Abstract
Carbon-carbon bonds, including those between sp3-hybridized carbons (alkyl-alkyl bonds), typically comprise much of the framework of organic molecules. In the case of sp3-hybridized carbons, the carbon can be stereogenic, and the particular stereochemistry can have implications for structure and/or function1,2,3. As a consequence, the development of methods that simultaneously construct alkyl-alkyl bonds and control stereochemistry is a highly important, as well as a challenging, objective. Herein, a new strategy for enantioselective alkyl-alkyl bond formation is described, wherein a racemic alkyl electrophile is coupled with an olefin in the presence of a hydrosilane, through the action of a chiral nickel catalyst. Families of racemic alkyl halides, including tertiary substrates, that have not previously proved to be suitable for enantioconvergent couplings with alkylmetal nucleophiles are shown to cross-couple with olefins with good enantioselectivity and yield under very mild conditions. Given the ready availability of olefins, this new approach opens the door to the development of ever more general and powerful methods for enantioconvergent alkyl-alkyl coupling.
The transition-metal-catalyzed enantioconvergent cross-coupling of a readily available racemic secondary alkyl electrophile with an alkylmetal nucleophile has begun to emerge as an effective strategy for addressing this two-fold task (Figure 1, Previous approach); however, to date, methods that proceed with high enantioselectivity and good yield have been described for only a small fraction of the possible permutations of electrophiles and nucleophiles4,5,6,7. In view of this deficiency, the development of alternate strategies for achieving enantioconvergent substitution reactions of racemic electrophiles has the potential to have a very substantial impact on organic synthesis. In this report, we provide the first demonstration that the reductive coupling of a racemic alkyl halide with an olefin8,9 serves as a powerful complement to the previous approach (Figure 1, This study). Indeed, this new strategy has some noteworthy advantages, specifically: olefins are typically more attractive coupling partners than are alkylmetal reagents; the single catalyst described herein is more versatile than any single catalyst yet described for electrophile/nucleophile couplings, enabling for the first time highly enantioselective cross-couplings of racemic tertiary electrophiles, as well as a wide variety of secondary electrophiles; and, the coupling conditions are mild.
Figure 1|. Transition-metal-catalyzed enantioconvergent alkyl-alkyl cross-coupling reactions of racemic alkyl electrophiles.

R = carbon substituent; X = leaving group; M = metal; Y = R or H; ee = enantiomeric excess.
Carbonyl groups that bear an α stereocentre occur in a variety of bioactive compounds10,11. and as a result the development of methods to generate such stereocentres in highly enantioenriched form has been the focus of intense interest. While early efforts concentrated largely on the use of stoichiometric chiral auxiliaries to control the desired stereochemistry, recent studies have increasingly centred on asymmetric catalysis12,13. including enantioconvergent alkyl-alkyl cross-couplings (electrophile/nucleophile)14.
Racemic secondary α-haloamides, which bear an acidic proton, have not been reported to serve as suitable partners in enantioselective electrophile/nucleophile cross-couplings. We have determined that a chiral nickel/bis(oxazoline) catalyst achieves the enantioconvergent coupling of a variety of such amides with an array of olefins, providing the desired products with generally high enantiomeric excess (ee) and good yield (Figure 2a, entries 1-19). The observation of both high enantioselectivity and good yield (e.g., entry 1: 94% ee, 84% yield), when the racemic electrophile is the limiting reagent, establishes that both enantiomers of the electrophile are being converted into the enantioenriched product, i.e., this is an enantioconvergent reaction, not a simple kinetic resolution. From a practical point of view, it is noteworthy that the coupling proceeds in only slightly diminished yield (and with no loss in ee) when run under an atmosphere of air, in the presence of one equivalent of water, with less catalyst, or with less olefin (66-78% yield; Figure 2a, entry 1). Furthermore, the coupling can be carried out on a gram scale with similar efficiency (94% ee, 88% yield).
Figure 2|. Enantioconvergent alkyl-alkyl cross-couplings of racemic secondary alkyl electrophiles with olefins.

