Abstract
Background
Lin28 is a gene involved in many biological processes, including development, glucose metabolism, and tumorigenesis. Let-7 miRNA is a tumor-suppressor gene that is frequently inactivated in cancer cells. The role of c-Myc (a target gene of let-7) and the Lin28-let-7-c-Myc pathway in the growth and malignancy of thyroid cancer is unclear. The purpose of the present study was to evaluate the expression of Lin28A, let-7a, and c-Myc in human papillary thyroid carcinoma (PTC) and to investigate their potential mechanisms in the progression of PTC.
Material/Methods
Lin28A and c-Myc expression were assessed in PTC tissues and PTC cell lines using immunohistochemistry, Western blotting, and real-time PCR. CCK-8 and Transwell assays were performed to evaluate PTC cell proliferation, migration, and invasion in cells in which the expression of Lin28A was downregulated by RNA interference or in which let-7a was overexpressed after transfection with let-7a mimics.
Results
The expression of Lin28A and c-Myc was upregulated in PTC tissues and cell lines, whereas the expression of let-7a was downregulated in PTC cell lines. Clinically, Lin28A was linked to a higher tumor/node/metastasis stage and the presence of lymph node metastases. Moreover, knockdown of Lin28A activated let-7a processing and inhibited the expression of the downstream gene c-Myc, suppressing cell proliferation, migration, and invasion. Similar results were obtained after let-7a overexpression.
Conclusions
The Lin28A/let-7a/c-Myc pathway is involved in cancer growth and malignant behavior in PTC and is a potential target for therapeutic intervention in this disease.
MeSH Keywords: Genes, myc; MicroRNAs; RNA-Binding Proteins; Thyroid Neoplasms
Background
Thyroid cancer is the most common malignant endocrine tumor, and papillary thyroid cancer (PTC) accounts for 80% of thyroid malignancies [1,2]. Most PTCs are indolent malignancies, with an estimated 5-year relative survival rate of approximately 95% [3]. However, PTC is clinically challenging because traditional treatment provides little benefit [4]. To develop effective treatments for PTC, it is important to fully understand the mechanisms involved in the development, progression, and metastasis of this disease.
Lin28 is a highly conserved RNA-binding protein that was originally regarded as a vital regulator of developmental timing in Caenorhabditis elegans [5]. The lethal-7 (let-7) microRNAs (miRNAs) are tumor suppressors and prognostic factors found in numerous types of cancer [6,7]. Lin28 can repress the biogenesis of mature let-7, binding to the terminal loops of pre-let-7 elements [8,9]. c-Myc, one of the downstream target genes of the Lin28/let-7 axis, is an important oncogene involved in the proliferation and migration of cancer cells [10,11]. Earlier studies indicated that the Lin28/let-7/c-Myc pathway played a significant role in the development and progression of some human cancers [12]. However, knowledge of this pathway in human PTC is limited. In this study, we investigated the involvement of the Lin28/let-7/c-Myc pathway in the progression of PTC.
Material and Methods
Patients and materials
A total of 87 formalin-fixed and paraffin-embedded tissue specimens were obtained from the First Affiliated Hospital of Shantou University Medical College. Among these, 57 were thyroid carcinoma radical surgery specimens from patients with PTC, and 30 were specimens from patients with nodular goiters (NG). Among the 57 patients with PTC, 48 were female and 9 were male. The mean age was 45.8±13.5 years (range 18–80 years). Tumor stage was identified using the criteria of the American Joint Committee on Cancer. No patient received preoperative chemotherapy or radiotherapy. Written informed consent was obtained from the patients or their families.
Immunohistochemistry for Lin28A and c-Myc
Immunohistochemistry (IHC) was performed on paraffin-embedded thyroid cancer tissue sections using mouse monoclonal antibodies against Lin28A (Cell Signaling Technology, Danvers, MA, USA) or rabbit monoclonal antibodies against human c-Myc (Zhongshan, Beijing, China) at a 1: 200 dilution. For the negative controls, isotype-matched antibodies were used. Tissue sections were observed under a microscope (CX21FS1, Olympus, Tokyo, Japan).
Cell culture
The Nthy-ori-3-1, TPC-1, and BCPAP cell lines were purchased from GuangZhou JENNIO Biotech Technology (Guangzhou, China). Nthy-ori-3-1 and TPC-1 cells were cultured in RPMI 1640 (GIBCO, Invitrogen, Carlsbad, CA, USA), supplemented with 10% fetal bovine serum (FBS) (GIBCO, Melbourne, Australia). BCPAP cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (GIBCO) containing 10% FBS of Australia origin. All the cells were cultured at 37°C in 5% CO2.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted using an mRNA extraction kit (Takara, Dalian, China) according to the manufacturer’s instructions, and the concentration and purity of the RNA were detected using ultraviolet spectrophotometry (Bio-Rad, Hercules, CA, USA). Reverse transcription was carried out using 1 μg of total RNA and a PrimeScript® RT Master Mix – Perfect Real-Time kit (Takara) according to the manufacturer’s instructions. Reverse transcription was performed under the following conditions: 37°C for 15 min followed by 85°C for 5 s and storage at 4°C. The qPCR reactions (25 μL total volume) contained 2 μL cDNA, 12.5 μL l× SYBR Premix Ex Taq (Takara), and 0.5 μL of each primer (10 μM). The 25-μL reactions were incubated in a 96-well optical plate at 95°C for 30 s, followed by 40 cycles at 95°C for 5 s and 60°C for 30 s using the CFX Connect Real-Time system (Bio-Rad). β-actin was used as the internal control and each experiment was repeated 3 times. The sequences of the qPCR primers used were as follows: Lin28A forward: 5′-TTGTCTTCTACCCTGCCCTCT-3′; Lin28A reverse: 5′-GAACAAGGGATGGAGGGTTTT-3′; c-Myc forward: 5′-CGTCCTCGGATTCTCTGCTC-3′; c-Myc reverse: 5′-GCTGGTGCATTTTCGGTTGT-3′. miRNA qPCR was performed using a Mir-X miRNA First-Stand Synthesis Kit (Clontech, Mountain View, CA, USA) according to the manufacturer’s instructions. U6 was used as the internal control. The primer sequence used for quantification of let-7a was 5′-UGAGGUAGUAGGUUGUAUAGUU-3′.
