Abstract
Objective:
Older age and lower education levels are known to be associated with worse neurocognitive (NC) performance in healthy adults, and individuals with HIV infection may experience accelerated brain/cognition aging. However, higher education may possibly protect against HIV-associated neurocognitive disorders (HAND). The aim of the current cross-sectional study was to assess the effect of age and education in an HIV-1 clade C infected adult population in urban Zambia.
Method:
Demographically corrected Zambian norms on a neuropsychological (NP) test battery were used to correct for normal age and education effects. The study assessed 286 HIV positive (+) males (37.1%) and females (62.9%) with a mean age of 41.35 (SD =8.56) and mean years of education = 10.16 (SD = 2.18). A comprehensive NP test battery was used to assess cognitive domains frequently affected by HIV: attention/working memory, learning/and delayed recall, executive function, verbal fluency, processing speed, verbal and visual episodic memory, and fine motor skills.
Results:
In younger HIV+ Zambians, higher education evidenced protective effects against NC impairments overall, and for the specific domains of executive functions, learning and speed of information processing. Impairment scores did not support accelerated overall brain aging although the restricted age range and relative youth of our total sample may have precluded detection of such tendencies.
Conclusions:
The present study raises the need to investigate factors that could be implicated in the poor neurocognitive performance among the younger, less educated HIV+ individuals in Zambia.
Keywords: HIV, neurocognitive functioning, age, education
General Scientific Summary
Zambia’s younger, less educated HIV+ individuals are at risk for cognitive impairment. Age and education interaction on cognitive performance was investigated in an HIV+ sample in Zambia. Younger, less educated HIV+ should be empowered in disease management in Zambia.
Antiretroviral therapy (ART) has reduced the worldwide HIV and AIDS- related mortality rates from a peak of 2.2 million in the mid-2000s to 1.8 million in 2010 and 1.1 million in 2015 (Negin, Mills, & Bärnighausen, 2012; UNAIDS, 2016). ART also has helped with a reduction in the prevalence of HIV-related dementia by as much as 50% (Bociąga-Jasik, Lickiewicz, Cieśla, Mach, & Garlicki, 2010; Cholewinńska & Szymańska, 2009; Rosca, Rosca, Simu, & Chirileanu, 2012; Vance, Fazeli, Ross, Wadley, & Ball, 2012). On the contrary, mild to moderate NP impairments have not declined; in fact, their prevalence actually may increase as people are living longer with HIV and its treatment (Heaton et al., 2010).
It has also been reported that the demographics of the HIV epidemic are steadily changing with increased proportions of middle-aged and older adults of 50 years or older living with the HIV infection (Gebo, 2008; Hardy & Vance, 2009; Justice, 2010). Globally, around 2.8 million adults aged 50 years and older were living with HIV in 2005. The United Nations General Assembly Special Session has noticed that the burden of HIV among those aged ≥50 years is frequently ignored and this represents a significant blind spot in the global response to the epidemic of HIV infection (Negin & Cumming, 2010).
Sub-Saharan Africa is also undergoing a demographic shift in terms of the number and age range of people living with HIV and AIDS: Various studies report that since more people have access to ART, mortality rates have begun to drop and HIV-positive people are surviving much longer. The successful scale up of ART in developing countries has moved from negligible levels in 2003 to more than 7.9 million in 2012 (Floyd et al., 2012; Negin & Cumming, 2010; Negin, Mills, & Bärnighausen, 2012; Reniers et al., 2009).
Since its first reported incidence of HIV in the 1980s, Zambia, a sub-Saharan African country, has been making strides to alleviate the effects of HIV and AIDS on its population. To that end, the Zambian government introduced ART free of charge at the two major hospitals since 2002. Free ART is currently offered at various primary health care centers and all government clinics in Zambia (Stringer et al., 2006).
However, as increasingly more people infected with HIV live longer, it is postulated that there will be a corresponding increase in milder forms of HAND. These forms have been named asymptomatic neurocognitive impairment, and mild neurocognitive disorders, which, according to some estimates, are affecting 30–60% of people living with HIV (Cholewińska & Szymańska, 2009; Heaton et al., 2010; Vance et al., 2012).
The aforementioned HIV-associated neurocognitive disorders affect various aspects of cognitive functioning including impairments in attention, concentration, learning, memory, psychomotor ability and speed of information processing (Kabuba, Menon, Franklin, Heaton, & Hestad, 2017; Negin & Cumming, 2010).