a, Secondary α-halocarbonyl compounds as electrophiles. b, Transformation into other families of enantioenriched compounds. c, Other families of electrophiles. d, Chain-walking and “directed” alkylation. e, Alkynes as coupling partners. aIodide as the leaving group. bReaction run in toluene at room temperature. cReaction run in toluene. dReaction run with 2 equiv NaI. eReaction run with 0.5 equiv (n-Bu)4NI.
The scope of this enantioconvergent cross-coupling is fairly broad with respect to the R substituent on the α carbon of the electrophile (Figure 2a, entries 1-4) and on the olefin (entries 5-14), providing the desired alkyl-alkyl coupling product in good ee and yield. A diverse array of functional groups, including an unactivated primary alkyl chloride and bromide, an acetal, an ester, a carbamate, a boronate ester, and an ether, are compatible with the reaction conditions (Figure 2a), as is an alcohol, an aldehyde, an aryl iodide, a benzofuran, an epoxide, an indole, a ketone, a secondary amine, and a thioether (see the Supporting Information). The stereochemistry of the chiral catalyst, rather than existing stereocentres on the olefin coupling partner, predominantly determines the stereochemistry of the coupling products in entries 11-14.
The scope with respect to the electrophile is not limited to changes in the α substituent of the original secondary amide (Figure 2a, entries 1-4); the carbonyl group can also be altered (entries 15-26). Thus, the same chiral nickel/bis(oxazoline) catalyst effects the cross-coupling of an array of racemic secondary amides (entries 15-19), tertiary amides (entries 20 and 21), lactams (entries 22-24), and esters (entries 25 and 26) with generally good enantiomeric excess and yield; other than tertiary amides14, none of these families of electrophiles have previously been shown to participate in enantioconvergent alkyl-alkyl couplings with an alkylmetal reagent as the nucleophile. In the case of the chiral amide illustrated in entries 18 and 19, the stereochemistry of the catalyst again primarily dictates the stereochemistry α to the carbonyl group in the cross-coupling product. The products of these enantioconvergent alkyl-alkyl couplings can be converted in a single step without significant racemization to other important families of enantioenriched compounds, including aryl and alkyl ketones, amines, alcohols, and carboxylic acids (Figure 2b, entries 27-31).
Furthermore, this chiral nickel/bis(oxazoline) catalyst can achieve enantioconvergent cross-couplings of substantially different families of racemic alkyl electrophiles to generate highly enantioenriched target structures. Fluorinated compounds are a class of molecules of substantial and growing interest in medicinal chemistry15. When the alkyl substituent of an α-halocarbonyl electrophile is replaced with a fluoro substituent, the catalyst effects selective substitution of the bromide in the presence of the fluoride, providing the desired product with good enantioselectivity (Figure 2c, entry 32: 89% ee). Furthermore, the catalyst can achieve asymmetric alkyl-alkyl bond formation not only when the bromide leaving group is α to a carbonyl group, but also when it is β (entry 33). Finally, the carbonyl group can be removed entirely (entries 34 and 35). Thus, the potential of this new approach to enantioconvergent alkyl-alkyl couplings of racemic alkyl electrophiles is manifest from the fact that, when alkylmetal reagents have been used as the cross-coupling partner, no single chiral catalyst has previously been shown to be effective for such a diverse range of secondary alkyl electrophiles4,5.
Interestingly, in the case of a 1,2-disubstituted olefin, the nickel catalyst is able to chain-walk to achieve exclusive n-alkylation (Figure 2d, entry 3616,17; for a mechanistic discussion, see below). On the other hand, the presence of a suitably positioned directing group18,19 can reverse the catalyst’s general preference for n-alkylation, leading primarily to the branched product (entry 37).
One of the most significant remaining challenges in the field of enantioconvergent alkyl-alkyl cross-couplings is the development of methods to couple racemic tertiary electrophiles with high enantioselectivity and yield to generate quaternary stereocentres20; success to date has largely been restricted to the use of enolate nucleophiles and/or allylic coupling partners21,22,23. In view of the limited progress in addressing this objective, we were pleased to determine that the same nickel/bis(oxazoline) catalyst that is effective for enantioconvergent couplings of racemic secondary alkyl halides (Figure 2) can be applied to corresponding reactions of tertiary electrophiles, specifically, α-halo-β-lactams, affording the desired products in excellent ee and generally good yield (Figure 3a). To the best of our knowledge, such a cross-coupling of a tertiary alkyl electrophile with an olefin has not previously been described, even to generate racemic product.