Western blotting
Proteins were extracted with RIPA containing phenylmethanesulfonylfluoride (PMSF; 100 μM, Panera, Guangzhou, China) and protease inhibitor (Biotool, Houston, TX, USA). Thereafter, the concentration of protein was determined using a bicinchoninic acid (BCA) kit (Panera). Sodium dodecyl sulfate (SDS)-loading buffer (Panera) was added to each sample. The proteins were resolved using 10% SDS-polyacrylamide gel electrophoresis, then transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, USA). The membranes were blocked with 10% skim milk at 20–25°C for 1 h, then incubated overnight with a primary antibody against human Lin28A, c-Myc, β-actin, or glyceraldehyde 3-phosphate dehydrogenase (GAPDH) at 4°C. All primary antibodies were purchased from Cell Signaling Technology and diluted to 1: 1000. After washing, membranes were incubated with a rabbit-anti-human IgG peroxide-conjugated antibody (1: 10 000, Jackson ImmunoResearch Laboratories, West Grove, PA, USA) at 20–25°C for 1 h. The antigen-antibody complexes were detected using an enhanced chemiluminescent (ECL) system (Bio-Rad). The experiments were repeated 3 times, and β-actin was used as a loading control.
Cell transfection
PTC cells were cultured to 70–90% confluence and transiently transected with short interfering RNAs targeting Lin28A (Lin28A-siRNAs), let-7a mimic, or negative control oligonucleotides (GenePharma, Suzhou, China) using Lipofectamine 3000 (Invitrogen) according to the manufacturer’s protocol. The siRNA sequences were as follows: Lin28A siRNA-1 sense: 5′-CAGUGGAGUUCACCUUUAATT-3′ and antisense: 5′-UUAAAGGUGAACUCCACUGTT-3′; Lin28A siRNA-2 sense: 5′-CAACCUACUUUCGAGAGGATT-3′ and antisense: 5′-UCCUCUCGAAAGUAGGUUGTT-3′. TPC-1 cells were transfected with 50 nM Lin28A-siRNAs, let-7a mimic or negative control (NC) and BCPAP cells were transfected with 30 nM Lin28A-siRNAs, let-7a mimic or NC.
Cell proliferation assay
Cell proliferation assays were conducted using a Cell Counting Kit-8 (CCK-8, DOJINDO, Kumamoto, Japan) according to the manufacturer’s instructions. The cells (5±103 cells/well) were seeded in 96-well plates. At the indicated time points, 10 μL of the CCK-8 solution was added to each well and the cells were incubated for 3 h. The optical density (OD) was measured at a wavelength of 450 nm using an auto-microplate reader (Thermo Fisher Scientific, Inc). Average OD values were used to estimate the number of cells in each group.
Cell migration and invasion assays
PTC cells (1×105 cells/well) in serum-free medium were plated in the upper inserts of 24-well Boyden chambers (Corning, New York, NY, USA). The lower chamber was filled with DMEM or RPMI 1640 supplemented with 10% FBS. After incubation at 37°C for 48 h, the cells on the upper surface of the filter were scraped off. The cells on the lower surface of the filter were washed, stained, and counted in at least 5 randomly selected fields per well under a microscope (magnification, 100×). Cell invasion assays were performed as described in the cell migration assays with the Transwell inserts coated with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA).
Statistical analysis
The SPSS software package version 19.0 (IBM, Armonk, NY, USA) was used for statistical analyses. All data are presented as the mean ± the standard deviation (SD) and were analyzed using the χ2 test. Differences between groups were evaluated by the t test (2 groups) and the one-way analysis of variance using the Student-Newman-Keuls post hoc test or an independent-samples t test (multiple-group comparison). All the experiments were repeated at least 3 times. Values of P<0.05 indicated statistically significant differences.
Results
Lin28A and c-Myc were overexpressed in PTC specimens
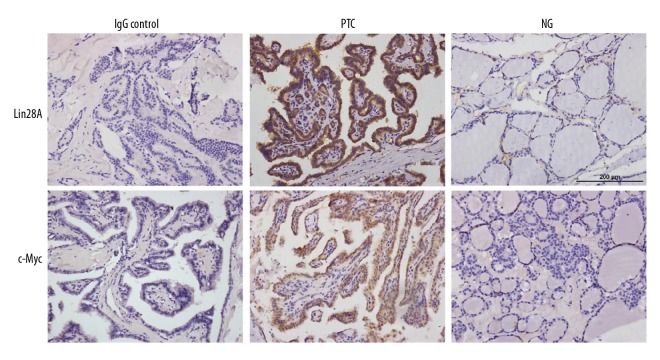
First, we investigated the expression of Lin28A and c-Myc in the 87 human thyroid tissue specimens. Lin28A was expressed mainly in the cytoplasm and was found in 27 (47.4%) of the 57 PTC cases and in 5 (16.7%) of the 30 NG samples (P=0.005; Table 1 and Figure 1). In addition, Lin28A was expressed in 66.7% (12/18) of the PTC samples from patients with lymph node metastases, whereas Lin28A was expressed less frequently in samples from patients without lymph node metastases (36.6%, P=0.047). Regarding the relationship between Lin28A expression and tumor/node/metastasis (TNM) classification, 75% of the samples from patients with PTC stage III–IV were positive for Lin28A, whereas only 40% of the samples from patients with stage I–II PTC were positive for Lin28A (P=0.031). Overall, more Lin28A was expressed in samples from patients at a late TNM stage and in tissues with lymph node metastases. There was no significant difference in Lin28A expression when considering sex, age, and tumor size as factors (Table 2).
