Abstract
Thyroid hormone (TH) is critical for vertebrate postembryonic development, a period around birth in mammals when plasma TH levels are high. Interestingly, TH receptors (TRs), especially TRα, are expressed prior to the synthesis and secretion of zygotic TH, suggesting the existence of unliganded TR during development. However, the role of unliganded TR during mammalian development has been difficult to study, in part due to the relatively weak phenotype of TR knockout mice. Amphibian metamorphosis resembles postembryonic development in mammals and is controlled by TH via TRs. Like in mammals, TRα gene is highly activated and is the major TR expressed prior to the synthesis of endogenous TH. By using TALEN (transcriptional activator like effector nucleases)-mediated gene editing approach, we and others have now shown that unliganded TRα has two independent functions during Xenopus premetamorphosis, i.e. inhibiting growth rate and slowing development. Furthermore, molecular and transgenic studies have shown that unliganded TRα accomplishes these via the recruitment of histone deacetylase (HDAC)-containing corepressor complexes to repress the expression of TH-inducible genes.
Keywords: amphibian metamorphosis, de-repression, thyroid hormone receptor, transcriptional regulation, Xenopus laevis, Xenopus tropicalis
Introduction
Thyroid hormone (TH) is not only important for development and maturation of many organs/tissues in vertebrate but also affects their function in the adult (Hetzel 1989; Lazar 1993; Tata 1993; Atkinson 1994; Freake & Oppenheimer 1995; Silva 1995; Franklyn & Gammage 1996; Shi 1999; Yen 2001). During mammalian development, the plasma levels of TH are high during the period around birth, also referred to as postembryonic development, when many organs such as the brain and intestine mature into the adult form (Hetzel 1989; Tata 1993; Shi 1999; Howdeshell 2002; Ishizuya-Oka & Shi 2011; Hasebe et al. 2013).
The dependence of mammalian embryos on the mother both before birth and during the neonatal period has made it difficult to study how TH signaling affects mammalian development. On the other hand, amphibian metamorphosis, which mimics the postembryonic development in mammals, is totally dependent on TH but independent of maternal influence. This and the ability to easily manipulate this process by controlling the availability of TH to the tadpoles offer an excellent opportunity to study how TH regulates postembryonic vertebrate development.
Thyroid hormone can both regulate gene expression through nuclear TH receptors (TRs) and influence cell behavior through cell surface and cytoplasmic binding protein via the so-called non-genomic pathways (Evans 1988; Lazar 1993; Tsai & O’malley 1994; Davis & Davis 1996; Yen 2001; Buchholz et al. 2006). Two highly conserved TR genes, TRα and TRβ, exist in all vertebrates (Laudet & Gronemeyer 2002). For TH inducible genes, TRs mainly bind to TH response elements (TREs) as heterodimers formed with 9-cis-retinoic acid receptors (RXRs) in/around the promoters of target genes even in the context of chromatin and repress their expression in the absence of TH. TH-binding to TR leads to chromatin disruption and the activation of these promoters (Wong et al. 1995, 1997, 1998; Sachs & Shi 2000; Hsia et al. 2001; Hsia & Shi 2002; Buchholz et al. 2005; Matsuura et al. 2012; Shi et al. 2012; Sun et al. 2014).
Expression studies have shown that zygotic expression of TR, especially TRα, occurs well before the synthesis and secretion of endogenous TH. In Xenopus laevis and Xenopus tropicalis, both TRα and RXRα genes are expressed at high levels by stage 45 when tadpole feeding begins while plasma TH is detectible only by stage 54, the onset of metamorphosis (Leloup & Buscaglia 1977; Yaoita & Brown 1990; Wong & Shi 1995; Wang et al. 2008). This and other studies have led to a dual function model for TR during frog development. Over the years, a number of laboratories have provided conclusive evidence in support of this model. Here, we will review some of these studies, with a focus on the role of unliganded TRα during premetamorphosis.
Mechanisms of TR function and the dual function model during frog development
Extensive biochemical, molecular, and cell culture studies have shown that TR regulates transcription by recruiting cofactors. For TH-inducible genes, unliganded TR recruits corepressor complexes, such as those containing histone deacetylase HDAC3 and the highly related TR-binding proteins N-CoR and SMRT (Burke & Baniahmad 2000; Glass & Rosenfeld 2000; Guenther et al. 2000; Li et al. 2000b; Zhang & Lazar 2000; Jones et al. 2001; Zhang et al. 2002; Ishizuka & Lazar 2003; Jones & Shi 2003; Yoon et al. 2003; Shi et al. 2012). This leads to local histone deacetylation and transcriptional inhibition (Fig. 1). In the presence of TH, liganded TR releases corepressor complexes and binds coactivator complexes to activate gene transcription. Many TR-coactivators form large complexes that are involved in chromatin disruption/remodeling and/or histone modifications. The best studied among them are SRC (steroid receptor coactivator)-1, 2, and 3, which complex with other proteins including CBP/p300 and protein arginine methyltransferase 1 (PRMT1) (Onate et al. 1995; Wong et al. 1995, 1997; Chen et al. 1997, 1999; Torchia et al. 1997; Li et al. 2000a; Zhang & Lazar 2000; Ito & Roeder 2001; Koh et al. 2001; Mckenna & O’malley 2001; Rachez & Freedman 2001; Sheppard et al. 2001; Yen 2001; Demarest et al. 2002; Huang et al. 2003; Matsuda et al. 2007, 2009; Heimeier et al. 2008; Matsuura et al. 2012; Shi et al. 2012).
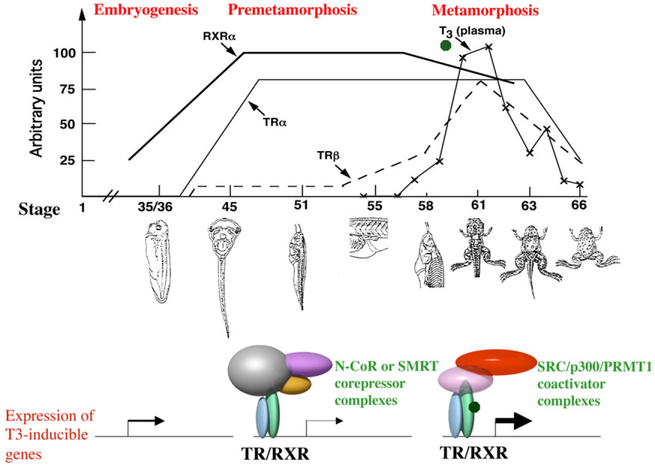
Fig. 1.