It is well documented that increasing age in itself is associated with declines in cognitive functioning. Equally, HIV infection is also associated with a risk of cognitive decline. Therefore, with the steady increase in the number of older adults living with HIV, there is need to understand the interaction of age and HIV-1 associated cognitive impairment (Hardy & Vance, 2009; Kissel, Pukay-Martin, & Bornstein, 2005; Valcour et al., 2004; Vance, 2009; Vance, McDougall, Wilson, Debiasi, & Cody, 2014).
The combined risks of HIV infection and older age are reported to increase the prevalence or severity of impairment beyond effects of either risk alone (Milanini & Valcour, 2017; Saylor et al., 2016). Previous studies have revealed that older HIV+ individuals are more likely to develop cognitive problems and a decline in functional ability (Farooqui & Farooqui, 2009; Sheppard et al., 2015). Neurocognitive impairment within HIV+ is said to significantly correlate with older age (Sheppard et al., 2015). Some scholars have postulated that this could be more directly related to ART toxicities and not HIV, however, comparative studies between drug-conservative groups and those on aggressive treatment demonstrates that uncontrolled HIV is essentially more detrimental than ART toxicities (Deeks & Phillips, 2009; Pathai, Bajillan, Landay, & High, 2014).
Additionally, concerns have been raised to the effect that HIV may precipitate an NP process similar to that observed in Alzheimer’s disease (Iudicello et al., 2012; Kuhlmann, Minihane, Huebbe, Nebel, & Rimbach, 2010; Valcour et al., 2004). This evidence thus further potentially poses an increased risk of developing cognitive problems for the older HIV+ individuals.
All these factors make it imperative to understand the increased risk of HIV infection and older age especially in sub-Saharan Africa were the number of people aging with HIV is increasing (Joska et al., 2011, 2012; Negin & Cumming, 2010).
Another key component related to cognitive functioning and HIV infection is education. There are currently conflicting results regarding aspects of education that are implicated in cognitive functioning in HIV-infected persons. Some studies involving HIV-1 positive subjects suggest that although educational attainment is an important factor in cognitive performance, years of education does not necessarily account for quality of education (Ryan et al., 2005). On the contrary, other studies have shown that level of education itself has an effect on cognitive functioning in infected groups (De Ronchi et al., 2002; Satz et al., 1993).
It is well established that level of education is an important element not only in normal people, but also in those with brain damage, affecting cognitive performance in various domains, especially for verbal performance tests. Higher education groups have been reported to present slower cognitive decline as a result of normal aging than the individuals with lower educational levels. This is because education may have a protective effect on cognitive functioning. Increased cognitive capacity, referred to as cognitive reserve, is typically used to explain the delay in cognitive and functional expression of neurodegenerative illnesses such as HIV (Ardila, 1998; Ardila, Ostrosky-Solis, Rosselli, & Gómez, 2000; Bornstein & Suga, 1988; Le Carret et al., 2003; Lezak, 1995; Ostrosky-Solis, Ardila, Rosselli, Lopez-Arango, & Uriel-Mendoza, 1998; Stern, 2002; Tombaugh, 2004; Welch, Doineau, Johnson, & King, 1996).
In normal populations, there are significant differences in cognitive functioning associated with levels of education. The effect of education is not linear but rather represents a negatively accelerated curve which tends to plateau. This is because the ceiling in NP tests are typically low (Ardila, 1998).
To determine the combined effects of HIV serostatus and education level on cognitive abnormalities, Satz et al. (1993), employed five NP tests (grooved pegboard, verbal fluency, symbol digit modalities, and Rey auditory verbal learning). There were abnormalities (38%) in those with less than 12 years of education compared with 17% cognitive abnormalities that were found in those with more than 12 years of education. They report that the interaction between education level and serostatus was evident even after possible confounding factors such as age, ethnicity and CD4 count level were controlled for. Their study had the strength of comprising a large sample size of 888 HIV+ and 855 HIV− participants. These results point to the potential interaction between level of education and HIV status on cognitive function.
In Zambia, the formal education system is broadly structured as basic education (1–9 years of education) and higher education (10–12 years of education). Basic education is a three- tiered system with lower basic (1–4 years), middle basic (5–7 years), and upper basic (8–9). The first two tiers are primary education while upper basic (8–9 years) is the first phase of secondary education and this is followed by high education (10–12 years). Students who complete 12 years of education are thereafter expected to pursue tertiary education offered in colleges and universities (UNESCO, 2011).