Table 1.
Lin28A expression in PTC and nodular goiter.
| Total | Lin28A | P-valve | ||
|---|---|---|---|---|
| Positive n | Positive percentage | |||
| PTC | 57 | 27 | 47.4 | |
| NG | 30 | 5 | 16.7 | 0.005 |
Figure 1.
Lin28A and c-Myc were overexpressed in PTC tissues. Representative IHC staining of Lin28A and c-Myc (brown staining) in human PTC and NG samples. Scale bar, 200 μm.
Table 2.
Clinical characteristics of Lin28A in PTC tissue.
| characteristic | Total | Lin28A | P-valve | |
|---|---|---|---|---|
| Positive n | Positive percentage | |||
| Gender | 0.848 | |||
| Male | 9 | 4 | 44.4 | |
| Female | 48 | 23 | 47.9 | |
| Age | 0.091 | |||
| <45 | 32 | 12 | 37.5 | |
| >45 | 25 | 15 | 60.0 | |
| Tumor size (cm) | 0.392 | |||
| <4.0 | 45 | 20 | 44.4 | |
| ≥4.0 | 12 | 7 | 58.3 | |
| Lymph node metastasis | 0.047 | |||
| Absent | 39 | 15 | 38.5 | |
| Present | 18 | 12 | 66.7 | |
| TNM | 0.031 | |||
| I–II | 45 | 18 | 40.0 | |
| III–IV | 12 | 9 | 75.0 | |
c-Myc was expressed mainly in the cytoplasm and was found in 22 (38.6%) of the 57 samples from patients with PTC and in 4 (13.3%) of the 30 NG samples (P=0.014; Table 3 and Figure 1). In addition, 66.7% (12/18) of the PTC samples from patients with lymph node metastases were positive for c-Myc, whereas the samples from patients without lymph node metastases expressed c-Myc less frequently (25.6%, P=0.003). There was no significant difference in c-Myc expression when considering sex, age, tumor size, and TNM stage as factors (Table 4).
Table 3.
c-Myc expression in PTC and nodular goiter.
| Total | c-Myc | P-valve | ||
|---|---|---|---|---|
| Positive n | Positive percentage | |||
| PTC | 57 | 22 | 38.6 | |
| NG | 30 | 4 | 13.3 | 0.014 |
Table 4.
Clinical characteristics of c-Myc in PTC tissue.
| characteristic | Total | c-Myc | P-valve | |
|---|---|---|---|---|
| Positive n | Positive percentage | |||
| Gender | 0.272 | |||
| Male | 9 | 2 | 22.2 | |
| Female | 48 | 20 | 41.7 | |
| Age | 0.066 | |||
| <45 | 32 | 9 | 28.1 | |
| >45 | 25 | 13 | 52.0 | |
| Tumor size (cm) | 0.361 | |||
| <4.0 | 45 | 16 | 35.6 | |
| ≥4.0 | 12 | 6 | 50.0 | |
| Lymph node metastasis | 0.003 | |||
| Absent | 39 | 10 | 25.6 | |
| Present | 18 | 12 | 66.7 | |
| TNM | 0.806 | |||
| I–II | 45 | 17 | 37.8 | |
| III–IV | 12 | 5 | 41.7 | |
Lin28A and c-Myc were upregulated and let-7a was downregulated in PTC
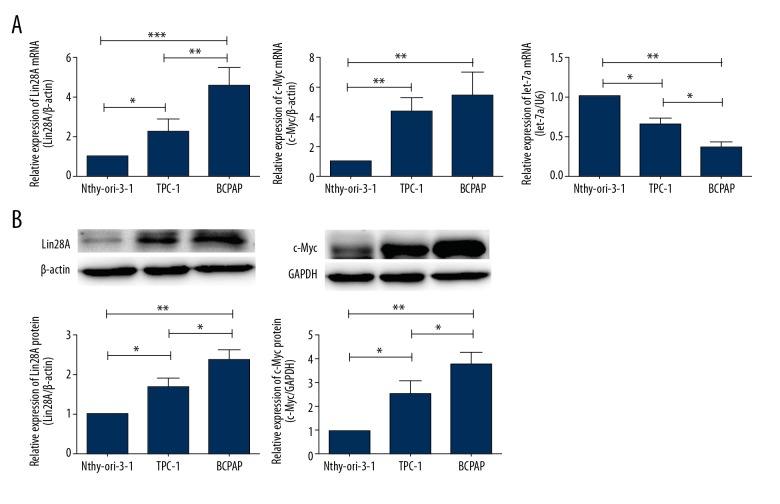
The levels of Lin28A and c-Myc mRNA in the 2 PTC cell lines investigated in this study (TPC-1 and BCPAP cells) were higher than those in normal thyroid cells (Nthy-ori-3-1, P<0.05 and P<0.01, respectively, Figure 2A). Additionally, more Lin28A mRNA was expressed in BCPAP cells than in TPC-1 cells (P<0.01, Figure 2A). There was no significant difference in the expression of c-Myc mRNA between TPC-1 and BCPAP cells. We obtained similar results when we investigated Lin28A and c-Myc protein expression. Lin28A levels were higher in BCPAP cells than in TPC-1 cells and the lowest in normal thyrocytes (P<0.05, Figure 2B). c-Myc was upregulated in PTC cells compared with that in normal thyrocytes (P<0.05, Figure 2B). In contrast to what we observed with mRNA expression, c-Myc protein levels were higher in BCPAP cells than in TPC-1 cells (P<0.05, Figure 2B). Conversely, the expression of let-7a was lower in PTC cells than in normal thyrocytes (P<0.05, Figure 2A). Among the 2 PTC cell lines investigated, the expression of let-7a was lower in BCPAP cells than in TPC-1 cells (P<0.05, Figure 2A).