Figure 3|. Enantioconvergent alkyl-alkyl cross-couplings of racemic tertiary alkyl electrophiles with olefins.
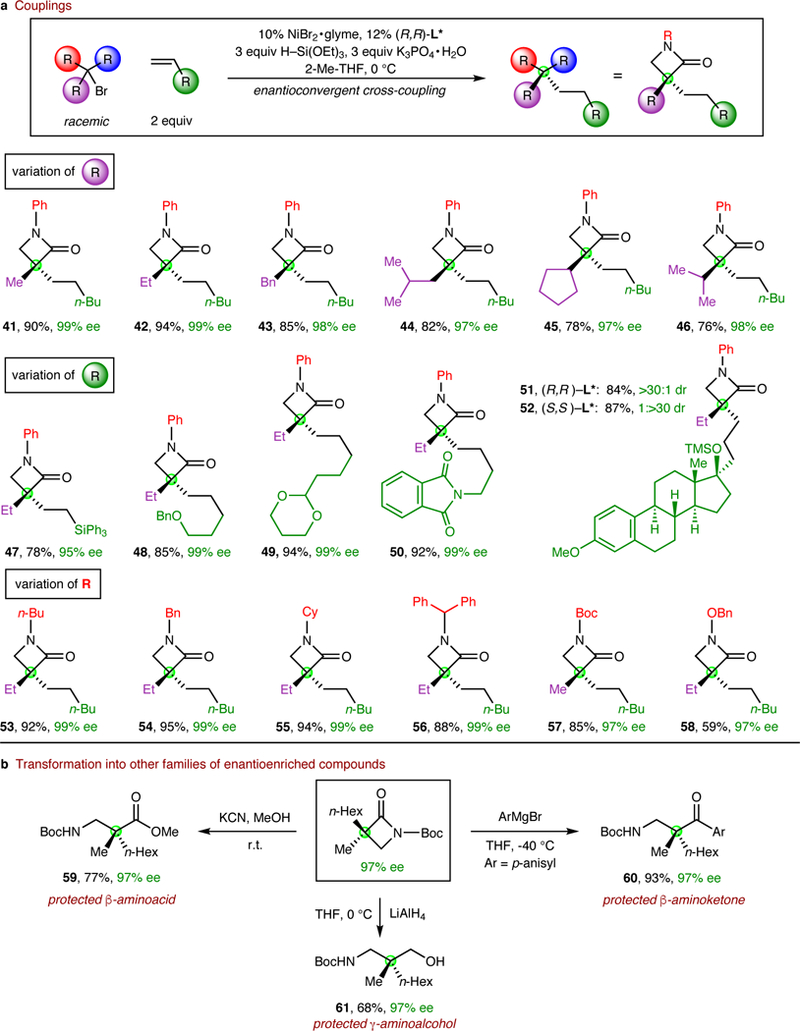
a, Couplings. b, Transformation into other families of enantioenriched compounds.
As illustrated in Figure 3a, high ee is observed in enantioconvergent alkyl-alkyl couplings of tertiary electrophiles that bear a variety of substituents. For example, the α-alkyl group of the racemic α-halo-β-lactam can range in steric demand from methyl to isopropyl (entries 41-46). A wide array of terminal olefins serve as suitable coupling partners, including substrates that contain a silane, an ether, an acetal, an imide, and a steroid subunit (entries 47-52). Furthermore, excellent enantioselectivity is observed regardless of whether the substituent on the nitrogen of the β-lactam is an aryl, alkyl, Boc, or alkoxy group (entries 53-58).
β-Lactams, including compounds that bear an α quaternary centre, are important not only as endpoints24,25,26,27, but also as intermediates in asymmetric synthesis28. Thus, β-aminoacids and β- aminoketones, as well as γ-aminoalcohols, all of which are significant targets due to their occurrence as subunits in bioactive compounds (e.g., dexmethylphenidate, tolperisone, and propranolol), can be generated from β-lactams without loss of ee (Figure 3b, entries 59-61).
