumor tissues and adjacent tissues. The correlations between HMGB1 and clinicopathological factors were analyzed with the chi-square test. Prognostic value of HMGB1 was evaluated with univariate analysis and multivariate analysis. By knocking down HMGB1 by siRNA, the functions of HMGB1 in progression of GBM cell lines were investigated by experiments in vitro.
Results
In our study, patients with high HMGB1 expression accounted for 42.2% of all the patients. High HMGB1 was correlated with low survival rates and was identified as an independent prognostic factor of GBM. Knockdown of intracellular HMGB1 remarkably decreased GBM cells proliferation and invasion. In hypoxia, intracellular HMGB1 of GBM cells was released out and activated AKT and ERK signaling pathways, thus promoting GBM cell invasion in this autocrine pathway.
Conclusions
HMGB1 is an independent prognostic biomarker for unfavorable prognosis of patients with GBM. Released HMGB1 of GBM cells can activate AKT and ERK signaling pathways and promote GBM cells invasion in this autocrine pathway, indicating that anti-HMGB1 therapy may be a promising treatment for GBM.
MeSH Keywords: Autocrine Communication, Glioblastoma, HMGB1 Protein, Neoplasm Invasiveness, Prognosis
Background
Glioblastoma multiforme (GBM) is the most common primary brain cancer and has a dismal outcome [1]. GBM is characterized by cellular invasiveness, proliferation, and high resistance to adjuvant therapy [2]. Multimodal treatments to GBM, including surgery followed by chemotherapy or radiotherapy, have achieved significant progress in recent years, but the prognosis of GBM remains unsatisfactory [1]. The median survival time of GBM patients after surgery is 15 to 18 months [3]. Several promising biomarkers or genetic alterations in GBM were identified with large-scale sequencing technologies, providing new insight into targeted therapies for GBM [4]. However, new diagnostic and prognostic biomarkers of GBM are still urgently needed because of the high malignancy of GBM.
High mobility group box 1 (HMGB1) is a DNA-binding protein actively released by cytokine stimulation or passively during cell death [5]. In nuclei, HMGB1 interacts with DNA without sequence specificity and functions as a structural protein of chromatin, regulating genome replication and recombination, mRNA transcription, and DNA repair [6]. As a secreted protein, the released HMGB1 stimulates many downstream signaling pathways by interacting with its receptors, such as TLR2, TLR4, and RAGE. Besides inflammation, HMGB1 is demonstrated to be involved in many processes such as cancer, trauma, and ischemia reperfusion injury [7,8]. The overexpression of HMGB1 was observed in various types of cancers, including gastric cancer, cholangiocarcinoma, lung cancer, and prostate cancer, and it was demonstrated to be associated with their progression or prognosis [9–11]. In 65 cases of glioma, high HMGB1 was considered be correlated with poor prognosis of glioma of all grades, but whether this conclusion applies to GBM patients is still in doubt. In conclusion, since emerging evidence indicates HMGB1 is a protein involved in cancer progression, the clinical significance and functions of HMGB1 in GBM have not been well investigated.
In our study, we investigated the expression of HMGB1 in 116 cases of GBM, and analyzed its correlation with clinicopathological factors and survival rates. Moreover, we assessed HMGB1 concentration in cell medium in normoxia and hypoxia. After silencing HMGB1 with siRNA, we explored the role of HMBG1 in cell signaling, proliferation, and invasion of GBM cells.
Material and Methods
Patients and follow-ups
The primary cohort consisted of 245 patients who underwent resection of GBM in Yidu Central Hospital or Jinan Central Hospital from 2002 to 2017. Pathological diagnosis as GBM was achieved by routine pathology. The test cohort was selected from the primary cohort if patients had available follow-ups and specimens for immunohistochemistry (IHC). The test cohort comprised 51 females and 65 males, with the median age as 47.9 years old. For mRNA detection, 12 pairs of GBM tissues and adjacent tumor tissues were obtained from surgery resection and were immediately preserved in liquid nitrogen. All specimens were obtained with prior consent of patients and the study was approved by the Ethics Committee of Yidu Central Hospital or Jinan Central Hospital. All tissues were confirmed according to the World Health Organization classification, 2007 version.