A model for the regulation of frog development by thyroid hormone (TH) and TH receptors (TRs). Top: Correlation of the levels of endogenous TH and mRNAs of TRα, TRβ and 9-cis-retinoic acid receptor (RXR) α genes with development. The embryos hatch around stage 35 and tadpole feeding begins at around stage 45 (Nieuwkoop & Faber 1956). The plasma concentrations (arbitrary units) of T3 are from (Leloup & Buscaglia 1977) (T3 or 3, 5, 3’-triiodothyronine is one of the two natural THs. The other one T4 or 3, 5, 3’, 5’-tetraiodothyronine, also known as thyroxine, binds to TR with weaker affinity). The mRNA levels for TRα (solid line), TRβ (broken line) and RXRα (bold line) are based on published data (Yaoita & Brown 1990; Wong & Shi 1995; Wang et al. 2008) and for clarity, plotted on arbitrary scales. Note that there are few morphological changes during premetamorphosis (stage 45–54) when there is little TH in the plasma. Metamorphosis begins after stage 54 (prometamorphosis: stage 54–58) and most dramatic changes occur at climax 58–66). Bottom: A molecular mechanism for the regulation of TH-inducible genes during development. The genes are expressed at the basal levels during embryogenesis when the levels of TH and TRs are low. After tadpole feeding begins at stage 45, TRα and RXRα expression increases dramatically. The resulting TR/RXR heterodimers bind to the TREs in the target genes and recruit corepressor complexes to repress gene expression prior to the synthesis of endogenous TH around stage 54. Subsequently, the rising TH concentration causes the release of the corepressor complexes and recruitment of coactivator complexes to the promoters due to TH binding to TR. This leads to the activation of TH-inducible genes and metamorphosis. Note that there are many different types of corepressor and coactivator complexes that can associate with TR and only exemplary ones are shown here.
Like in mammals, there are two TR genes, TRα and TRβ, in Xenopus laevis and Xenopus tropicalis (Yaoita & Brown 1990; Wang et al. 2008). There is TRβ mRNA expressed during early development, while the expression of TRα and RXRα is activated around hatching (stage 35) and reaches high levels by stage 45 (Fig. 1) (Yaoita & Brown 1990; Kanamori & Brown 1992; Wang et al. 2008), the onset of tadpole feeding (Nieuwkoop & Faber 1956). Based on this and the molecular mechanism of TR action, we have previously proposed a dual function model for TR during the frog development (Fig. 1) (Shi et al. 1996; Sachs et al. 2000). That is, TH-inducible genes are repressed by unliganded TR in the form of TRα /RXRα heterodimers by the end of embryogenesis when a free feeding tadpole is formed at stage 45. This is accomplished through the recruitment of corepressor complexes containing HDACs to ensure proper tadpole growth and prevents premature metamorphic organ transformations, thus regulating the timing of metamorphosis. After stage 54, the availability of endogenously synthesized TH leads the release of the corepressor complexes and concurrent recruitment of the coactivator complexes. The coactivator complexes in turn remodel chromatin and/or modify histones via acetylation and methylation, etc., to activate the genes, leading to tadpole metamorphosis (Fig. 1).
TR is necessary and sufficient for metamorphosis
A prerequisite of the dual function model above is that TR plays an essential role in metamorphosis. Indeed, a number of in vivo studies, including transgenic analyses with mutant TRs have provided strong evidence to support this (Puzianowska-Kuznicka et al. 1997; Sachs & Shi 2000; Schreiber et al. 2001; Das et al. 2002; Buchholz et al. 2003, 2004, 2005; Nakajima & Yaoita 2003; Schreiber & Brown 2003; Hasebe et al. 2011). In particular, overexpression of a dominant negative TR that lacks TH binding ability but retains the ability to heterodimerize with RXR and repress gene expression inhibits not only TH-induced gene activation but more importantly also the metamorphic transformations in all organs/tissues of Xenopus laevis tadpoles (Schreiber et al. 2001; Das et al. 2002; Buchholz et al. 2003; Nakajima & Yaoita 2003; Schreiber & Brown 2003). Conversely, premetamorphic tadpoles transgenic for a dominant positive TR under the control of a heat shock-inducible promoter undergo metamorphosis upon heat shock treatment even in the absence of TH (Buchholz et al. 2004; Hasebe et al. 2011). This is accompanied by the specific activation of TH-inducible genes. Thus, TR is not only necessary but also sufficient to mediate the effects of TH during Xenopus metamorphosis.
A role of TRα in regulating both metamorphic rate and timing
A critical test for the dual function model is to demonstrate a role of endogenous TR during Xenopus development. While it is possible to knockdown the expression of endogenous genes during metamorphosis by using antisense morpholino oligonucleotides (Matsuda & Shi 2010), such studies are difficult to carry out and the knockdown is often very limited. The recent development of TALEN and CRISPR-nuclease mediated knockout/knockdown has essentially revolutionized genetic studies of postembryonic development, especially in amphibians where there had been no effective knockout strategy (Lei et al. 2012, 2013; Blitz et al. 2013; Nakayama et al. 2013; Guo et al. 2014; Wang et al. 2015; Wen et al. 2015). Making use of the TALEN technology, we and the Buchholz’s laboratory recently investigated the function of endogenous TRα during Xenopus tropicalis development (Choi et al. 2015; Wen & Shi 2015; Yen 2015). Both groups independently developed a TALEN nuclease targeting TRα and introduced the nucleases into fertilized eggs by microinjecting the corresponding mRNAs. We observed that injecting into fertilized egg prior to the first cell division, mRNAs encoding the two arms of the TRα TALEN nuclease, but not when either TALEN arm was replaced with a non-specific TALEN arm, led to genetic alterations at the TRα target site with about 90% efficiency in the resulting animals, enabling the phenotypic analysis with the resulting F0-generation animals (Wen & Shi 2015). A different approach was taken by the Buchholz’s group (Choi et al. 2015). They coinjected mRNAs encoding the TALEN nuclease and mCherry into one cell of the two-cell stage embryos. This enabled them to observe the effect of the knockdown in one side of the embryo when compared to the other, wild type side. Both groups observed that TRα knockdown led to precocious limb development (Fig. 2B). The Buchholz’s group further confirmed this phenotype by analyzing F1 animals produced from mating two F0 animals generated from the mRNA injection, where they observed that only when both copies of the TRα gene were mutated, were the limbs developed precociously (Choi et al. 2015).