Figure 2.
Lin28A and c-Myc were overexpressed in PTC cell lines. (A) qPCR analysis was used to detect the mRNA expression of Lin28A, c-Myc, and let-7a. The expression of Lin28A and c-Myc was higher in TPC-1 and BCPAP PTC cells compared with that in normal human thyrocytes (Nthy-ori-3-1 cells). Let-7a miRNA was expressed at lower levels in PTC cells than in Nthy-ori-3-1 cells. (B) Western blot analysis was used to detect the protein levels of Lin28A and c-Myc in normal human thyrocytes, TPC-1 and BCPAP cells. The analysis showed increased Lin28A and c-Myc levels in PTC cells. β-actin and GAPDH were detected as loading controls. Data are expressed as means ±SD of 3 independent experiments. * P<0.05; ** P<0.01; *** P<0.001.
Relationships among Lin28A, let-7a, and c-Myc expression in PTC
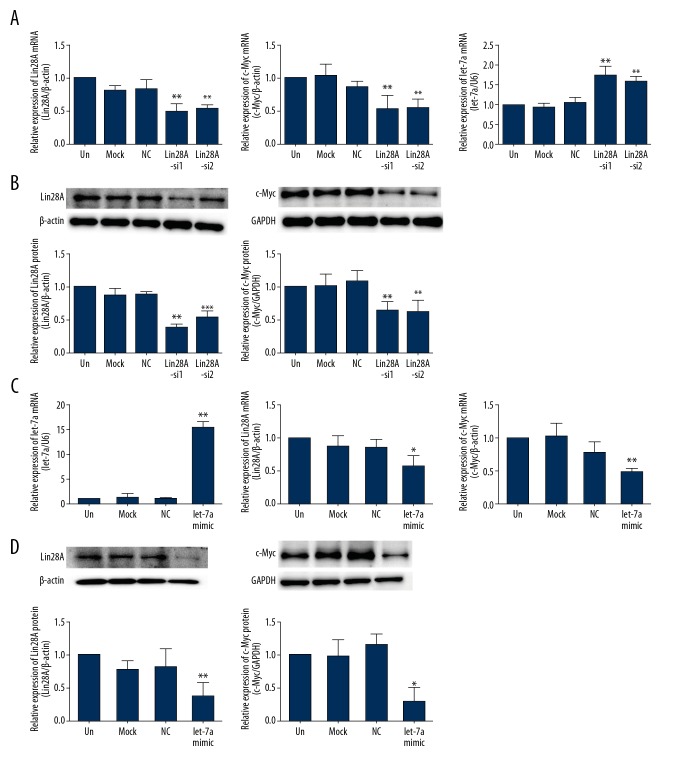
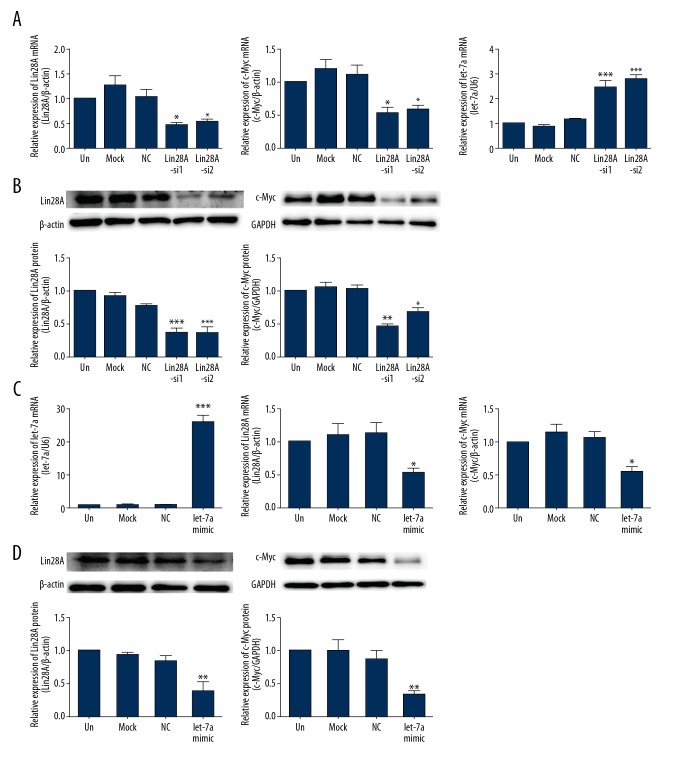
Lin28 was previously found to inhibit the processing of let-7 in mammalian cells, preventing its accumulation [13]. To assess whether Lin28A regulated let-7a in PTC cells, we suppressed Lin28A expression in TPC-1 (Figure 3A, 3B) and BCPAP (Figure 4A, 4B) cells using RNA interference. Lin28A knockdown was associated with upregulation of let-7a mRNA in TPC-1 (P<0.01, Figure 3A) and BCPAP (P<0.001, Figure 4A) cells compared with that in control cells. Meanwhile, the expression of c-Myc decreased in the same cells, both at the mRNA (P<0.01, Figure 3A and P<0.05, Figure 4A) and protein (P<0.01, Figure 3B and P<0.01, Figure 4B) levels.
Figure 3.
Relationship among Lin28A, c-Myc, and let-7a in TPC-1 cells. (A) qPCR analysis was used to detect the expression of Lin28A, c-Myc, and let-7a in TPC-1 cells transfected with Lin28A-siRNAs. Knockdown of Lin28A cells was associated with lower c-Myc expression and higher let-7a expression. (B) Western blot analysis was used to detect the protein expression of Lin28A and c-Myc in TPC-1 cells transfected with Lin28A-siRNAs. Knockdown of Lin28A was associated with lower c-Myc levels. (C) qPCR analysis was used to detect the mRNA expression of let-7a, Lin28A, and c-Myc in TPC-1 cells transfected with let-7a mimics. The overexpression of let-7a was associated with a decrease in Lin28A and c-Myc expression. (D) Western blot analysis was used to detect Lin28A and c-Myc protein expression in TPC-1 cells transfected with let-7a mimics. The expression of Lin28A and c-Myc was downregulated by the overexpression of let-7a. Data are expressed as means ±SD of 3 independent experiments. * P<0.05; ** P<0.01; *** P<0.001, compared with media-only group (un).