Immunohistochemical detection
HMGB1 expression in GBM tissues was visualized by IHC according to the method described in a previous report [12]. In brief, anti-HMGB1 antibodies at 1: 100 dilution (CellSignaling Technology, Danvers, MA, USA) were used for incubation overnight at 4°C. After washing in phosphate-buffered saline (PBS) and incubating in corresponding secondary antibodies (Sangon, Shanghai, China) at room temperature for 30 min, specimens were incubated in horseradish peroxidase-labeled working solution (Sangon, Shanghai, China) for 20 min, and finally the 3,3′-diaminobenzidine was applied for visualization.
The IHC results were semi-quantified by 2 senior pathologists by calculating the IHC score. The pathologists were unaware of the clinical data. The total IHC score was defined as: total score=score of positive cells×score of staining intensity. The score of positive cells was defined as: 1, <25% of positive cells; 2, 25–50% of positive cells; 3, 50–75% of positive cells; and 4, 75–100% of positive cells. The score of staining intensity from 0 to 4 referred to negative staining, weak staining, medium staining, and strong staining, respectively. The high-expression and low-expression groups were identified by the cut-offs of the IHC score, which was defined by the ROC curves [13].
Cells culture and agents
Human GBM cell lines U251 MG, U118 MG, and A172 were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and cultured in DMEM containing 10% fetal bovine serum (FBS) supplemented with streptomycin (100 μg/ml) and penicillin (100 U/ml). All reagents were purchased from Sigma-Aldrich Company unless specified otherwise. Applied antibodies were: primary antibodies of ERK, AKT, phosphorylated ERK (Tyr202/204), AKT (Ser473) (CellSignaling Technology, Danvers, MA, USA), and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Human recombinant HMGB1 was from Sino Biotech (Beijing, China).
Western blot analysis
Cells were lysed with RIPA lysis and the whole-cell lysates were used for electrophoresis. After electrophoresis, proteins were transferred to a nitrocellulose filter membrane. Primary antibodies were used to incubate the membrane at 4°C overnight, and the corresponding secondary antibodies were applied at room temperature for 1 h. Finally, enhanced chemiluminescent (ECL) agent was used for visualization of proteins.
HMGB1 knockdown
Knockdown of HMGB1 was accomplished by small interfering RNA (siRNA) purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Transfection of siRNA was performed with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The siRNA sequences were designed as follows: siHMGB1-1, sense strand: 5′-CCUGUCCAUUGGUGAUGUUTT-3′; anti-sense strand: 5′-AACAUCACCAAUGGACAGGTT-3′.
In vitro cell proliferation and invasion assay
Cell proliferation was evaluated with MTT assay according to methods described in a previous report [14]. Briefly, equal numbers of cells were plated into 96-well plates and incubated for 48 h with or without recombinant HMBG1 stimulation, then 10 mg/ml MTT was added to each well and the crystals at the bottom were resolved by 100 μl DMSO after 6 h. The optical density (OD) at 570 nm of the control group was set as the baseline, and the proliferation index was calculated by ratio to the control.
Invasion of GBM cells was detected with matrigel invasion assay (BD, Franklin Lakes, NJ, USA). In brief, GBM cells were placed on top of the upper chamber with medium with or without recombinant HMGB1 (rHMGB1) in the bottom chamber. All chamber assays were analyzed 14 h after cell plating. Cells in the upper chamber were removed by wiping with a cotton swab, and cells in the bottom chamber were fixed in methanol and stained with hematoxylin. Cells were counted in at least 8 random visual fields. The invaded cells of the control group were set as baseline and the number of cells in the other groups were standardized to the baseline.
Conditioned medium
Conditioned medium of GBM cell lines were obtained after culture in normoxia or hypoxia experiments with 20% or 1% O2 for 6 h. After centrifuging at 1000 g for 15 min to remove cell debris, the conditioned medium was centrifuged at 20 000 g at 4°C for 30 min, and the participation was collected and re-suspended with normal medium.
RNA extraction and RT-PCR
The total mRNA was extracted with TRIzol reagent (Thermo Fisher, Waltham, MA, USA). Quantitative PCR was performed with SYBR Green Master Mix and StepOnePlus system (Applied Biosystem, Waltham, MA, USA). The ΔΔCt method was used to calculate the relative expression with GAPDH. The primer sequences of RT-PCR were used:
HMGB1, forward TTCATTTCTCTTTCATAACGGG,
reverse TCTAAGAAGTGCTCAGAGAGGTG,
GAPDH, forward GAGTCAACGGATTTGGTCGT,
reverse GACAAGCTTCCCGTTCTCAG.