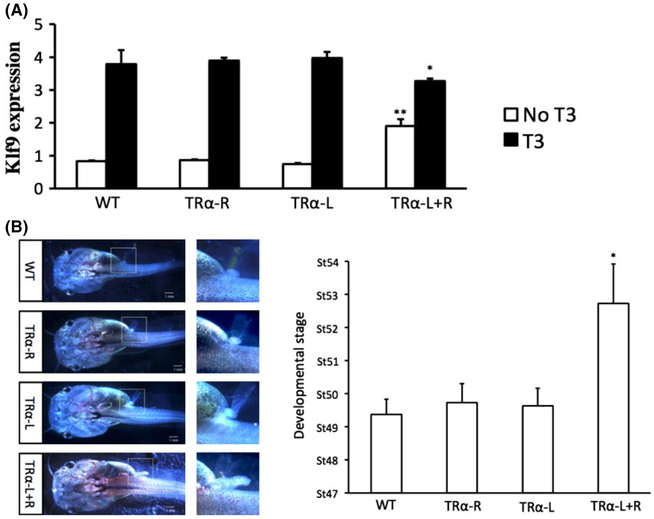
Fig. 2.
(A) Thyroid hormone (TH) receptor α (TRα) knockdown increases the expression of the TH-inducible gene Klf9 in premetamorphic tadpoles but reduces its induction by TH treatment of Xenopus tropicalis tadpoles. TRα was knocked down by injecting mRNAs encoding TALEN left (L) and right (R) arms into fertilized eggs. WT: no TALEN mRNA injection, TRα-R: TRα TALEN-R and control-L mRNAs injected, TRα-L: TRα TALEN-L arm and control-R mRNAs injected, and TRα-L+R:TRα TALEN-L and -R mRNAs injected. Premetamorphic tadpoles were treated with 10 nmol/L TH for 18 h and total RNA was isolated for reverse transcription-polymerase chain reaction (RT–PCR) analysis for the expression of Klf9. Note that its expression was higher in the TRα TALEN injected tadpoles in the absence of TH but lower in the presence of TH, respectively, when compared to the WT and control groups, suggesting that both the repression by unliganded TR and activation by TH-bound TR were reduced when TRα was knocked down. ** and * indicate significant difference between the TRα knockdown group compared to all three other groups (**P < 0.01, *P < 0.05). See (Wen & Shi 2015) for details. (B) TRα knockdown accelerates premetamorphic development. The animals were staged at the age of 20 days post-fertilization. Left panel: Representative animals with the area in dashed box magnified to show the hindlimb size and morphology. Note the more developed hindlimb for the TRα knockdown animal. Right panel shows the average developmental stages of the animals with different TALEN mRNA injections. Note that the TRα TALEN-injected group developed on average 3.5 stages more advanced compared to the other three groups. *Indicates significant difference between the TRα knockdown group compared to all three other groups (P < 0.05). See (Wen & Shi 2015) for details.
Limb morphology is the standard criterion for staging tadpoles between stage 48, shortly after the onset of feeding, and stage 54, the onset of metamorphosis (Nieuwkoop & Faber 1956). By staging the knockdown animals, both group found that TRα knockdown accelerated premetamorphic tadpole development by a few stages (Fig. 2C) (Choi et al. 2015; Wen & Shi 2015). Importantly and consistent with the dual function model, the expression of several well known TH-inducible genes were de-repressed (Fig. 2A). Furthermore, the knockdown/knockout animals were resistant to treatment of exogenous TH in terms of both morphological changes and the regulation of TH response genes (Choi et al. 2015; Wen & Shi 2015). In addition, when the knockdown animals were allowed to undergo natural metamorphosis, it was found that TRα knockdown drastically slowed down natural metamorphosis as measured by the time required for the animals to advance from stage 54, the onset of metamorphosis, to stage 58, the early metamorphic climax (Wen & Shi 2015). Thus, unliganded TRα indeed plays a critical role in regulating the timing of metamorphosis initiation, while when TH is present, TRα also regulates the rate of metamorphosis progression. This finding also complements earlier studies showing that TR overexpression induces precocious cell death of tadpole tail muscle and thus accelerates metamorphosis (Okada et al. 2012).
A novel role of unliganded TR in regulating premtamorphic tadpole growth
A surprising finding of the above studies is that TRα knockdown tadpoles grow faster during premetamorphosis, with the knockdown ones larger when compared to age-matched wild type siblings (Fig. 3A,B) (Wen & Shi 2015). Interestingly, the two growth hormone (GH) genes are found to be upregulated in the knockdown animals. In mammals, GH is activated at the transcriptional level by TH (Santos et al. 1987; Kanamori & Brown 1992). Thus, it is likely that GH is similarly regulated, i.e. repressed by TR in the absence of TH in premetamorphic Xenopus tropicalis tadpoles, and TRα knockdown thus leads to de-repression of GH genes, thereby causing faster growth of the premetamorphic tadpoles.
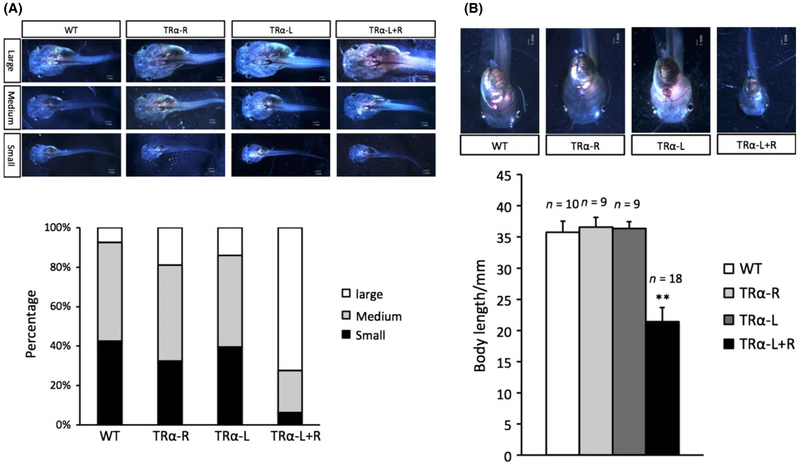
Fig. 3.
(A) Thyroid hormone (TH) receptor α (TRα) knockdown tadpoles have faster growth during premetamorphosis in Xenopus tropicalis. Tadpoles with different TALEN mRNA injections as in Figure 2 were classified in three categories: large size, medium size, and small size (top panel), at the age of 20 days post-fertilization and the percentage in each category is shown in a bar graph (bottom panel). (B) TRα knockdown tadpoles are smaller than the wild type siblings at the onset of prometamorphosis (stage 54). Top: Representative photos of stage 54 (the onset of metamorphosis) tadpoles. Tadpoles were staged based on hindlimb morphology. All animals are at the same stage but are of different ages. Bottom: The average size (body length) of the animals at stage 54 for different groups. The letter n indicates the number of tadpoles for each group. Tadpole body length was measured from jaw to tail tips. **Indicates significant difference between the TRα knockdown group compared to all three other groups (P < 0.05). See Fig. 2 and (Wen & Shi 2015) for details.
