Abstract
Leukoaraiosis, also referred to as white-matter hyperintensities (WMHs) or age-related white matter changes, is the most frequently seen lesion on brain magnetic resonance images (MRI) in the elderly. LA is a subject of intense research interest, and is correlated with stroke, cognitive impairment or dementia, disturbances, affective disorders, and poor prognoses. Rapid advances in neuroimaging have enabled greater understanding of LA associated with aging-related cognitive decline or dementia. Recently, the techniques of multimodal MRI, such as structural MRI (sMRI), resting-state functional MRI (rs-MRI), cerebrovascular reactivity (CVR), diffusion tensor imaging (DTI), magnetic resonance spectroscopy (MRS), and dynamic contrast-enhanced MRI (DCE-MRI), have been used to explore the underlying mechanism of cognitive impairment in patients with LA. These multimodal MRI techniques may provide further insights into the structural and functional changes of LA with cognitive dysfunction.
MeSH Keywords: Cognition, Diffusion Tensor Imaging, Leukoaraiosis, Magnetic Resonance Imaging, Magnetic Resonance Spectroscopy
Background
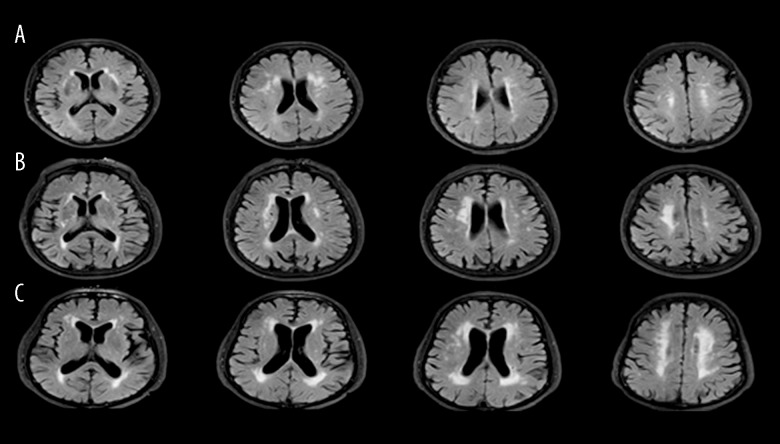
Leukoaraiosis (LA), also referred to as white matter lesions (WMLs) or age-related white matter changes, describes diffuse white-matter hyperintensities (WMHs) on MRI scans, and is often seen in the elderly. The 3 severity degrees were assessed according to the modified scale of Fazekas et al. (Figure 1). As a radiographic marker of cerebral small vessel disease (CSVD), the pathogenesis of LA includes demyelination, neuronal loss, loss of glial cells, axon destruction, reactive gliosis, and arteriolosclerosis [1]. It has been increasingly recognized to be correlated with cognitive dysfunction or dementia, stroke, and some neurodegenerative disorders. The effects of LA on cognitive function are insidious and can be difficult to detect at an early stage but are nevertheless crucial. There is a growing body of evidence that the burdens of LA influence cognitive function in multiple domains, mainly attention/executive function, processing speed, and memory [2]. However, the underlying pathogenesis remains unclear, which may be due to the dysfunction of fronto-subcortical pathways [3].
Figure 1.
The 3 severity grades of ischemic leukoaraiosis according to Fazekas scale (A, mild; B, moderate; C, severe).
The purpose of this study was to summarize the pathogenesis of LA and its impact on cognition, and also to evaluate different imaging techniques to explore the pathophysiology of cognitive decline. Table 1 summarizes these advanced MRI techniques and also shows the pathophysiology of cognitive decline in LA. Our review highlights the potential of these recent developments to achieve faster adoption of these technologies in LA studies. Furthermore, the techniques of multimodal MRI are critical to assessing the relationship between brain structural and functional changes in LA with cognitive decline.
Table 1.