According to the Zambia National Education Profile (2014), the net enrollment rate for primary school is at 94% and the primary completion rate is 91%. The enrollment rate for lower secondary education rate is 68% while the transition rate to higher education is 56%.
In light of the foregoing, the current study sought to investigate whether age and education has an effect on the cognitive functioning of the HIV+ adult population in Zambia. The current study had the strength of employing a comprehensive NP test battery measuring seven cognitive domains. Normal effects of age and education were corrected for using a previously collected large sample of healthy HIV− adult Zambians (Hestad et al., 2016).
The main aim of the current study was to establish how neuro-cognitive functioning is affected by age and level of education in people infected with HIV-1 clade C. Specifically, we wanted to find out the possible neurocognitive differences between HIV+ young adults and HIV+ older adults. Furthermore, the study sought to determine if there are neurocognitive differences between HIV+ participants with more years of formal education versus those with fewer years of formal education.
We hypothesized that there would be a main effect for age and education on NP performance in our HIV+ sample. We expect that the older HIV+ participants will have poorer results on demographically corrected NP test scores (from published norms), compared with the younger HIV+ participants and, furthermore, we expect that the HIV+ participants with higher levels of education would have better scores than the HIV+ participants who had lower levels of education.
Method
Participants
The sample was drawn from six urban clinics in the Zambian capital city of Lusaka. The clinics sampled were; Chilenje, Chipata, Kabwata, Kalingalinga, Matero Main, and Matero Referral clinic. All of the clinics sampled are under the management of the Lusaka District Health Management Team in Zambia. These clinics were chosen because of the presence of the antiretroviral centers, which routinely provides HIV counseling and testing services as well as providing treatment.
Participants were eligible to participate in the study if they were: HIV sero-positive and on antiretroviral treatment. Information about HIV status was based on the participants’ medical files. They also were required to have a minimum of 5 years of formal education. English is the primary language of instruction in the Zambian school system, and the testing was performed in English (Hestad et al., 2016). Participants were also required to be between 20 and 65 years of age (to conform to the age range of the HIV− controls who participated in the Zambian NP norming study; Hestad et al., 2016).
Potential participants were excluded from the study if they had a history of non-HIV related neurological disorders such as epilepsy or closed head injury. This information was obtained by means of the Neurobehavioural Medical Screen Form, which assesses past medical and neurological histories (Kabuba et al., 2017). Participants with a history of drug abuse were also excluded from the study. This information was obtained using a structured interview (Heaton, Miller, Taylor, & Grant, 2004; Kabuba, Menon, & Hestad, 2011). Furthermore, Individuals with obvious physical disabilities were excluded from the study to minimize the possibility of test performance requiring motor dexterity being impaired due to the handicap.
The target sample size was 324 participants, but only 286 HIV+ participants were included in the current study because 38 participants with pulmonary tuberculosis were excluded due to the possible effect of tuberculosis on NP functioning (Robertson, Liner, & Heaton, 2009).
Procedure
The recruitment process was carried out with the assistance of nurses at each clinic who identified participants who routinely seek ART meeting the inclusion criteria. Once informed consent was obtained, the participants were referred to one of 10 neuropsychology master of science students from University of Zambia, who had received extensive training in interviewing, as well as administration and scoring of the NP tests. The testing process for each participant took approximately 2 hr 30 min (Kabuba et al., 2017).
The first part of the testing process involved obtaining the participants’ demographic characteristics, medical, and psychiatric information based on self-report. Medical details were confirmed by the patients’ medical records provided by the medical personnel. Administration of NP tests was carried out in the same order for all participants, and they were compensated the Zambian Kwacha equivalent of $5 (USD) for transport and refreshment allowance at the end of the testing process (Kabuba et al., 2017).
Measures
Cognitive functioning.