The fact that TRα knockdown causes both faster growth and accelerated development raises the interesting question whether the two effects are dependent. It is reasonable to expect growth and development are tightly related with faster growing animals reaching metamorphosis sooner. In this case, one would expect that the wild type and knockdown tadpoles are similar in size at the onset of metamorphosis (stage 54), although requiring different lengths of time to reach it. Surprisingly, when stage 54 animals are compared, TRα knockdown tadpoles are actually smaller than the wild type siblings (Fig. 3A,B). Thus, the increased growth rate due to TRα knockdown appears to be uncoupled from the acceleration of tadpole development in the knockdown animals. It further suggests that TRα plays a much more important role in slowing development of the animals toward metamorphosis but a minor role in repressing growth (Fig. 4). Thus, even though at the end of embryogenesis and onset of tadpole feeding, the knockdown and wild type animals of the same age are similar in size (Fig. 4), as feeding begins, TRα knockdown animals grow in size faster than wild type siblings and become bigger than the wild type ones when compared at the same age (Fig. 4, at any time point along the horizontal axis prior to reaching stage 54). However, these knockdown animals develop (advancement in developmental stages) much faster, reaching stage 54 at much younger age. The wild type animals grow slower and take longer time to reach stage 54 (i.e. at older age). This gives them extra growth time before initiating metamorphosis (stage 54). The final outcome is that the faster growing TRα knockdown tadpoles are nonetheless smaller at the onset of metamorphosis when compared to the wild type siblings (Fig. 4, the vertical axis value for the animals at stage 54).
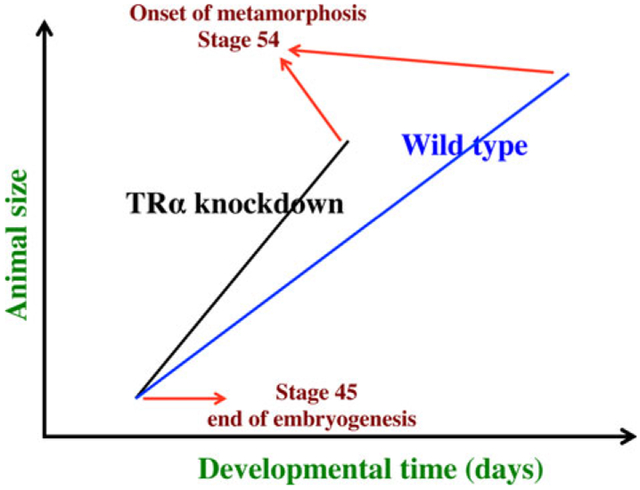
Fig. 4.
Schematics showing the dual effects of thyroid hormone (TH) receptor α (TRα) knockdown on premetamorphic development in Xenopus tropicalis. TRα knockdown has little effect on embryogenesis and the resulting tadpoles are normal by feeding stage (stage 45). Once feeding begins, the animals grow differently, with the knockdown ones growing faster, and thus larger when compared to wild type siblings at the same age (in days) (compared with the vertical axis values of the lines for the knockdown and wild type animals at any given position along the horizontal axis between stages 45 and 54). The knockdown animals also have faster development, reaching developmentally more advanced stages when compared to wild type siblings at the same age (in days). Thus, the knockdown animals reach stage 54, the onset of metamorphosis, at a younger age (see the horizontal axis locations for the upper end of the lines). Interestingly, when the animals at stage 54 are compared, the wild type ones are actually larger than the knockdown siblings even though the latter grow faster. This is because the wild type animals take longer to reach metamorphosis (stage 54). The extra growth time needed to reach stage 54 enables the wild type ones to catch up and surpass the knockdown ones in size. These results indicate that the effects of TR on growth and development are not dependent on each other (as otherwise, the size of the animals at stage 54 would be identical between the wild type and knockdown ones).
A role of HDAC-corepressor complexes in the control of metamorphic timing by unliganded TR
Molecular studies have shown that unliganded TR recruits HDAC containing corepressor complexes, in particular the N-CoR (nuclear receptor co-repressor) and SMRT (silencing mediator for retinoid and thyroid hormone receptors)-containing HDAC-complexes to target genes. Indeed, chromatin immunoprecipitation (ChIP) analyses have shown that indeed N-CoR and SMRT complexes are recruited by TR in premetamorphic tadpoles to endogenous target genes (Sachs et al. 2002; Tomita et al. 2004) and importantly, upon TH treatment of premetamorphic tadpoles or during natural metamorphosis, such complexes are released and local histone acetylation levels increase (Sachs et al. 2002; Tomita et al. 2004), supporting a role of the corepressor complexes and histone deacetylation in the repression of TH target genes and premetamorphic animal development.
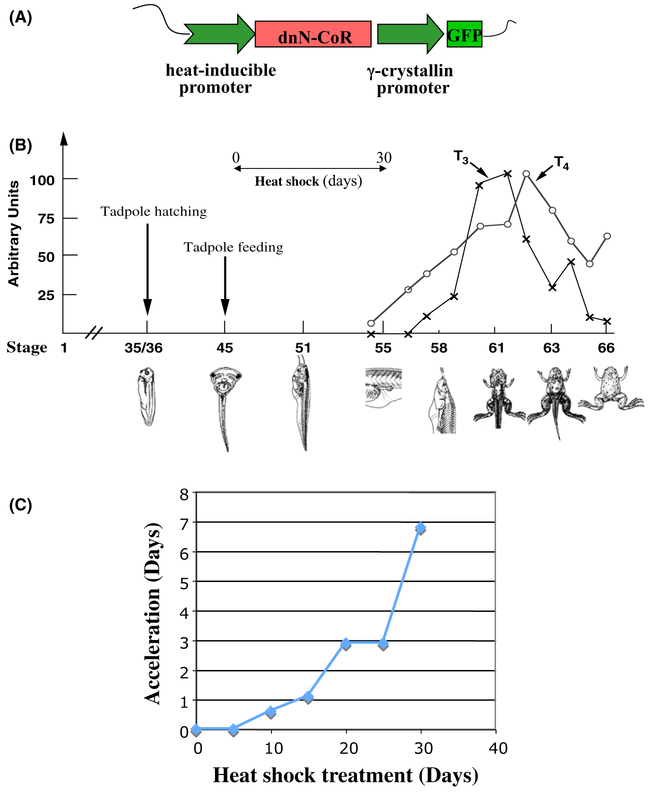
To investigate the role of corepressor complexes in premetamorphic tadpoles, we have generated a dominant negative form (dnN-CoR) of the Xenopus laevis N-CoR that contained only the receptor interacting domain (ID) near the C-terminus of N-CoR and introduced it into tadpoles via transgenesis by placing it under the control of a heat shock-inducible promoter (Fig. 5A). Heat shock treatment of the transgenic tadpoles leads to overexpression of the dnN-CoR, which should bind to endogenous TR during the developmental stages 45–54 when TR expression is high while TH levels are low (Fig. 1), a window period of about 30 days (Fig. 5B) (Nieuwkoop & Faber 1956). Thus, it should disrupt the recruitment of endogenous corepressor complexes to the target genes. When transgenic and their wild type siblings were subjected to daily heat shock treatment, starting at stage 46, endogenous TH response genes were indeed derepressed and more importantly, transgenic animals developed faster, reaching stage 54, the onset of metamorphosis, by as much as 7 days earlier than the wild type siblings during the 30 day experiment (Fig. 5C) (Sato et al. 2007). Thus, the recruitment of the endogenous HDAC-containing corepressor complexes by unliganded TR is required for gene repression in premetamorphic tadpoles and for proper timing of the initiation of metamorphosis.