A summary of the multimodal MRI to assess leukoaraiosis with cognitive impairment.
| Multimodal MRI techniques | Main observation targets | The main findings | |
|---|---|---|---|
| 1 | Structural Magnetic Resonance Imaging (sMRI) | Gray-matter atrophy, white-matter lesions, and whole-brain atrophy | Decreased gray-matter density in frontal lobes, hippocampus, and cingulate cortex; lower cortical thickness in multimodal integration regions, such as frontotemporal regions |
| 2 | Blood oxygen level-dependent (BOLD) contrast | Identify the intrinsic spontaneous brain neural activity during resting state to investigate the brain function, such as amplitude of low-frequency fluctuations (ALFF) and default-mode network (DMN) | Significant decrease in ALFF in the left parahippocampal gyrus (PHG) and an increased ALFF in right superior orbital frontal gyrus (SOFG); increased functional connectivity (FC) between the right insular region and the right SOFG and between the right calcarine cortex and the left PHG; significant differences in functional alterations of the DMN |
| 3 | Cerebrovascular reactivity (CVR) measurements | Cerebral autoregulation and vasodilatory capacity | Impaired CVR may contribute to the progression of white matter disease |
| 4 | Diffusion tensor imaging (DTI) | Determine the integrity and lesions of white-matter tracts, such as fractional anisotropy (FA) and mean diffusivity (MD) | Dysfunction of white matter integrity; DTI parameters (especially FA) could serve as a potent imaging indicator for detecting the invisible alteration of white matter integrity |
| 5 | Dynamic contrast enhancement (DCE) MRI | Evaluate the permeability and integrity of blood-brain barrier (BBB); quantitative tissue perfusion such as blood volume, mean transit time, mean transit time | Higher BBB permeability was associated with higher LA burden and cognitive decline |
| 6 | Magnetic resonance spectroscopy (MRS) | Identify changes in cerebral metabolites such as the ratios of N-acetyl aspartate (NAA)/choline (Cho) and NAA/creatine (Cr) | Metabolic changes suggested that MRS can be explored as a marker for cognitive dysfunction in patients with LA |
Structural Magnetic Resonance Imaging (sMRI)
WMHs and brain atrophy often coexist in the elderly. Voxel-based morphometry (VBM) is an automated technique widely used for quantitative measurements of white matter in separate regions. It utilizes T1-weighted images to perform voxel-wise statistical tests to discover subtle brain volume changes [4]. Understanding the specific relationship between LA and whole-brain structure may shed light on the pathophysiology of cognitive disorders in LA. There was a link between regionally specific brain atrophy and cognitive dysfunction in LA [5]. It was reported that LA patients had significantly reduced gray matter volume in the right supramarginal gyrus, right angular gyrus, right middle temporal gyrus, and right anterior cingulum [6]. This was in line with another study that found the LA group had a significantly decreased gray-matter density in left middle frontal gyrus and in the anterior and middle cingulate cortex [7]. LA may independently predict brain structural changes relevant to cognitive function, especially atrophy of the hippocampus and frontal lobes. The Cardiovascular Health Study also found that LA was inversely correlated with gray mater volume, with the greatest volume loss in the frontal cortex [8]. Recently, positive associations were also found between WMHs load and the voxel-based model of gray matter, specifically in the right anterior prefrontal cortex and the medial prefrontal cortex and cingulate gyrus [9]. However, more longitudinal data are needed to confirm the exact relationship between the load of LA and the decline in cognitive function.