We have begun to investigate the mechanism of this new catalytic enantioconvergent alkyl-alkyl cross-coupling process. One possible pathway is that the olefin undergoes nickel-catalyzed hydrosilylation29 and that the resulting alkylsilane serves as a nucleophile in a conventional electrophile/nucleophile (Hiyama-type) cross-coupling. To test this possibility, we have subjected the putative alkylsilane to the reaction conditions, and we have observed essentially no cross-coupling, which rules out this pathway (Figure 4a).
Figure 4|. Mechanism.
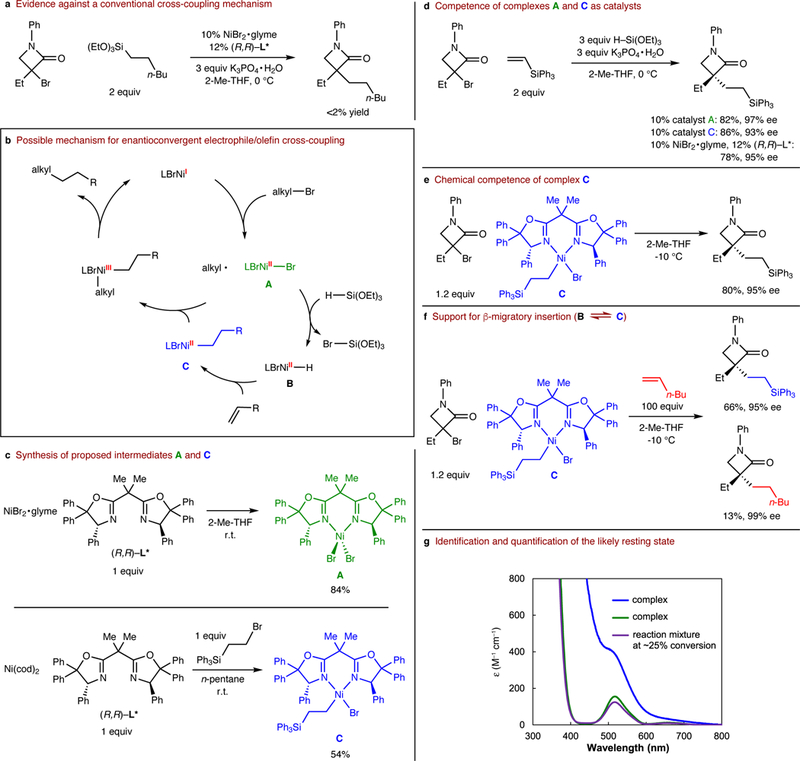
a, Evidence against a conventional cross-coupling mechanism. b, Possible mechanism for enantioconvergent electrophile/olefin cross-coupling (for the sake of simplicity, all steps are drawn as irreversible; H-Si(OEt)3 represents the hydrosilane activated by K3PO4•H2O). c, Synthesis of proposed intermediates A and C. d, Competence of complexes A and C as catalysts. e, Chemical competence of complex C. f, Support for β-migratory insertion. g, Identification and quantification of the likely resting state via UV-vis spectroscopy (coupling partners illustrated in d, using10% NiBr2•glyme/12% (R,R)-L*, at ∼25% conversion).
Our current hypothesis is that enantioconvergent electrophile/olefin cross-coupling proceeds through the pathway illustrated in Figure 4b, which builds on a mechanistic study of an enantioconvergent electrophile/nucleophile cross-coupling30. Thus, LBrNiII-Br (A) reacts with the hydrosilane to generate a nickel-hydride complex (LBrNiII-H, B). Olefin complexation followed by β-migratory insertion then provides a nickel-alkyl complex (LBrNiII-CH2CH2R, C), which enters the reaction cycle for electrophile/nucleophile cross-coupling.
Using EPR spectroscopy, we have examined a cross-coupling in progress, and we have determined that the reaction mixture is EPR-silent, which is consistent with the resting state of the catalytic cycle being one or more nickel(II) complexes such as A-C, rather than nickel(I) and nickel(III) complexes (Figure 4b). We have independently synthesized nickel(II) complexes A and C (Figure 4c), and we have crystallographically characterized complex A.