Figure 4.
Relationship among Lin28A, c-Myc, and let-7a in BCPAP cells. (A) qPCR analysis was used to detect the mRNA expression of Lin28A, c-Myc, and let-7a in BCPAP cells transfected with Lin28A-siRNAs. The knockdown of Lin28A was associated with lower c-Myc and higher let-7a expression. (B) Western blot analysis was used to detect the protein expression of Lin28A and c-Myc expression in BCPAP cells transfected with Lin28A-siRNAs. The knockdown of Lin28A was associated with lower c-Myc levels. (C) qPCR analysis was used to detect the mRNA expression of let-7a, Lin28A, and c-Myc in BCPAP cells transfected with let-7a mimics. The overexpression of let-7a was associated with a decrease in Lin28A and c-Myc mRNA expression. (D) Western blot analysis was used to detect Lin28A and c-Myc protein expression in BCPAP cells transfected with let-7a mimics. The expression of Lin28A and c-Myc was downregulated by the overexpression of let-7a. Data are expressed as means ±SD of 3 independent experiments. * P<0.05; ** P<0.01; *** P<0.001, compared with media-only group (un).
To explore whether let-7a exerted its effect upstream of c-Myc to regulate the expression of Lin28A, we overexpressed let-7a in TPC-1 and BCPAP cells. The transfection of let-7a mimics was associated with increased let-7a levels (P<0.001, Figure 3C and P<0.001, Figure 4C). We found that augmentation of let-7a expression suppressed Lin28A and c-Myc levels in TPC-1 and BCPAP cells relative to that in control cells (Figures 3C, 3D, 4C, 4D). These data may indicate the existence, in PTC, of a double-negative feedback loop between Lin28 and let-7, involving c-Myc.
Inhibition of Lin28A or overexpression of let-7a suppressed PTC cell proliferation
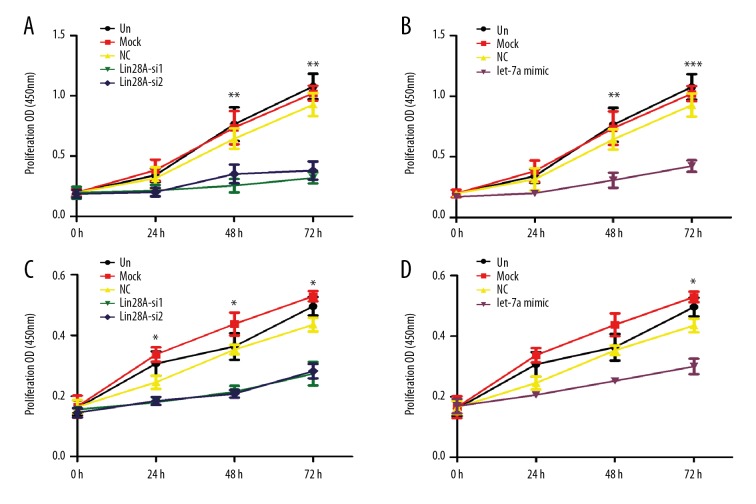
The effects of Lin28A and let-7a on the proliferation of PTC cells are not known. To evaluate these phenomena, we first performed CCK-8 assays in TPC-1 and BCPAP cells upon downregulation of Lin28A. Both TPC-1 and BCPAP cells transfected with Lin28A-siRNA exhibited lower rates of growth compared with control cells (Figure 5A, 5C). Thereafter, we investigated whether let-7a also regulated PTC cell growth. The transfection of let-7a mimics inhibited the proliferation of TPC-1 and BCPAP cells (Figure 5B, 5D). These data demonstrate that downregulation of Lin28A or overexpression of let-7a inhibited the proliferation of PTC cells.
Figure 5.
Inhibition of Lin28A or overexpression of let-7a suppressed the proliferation of PTC cells. The numbers of Lin28A-silenced TPC-1 cells (A), let-7a-overexpressing TPC-1 cells (B), Lin28A-silenced BCPAP cells (C), and let-7a-overexpressing BCPAP cells (D) were determined individually at the indicated time points (0–72 h) by CCK-8 assay. Data are shown as means ±SD of 3 independent experiments. * P<0.05; ** P<0.01; *** P<0.001, compared with media-only group (un).