Previous VBM studies have suggested that cortical atrophy is regionally distributed in LA, but few studies have assessed cortical thickness in LA. It was reported that cortical thickness was significantly lower in multimodal integration regions in LA, which suggested that LA patients were more likely to exhibit cortical thinning, especially in multimodal integration and recognition-related regions. The current morphometry data provided further evidence for LA-associated structural plasticity [10]. Another study of cortical thickness also found that higher WMHs load was related to lower cortical thickness in frontotemporal regions. Network analyses also revealed that measures of network disruption were associated with WMHs and cognitive performance. Cognitive performance was related to cortical thickness in frontotemporal regions and network measures [11]. The evidence that cortical thickness has an independent influence on the cognitive impairments suggests that the association between WMHs and cognitive impairments may be mediated by cortical thinning [12]. The above findings have some important implications in understanding the relationship between WMHs, cortical morphology, and the possible accompanying cognitive decline and dementia.
Resting-State fMRI(rs-fMRI)
The technique of rs-fMRI can identify the intrinsic spontaneous brain neural activity during resting state without task performance, which is increasingly used to investigate brain function. The amplitude of low-frequency fluctuations (ALFF) from rs-fMRI signals can be used to detect such intrinsic spontaneous brain activity and provide valuable insights into the pathophysiology of central nervous system disease. Patients with LA exhibited significant cognitive impairment, which was related with different amplitude fluctuations of rs-fMRI signals [13–15]. Recently, a study from China also showed that LA caused a significant decrease in the ALFF in the left parahippocampal gyrus (PHG) and increased ALFF in the right superior orbital frontal gyrus (SOFG). LA also showed an increased functional connectivity (FC) between the right insular region and the right SOFG and between the right calcarine cortex and the left PHG. This work further enhances understanding of the pathophysiology of LA, which is associated with widespread cerebral function deficits detected by the technique of rs-fMRI [16].
LA consists of acquired lesions that accumulate and disrupt neuron-to-neuron connectivity. Resting state networks (RSNs) are spatially coherent patterns in the human brain and their interactions sustain our daily function. The default-mode network (DMN) is closely related to selective extraction and classification of episodic memory and self-cognition, and it plays an important role in cognitive function [17]. Patients with LA-associated subcortical vascular cognitive impairment (SVCI) exhibited significant differences in the functional alterations of the DMN, which might be a feature distinguishing between SVCI and amnestic mild cognitive impairment (aMCI) patients [18]. As for the sensorimotor network (SMN), patients with LA also have impaired sensorimotor integration caused by interfering with the communication or coordination of these aforementioned regions related to the SMN [19]. Another study assessed alterations in FC patterns based on several well-defined RSNs, including DMN, SMN, dorsal attention network (DAN), frontal-parietal control network (FPCN), and auditory network (AN). The findings of wide alterations of inter-network connectivity mainly involving the SMN, DMN, FPCN and DAN highlight the importance of FC in understanding the effects of LA on cognitive dysfunction [20]. Furthermore, the dysfunction of RSNs might be a consequence of decreased white matter structural connectivity, which further affects cognitive performance [21]. Thus, the technique of rs-fMRI could be quite useful in exploring specific cognitive dysfunction by detecting spontaneous brain neural activity and FC.
Cerebrovascular Reactivity (CVR)
The impaired cerebral autoregulation and vasodilatory capacity may play in role in the pathogenesis of LA. Cerebrovascular reactivity (CVR) is an indicator of cerebrovascular reserve and provides important information about vascular health in a range of brain conditions and disorders. CVR can be assessed by a variety of ultrasonographic, nuclear medicine, and neuro-radiologic techniques, which also include MRI [22]. The breath-hold CVR mapping is an essential component of quality control analysis in clinical fMRI [23]. Currently available studies on CVR in patients with CSVD have been summarized in a recent meta-analysis [24]. Recently, impaired CVR has been associated with subtle changes in the tissue integrity of NAWM, suggesting that impaired CVR contributes to the progression of white matter disease [25]. Cerebral autoregulation and CO2 reactivity are 2 distinct processes related to blood pressure levels and duration of hypertension. Greater nocturnal dipping was associated with higher cerebral autoregulatory index (ARI) values, suggesting preservation of autoregulation in patients with ischemic subcortical white matter disease, with increased vulnerability to reduced cerebral perfusion [26]. Evaluation of CVR is important in determining vascular chronic damage to the brain and neurocognitive dysfunction by a variety of mechanisms, including white matter lesion, brain atrophy, and impairment of cerebral connectivity [27]. CVR mapping is an evolving standard for clinical functional imaging and needs to be further clarified.