Our studies of complexes A and C are consistent with the mechanism illustrated in Figure 4b. When used in place of NiBr2•glyme/(R,R)-L*, both complexes furnish the cross-coupling product with ee and yield that are similar to the catalyst generated in situ under our standard conditions (Figure 4d). Furthermore, nickel-alkyl complex C is chemically competent, reacting with an electrophile in a stoichiometric coupling to provide the product with the expected enantioselectivity and in good yield (Figure 4e; this process is inhibited by the addition of TEMPO, a radical trap31); we anticipate that a Ni(I) complex (generated by reductive elimination of Ni(II) → Ni(0), followed by comproportionation with Ni(II) to form Ni(I)) serves as an initiator of this reaction.30 When this reaction is conducted in the presence of an olefin (1-hexene), a mixture of two alkylation products is observed (Figure 4f), which supports the accessibility of nickel hydride B and its β-migratory insertion, key aspects of the proposed mechanism (Figure 4b). Finally, analysis via ultraviolet-visible (UV-vis) spectroscopy of a coupling reaction in progress is consistent with the suggestion that ∼80% of the nickel in the reaction mixture is present as complex A (Figure 4g; no evidence for complex C).
In summary, we have described a new strategy for enantioconvergent alkyl-alkyl cross-couplings, specifically, nickel-catalyzed couplings of racemic secondary and tertiary alkyl electrophiles with olefins. This approach does not merely complement the traditional electrophile/nucleophile approach for achieving such transformations, since electrophile/olefin cross-couplings can have significant advantages arising from the ready availability of olefins, broader scope, and milder conditions. Furthermore, we anticipate that this strategy will be applicable to enantioconvergent cross-couplings with a variety of unsaturated compounds other than olefins (e.g., Figure 2e), thereby opening the door to a wide array of powerful new methods for asymmetric catalysis.
Supplementary Material
Acknowledgements
Support has been provided by the National Institutes of Health (National Institute of General Medical Sciences, R01-GM62871) and the Gordon and Betty Moore Foundation (Caltech Center for Catalysis and Chemical Synthesis). We thank L. M. Henling, D. G. VanderVelde, and S. C. Virgil for assistance and helpful discussions.
Footnotes
Author Information. The authors declare no competing financial interests.
Supplementary Information is available in the online version of the paper.
Data Availability. The data that support the findings of this study are available within the paper, in the Supplementary Information (experimental procedures and characterization data), and from the Cambridge Crystallographic Data Centre (crystallographic data are available free of charge under reference CCDC 1822790-1822793, 1839344-1839346, and 1861568).
References
- 1.Carreira EM & Yamamoto H, Eds. Comprehensive Chirality (Academic, 2012). [Google Scholar]
- 2.Lin G-Q, You Q-D & Cheng J-F, Eds. Chiral Drugs: Chemistry and Biological Action (Wiley, 2011). [Google Scholar]
- 3.Lovering F, Bikker J & Humblet C Escape from flatland: Increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Choi J & Fu GC Transition metal-catalyzed alkyl-alkyl bond formation: Another dimension in cross-coupling chemistry. Science 356, eaaf7230 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu GC Transition-metal catalysis of nucleophilic substitution reactions: A radical alternative to SN1 and SN2 processes. ACS Cent. Sci. 3, 692–700 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwasaki T & Kambe N Coupling reactions between sp3-carbon centers. Comprehensive Organic Synthesis 3, 337–391 (2014). [Google Scholar]