Lin28A and let-7a affected tumor migration and invasion in PTC
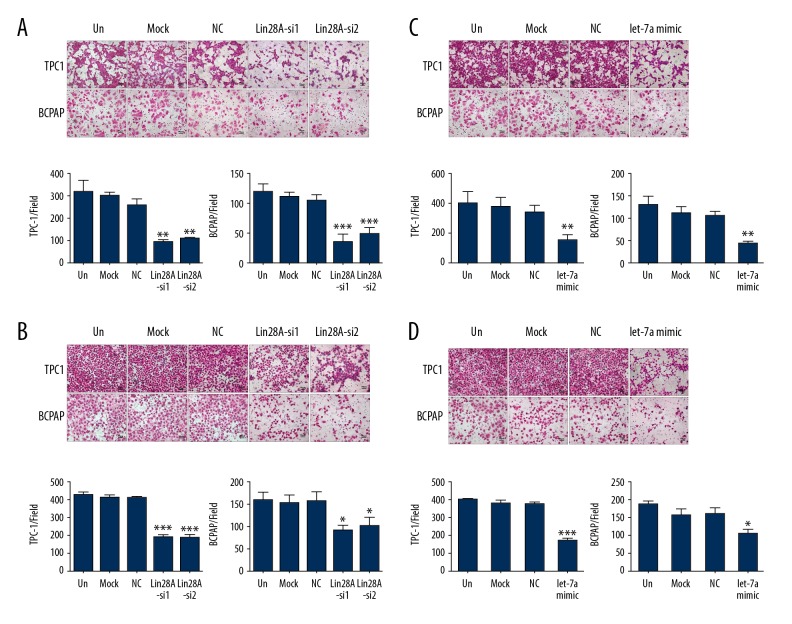
PTC cell migration was investigated following Lin28A knockdown. The migration of TPC-1 and BCPAP cells was inhibited after Lin28A silencing (Figure 6A). Similar results were obtained upon overexpression of let-7a through the transfection of let-7a mimics (Figure 6C). To confirm that Lin28A functions as an oncogene in PTC, we explored the influence of Lin28A on the invasion of TPC-1 and BCPAP cells in vitro. We found that the ability of TPC-1 and BCPAP cells to invade was significantly reduced after Lin28A downregulation (Figure 6B).
Figure 6.
Inhibition of Lin28A or overexpression of let-7a suppressed PTC cell migration and invasion. Representative images and relative quantification of Transwell migration assay (A) and invasion assay (B) in Lin28A-silenced TPC-1 and BCPAP cells. Representative images and relevant quantification of Transwell migration assay (C) and invasion assay (D) in TPC-1 and BCPAP cells overexpressing let-7a. ** P<0.01; ** P<0.01; *** P<0.001, compared with media-only group (un).
We demonstrated above that ectopic let-7a suppressed the expression of Lin28A. To further demonstrate that the effect of Lin28A on the invasion of PTC cells occurred through let-7a, we upregulated let-7a in these cells. Our results indicated that ectopic expression of let-7a markedly inhibited TPC-1 cell invasion (P<0.001, Figure 6D) and BCPAP (P<0.05, Figure 6D). Taken together, these data confirm that Lin28A promotes PTC cell invasion, whereas let-7a suppressed it.
Discussion
Mammalian Lin28 has 2 paralogs, Lin28A and Lin28B, which are highly expressed in most tumor tissues and cell lines. In addition, both are involved in cancer differentiation, invasion, and metastasis [12, 14]. One recent study indicated that Lin28 plays a role in paclitaxel resistance in breast cancer [15]. Additionally, overexpression of Lin28 was reported in cancers of the prostate, ovary, liver, kidney, and colon [16–23]. In the present study we discovered that Lin28A was highly expressed in PTC tissues and cell lines. In addition, the expression of Lin28A was correlated with lymph node metastasis and TNM stage in PTC. The PTC cell line, TPC-1, expressed a rearranged form of RET, and BCPAP cells harbored the BRAF V600E gene mutation [24,25]. Studies have suggested that BCPAP and TPC-1 cells displayed notable differences in their proliferation rates (higher in BCPAP than in TPC-1 cells) and that BCPAP cells produced tumors with aggressive clinical behaviors in orthotropic nude mice [26]. This may be why more Lin28A was expressed in BCPAP cells than in TPC-1 cells.
The let-7 family of miRNA was first identified in C. elegans; its members are tumor suppressors and are downregulated in some cancers, such as gastric, colon, and lung cancers [6,27–29]. A recent study showed that let-7a is involved in PTC tumorigenesis by negatively regulating AKT2 [30]. Our study indicated that let-7a was expressed at low levels in PTC cell lines compared to a normal thyroid cell line and was expressed the least in BCPAP cells. c-Myc is a member of the Myc gene family. It is a common oncogene and a highly pleiotropic transcription factor known to control proliferation, metabolism, differentiation, and apoptosis [31,32]. c-Myc is a helix-loop-helix leucine zipper transcription factor that is known to be abnormally expressed in human malignancies and is a proven downstream target gene of the Lin28/let-7 axis [11,33–35]. In human cancers, its expression is deregulated, and it is considered an indicator of poor prognosis [36–38]. In our study, c-Myc was highly expressed in PTC tissues and PTC cell lines. Clinically, its expression is correlated with lymph node metastasis.
Previous studies have indicated that Lin28 binds to the terminal loop of the precursors of the let-7 family miRNAs and blocks their processing into mature miRNAs [18,39]. In addition, let-7 suppressed the translation of Lin28 by binding to the 3′ UTR of Lin28 [13,40]. Lin28 also de-repressed c-Myc by inhibiting let-7, and c-Myc transcriptionally activated Lin28 [41,42]. Therefore, the Lin28/let-7/c-Myc axis might play a significant role in the characteristic miRNA expression profiles observed in various tumors [43,44]. To prove that the Lin28A/let-7a/c-Myc pathway exists in PTCs and affects the biological behavior of PTC cells, we first silenced the expression of Lin28A and detected the expressions of c-Myc and let-7a. Knockdown of Lin28A was associated with a decrease in c-Myc expression and an increase in let-7a levels. We also found that overexpression of let-7a, after transfection with let-7 mimics, noticeably downregulated Lin28A and c-Myc expression. Pan et al. demonstrated that Lin28 inhibited the expression of let-7, suppressed cell proliferation, and promoted cell cycle progression in human small cell lung cancer cells [45]. A breast cancer study indicated that the migration of Lin28-silenced MDA-MB-231 cells was markedly reduced and the opposite effect was observed upon Lin28 overexpression [46]. Similarly, we found that knockdown of Lin28A diminished PTC cell proliferation, migration, and invasion. Moreover, our study indicates that overexpression of let-7a impaired cellular proliferation, migration, and invasion. Further studies are required to investigate the role of the Lin28A/let-7a/c-Myc pathway in other types of thyroid cancer.
