ABSTRACT
Plant ONE-HELIX PROTEINS (OHPs) are part of the light-harvesting complex superfamily whose members are involved in various processes related to sensing and capturing light as well as light protection. We recently showed the requirement of a functional OHP1-OHP2 heterodimer for efficient D1 synthesis. Interestingly, while the ohp1 knockout mutant showed a strong defect in accumulation of the photosystem II and is hardly viable, virus-induced gene silencing of OHP1 had no detectable impact on plant growth and performance under standard growth conditions. However, in vivo labeling assays with 35S-methionine indicate a reduced D1 synthesis rate. Here, we show that VIGS-OHP1 plants are more susceptible towards elevated light intensities than control plants. This underlines an obligatory function of OHP1 for light acclimation.
Keywords: OHP1, one-helix protein, Arabidopsis, high light acclimation, photosynthesis and repair of photosynthetic complexes under light stress
Emerging insights into the function of the Arabidopsis one-helix proteins (OHPs) emphasize the important impact of OHPs on the accumulation of the photosystem II (PSII) core complex proteins1-3 rather than a functional association of OHP2 with PSI, which was initially proposed.4 However, a high light (HL)-induced increase in OHP1 transcripts was reported2,5 and OHP1 was postulated to be functionally correlated with the response to light stress. As it is currently assumed that both OHP variants act as OHP1-OHP2 heterodimers, their function depends on the mutual stability of both proteins.1 Virus-induced gene silencing (VIGS) of OHP2 phenotypically and biochemically resembled the ohp2 T-DNA insertion mutant lines. They were characterized by an almost undetectably slow D1 synthesis rate, leading to a specific destabilization of PSII core proteins and PSII complexes.1 In contrast, VIGS-OHP1 plants showed no phenotypical modification relative to the VIGS-GFP (green fluorescent protein) control seedlings under standard growth conditions as well as control-like levels of PSII proteins, although also in these plants the D1 synthesis rate was decreased.1
We continued to explore the impact of OHP1 on the D1 synthesis rate and stability and exposed the VIGS-plants to light stress conditions: Leaf discs of VIGS-plants were excised and exposed to HL (850 µmol photons s−1 m−2) while floating on ultrapure water and being protected from the heat of the fluorescent lamps by a 5 cm water layer in a transparent container to prevent withering of the samples. Samples were harvested at the beginning as well as after 2, 6 and 24 hours of HL exposure and chlorophyll fluorescence was measured with a PAM imager after a 10 minute dark incubation (Figure 1(a)). Both VIGS-GFP and VIGS-OHP1 showed a similar Fv/Fm ratio at the beginning of the experiment, but in VIGS-OHP1 the Fv/Fm ratio decreased much faster upon HL exposure than in the VIGS-GFP control (Figure 1a, b)). After 24 hours of HL exposure the Fv/Fm ratio in both samples partially recovered, but still retained lower in VIGS-OHP1. To assess D1 levels, total proteins were separated by SDS-PAGE and analyzed by immunoblotting (Figure 1(c)). Whereas VIGS-GFP seedlings contained a stable amount of D1 during 24 hours of HL, D1 remarkably decreased after 6 hours of treatment in VIGS-OHP1 seedlings and remained at a low level until the end of the experiment. As reported earlier,1 the OHP2 content decreased in VIGS-OHP1 leaf samples, while the content of OHP2 and OHP1 slightly increased in control seedlings during HL stress (Figure 1(c)).
Figure 1.
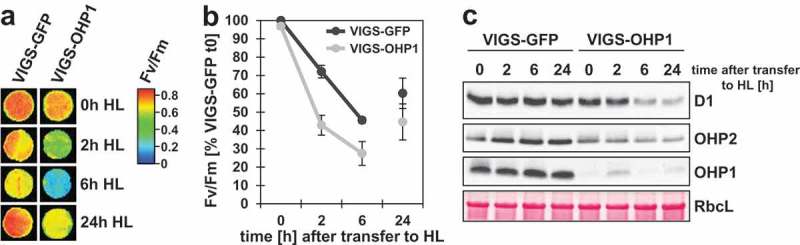
VIGS-OHP1 plants are susceptible to high light intensities.