Fig. 5.
Transgenic expression of a dominant negative (dn) corepressor leads to premature metamorphosis in Xenopus laevis. (A) Schematic representation of dnN-CoR transgenic construct. The dnN-CoR was placed under the control of a heat shock-inducible promoter in a plasmid that also contained the γ-crystallin promoter driving the expression of green fluorescent protein (GFP) for identification of transgenic tadpoles. (B) Experimental strategy. The transgenic and wild type animals are subjected to daily heat shock treatment at the onset of feeding, around stage 45, until they reach stage 54, the onset of metamorphosis when endogenous T3 and T4 becomes available (Leloup & Buscaglia 1977), which bind to TR to prevent the binding of dnN-coR to TR. (C) Transgenic tadpoles expressing the dnN-CoR initiate metamorphosis earlier than wild type siblings. Transgenic F1 generation tadpoles and wild type siblings at stage 46 were treated daily with heat shock to induce dnN-CoR expression in the transgenic but not wild type animals and their developmental stages were determined every 5 days for 30 days (note that at the end of the 30-day experiments, transgenic animals had initiated metamorphosis, i.e. passed stage 54, and thus the experiment was ended since the dnN-CoR would have no effect on TR when TH is present). The time (days) needed for the wild type animals to catch up the stage of the transgenic siblings at different time points of the heat shock treatment was calculated based on the staging by (Nieuwkoop & Faber 1956) and plotted here. See Fig. 1 and (Sato et al. 2007) for more details.
Conclusions
Ever since the discovery that TR can bind to TREs both in the presence and absence of TH and can repress gene expression in the absence of TH, it has been suggested that unliganded TR may play a role in vertebrate development. The external development of amphibians offers the best opportunity to test this hypothesis. Indeed, a number of earlier transgenic and molecular studies have provided strong evidence for such a role by unliganded TR during premetamorphosis. Recent knockout/knockdown studies have now provided conclusive evidence that unliganded TRα represses target genes to prevent precocious metamorphosis and that TRα further regulates the rate of metamorphosis when TH is present. Surprisingly, these studies have also revealed a novel function for unliganded TRα. That is, unliganded TR regulates the rate of animal growth prior to metamorphosis in a manner that is uncoupled from its effect on developmental progression toward metamorphosis.
Anuran metamorphosis resembles the postembryonic development in mammal. It is very likely that premetamorphosis in anurans will share similarity with the period of mammalian development prior to the rise of endogenous TH. Indeed, as in amphibians, TR is expressed prior to the maturation of the thyroid gland in mammals, suggesting that similar functions for unliganded TR may exist in mammals. Findings from a number of studies in mammals are consistent with such a view, including the effect of TRα knockout on heart gene expression and development (Mai et al. 2004) and the effect of TRβ deletion/mutations on the auditory and visual systems (Refetoff et al. 1967; Brucker-Davis et al. 1996; Forrest et al. 1996; Ng et al. 2001; Griffith et al. 2002; Jones et al. 2003). Similarly, deleting the gene encoding the TH-inactivating enzyme, type 3 deiodinase, also causes auditory defects (Ng et al. 2009), arguing for the importance of low TH levels (thus leaving more TR unliganded). Finally, mutations in N-CoR and SMRT, two of the best-studied corepressors that bind to unliganded TR, in mice that disrupt corepressor-TR interaction or deacetylase activation lead to derepression of TH-inducible genes, supporting a role of corepressor recruitment by unliganded TR in gene regulation in vivo in mammals (Astapova et al. 2008; You et al. 2010; Pei et al. 2011). Thus, it is very likely that unliganded TR may have similar functions during mammalian development, such as coordinating organ growth and development (Choi et al. 2015; Wen & Shi 2015; Yen 2015).
Acknowledgment
The work in the authors’ laboratory has been supported by the Intramural Research Program of NICHD, NIH.