- 7.Geist E, Kirschning A & Schmidt T sp3-sp3 Coupling reactions in the synthesis of natural products and biologically active molecules. Nat. Prod. Rep. 31, 441–448 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Lu X et al. Practical carbon-carbon bond formation from olefins through nickel-catalyzed reductive olefin hydrocarbonation. Nat. Commun. 7, 11129 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y-M, Bruno NC, Placeres AL, Zhu S & Buchwald SL Enantioselective synthesis of carbo- and heterocycles through a CuH-catalyzed hydroalkylation approach. J. Am. Chem. Soc. 137, 10524−10527 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tobert JA Lovastatin and beyond: The history of the HMG-CoA reductase inhibitors. Nat. Rev. Drug Discov. 2, 517–526 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Ganellin CR Discovery of the cholesterol absorption inhibitor, ezetimibe In Introduction to Biological and Small Molecule Drug Research and Development, Ganellin CR, Roberts SM & Jefferis R, Eds. (Elsevier, 2013) pp. 339–416. [Google Scholar]
- 12.Stoltz BM et al. Alkylations of enols and enolates. Comprehensive Organic Synthesis 3, 1–55 (2014). [Google Scholar]
- 13.MacMillan DWC & Watson AJB α-Functionalization of carbonyl compounds. Science of Synthesis: Stereoselective Synthesis 3, 675–745 (2011). [Google Scholar]
- 14.Fischer C & Fu GC Asymmetric nickel-catalyzed Negishi cross-couplings of secondary α-bromo amides with organozinc reagents. J. Am. Chem. Soc. 127, 4594–4595 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Gouverneur V & Müller K Fluorine in Pharmaceutical and Medicinal Chemistry (Imperial College Press, 2012). [Google Scholar]
- 16.Juliá-Hernández F, Moragas T, Cornella J & Martin R Remote carboxylation of halogenated aliphatic hydrocarbons with carbon dioxide. Nature 545, 84–88 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Zhou F, Zhu J, Zhang Y & Zhu S NiH-Catalyzed Reductive Relay Hydroalkylation: A Strategy for the Remote C(sp3)-H Alkylation of Alkenes. Angew. Chem. Int. Ed. 57, 4058–4062 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Hoveyda AH, Evans DA & Fu GC Substrate-Directable Chemical Reactions. Chem. Rev. 93, 1307–1370 (1993). [Google Scholar]
- 19.Derosa J, Tran VT, Boulous MN, Chen JS & Engle KM Nickel-Catalyzed β,γ-Dicarbofunctionalization of Alkenyl Carbonyl Compounds via Conjunctive Cross-Coupling. J. Am. Chem. Soc. 139, 10657–10660 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Quasdorf KW & Overman LE Catalytic enantioselective synthesis of quaternary carbon stereocentres. Nature 516, 181–191 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding C-H & Hou X-L Nucleophiles with allyl metal complexes. Comprehensive Organic Synthesis 4, 648–698 (2014). [Google Scholar]
- 22.Murakata M, Jono T, Mizuno Y & Hoshino O Construction of chiral quaternary carbon centers by catalytic enantioselective radical-mediated allylation of α-iodolactones using allyltributyltin in the presence of a chiral Lewis acid. J. Am. Chem. Soc. 119, 11713–11714 (1997). [Google Scholar]
- 23.Ma S, Han X, Krishnan S, Virgil SC & Stoltz BM Catalytic enantioselective stereoablative alkylation of 3-halooxindoles: Facile access to oxindoles with C3 all-carbon quaternary stereocenters. Angew. Chem. Int. Ed. 48, 8037–8041 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Banik BK, Ed. β-Lactams: Unique Structures of Distinction for Novel Molecules (Springer, 2013). [Google Scholar]
- 25.Decuyper L et al. Antibacterial and β-lactamase inhibitory activity of monocyclic β-lactams. Med. Res. Rev. 38, 426–503(2018). [DOI] [PubMed] [Google Scholar]
- 26.Galletti P & Giacomini D Monocyclic β-lactams: New structures for new biological activities. Curr. Med. Chem. 18, 4265–4283 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Chrusciel RA et al. Therapeutic compounds and compositions. WO Patent 2015/120062 A2, August 13, 2015. [Google Scholar]
- 28.Ojima I, Zuniga ES & Seitz JD Advances in the use of enantiopure β-lactams for the synthesis of biologically active compounds of medicinal interests In β-Lactams: Unique Structures of Distinction for Novel Molecules, Banik BK, Ed. (Springer, 2013), pp. 1–64. [Google Scholar]
- 29.Du X & Huang Z Advances in base-metal-catalyzed alkene hydrosilylation. ACS Catal. 7, 1227–1243 (2017). [Google Scholar]
- 30.Schley ND & Fu GC Nickel-catalyzed Negishi arylations of propargylic bromides: A mechanistic investigation. J. Am. Chem. Soc. 136, 16588–16593 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henry-Riyad H et al. 2,2,6,6-Tetramethylpiperidin-1-oxyl In Handbook of Reagents for Organic Synthesis, Fuchs PL, Ed. (Wiley, 2013,) pp. 620–626. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