Statistical analysis
All data were analyzed with SPSS22.0software. The correlations between HMGB1 expression and the clinicopathological factors were analyzed by chi-square test. The survival curves were displayed with Kaplan-Meier method and the statistical differences between different groups were calculated with the log-rank test. The Cox proportional hazards regression model was used to identify the independent prognostic factors. The difference between HMGB1 mRNA in the adjacent tissues and tumor tissues was compared by paired t test. P<0.05 was considered statistically significant.
Results
Expression of HMGB1 in GBM tissues was higher than in adjacent tissues
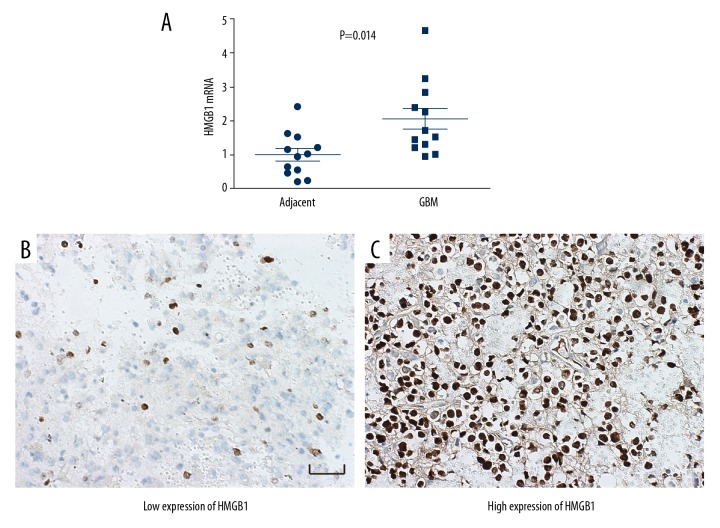
In our study, to compare the difference between GBM tissues and the corresponding adjacent tissues, the mRNA level of HMGB1 was detected with qRT-PCR. A total of 12 pairs of GBM tissues and adjacent tissues were collected prospectively. The mRNA levels of HMGB1 in tumor tissues were significantly higher than in adjacent tissues, indicating that high HMGB1 promotes the oncogenesis of GBM (Figure 1A). Moreover, in paraffin-embedded specimens, HMGB1 expression was detected with IHC and evaluated by the IHC score, which was detailed in the Materials and Methods section above. According to the cut-off of the IHC score, the cohort was divided into subgroups with low and high expression of HMGB1 (Figure 1B, 1C). The percentages of patients with low and high expression of HMGB1 were 57.8% and 42.2%, respectively.
Figure 1.
HMGB1 expression in GBM tissues and adjacent tissues. (A) The mRNA levels of HMGB1 in GBM tissues and the adjacent tissues were detected with RT-PCR. HMGB1 mRNA in GBM was significantly higher than that in adjacent tissues. (B, C) Representative images of low expression and high expression of HMGB1. Scale bar: 50 μM.
Correlation between HMGB1 and clinicopathological factors
The correlation between HMGB1 expression and clinicopathological factors were analyzed with the chi-square test (Table 1). Interestingly, high expression of HMGB1 was significantly associated with high percentage of Ki67, indicating that cells with high HMGB1 have more aggressive behavior.
Table 1.
Correlation between HMGB1 expression and clinicopathological factors.
| Parameters | HMGB1 | P* | |
|---|---|---|---|
| Low | High | ||
| Age | 0.850 | ||
| ≤50 | 39 | 27 | |
| >50 | 28 | 22 | |
| Sex | 0.852 | ||
| Male | 37 | 28 | |
| Female | 30 | 21 | |
| KPS | 0.345 | ||
| <70 | 31 | 18 | |
| ≥70 | 36 | 31 | |
| Extent of resection | 0.574 | ||
| Gross total resection (95%) | 37 | 24 | |
| Subtotal resection | 30 | 25 | |
| Ki67 | 0.002 | ||
| <10% | 55 | 27 | |
| ≥10% | 12 | 22 | |
Means calculated by Chi-Square test.