Conclusions
In the present study we found that high expression of Lin28A was associated with lymph node metastasis and late TNM stage in PTC and that the expression of Lin28A and c-Myc was correlated. Moreover, we showed that the Lin28A/let-7a/c-Myc pathway affected the growth and malignant behavior of PTC. These findings demonstrate the multifactorial nature of the Lin28A/let-7a/c-Myc pathway and indicate that it might be a target for therapeutic intervention in PTC in the future.
Acknowledgements
We thank members of the Laboratory of Molecular Cardiology, First Affiliated Hospital, Shantou University Medical College for their technical assistance.
Abbreviations
- PTC
papillary thyroid carcinoma
- NG
nodular goiter
- mRNA
messenger RNA
- miRNA
microRNA
- qRT-PCR
quantitative real-time polymerase chain reaction
- CCK-8
cell counting kit-8
Footnotes
Source of support: This study was supported by grants from the Natural Science Foundation of China (81672640), the Department of Education, Guangdong Government under the Top-tier University Development Scheme for Research and Control of Infectious Diseases (2015045), the Sail Plan Training High-Level Personnel Project of Guangdong Province (174019), the Guangdong Natural Science Foundation (2014A030313484), the Science and Technology Planning Project of Guangdong Province (2013B021800174 and 2013B021800175), the Educational Commission of Guangdong Province (2013kjcx0077), and the Science and Technology Planning Project of Shantou City ([2013]244-63)
Cconflicts of interest
None.
References
- 1.Nix P, Nicolaides A, Coatesworth AP. Thyroid cancer review 1: Presentation and investigation of thyroid cancer. Int J Clin Pract. 2005;59:1340–44. doi: 10.1111/j.1368-5031.2005.00671.x. [DOI] [PubMed] [Google Scholar]
- 2.Dal Maso L, Bosetti C, La Vecchia C, et al. Risk factors for thyroid cancer: An epidemiological review focused on nutritional factors. Cancer Causes Control. 2009;20:75–86. doi: 10.1007/s10552-008-9219-5. [DOI] [PubMed] [Google Scholar]
- 3.Minna E, Romeo P, Dugo M, et al. miR-451a is underexpressed and targets AKT/mTOR pathway in papillary thyroid carcinoma. Oncotarget. 2016;7(11):12731–47. doi: 10.18632/oncotarget.7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McLeod DS, Sawka AM, Cooper DS. Controversies in primary treatment of low-risk papillary thyroid cancer. Lancet. 2013;381:1046–57. doi: 10.1016/S0140-6736(12)62205-3. [DOI] [PubMed] [Google Scholar]
- 5.Moss EG, Lee RC, Ambros V. The cold shock domain protein LIN-28 controls developmental timing in C. elegans and is regulated by the lin-4 RNA. Cell. 1997;88:637–46. doi: 10.1016/s0092-8674(00)81906-6. [DOI] [PubMed] [Google Scholar]
- 6.Takamizawa J, Konishi H, Yanagisawa K, et al. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004;64:3753–56. doi: 10.1158/0008-5472.CAN-04-0637. [DOI] [PubMed] [Google Scholar]
- 7.Shell S, Park SM, Radjabi AR, et al. Let-7 expression defines two differentiation stages of cancer. Proc Natl Acad Sci USA. 2007;104:11400–5. doi: 10.1073/pnas.0704372104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang Y. A mirror of two faces: Lin28 as a master regulator of both miRNA and mRNA. Wiley Interdisciplinary Reviews: RNA. 2012;3:483–94. doi: 10.1002/wrna.1112. [DOI] [PubMed] [Google Scholar]
- 9.Heo I, Joo C, Cho J, et al. Lin28 mediates the terminal uridylation of let-7 precursor MicroRNA. Mol Cell. 2008;32:276–84. doi: 10.1016/j.molcel.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Cappellen D, Schlange T, Bauer M, et al. Novel c-MYC target genes mediate differential effects on cell proliferation and migration. EMBO Rep. 2006;8:70–76. doi: 10.1038/sj.embor.7400849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyerinas B, Park SM, Shomron N, et al. Identification of let-7-regulated oncofetal genes. Cancer Res. 2008;68:2587–91. doi: 10.1158/0008-5472.CAN-08-0264. [DOI] [PubMed] [Google Scholar]
- 12.Jiang S, Baltimore D. RNA-binding protein Lin28 in cancer and immunity. Cancer Lett. 2016;375:108–13. doi: 10.1016/j.canlet.2016.02.050. [DOI] [PubMed] [Google Scholar]
- 13.Newman MA, Thomson JM, Hammond SM. Lin-28 interaction with the Let-7 precursor loop mediates regulated microRNA processing. RNA. 2008;14:1539–49. doi: 10.1261/rna.1155108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thornton JE, Gregory RI. How does Lin28 let-7 control development and disease? Trends Cell Biol. 2012;22:474–82. doi: 10.1016/j.tcb.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lv K, Liu L, Wang L, et al. Lin28 mediates paclitaxel resistance by modulating p21, Rb and Let-7a miRNA in breast cancer cells. PLoS One. 2012;7:e40008. doi: 10.1371/journal.pone.0040008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tummala R, Nadiminty N, Lou W, et al. Lin28 promotes growth of prostate cancer cells and activates the androgen receptor. Am J Pathol. 2013;183:288–95. doi: 10.1016/j.ajpath.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piskounova E, Polytarchou C, Thornton JE, et al. Lin28A and Lin28B inhibit let-7 microRNA biogenesis by distinct mechanisms. Cell. 2011;147:1066–79. doi: 10.1016/j.cell.2011.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viswanathan SR, Daley GQ. Lin28: A microRNA regulator with a macro role. Cell. 2010;140:445–49. doi: 10.1016/j.cell.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Karaayvaz M, Pal T, Song B, et al. Prognostic significance of miR-215 in colon cancer. Clin Colorectal Cancer. 2011;10:340–47. doi: 10.1016/j.clcc.