References
- Astapova I, Lee LJ, Morales C, Tauber S, Bilban M & Hollenberg AN 2008. The nuclear corepressor, NCoR, regulates thyroid hormone action in vivo. Proc. Natl. Acad. Sci. U.S.A 105, 19544–19549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson BG 1994. Metamorphosis: model systems for studying gene expression in postembryonic development. Dev. Genet. 15, 313–319. [Google Scholar]
- Blitz IL, Biesinger J, Xie X & Cho KW 2013. Biallelic genome modification in F(0) Xenopus tropicalis embryos using the CRISPR/Cas system. Genesis 51, 827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brucker-Davis F, Skarulis MC, Pikus A, Ishizawar D, Mastroianni M-A, Koby M & Weintraub BD 1996. Prevalence and mechanisms of hearing loss in patients with resistance to thyroid hormone (RTH). J. Clin. Endocrinol. Metab. 81, 2768–2772. [DOI] [PubMed] [Google Scholar]
- Buchholz DR, Hsia VS-C, Fu L & Shi Y-B 2003. A dominant negative thyroid hormone receptor blocks amphibian metamorphosis by retaining corepressors at target genes. Mol. Cell. Biol. 23, 6750–6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz DR, Tomita A, Fu L, Paul BD & Shi Y-B 2004. Transgenic analysis reveals that thyroid hormone receptor is sufficient to mediate the thyroid hormone signal in frog metamorphosis. Mol. Cell. Biol. 24, 9026–9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz DR, Paul BD & Shi YB 2005. Gene-specific changes in promoter occupancy by thyroid hormone receptor during frog metamorphosis. Implications for developmental gene regulation. J. Biol. Chem. 280, 41222–41228. [DOI] [PubMed] [Google Scholar]
- Buchholz DR, Paul BD, Fu L & Shi YB 2006. Molecular and developmental analyses of thyroid hormone receptor function in Xenopus laevis, the African clawed frog. Gen. Comp. Endocrinol. 145, 1–19. [DOI] [PubMed] [Google Scholar]
- Burke LJ & Baniahmad A 2000. Co-repressors 2000. FASEB J 14, 1876–1888. [DOI] [PubMed] [Google Scholar]
- Chen H, Lin RJ, Schiltz RL, Chakravarti D, Nash A, Nagy L, Privalsky ML, Nakatani Y & Evans RM 1997. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell 90, 569–580. [DOI] [PubMed] [Google Scholar]
- Chen D, Ma H, Hong H, Koh SS, Huang SM, Schurter BT, Aswad DW & Stallcup MR 1999. Regulation of transcription by a protein methyltransferase. Science 284, 2174–2177. [DOI] [PubMed] [Google Scholar]
- Choi J, Suzuki KI, Sakuma T, Shewade L, Yamamoto T & Buchholz DR 2015. Unliganded thyroid hormone receptor alpha regulates developmental timing via gene repression as revealed by gene disruption in Xenopus tropicalis. Endocrinology 156, 735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das B, Schreiber AM, Huang H & Brown DD 2002. Multiple thyroid hormone-induced muscle growth and death programs during metamorphosis in Xenopus laevis. Proc. Natl Acad. Sci. U.S.A 99, 12230–12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis PJ & Davis FB 1996. Nongenomic actions of thyroid hormone. Thyroid 6, 497–504. [DOI] [PubMed] [Google Scholar]
- Demarest SJ, Martinez-Yamout M, Chung J, Chen H, Xu W, Dyson HJ, Evans RM & Wright PE 2002. Mutual synergistic folding in recruitment of CBP/p300 by p160 nuclear receptor coactivators. Nature 415, 549–553. [DOI] [PubMed] [Google Scholar]
- Evans RM 1988. The steroid and thyroid hormone receptor superfamily. Science 240, 889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest D, Erway LC, Ng L, Altschuler R & Curran T 1996. Thyroid hormone receptor beta is essential for development of auditory function. Nat. Genet. 13, 354–357. [DOI] [PubMed] [Google Scholar]
- Franklyn JA & Gammage MD 1996. Thyroid disease: effects on cardiovascular function. Trends Endocrinol. Metab. 7, 50–54. [DOI] [PubMed] [Google Scholar]
- Freake HC & Oppenheimer JH 1995. Thermogenesis and thyroid function. Annu. Rev. Nutr. 15, 263–291. [DOI] [PubMed] [Google Scholar]
- Glass CK & Rosenfeld MG 2000. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 14, 121–141. [PubMed] [Google Scholar]
- Griffith AJ, Szymko YM, Kaneshige M, Quinonez RE, Kaneshige K, Heintz KA, Mastroianni MA, Kelley MW & Cheng SY 2002. Knock-in mouse model for resistance to thyroid hormone (RTH): an RTH mutation in the thyroid hormone receptor beta gene disrupts cochlear morphogenesis. J. Assoc. Res. Otolaryngol. 3, 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther MG, Lane WS, Fischle W, Verdin E, Lazar MA & Shiekhattar R 2000. A core SMRT corepressor complex containing HDAC3 and TBL1, a WD40-repeat protein linked to deafness. Genes Dev. 14, 1048–1057. [PMC free article] [PubMed] [Google Scholar]
- Guo X, Zhang T, Hu Z, Zhang Y, Shi Z, Wang Q, Cui Y, Wang F, Zhao H & Chen Y 2014. Efficient RNA/Cas9-mediated genome editing in Xenopus tropicalis. Development 141, 707–714. [DOI] [PubMed] [Google Scholar]
- Hasebe T, Buchholz DR, Shi YB & Ishizuya-Oka A 2011. Epithelial-connective tissue interactions induced by thyroid hormone receptor are essential for adult stem cell development in the Xenopus laevis intestine. Stem Cells 29, 154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasebe T, Fu L, Miller TC, Zhang Y, Shi YB & Ishizuya-Oka A 2013. Thyroid hormone-induced cell-cell interactions are required for the development of adult intestinal stem cells. Cell Biosci. 3, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimeier RA, Hsia VS-C & Shi Y-B 2008. Participation of BAF57 and BRG1-containing chromatin remodeling complexes in thyroid hormone-dependent gene activation during vertebrate development. Mol. Endocrinol. 22, 1065–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetzel BS 1989. The Story of Iodine Deficiency: An International Challenge in Nutrition. Oxford University Press, Oxford. [Google Scholar]
- Howdeshell KL 2002. A model of the development of the brain as a construct of the thyroid system. Environ. Health Perspect 110, 337–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia VS-C & Shi Y-B 2002. Chromatin disruption and histone acetylation in the regulation of HIV-LTR by thyroid hormone receptor. Mol. Cell. Biol 22, 4043–4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia VS-C, Wang H & Shi Y-B 2001. Involvement of chromatin and histone acetylation in the regulation of HIV-LTR by thyroid hormone receptor. Cell Res 11, 8–16. [DOI] [PubMed] [Google Scholar]
- Huang Z-Q, Li J, Sachs LM, Cole PA & Wong J 2003. A role for cofactor-cofactor and cofactor-histone interactions in targeting p300, SWI/SNF and Mediator for transcription. EMBOJ. 22, 2146–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka T & Lazar MA 2003. The N-CoR/histone deacetylase 3 complex is required for repression by thyroid hormone receptor. Mol. Cell. Biol. 23, 5122–5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuya-Oka A & Shi YB 2011. Evolutionary insights into postembryonic development of adult intestinal stem cells. Cell Biosci. 1, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M & Roeder RG 2001. The TRAP/SMCC/Mediator complex and thyroid hormone receptor function. Trends Endocrinol. Metab. 12, 127–134. [DOI] [PubMed] [Google Scholar]