High HMGB1 expression was associated with poor prognosis of GBM
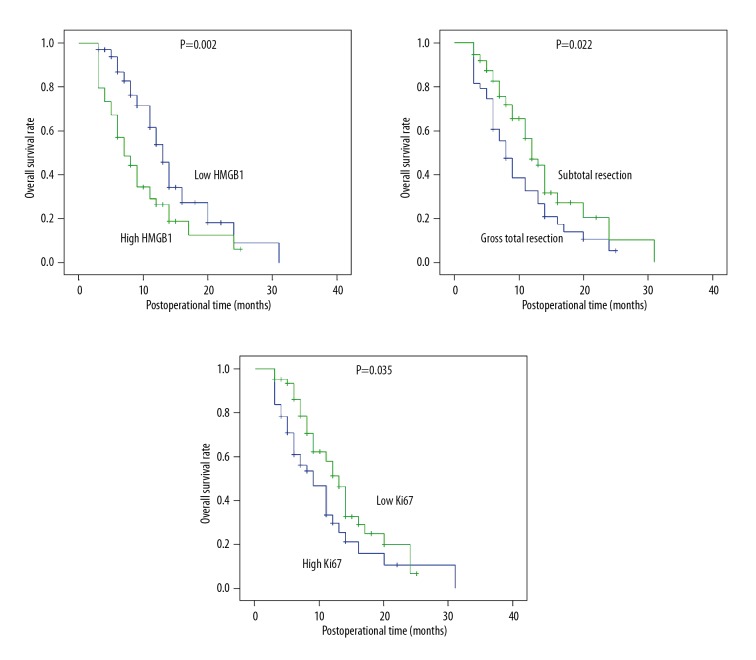
The prognostic value of HMGB1 in GBM was evaluated with univariate analysis followed by multivariate analysis (Table 2). Kaplan-Meier univariate analysis was first performed to investigate the correlation between the survival rates and all of the clinicopathological factors. High expression of HMGB1 was significantly correlated with lower survival curves (P=0.002) (Figure 2A). Moreover, resection margin (P=0.022) (Figure 2B) and Ki67 percentage (P=0.035) (Figure 2C) were also associated with poorer prognosis.
Table 2.
Prognostic value of HMGB1 was analyzed with univariate and multivariate analysis.
| Parameters | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| 1-year OS(%) | P* | HR | 95%CI | P# | |
| Age | |||||
| ≤50 | 47.2 | ||||
| >50 | 33.7 | 0.836 | |||
| Gender | |||||
| Male | 47.6 | ||||
| Female | 40.8 | 0.880 | |||
| KPS | |||||
| <70 | 39.8 | ||||
| ≥70 | 42.4 | 0.223 | |||
| Extent of resection | |||||
| Gross total resection | 51.1 | 1 | |||
| Subtotal resection | 29.7 | 0.022 | 2.16 | 1.31–3.55 | 0.003 |
| Ki67 | |||||
| <10% | 45.6 | 1 | |||
| ≥10% | 33.2 | 0.035 | 1.39 | 0.83–2.32 | 0.206 |
| HMGB1 | |||||
| Low | 53.9 | 1 | |||
| High | 26.6 | 0.002 | 2.18 | 1.30–3.66 | 0.003 |
Means calculated by Log-rank test;
means calculated by Cox proportional hazards regression.
Figure 2.
Survival curves of HMGB1 expression, surgical margin, and Ki67. The overall survival curves were stratified with HMGB1 expression, surgical margin, and Ki67 status. High HMGB1, gross total resection, and high Ki67 were significantly associated with lower overall survival rates.
Independent prognostic biomarkers were further identified by multivariate analysis with Cox regression hazard model. HMGB1 was identified as an independent biomarker indicating poor prognosis of GBM (P=0.003, HR=2.18, 95%CI=1.30–3.66). Resection margin was also an independent prognostic factor of GBM.