Diffusion Tensor Imaging (DTI)
DTI is ideally suited to investigate the cortical disconnection as it provides indices of structural integrity within interconnected neural networks [28]. DTI is widely used to assess the microstructural integrity of white matter and to provide information about the structural characteristics of white matter. A growing body of evidence indicates that cognitive function is strongly associated with white matter integrity detected by DTI [29]. Furthermore, the parameters of fractional anisotropy (FA) could serve as a potent biomarker to detect the invisible alteration of white matter integrity and to explore its potential cognitive relevance in LA [30]. A recent tract-based spatial statistics (TBSS) study also showed that the atrophy and reduced diffusion anisotropy of the corpus callosum may indicate diffuse deep white matter damage in LA, which may explain global cognitive impairment and progression of vascular dementia [31]. Another voxel-based analysis study revealed that FA and whole-brain mean diffusivity (MD) are sensitive tools for use in evaluating its relationship with cognition in patients with LA [32]. Our study also demonstrated that DTI may provide some important information about the cognitive dysfunction in patients with LA, which may be largely attributed to the “disconnection” of cortico-subcortical pathways [33].
Dynamic Contrast-Enhanced MRI (DCE-MRI)
Blood-brain barrier (BBB) disturbance has been proposed to play a pivotal role in the pathophysiology of LA. However, the relationship of LA and quantification of regional BBB permeability has not been well clarified. BBB leakage increased with hypertension and the burden of both WMHs and normal-appearing white matter (NAWM) [34]. It was also reported that white matter permeability was significantly higher in LA, and the increased BBB permeability in NAWM also supported a close relationship between BBB disruption and the progression of LA [35]. Absolute blood pressure levels and their variations over time (i.e., blood pressure variability) were significantly associated with white matter disease burden and cognitive impairment [36–39]. Recently, we also found that higher BBB permeability was associated with higher WMHs burden and cognitive decline. Our study further indicates that the compromised BBB integrity may be a critical contributor to the pathogenesis of LA and cognitive impairment [40]. As a result, DCE-MRI may be helpful to evaluate the permeability of BBB and its relationship with cognitive impairment. Future studies are needed to determine the relationship between BBB damage and development of WMHs [41].
Magnetic Resonance Spectroscopy (MRS)
The technique of MRS facilitates non-invasive imaging to identify metabolic changes in brain tissue. It was reported that the estimates of neuro-metabolite levels provide additional and useful information on cognitive function in LA [42]. Our study found that this relationship between cognitive function and metabolic changes suggests that MRS can be explored as a marker for cognitive dysfunction in patients with LA [43]. The combined DTI and MRS study also found the techniques of MRS can be used to investigate pathological changes in the anterior and posterior periventricular white matter, which may be correlated with executive function changes in patients with LA [29]. The MRS studies were consistent with neuronal loss in patients with LA, and cognitive dysfunction was also correlated with MRS-indexed biochemical changes.
Conclusions
To date, the pathophysiology of LA has not been clearly determined. LA is associated with increased risk of cognitive impairment; however, the underlying pathological changes and their relationship to cognitive impairments are obscure. In the present review, we suggest that the techniques of multimodal MRI are critical to examining the relationship between brain structural and functional changes in LA with cognitive decline. However, confirmation of our hypothesis and the exact relationship between LA and cognitive deficit requires further investigation.
Footnotes
Source of support: This work was supported by the National Natural Science Foundation of China (81301016, 81371201) and the Beijing Municipal Administration of Hospitals’ Youth Programme (QML20150303)
Conflicts of interest
None.