- Jones PL & Shi Y-B 2003. N-CoR-HDAC corepressor complexes: roles in transcriptional regulation by nuclear hormone receptors In: Current Topics in Microbiology and Immunology: Protein Complexes That Modify Chromatin, Vol. 274 (ed. Workman JL), pp. 237–268. Springer-Verlag, Berlin. [DOI] [PubMed] [Google Scholar]
- Jones PL, Sachs LM, Rouse N, Wade PA & Shi YB 2001. Multiple N-CoR complexes contain distinct histone deacetylases. J. Biol. Chem. 276, 8807–8811. [DOI] [PubMed] [Google Scholar]
- Jones I, Srinivas M, Ng L & Forrest D 2003. The thyroid hormone receptor beta gene: structure and functions in the brain and sensory systems. Thyroid 13, 1057–1068. [DOI] [PubMed] [Google Scholar]
- Kanamori A & Brown DD 1992. The regulation of thyroid hormone receptor beta genes by thyroid hormone in Xenopus laevis. J. Biol. Chem. 267, 739–745. [PubMed] [Google Scholar]
- Koh SS, Chen DG, Lee YH & Stallcup MR 2001. Synergistic enhancement of nuclear receptor function by p160 coactivators and two coactivators with protein methyltransferase activities. J. Biol. Chem. 276, 1089–1098. [DOI] [PubMed] [Google Scholar]
- Laudet V & Gronemeyer H 2002. The Nuclear Receptor Facts-Book. Academic Press, San Diego. [Google Scholar]
- Lazar MA 1993. Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr. Rev. 14, 184–193. [DOI] [PubMed] [Google Scholar]
- Lei Y, Guo X, Liu Y, Cao Y, Deng Y, Chen X, Cheng CH, Dawid IB, Chen Y & Zhao H 2012. Efficient targeted gene disruption in Xenopus embryos using engineered transcription activator-like effector nucleases (TALENs). Proc. Natl Acad. Sci. U.S.A 109, 17484–17489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Guo X, Deng Y, Chen Y &Zhao H 2013. Generation of gene disruptions by transcription activator-like effector nucleases (TALENs) in Xenopus tropicalis embryos. Cell Biosci 3, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leloup J & Buscaglia M 1977. La triiodothyronine: hormone de la metamorphose des amphibiens. C.R. Acad. Sci 284, 2261–2263. [Google Scholar]
- Li J, O’malley BW & Wong J 2000a. p300 requires its histone acetyltransferase activity and SRC-1 interaction domain to facilitate thyroid hormone receptor activation in chromatin. Mol. Cell. Biol. 20, 2031–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang J, Wang J, Nawaz Z, Liu JM, Qin J &Wong J 2000b. Both corepressor proteins SMRT and N-CoR exist in large protein complexes containing HDAC3. EMBO J. 19, 4342–4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai W, Janier MF, Allioli N, Quignodon L, Chuzel T, Fla-mant F & Samarut J 2004. Thyroid hormone receptor alpha is a molecular switch of cardiac function between fetal and postnatal life. Proc. Natl Acad. Sci. U.S.A 101, 10332–10337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H & Shi YB 2010. An essential and evolutionarily conserved role of protein arginine methyltransferase 1 for adult intestinal stem cells during postembryonic development. Stem Cells 28, 2073–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Paul BD, Choi CY & Shi Y-B 2007. Contrasting effects of two alternative splicing forms of coactivator-associated arginine methyltransferase 1 on thyroid hormone receptor-mediated transcription in Xenopus laevis. Mol. Endocrinol. 21, 1082–1094. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Paul BD, Choi CY, Hasebe T & Shi Y-B 2009. Novel functions of protein arginine methyltransferase 1 in thyroid hormone receptor-mediated transcription and in the regulation of metamorphic rate in Xenopus laevis. Mol. Cell. Biol. 29, 745–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura K, Fujimoto K, Das B, Fu L, Lu CD & Shi YB 2012. Histone H3K79 methyltransferase Dot1L is directly activated by thyroid hormone receptor during Xenopus metamorphosis. Cell Biosci. 2, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckenna NJ & O’malley BW 2001. Nuclear receptors, coregulators, ligands, and selective receptor modulators: making sense of the patchwork quilt. Ann. N. Y. Acad. Sci. 949, 3–5. [DOI] [PubMed] [Google Scholar]
- Nakajima K & Yaoita Y 2003. Dual mechanisms governing muscle cell death in tadpole tail during amphibian metamorphosis. Dev. Dyn. 227, 246–255. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Fish MB, Fisher M, Oomen-Hajagos J, Thomsen GH & Grainger RM 2013. Simple and efficient CRISPR/Cas9-mediated targeted mutagenesis in Xenopus tropicalis. Genesis 51, 835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L, Hurley JB, Dierks B, Srinivas M, Saltó C, Vennström B, Reh TA & Forrest D 2001. A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nat. Genet. 27, 94–98. [DOI] [PubMed] [Google Scholar]
- Ng L, Hernandez A, He W, Ren T, Srinivas M, Ma M, Galton VA, St Germain DL & Forrest D 2009. A protective role for type 3 deiodinase, a thyroid hormone-inactivating enzyme, in cochlear development and auditory function. Endocrinology 150, 1952–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop PD & Faber J 1956. Normal Table of Xenopus Laevis. North Holland Publishing, Amsterdam. [Google Scholar]
- Okada M, Nakajima K & Yaoita Y 2012. Translational regulation by the 5’-UTR of thyroid hormone receptor alpha mRNA. J. Biochem. 151, 519–531. [DOI] [PubMed] [Google Scholar]
- Onate SA, Tsai SY, Tsai MJ & O’malley BW 1995. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science 270, 1354–1357. [DOI] [PubMed] [Google Scholar]
- Pei L, Leblanc M, Barish G, Atkins A, Nofsinger R, Whyte J, Gold D, He M, Kawamura K, Li HR, Downes M, Yu RT, Powell HC, Lingrel JB & Evans RM 2011. Thyroid hormone receptor repression is linked to type I pneumocyte-associated respiratory distress syndrome. Nat. Med. 17, 1466–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzianowska-Kuznicka M, Damjanovski S & Shi Y-B 1997. Both thyroid hormone and 9-cis retinoic acid receptors are required to efficiently mediate the effects of thyroid hormone on embryonic development and specific gene regulation in Xenopus laevis. Mol. Cell. Biol. 17, 4738–4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachez C & Freedman LP 2001. Mediator complexes and transcription. Curr. Opin. Cell Biol. 13, 274–280. [DOI] [PubMed] [Google Scholar]
- Refetoff S, Dewind LT & Degroot LJ 1967. Familial syndrome combining deaf-mutism, stuppled epiphyses, goiter and abnormally high PBI: possible target organ refractoriness to thyroid hormone. J. Clin. Endocrinol. Metab. 27, 279–294. [DOI] [PubMed] [Google Scholar]