Intracellular HMGB1 promoted GBM cell proliferation and invasion
In clinical analysis, we observed that high HMGB1 led to poorer prognosis of GBM, so we performed experiments in vitro to explore the underlying mechanisms. The intrinsic expression of HMGB1 in different GBM cell lines was detected with Western blotting. Cell line U118 had the highest expression of HMGB1 and U251 had the lowest expression among the 3 cell lines (Figure 3A). With siRNA, we silenced the HMGB1 expression in U118 cells (Figure 3B) and detected the influence of HMGB1 knockdown on GBM cells proliferation and invasion. Compared to cells with normal HMGB1 expression, HMGB1 knockdown significantly decreased U118 cell proliferation (Figure 3C). The attenuation of invasion was more remarkable compared with proliferation. Invasion of U118 cells was notably impaired in cells transfected with HMGB1 siRNA (Figure 3D).
Figure 3.
Intracellular HMGB1 could promote GBM cells proliferation and invasion. (A) The intrinsic expressions of HMGB1 in GBM cell lines U251MG, U118MG, and A174 were detected with Western blotting. (B) HMGB1 expression in U118 was successfully silenced by siRNA. (C) Proliferation of U118 cells transfected with HMGB1 siRNA was remarkably impaired. (D) Cell invasion of U118 after HMGB1 knockdown was significantly attenuated compared with the control group. (E) Representative images of invaded cells of different groups.
HMGB1 was released and stimulated AKT signaling
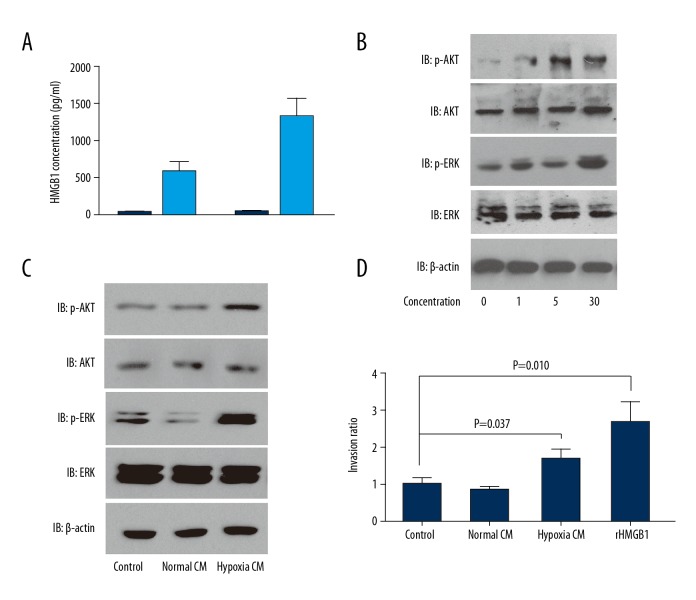
Intracellular HMGB1 is released as a cytokine and also functions as a nuclear protein, so we further investigated whether HMGB1promotes GBM cell proliferation and invasion via functioning as a nuclear protein or a cytokine. First, we assessed the HMGB1 concentration in medium of U118 and U251 cells under hypoxia condition (Figure 4A). The medium concentration without hypoxia stimulation ranged from 10 to 30 pg/ml, but increased to 600 to 1600 pg/ml in hypoxia, indicating that hypoxia promoted HMGB1 release from GBM cells. With human rHMGB1, we demonstrated that extracellular HMGB1 activated the phosphorylation of AKT and ERK, which is essential in tumor cells proliferation and invasion (Figure 4B). HMGB1 with the concentration of more than 1 ng/ml was enough to stimulate the phosphorylation of AKT and ERK. Moreover, the conditioned medium (CM) of U118 cells in normal condition or hypoxia condition was used to incubate U118 for 30 min. The conditioned medium notably stimulated the phosphorylation of AKT and ERK, suggesting that GBM cells are activated by release of HMGB1 in an autocrine pathway (Figure 4C). The effects of CM and rHMGB1 on U118 cell invasion were also investigated. As a result, both CM in hypoxia and rHMGB1 enhanced the invasion of GBM cells (Figure 4D). These results prove that intracellular HMGB1 is released in extreme environments like hypoxia, which are common in the tumor microenvironment. The released HMGB1 can promote GBM progression in this autocrine pathway.
Figure 4.
Intracellular HMGB1 were released from and activated AKT and ERK signaling. (A) HMGB1 was released from U251 or U118 cells in condition of hypoxia. (B) Human recombinant HMGB1 at different concentrations stimulated AKT and ERK signaling of U118 cells. (C) Conditioned medium of hypoxia promoted phosphorylation of AKT and ERK of U118. (D) Conditioned medium of hypoxia and recombinant HMGB1 promoted invasion of U118 cells.