References
- 1.Hase Y, Horsburgh K, Ihara M, et al. White matter degeneration in vascular and other ageing-related dementias. J Neurochem. 2018;144:617–33. doi: 10.1111/jnc.14271. [DOI] [PubMed] [Google Scholar]
- 2.Te M, Zhao E, Zheng X, et al. Leukoaraiosis with mild cognitive impairment. Neurol Res. 2015;37:410–14. doi: 10.1179/1743132815Y.0000000028. [DOI] [PubMed] [Google Scholar]
- 3.Kim TW, Kim YH, Kim KH, et al. White matter hyperintensities and cognitive dysfunction in patients with infratentorial stroke. Ann Rehabil Med. 2014;38:620–27. doi: 10.5535/arm.2014.38.5.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yin RH, Tan L, Liu Y, et al. Multimodal voxel-based meta-analysis of white matter abnormalities in Alzheimer’s disease. J Alzheimers Dis. 2015;47:495–507. doi: 10.3233/JAD-150139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mok VC, Liu T, Lam WW, et al. Neuroimaging predictors of cognitive impairment in confluent white matter lesion: Volumetric analyses of 99 brain regions. Dement Geriatr Cogn Disord. 2008;25:67–73. doi: 10.1159/000111692. [DOI] [PubMed] [Google Scholar]
- 6.Duan D, Li C, Shen L, et al. Regional gray matter atrophy coexistent with occipital periventricular white matter hyper intensities. Front Aging Neurosci. 2016;8:214. doi: 10.3389/fnagi.2016.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng Y, Li S, Zhuang Y, et al. Density abnormalities in normal-appearing gray matter in the middle-aged brain with white matter hyperintense lesions: A DARTEL-enhanced voxel-based morphometry study. Clin Interv Aging. 2016;11:615–22. doi: 10.2147/CIA.S98409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raji CA, Lopez OL, Kuller LH, et al. White matter lesions and brain gray matter volume in cognitively normal elders. Neurobiol Aging. 2012;33:834.e7–16. doi: 10.1016/j.neurobiolaging.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Marco M, Manca R, Mitolo M, et al. White matter hyperintensity load modulates brain morphometry and brain connectivity in healthy adults: a neuroplastic mechanism? Neural Plast. 2017;2017 doi: 10.1155/2017/4050536. 4050536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhuang Y, Zeng X, Wang B, et al. Cortical surface thickness in the middle-aged brain with white matter hyperintense lesions. Front Aging Neurosci. 2017;9:225. doi: 10.3389/fnagi.2017.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuladhar AM, Reid AT, Shumskaya E, et al. Relationship between white matter hyperintensities, cortical thickness, and cognition. Stroke. 2015;46:425–32. doi: 10.1161/STROKEAHA.114.007146. [DOI] [PubMed] [Google Scholar]
- 12.Zi W, Duan D, Zheng J. Cognitive impairments associated with periventricular white matter hyperintensities are mediated by cortical atrophy. Acta Neurol Scand. 2014;130:178–87. doi: 10.1111/ane.12262. [DOI] [PubMed] [Google Scholar]
- 13.Li C, Yang J, Yin X, et al. Abnormal intrinsic brain activity patterns in leukoaraiosis with and without cognitive impairment. Behav Brain Res. 2015;292:409–13. doi: 10.1016/j.bbr.2015.06.033. [DOI] [PubMed] [Google Scholar]
- 14.Li Q, Zhao LQ, Hu FY. Characteristics of cognitive impairment and the resting state functional MRI in patients with leukoaraiosis. Zhonghua Yi Xue Za Zhi. 2017;97:3529–33. doi: 10.3760/cma.j.issn.0376-2491.2017.45.003. [DOI] [PubMed] [Google Scholar]