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nam Y, Chen C, Gregory RI, et al. Molecular basis for interaction of let-7 microRNAs with Lin28. Cell. 2011;147:1080–91. doi: 10.1016/j.cell.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King CE, Cuatrecasas M, Castells A, et al. LIN28B promotes colon cancer progression and metastasis. Cancer Res. 2011;71:4260–68. doi: 10.1158/0008-5472.CAN-10-4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen LH, Robinton DA, Seligson MT, et al. Lin28b is sufficient to drive liver cancer and necessary for its maintenance in murine models. Cancer Cell. 2014;26:248–61. doi: 10.1016/j.ccr.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rakheja D, Chen KS, Liu Y, et al. Somatic mutations in DROSHA and DICER1 impair microRNA biogenesis through distinct mechanisms in Wilms tumours. Nat Commun. 2014;2:4802. doi: 10.1038/ncomms5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Staveren WC, Solis DY, Hebrant A, et al. Human cancer cell lines: Experimental models for cancer cells in situ? For cancer stem cells? Biochim Biophys Acta. 2009;1795:92–103. doi: 10.1016/j.bbcan.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol. 2011;7:569–80. doi: 10.1038/nrendo.2011.142. [DOI] [PubMed] [Google Scholar]
- 26.Coperchini F, Pignatti P, Leporati P, et al. Normal human thyroid cells, BCPAP, and TPC-1 thyroid tumor cell lines display different profile in both basal and TNF-alpha-induced CXCL8 secretion. Endocrine. 2016;54:123–28. doi: 10.1007/s12020-015-0764-x. [DOI] [PubMed] [Google Scholar]
- 27.Reinhart BJ, Slack FJ, Basson M, et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–6. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- 28.Tang R, Yang C, Ma X, et al. MiR-let-7a inhibits cell proliferation, migration, and invasion by down-regulating PKM2 in gastric cancer. Oncotarget. 2016;7:5972–84. doi: 10.18632/oncotarget.6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akao Y, Nakagawa Y, Naoe T. let-7 microRNA functions as a potential growth suppressor in human colon cancer cells. Biol Pharm Bull. 2006;29:903–6. doi: 10.1248/bpb.29.903. [DOI] [PubMed] [Google Scholar]
- 30.Zhou B, Shan H, Su Y, et al. Let-7a inhibits migration, invasion and tumor growth by targeting AKT2 in papillary thyroid carcinoma. Oncotarget. 2017;8(41):69746–55. doi: 10.18632/oncotarget.19261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nasi S, Ciarapica R, Jucker R, et al. Making decisions through Myc. FEBS Lett. 2001;490:153–62. doi: 10.1016/s0014-5793(01)02118-4. [DOI] [PubMed] [Google Scholar]
- 32.Evan GI, Wyllie AH, Gilbert CS, et al. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–28. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- 33.Cole MD, McMahon SB. The Myc oncoprotein: a critical evaluation of transactivation and target gene regulation. Oncogene. 1999;18:2916–24. doi: 10.1038/sj.onc.1202748. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez PC, Frank SR, Wang L, et al. Genomic targets of the human c-Myc protein. Genes Dev. 2003;17:1115–29. doi: 10.1101/gad.1067003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Z, Van Calcar S, Qu C, et al. A global transcriptional regulatory role for c-Myc in Burkitt’s lymphoma cells. Proc Natl Acad Sci USA. 2003;100:8164–69. doi: 10.1073/pnas.1332764100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bourhis J, Le MG, Barrois M, et al. Prognostic value of c-myc proto-oncogene overexpression in early invasive carcinoma of the cervix. J Clin Oncol. 1990;8:1789–96. doi: 10.1200/JCO.1990.8.11.1789. [DOI] [PubMed] [Google Scholar]
- 37.Herms J, Neidt I, Luscher B, et al. C-MYC expression in medulloblastoma and its prognostic value. Int J Cancer. 2000;89:395–402. [PubMed] [Google Scholar]
- 38.Lonn U, Lonn S, Nilsson B, et al. Prognostic value of erb-B2 and myc amplification in breast cancer imprints. Cancer. 1995;75:2681–87. doi: 10.1002/1097-0142(19950601)75:11<2681::aid-cncr2820751107>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 39.Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microRNA processing by Lin28. Science. 2008;320:97–100. doi: 10.1126/science.1154040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rybak A, Fuchs H, Smirnova L, et al. A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nat Cell Biol. 2008;10:987–93. doi: 10.1038/ncb1759. [DOI] [PubMed] [Google Scholar]
- 41.Chang TC, Zeitels LR, Hwang HW, et al. Lin-28B transactivation is necessary for Myc-mediated let-7 repression and proliferation. Proc Natl Acad Sci USA. 2009;106:3384–89. doi: 10.1073/pnas.0808300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dangi-Garimella S, Yun J, Eves EM, et al. Raf kinase inhibitory protein suppresses a metastasis signalling cascade involving LIN28 and let-7. EMBO J. 2009;28:347–58. doi: 10.1038/emboj.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–38. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 44.Nadiminty N, Tummala R, Lou W, et al. MicroRNA let-7c is downregulated in prostate cancer and suppresses prostate cancer growth. PLoS One. 2012;7:e32832. doi: 10.1371/journal.pone.0032832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pan L, Gong Z, Zhong Z, et al. Lin-28 reactivation is required for let-7 repression and proliferation in human small cell lung cancer cells. Mol Cell Biochem. 2011;355:257–63. doi: 10.1007/s11010-011-0862-x. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y, Li H, Feng J, et al. Lin28 induces epithelial-to-mesenchymal transition and stemness via downregulation of let-7a in breast cancer cells. PLoS One. 2013;8:e83083. doi: 10.1371/journal.pone.0083083. [DOI] [PMC free article] [PubMed] [Google Scholar]