Discussion
GBM is the most aggressive and common brain tumor worldwide. Although several biomarkers are considered as promising targets, such as EGFR, there has been little progress in developing targeted therapy against GBM [15]. Several drugs are in clinical trials, but there is no available drug currently in clinical use [16]. New targeted therapy is based on finding new biomarkers. Effective biomarkers, both diagnostic and prognostic, not only stimulate interest in exploration of new drugs, but also provide new insight into traditional classification, which can help select high-risk patients and guide treatment more precisely. Here, we identified HMGB1 as a prognostic biomarker of GBM, indicating that anti-HMGB1 therapy may be a promising treatment for GBM patients. At present, there is no specific inhibitor of HMGB1. However, the neutralizing antibody of HMGB1 can antagonize extracellular HMGB1, and the antitumor effect of the neutralizing antibody of HMGB1 has been demonstrated by in vivo experiments [17].
As a secreted protein, HMGB1 functions as a proinflammatory factor via binding with its receptors, including RAGE, TLR2, TLR4, TLR9, Mac-1, CXCR4, CXCR12, TIM-3, and CD24-Siglec-10 (Siglec-G in mice). After the interaction between HMGB1 and these factors, a series of signaling pathways, such as MAPKs, PI3K/Akt, and NF-κB, are activated. Interestingly, sustained stimulation of the PI3K/Akt pathway is a molecular feature of GBM, and we also observed that extracellular HMGB1 activated the PI3K/Akt pathway of GBM cells. However, we did not identify the key receptor responsible for interacting with extracellular HMGB1 and initiating PI3K/Akt in GBM cells. This is an interesting topic and needs further experiments. In normal condition, HMGB1 is mostly secreted by immune cells. However, HMGB1 can be released from most dying cells. Hypoxia is a common situation in the tumor microenvironment, and HMGB1 has been proved to be released from other tumor cells in hypoxia and to promote progression [18]. Our study confirmed that HMGB1 can be released from GBM cells under hypoxia and proved the oncogenic role of HMGB1 in GBM.
HMGB1 is involved in many biological processes, including DNA repair, proliferation, migration, autophagy, transcription, and secretion. Although HMGB1 was proved to promote cancer progression in various kinds of cancers, the role of HMGB1 in cancer treatment is still controversial [19]. HMGB1 in glioma cells was demonstrated to be released into circulation and can be regarded as a biomarker of treatment efficacy [20]. Moreover, miRNA-129-2 was proved to suppress glioma progression by targeting HMGB1 [21]. In a previous study, HMGB1 was reported to be associated with poor prognosis in 65 glioma samples [22]. However, this conclusion is not convincing because glioma has high heterogeneity among different grades, and the patient numbers were relatively low. In the present study, we focused on GBM, the grade IV glioma, and collected a total of 116 cases in 2 different medical centers. Furthermore, our study shows the underlying mechanisms by proving that high intracellular HMGB1 promotes GBM proliferation and invasion in an autocrine pathway. Therapies to blockage of HMGB1 release or directly target HMGB1 may be promising treatment options.
Conclusions
HMGB1 is an independent prognostic biomarker for unfavorable prognosis of patients with GBM. Released HMGB1 of GBM cells can activate AKT and ERK signaling pathways and promotes GBM cell invasion in this autocrine pathway, indicating that anti-HMGB1 therapy may be a promising treatment for GBM.
Footnotes
Source of support: Departmental sources
Conflicts of interest
None.