- 15.Ding X, Wu J, Zhou Z, et al. Specific locations within the white matter and cortex are involved in the cognitive impairments associated with periventricular white matter lesions (PWMLs) Behav Brain Res. 2015;289:9–18. doi: 10.1016/j.bbr.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 16.Cheng R, Qi H, Liu Y, et al. Abnormal amplitude of low-frequency fluctuations and functional connectivity of resting-state functional magnetic resonance imaging in patients with leukoaraiosis. Brain Behav. 2017;7:e00714. doi: 10.1002/brb3.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin M, Pelak VS, Cordes D. Aberrant default mode network in subjects with amnestic mild cognitive impairment using resting-state functional MRI. Magn Reson Imaging. 2012;30:48–61. doi: 10.1016/j.mri.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Wang C, Liang H, et al. Resting-state functional magnetic resonance imaging in patients with leukoaraiosis-associated subcortical vascular cognitive impairment: A cross-sectional study. Neurol Res. 2016;38:510–17. doi: 10.1080/01616412.2016.1177929. [DOI] [PubMed] [Google Scholar]
- 19.Wu X, Lai Y, Zhang Y, et al. Breakdown of sensorimotor network communication in leukoaraiosis. Neurodegener Dis. 2015;15:322–30. doi: 10.1159/000435918. [DOI] [PubMed] [Google Scholar]
- 20.Ding JR, Ding X, Hua B, et al. Altered connectivity patterns among resting state networks in patients with ischemic white matter lesions. Brain Imaging Behav. 2017 doi: 10.1007/s11682-017-9793-9. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang Y, Sun X, Xu S, et al. Preclinical cerebral network connectivity evidence of deficits in mild white matter lesions. Front Aging Neurosci. 2016;8:27. doi: 10.3389/fnagi.2016.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu P, De Vis JB, Lu H. Cerebrovascular reactivity (CVR) MRI with CO2 challenge: A technical review. Neuroimage. 2018 doi: 10.1016/j.neuroimage.2018.03.047. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pillai JJ, Mikulis DJ. Cerebrovascular reactivity mapping: An evolving standard for clinical functional imaging. Am J Neuroradiol. 2015;36:7–13. doi: 10.3174/ajnr.A3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blair GW, Doubal FN, Thrippleton MJ, et al. Magnetic resonance imaging for assessment of cerebrovascular reactivity in cerebral small vessel disease: A systematic review. J Cereb Blood Flow Metab. 2016;36:833–41. doi: 10.1177/0271678X16631756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sam K, Peltenburg B, Conklin J, et al. Cerebrovascular reactivity and white matter integrity. Neurology. 2016;87:2333–39. doi: 10.1212/WNL.0000000000003373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birns J, Jarosz J, Markus HS, et al. Cerebrovascular reactivity and dynamic autoregulation in ischaemic subcortical white matter disease. J Neurol Neurosurg Psychiatry. 2009;80:1093–98. doi: 10.1136/jnnp.2009.174607. [DOI] [PubMed] [Google Scholar]
- 27.Lattanzi S, Carbonari L, Pagliariccio G, et al. Neurocognitive functioning and cerebrovascular reactivity after carotid endarterectomy. Neurology. 2018;90:e307–15. doi: 10.1212/WNL.0000000000004862. [DOI] [PubMed] [Google Scholar]
- 28.Bennett IJ, Madden DJ. Disconnected aging: Cerebral white matter integrity and age-related differences in cognition. Neuroscience. 2014;276:187–205. doi: 10.1016/j.neuroscience.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li C, Ling X, Liu S, et al. Abnormalities of magnetic resonance spectroscopy and diffusion tensor imaging are correlated with executive dysfunction in patients with ischemic leukoaraiosis. J Clin Neurosci. 2012;19:718–22. doi: 10.1016/j.jocn.2011.07.052. [DOI] [PubMed] [Google Scholar]