(a) Measurement of Fv/Fm ratios in leaf discs of VIGS-GFP and VIGS-OHP1 plants after exposure to high light (850 µmol photons s−1 m−2). False colors indicate Fv/Fm ratios and numerical values of the color code are indicated. One representative leaf disc for each time-point is shown. (b) Numeric representation of Fv/Fm ratios displayed in A. Data are normalized to Fv/Fm in VIGS-GFP at time-point t0, which is set to 100%. Mean values and standard deviations of three biological replicates are shown. B, Western blot analysis of OHP variants and D1 from leaf discs of VIGS-GFP and VIGS-OHP1 plants exposed to HL as described above. Ponceau S staining of the large subunit of RuBisCO (RbcL) is shown as the loading control.
The increased OHP1 content during the short-term HL exposure is in line with previous reports indicating a HL-dependent induction of OHP1 expression.2,5 When re-monitoring the expression of OHP1 and OHP2 by RT-qPCR, the VIGS-GFP leaf discs showed twice the amount of both transcripts after 24 hours of HL (Figure 2). We compared this result with other known HL-inducible genes, here for example ELIP1, which encodes EARLY LIGHT-INDUCIBLE PROTEIN 1, another member of the LHC protein family. However, the ELIP1 transcripts accumulated more than 100-fold after 24 hours of HL exposure (Figure 2). In comparison, the twofold increased levels of the OHP1 transcript seemed to be marginal. Thus, it remains open whether the observed increased OHP content (Figure 1(c)) corresponds to increasing transcript levels or is caused by HL-triggered accumulation or stabilization of OHPs. A decrease of the Fv/Fm ratio upon HL exposure, which indicates a lower maximum quantum efficiency of PSII, was determined for both lines, VIGS-GFP and VIGS-OHP1 (Figure 1(a, b)). Generally, a decreasing Fv/Fm ratio counts for stress exposure, such as HL, and is caused by damage or inactivation of PSII, i.e. photoinhibition, or induced non-photochemical quenching.6 The faster decreasing Fv/Fm ratios of VIGS-OHP1 compared to those of VIGS-GFP seedlings indicate a higher susceptibility of PSII towards excessive amounts of light in seedlings with OHP deficiency. In-vivo labeling assays with 35S-methionine unveiled a lower synthesis rate of D1 in VIGS-OHP1,1 which is consequently also consistent with a strong decline of the D1 content after the HL periods (Figure 1(c)). In conclusion, impaired D1 recycling and/or lower stability correlate with the deficiency of OHP1 under HL conditions.
Figure 2.
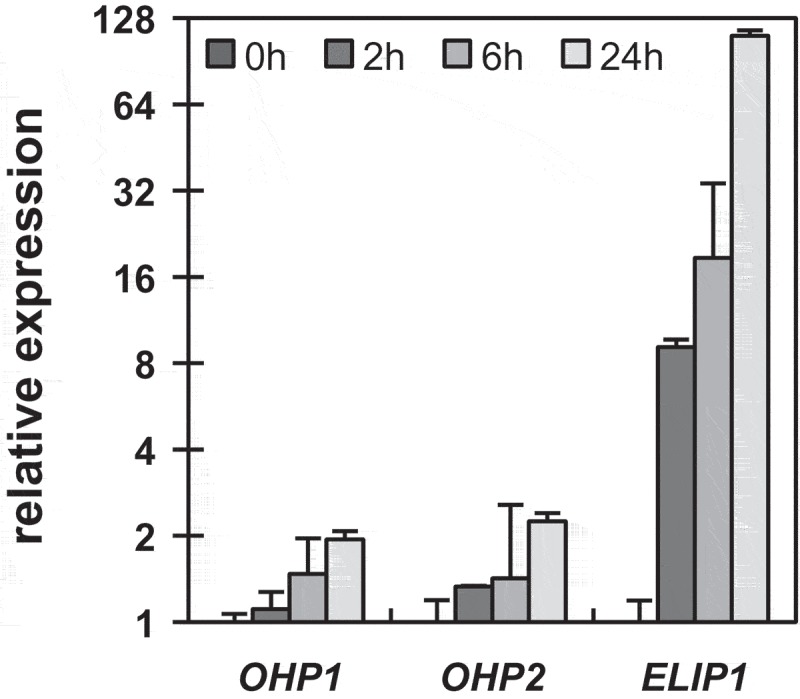
Light-induced expression of OHP transcripts.