References
- 1.Alifieris C, Trafalis DT. Glioblastoma multiforme: Pathogenesis and treatment. Pharmacol Ther. 2015;152:63–82. doi: 10.1016/j.pharmthera.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Baumert BG, Hegi ME, van den Bent MJ, et al. Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033-26033): A randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2016;17:1521–32. doi: 10.1016/S1470-2045(16)30313-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim M, Xia Y, Bettegowda C, Weller M. Current state of immunotherapy for glioblastoma. Nat Rev Clin Oncol. 2018;15(7):422–42. doi: 10.1038/s41571-018-0003-5. [DOI] [PubMed] [Google Scholar]
- 4.Westphal M, Lamszus K. Circulating biomarkers for gliomas. Nat Rev Neurol. 2015;11:556–66. doi: 10.1038/nrneurol.2015.171. [DOI] [PubMed] [Google Scholar]
- 5.Sims GP, Rowe DC, Rietdijk ST, et al. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–88. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 6.Prasad R, Liu Y, Deterding LJ, et al. HMGB1 is a cofactor in mammalian base excision repair. Mol Cell. 2007;27:829–41. doi: 10.1016/j.molcel.2007.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang D, Kang R, Zeh HJ, 3rd, Lotze MT. High-mobility group box 1 and cancer. Biochim Biophys Acta. 2010;1799:131–40. doi: 10.1016/j.bbagrm.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirsiger S, Simmen HP, Werner CM, et al. Danger signals activating the immune response after trauma. Mediators Inflamm. 2012;2012 doi: 10.1155/2012/315941. 315941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung HW, Lim JB. High-mobility group box-1 contributes tumor angiogenesis under interleukin-8 mediation during gastric cancer progression. Cancer Sci. 2017;108:1594–601. doi: 10.1111/cas.13288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang S, Xu L, Yang T, Wang F. High-mobility group box-1 and its role in angiogenesis. J Leukoc Biol. 2014;95:563–74. doi: 10.1189/jlb.0713412. [DOI] [PubMed] [Google Scholar]
- 11.Xu YF, Ge FJ, Han B, et al. High-mobility group box 1 expression and lymph node metastasis in intrahepatic cholangiocarcinoma. World J Gastroenterol. 2015;21:3256–65. doi: 10.3748/wjg.v21.i11.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Y, Yang X, Li Z, et al. Sprouty2 correlates with favorable prognosis of gastric adenocarcinoma via suppressing FGFR2-induced ERK phosphorylation and cancer progression. Oncotarget. 2017;8:4888–900. doi: 10.18632/oncotarget.13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu H, Xu Y, Zhang Q, et al. Prognostic significance of TBL1XR1 in predicting liver metastasis for early stage colorectal cancer. Surg Oncol. 2017;26:13–20. doi: 10.1016/j.suronc.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Wang HM, Xu YF, Ning SL, et al. The catalytic region and PEST domain of PTPN18 distinctly regulate the HER2 phosphorylation and ubiquitination barcodes. Cell Res. 2014;24:1067–90. doi: 10.1038/cr.2014.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weller M, Pfister SM, Wick W, et al. Molecular neuro-oncology in clinical practice: a new horizon. Lancet Oncol. 2013;14:e370–79. doi: 10.1016/S1470-2045(13)70168-2. [DOI] [PubMed] [Google Scholar]
- 16.Bai RY, Staedtke V, Riggins GJ. Molecular targeting of glioblastoma: Drug discovery and therapies. Trends Mol Med. 2011;17:301–12. doi: 10.1016/j.molmed.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo Y, Ohmori H, Fujii K, et al. HMGB1 attenuates anti-metastatic defence of the liver in colorectal cancer. Eur J Cancer. 2010;46:791–99. doi: 10.1016/j.ejca.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Tohme S, Yazdani HO, Liu Y, et al. Hypoxia mediates mitochondrial biogenesis in hepatocellular carcinoma to promote tumor growth through HMGB1 and TLR9 interaction. Hepatology. 2017;66:182–97. doi: 10.1002/hep.29184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He SJ, Cheng J, Feng X, et al. The dual role and therapeutic potential of high-mobility group box 1 in cancer. Oncotarget. 2017;8:64534–50. doi: 10.18632/oncotarget.17885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Candolfi M, Yagiz K, Foulad D, et al. Release of HMGB1 in response to proapoptotic glioma killing strategies: Efficacy and neurotoxicity. Clin Cancer Res. 2009;15:4401–14. doi: 10.1158/1078-0432.CCR-09-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y, Huang JQ, Zhang X, Shen LF. MiR-129-2 functions as a tumor suppressor in glioma cells by targeting HMGB1 and is down-regulated by DNA methylation. Mol Cell Biochem. 2015;404:229–39. doi: 10.1007/s11010-015-2382-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang XJ, Zhou SL, Fu XD, et al. Clinical and prognostic significance of high-mobility group box-1 in human gliomas. Exp Ther Med. 2015;9:513–18. doi: 10.3892/etm.2014.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]