- Sachs LM & Shi Y-B 2000. Targeted chromatin binding and histone acetylation in vivo by thyroid hormone receptor during amphibian development. Proc. Natl. Acad. Sci. U.S.A 97, 13138–13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs LM, Damjanovski S, Jones PL, Li Q, Amano T, Ueda S, Shi YB & Ishizuya-Oka A 2000. Dual functions of thyroid hormone receptors during Xenopus development. Comp Biochem Physiol B Biochem Mol Biol 126, 199–211. [DOI] [PubMed] [Google Scholar]
- Sachs LM, Jones PL, Havis E, Rouse N, Demeneix BA & Shi Y-B 2002. N-CoR recruitment by unliganded thyroid hormone receptor in gene repression during Xenopus laevis development. Mol. Cell. Biol. 22, 8527–8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos A, Perez-Castillo A, Wong NC & Oppenheimer JH 1987. Labile proteins are necessary for T3 induction of growth hormone mRNA in normal rat pituitary and rat pituitary tumor cells. J. Biol. Chem. 262, 16880–16884. [PubMed] [Google Scholar]
- Sato Y, Buchholz DR, Paul BD & Shi Y-B 2007. A role of unliganded thyroid hormone receptor in postembryonic development in Xenopus laevis. Mech. Dev. 124, 476–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber AM & Brown DD 2003. Tadpole skin dies autonomously in response to thyroid hormone at metamorphosis. Proc. Natl Acad. Sci. U.S.A 100, 1769–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber AM, Das B, Huang H, Marsh-Armstrong N & Brown DD 2001. Diverse developmental programs of Xenopus laevis metamorphosis are inhibited by a dominant negative thyroid hormone receptor. Proc. Natl. Acad. Sci. U.S.A 98, 10739–10744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard HM, Harries JC, Hussain S, Bevan C &Heery DM 2001. Analysis of the steroid receptor coactivator 1 (SRC1)-CREB binding protein interaction interface and its importance for the function of SRC1. Mol. Cell. Biol 21, 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y-B 1999. Amphibian Metamorphosis: From Morphology to Molecular Biology. John Wiley & Sons Inc, New York. [Google Scholar]
- Shi Y-B, Wong J, Puzianowska-Kuznicka M & Stolow M 1996. Tadpole competence and tissue-specific temporal regulation of amphibian metamorphosis: roles of thyroid hormone and its receptors. BioEssays 18, 391–399. [DOI] [PubMed] [Google Scholar]
- Shi YB, Matsuura K, Fujimoto K, Wen L & Fu L 2012. Thyroid hormone receptor actions on transcription in amphibia: the roles of histone modification and chromatin disruption. Cell Biosci. 2, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JE 1995. Thyroid hormone control of thermogenesis and energy balance. Thyroid 5, 481–492. [DOI] [PubMed] [Google Scholar]
- Sun G, Fu L & Shi Y-B 2014. Epigenetic regulation of thyroid hormone-induced adult intestinal stem cell development during anuran metamorphosis. Cell Biosci. 4, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata JR 1993. Gene expression during metamorphosis: an ideal model for post-embryonic development. BioEssays 15, 239–248. [DOI] [PubMed] [Google Scholar]
- Tomita A, Buchholz DR & Shi Y-B 2004. Recruitment of N-CoR/SMRT-TBLR1 corepressor complex by unliganded thyroid hormone receptor for gene repression during frog development. Mol. Cell. Biol. 24, 3337–3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchia J, Rose DW, Inostroza J, Kamei Y, Westin S, Glass CK & Rosenfeld MG 1997. The transcriptional co-activator p/CIP binds CBP and mediates nuclear-receptor function. Nature 387, 677–684. [DOI] [PubMed] [Google Scholar]
- Tsai MJ & O’malley BW 1994. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu. Rev. Biochem. 63, 451–486. [DOI] [PubMed] [Google Scholar]
- Wang X, Matsuda H & Shi Y-B 2008. Developmental regulation and function of thyroid hormone receptors and 9-cis retinoic acid receptors during Xenopus tropicalis metamorphosis. Endocrinology 149, 5610–5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Shi Z, Cui Y, Guo X, Shi YB & Chen Y 2015. Targeted gene disruption inXenopus laevis using CRISPR/Cas9. Cell Biosci 5, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L & Shi YB 2015. Unliganded thyroid hormone receptor alpha controls developmental timing in Xenopus tropicalis. Endocrinology 156, 721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Fu L, Guo X, Chen Y & Shi YB 2015. Histone methyltransferase Dot1 L plays a role in postembryonic development in Xenopus tropicalis. FASEB J 29, 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J & Shi Y-B 1995. Coordinated regulation of and transcriptional activation by Xenopus thyroid hormone and retinoid X receptors. J. Biol. Chem 270, 18479–18483. [DOI] [PubMed] [Google Scholar]
- Wong J, Shi YB & Wolffe AP 1995. A role for nucleosome assembly in both silencing and activation of the Xenopus TR beta A gene by the thyroid hormone receptor. Genes Dev. 9, 2696–2711. [DOI] [PubMed] [Google Scholar]
- Wong J, Shi Y-B & Wolffe AP 1997. Determinants of chromatin disruption and transcriptional regulation instigated by the thyroid hormone receptor: hormone-regulated chromatin disruption is not sufficient for transcriptinal activation. EMBO J. 16, 3158–3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J, Patterton D, Imhof D, Guschin D, Shi Y-B & Wolffe AP 1998. Distinct requirements for chromatin assembly in transcriptional repression by thyroid hormone receptor and histone deacetylase. EMBOJ. 17, 520–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaoita Y & Brown DD 1990. A correlation of thyroid hormone receptor gene expression with amphibian metamorphosis. Genes Dev. 4, 1917–1924. [DOI] [PubMed] [Google Scholar]
- Yen PM 2001. Physiological and molecular basis of thyroid hormone action. Physiol. Rev 81, 1097–1142. [DOI] [PubMed] [Google Scholar]
- Yen PM 2015. Unliganded TRs regulate growth and developmental timing during early embryogenesis: evidence for a dual function mechanism of TR action. Cell Biosci 5, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H-G, Chan DW, Huang ZQ, Li J, Fondell JD, Qin J & Wong J 2003. Purification and functional characterization of the human N-CoR complex: the roles of HDAC3, TBL1 and TBLR1. EMBO J 22, 1336–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You SH, Liao X, Weiss RE & Lazar MA 2010. The interaction between nuclear receptor corepressor and histone deacetylase 3 regulates both positive and negative thyroid hormone action in vivo. Mol. Endocrinol 24, 1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J & Lazar MA 2000. The mechanism of action of thyroid hormones. Annu. Rev. Physiol 62, 439–466. [DOI] [PubMed] [Google Scholar]
- Zhang J, Kalkum M, Chait BT & Roeder RG 2002. The N-CoR-HDAC3 nuclear receptor corepressor complex inhibits the JNK pathway through the integral subunit GPS2. Mol. Cell 9, 611–623. [DOI] [PubMed] [Google Scholar]