- 30.Zhong G, Zhang R, Jiaerken Y, et al. Better correlation of cognitive function to white matter integrity than to blood supply in subjects with leukoaraiosis. Front Aging Neurosci. 2017;9:185. doi: 10.3389/fnagi.2017.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otsuka Y, Yamauchi H, Sawamoto N, et al. Diffuse tract damage in the hemispheric deep white matter may correlate with global cognitive impairment and callosal atrophy in patients with extensive leukoaraiosis. Am J Neuroradiol. 2012;33:726–32. doi: 10.3174/ajnr.A2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Della Nave R, Foresti S, Pratesi A, et al. Whole-brain histogram and voxel-based analyses of diffusion tensor imaging in patients with leukoaraiosis: Correlation with motor and cognitive impairment. Am J Neuroradiol. 2007;28:1313–19. doi: 10.3174/ajnr.A0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan JL, Wang SK, Guo XJ, et al. Disconnections of cortico-subcortical pathways related to cognitive impairment in patients with leukoaraiosis: A preliminary diffusion tensor imaging study. Eur Neurol. 2017;78:41–47. doi: 10.1159/000477899. [DOI] [PubMed] [Google Scholar]
- 34.Munoz Maniega S, Chappell FM, Valdes Hernandez MC, et al. Integrity of normal-appearing white matter: Influence of age, visible lesion burden and hypertension in patients with small-vessel disease. J Cereb Blood Flow Metab. 2017;37:644–56. doi: 10.1177/0271678X16635657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huisa BN, Caprihan A, Thompson J, et al. Long-term blood-brain barrier permeability changes in Binswanger disease. Stroke. 2015;46:2413–18. doi: 10.1161/STROKEAHA.115.009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Z, Zhao Y, Zhang H, et al. Excessive variability in systolic blood pressure that is self-measured at home exacerbates the progression of brain white matter lesions and cognitive impairment in the oldest old. Hypertens Res. 2016;39:245–53. doi: 10.1038/hr.2015.135. [DOI] [PubMed] [Google Scholar]
- 37.Lattanzi S, Brigo F, Vernieri F, et al. Visit-to-visit variability in blood pressure and Alzheimer’s disease. J Clin Hypertens (Greenwich) 2018;20:918–24. doi: 10.1111/jch.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lattanzi S, Luzzi S, Provinciali L, et al. Blood pressure variability in Alzheimer’s disease and frontotemporal dementia: The effect on the rate of cognitive decline. J Alzheimers Dis. 2015;45:387–94. doi: 10.3233/JAD-142532. [DOI] [PubMed] [Google Scholar]
- 39.Yamaguchi Y, Wada M, Sato H, et al. Impact of ambulatory blood pressure variability on cerebral small vessel disease progression and cognitive decline in community-based elderly Japanese. Am J Hypertens. 2014;27:1257–67. doi: 10.1093/ajh/hpu045. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Li M, Zhang X, et al. Higher blood-brain barrier permeability is associated with higher white matter hyperintensities burden. J Neurol. 2017;264:1474–81. doi: 10.1007/s00415-017-8550-8. [DOI] [PubMed] [Google Scholar]
- 41.Taheri S, Gasparovic C, Huisa BN, et al. Blood-brain barrier permeability abnormalities in vascular cognitive impairment. Stroke. 2011;42:2158–63. doi: 10.1161/STROKEAHA.110.611731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gasparovic C, Prestopnik J, Thompson J, et al. 1H-MR spectroscopy metabolite levels correlate with executive function in vascular cognitive impairment. J Neurol Neurosurg Psychiatry. 2013;84:715–21. doi: 10.1136/jnnp-2012-303878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang S, Yuan J, Guo X, et al. Neurochemical correlates of cognitive dysfunction in patients with leukoaraiosis: A proton magnetic resonance spectroscopy study. Neurol Res. 2012;34:989–97. doi: 10.1179/1743132812Y.0000000104. [DOI] [PubMed] [Google Scholar]