RT-qPCR analysis of OHP1, OHP2 and ELIP1 transcripts in VIGS-GFP plants during exposure to HL. Data were analyzed by the ΔΔCt-method and expression was normalized to the first time-point (t0). A mean value of three biological replicates is shown, error bars represent standard deviation.
Besides the eukaryotic OHPs, the other members of the LHC superfamily possess one or two LHC-transmembrane helices. The cross-like configuration of these helices in the LHCPs and presumably also ELIPs contributes to the stability of the proteins.7 By analogy, the two OHP variants are proposed to interact with each other to allow binding of chlorophyll and/or precursors between the two transmembrane domains. It is proposed that the heterodimer formation is crucial for stability and function of both OHPs. Derived from the ohp1 and the HL-exposed VIGS-OHP1 phenotype, OHP1 is predicted to be indispensable for effective functioning of the OHP1-OHP2 heterodimer during early seedling development and in adult plants under light stress. The early developmental stage is characterized by a rapid assembly of the photosynthetic complexes in a vulnerable environment, which resembles a light-sensitive state. Light stress conditions in adult plants require a high rate of the D1 repair cycle.8 The complete set of (pigment supplying) auxiliary factors, including OHPs and HCF2449 might be essential for acclimation of plants at both conditions. Thus, the seedling-lethal phenotype of the ohp1 mutant and the stronger susceptibility of photosynthesis to HL-stress in the VIGS-OHP1 lines are explained with impaired action of the OHP heterodimer. Ultimately, the putative pigment-binding ability of the OHP-complex favors the hypothesis that the OHP1-OHP2 heterodimer formation is required to deliver chlorophyll for newly synthesized D1 proteins and to quench excitation energy of chlorophyll during a salvage pathway.10–13
Materials and methods
Plant material and growth conditions, vigs-assay
Arabidopsis wild-type plants (ecotype Col-0) were grown on soil in a growth chamber under long day conditions (16h photoperiod, 100µmol photons s−1 m−2, 20°C). The VIGS-assay was performed with 12-day-old seedlings as previously described.1
Light-stress assay
Leaf discs (7mm diameter) were excised from 5-week-old VIGS plants and incubated in HL (850 µmol photons s−1 m−2) for up to 24 hours floating on ultrapure water. To shield the discs from heat radiation of the lamps, a 5cm thick water basin was placed on top of the samples. Samples were harvested at indicated time-points.
Chlorophyll fluorescence measurements
Chlorophyll fluorescence kinetics of leaf discs were measured in a PAM-imager chamber (FluorCam 700MF, Photon Systems Instruments) after ten minutes of dark incubation. Fv/Fm ratios were normalized to time-point T0 of VIGS-GFP and displayed in per cent.
SDS-PAGE, Western blot
Total leaf protein extraction, SDS-PAGE, Western blotting and chemiluminescence detection was performed exactly as previously described.1
RNA extraction, RT-qPCR
Total RNAs were extracted by a citric acid based extraction method14 and cDNA-synthesis, reverse transcription and RT-qPCR was performed exactly as described before.1 Data were analyzed by the ΔΔCt-method.15
Funding Statement
This work was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) to B.G. (Gr936 14-1, subproject of the DFG-Research Unit FOR2092).
Abbreviations
GFPgreen fluorescent protein
HLhigh light
OHPone-helix protein
PAMpulse-amplitude modulation
PSphotosystem
RbcLlarge subunit of Ribulose-1,5-bisphosphate carboxylase/oxygenase
VIGSvirus-induced gene silencing
References
- 1.Hey D, Grimm B.. ONE-HELIX PROTEIN 2 (OHP2) is required for the stability of OHP1 and assembly factor HCF244 and is functionally linked to PSII biogenesis. Plant Physiol. 2018;177:1453–1472. doi: 10.1104/pp.18.00540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Myouga F, Takahashi K, Tanaka R, Nagata N, Kiss AZ, Funk C, Nomura Y, Nakagami H, Jansson S, Shinozaki K, et al. Stable accumulation of photosystem II requires ONE-HELIX PROTEIN1 (OHP1) of the light harvesting-like family. Plant Physiol. 2018;176:2277–2291. doi: 10.1104/pp.17.01782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck J, Lohscheider JN, Albert S, Andersson U, Mendgen KW, Rojas-Stütz MC, Adamska I, Funck D. Small one-helix proteins are essential for photosynthesis in Arabidopsis. Front Plant Sci. 2017;8:7. doi: 10.3389/fpls.2017.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson U. Light stress-induced one-helix protein of the chlorophyll a/b-binding family associated with photosystem I. Plant Physiol. 2003;132:811–820. doi: 10.1104/pp.102.019281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. doi: 10.1023/a:1006365213954. Jansson S, Andersson J, Kim SJ, Jackowski G. An Arabidopsis thaliana proteinhomologous to cyanobacterial high-light-inducible proteins. Plant Mol Biol. 2000;42(2):345–351. [DOI] [PubMed] [Google Scholar]
- 6.Murchie EH, Lawson T. Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. J Exp Bot. 2013;64:3983–3998. doi: 10.1093/jxb/ert208. [DOI] [PubMed] [Google Scholar]
- 7.Kühlbrandt W, Wang DN, Fujiyoshi Y. Atomic model of plant light-harvesting complex by electron crystallography. Nature. 1994;367:614–621. doi: 10.1038/367614a0. [DOI] [PubMed] [Google Scholar]
- 8.Aro EM, McCaffery S, Anderson JM. Photoinhibition and D1 protein degradation in Peas acclimated to different growth irradiances. Plant Physiol. 1993;103:835–843. doi: 10.1104/pp.103.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Komenda J, Sobotka R. Cyanobacterial high-light-inducible proteins - protectors of chlorophyll-protein synthesis and assembly. Biochim Biophys Acta 2016;1857:288–295. doi: 10.1016/j.bbabio.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Shukla MK, Llansola-Portoles MJ, Tichý M, Pascal AA, Robert B, Sobotka R. Binding of pigments to the cyanobacterial high-light-inducible protein HliC. Photosyn Res. 2018;137:29–39. doi: 10.1007/s11120-017-0475-7. [DOI] [PubMed] [Google Scholar]
- 11.Staleva H, Komenda J, Shukla MK, Šlouf V, Kaňa R, Polívka T, Sobotka R. Mechanism of photoprotection in the cyanobacterial ancestor of plant antenna proteins. Nat Chem Biol. 2015;11:287–291. doi: 10.1038/nchembio.1755. [DOI] [PubMed] [Google Scholar]
- 12.Lin YP, Endow JK, Dufour J, Zhu J, Inoue K. Analysis of an Arabidopsis heat-sensitive mutant reveals that chlorophyll synthase is involved in reutilization of chlorophyllide during chlorophyll turnover. Plant J. 2014;80:14–26. doi: 10.1111/tpj.12655. [DOI] [PubMed] [Google Scholar]
- 13.Komenda J, Sobotka R, Nitschke W, Baymann F, Neehaul Y, Hellwig P, Richers S, Vonck J, Bott M, Hunte C. Cyanobacterial high-light-inducible proteins - protectors of chlorophyll-protein synthesis and assembly. Biochim Biophys Acta. 2016;1857:288–295. doi: 10.1016/j.bbabio.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Onate-Sanchez L, Vicente-Carbajosa J, Stern AF, Byrd JR, Cherlin EJ, Wang Y, Yuan C, Nembhard I, Brush JE, Krumholz HM. DNA-free RNA isolation protocols for Arabidopsis thaliana, including seeds and siliques. BMC Res Notes. 2008;1:93. doi: 10.1186/1756-0500-1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucl Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]