Cognitive functioning was measured using an NP test battery that measures cognition across the seven ability domains that have been identified as frequently affected in HIV-associated neurocognitive disorders (Antinori et al., 2007): Executive functioning (Stroop Color–Word Interference trial, Category Test errors, WI Card Sorting Test–64 total errors, and Color Trails 2), working memory/attention (Paced Auditory Serial Addition Test–50 and Wechsler Memory Scale-III Spatial Span Test), speed of information processing (Wechsler Adult Intelligence Scale-III [WAIS-III], Digit Symbol, WAIS-III Symbol Search, Trails A, Color Trails 1, Stroop Color Naming and Stroop Word Naming), verbal fluency (letter fluency, animal fluency, and action fluency), learning (Hopkins Verbal Learning Test—Revised) <VLT-R] and Brief Visuospatial Learning Test—Revised) [BVMT-R], delayed recall (HVLT-R delay and BVMT-R delay), and complex motor function (grooved pegboard [dominant and nondominant hands]). The test battery is appropriate for the current study because it measures the domains typically affected by HIV. It is also an internationally well-recognized NP assessment tool that has been translated into multiple languages around the world (Heaton et al., 2010; Heaton, Marcotte, et al., 2004; Hestad et al., 2012; Kabuba et al., 2011; Kanmogne et al., 2010). The battery has been previously adapted and normed for the Zambian population (Hestad et al., 2012).
Test items such as those on the HVLT-R were adapted to make them more culturally appropriate for Zambia (Hestad et al., 2012).
Age in the current study was a continuous variable, illustrated as lower and upper quartiles distributions of the data: “young” for ages 35 years and below, and “old” for ages 47 years and above.
Education was a continuous variable, illustrated as a lower and upper quartile distribution of the data; less than 9 years of education were considered in the category of “low education level,” while those with 12 years and more in the category of “high education.”
Disease characteristics.
Participants were categorized according to whether their HIV viral loads were detectable or undetectable. Viral load was detected at 50 copies/mL. This information is considered here because previous studies have reported HIV viral load detectability as an induction of effective therapy (Ellis et al., 1997; McArthur et al., 1997).
Nadir CD4 was taken at the point of the participant’s eligibility for ART and current CD4 taken at the time of data collection for the current study. Information on duration of ART was obtained from the patients’ medical files.
AIDS status constituted participants with AIDS and those without AIDS; this information was obtained from the participants’ medical files. In Zambia, AIDS is typically diagnosed when an HIV-infected individual has a CD4 count below 200 cells/mm3 of blood, weakening the immune system and putting the individual at risk of illnesses and infections that are AIDS defining according to the World Health Organization (WHO) stages (WHO Stages 3 and 4 condition; Childs et al., 1999; Zambia Ministry of Health, 2010).
Nutritional information indexed by body mass index (BMI) was collected because it has been linked to NP functioning (Joska et al., 2011).
Ethical Consideration
The study was approved by the University of Zambia Biomedical Research Ethics Committee and Ministry of Health. The research protocol was also formally approved by the Lusaka District Health Management Team, which oversees the clinics in Lusaka and the Ministry of Health.
Written informed consent was obtained from all research participants. Participants were given the opportunity to take breaks or withdraw from the study at any time with no penalty incurred. All the data remained anonymous; the participants were assigned an arbitrary code for analysis purposes.
Data Management
The raw data obtained on the NP test battery was converted into demographically corrected T scores based on data previously collected from 324 healthy Zambians (Hestad et al., 2016). The 324 healthy sample comprised 157 (48.5%) males and 167 (51.5%) females, with an average age of 38.5 (SD = 12.80) years and an average education level of 11.0 (SD = 2.58) years. The norming process was similar to that used for the United States normative data (Heaton, Miller, et al., 2004). The raw scores were corrected for the effects of age, education, sex, and urban/rural background by first converting them to normally distributed scaled scores with a mean of 10 and SD of 3; the scaled scores were then converted to demographically corrected T scores with a mean of 50 and SD of 10. Detailed procedures and results of the norming study have been reported elsewhere (Hestad et al., 2016).
Global deficit scores were used to determine the neurocognitive impairment status and impairment levels of the participants (Blackstone et al., 2012). Global deficit score is a mean of impairment ratings that uses demographically corrected T scores on a 5-point scale from 0 (normal/0 deficits) to 5 (severely impaired). A deficit score of 0 means normal performance (T score ≥40), whereas a deficit score of 1 means mild impairment (T score = 35–39), 2 means mild-to-moderate impairment (T score = 30–34), 3 means moderate impairment (T score = 25–29), 4 means moderate-to-severe impairment (T score = 20–24), and 5 means severe impairment (T score <11021]20). Participants were classified as impaired in an ability domain if they had an average deficit score ≥0.5 on that particular cognitive domain. This average is called the domain deficit score. Participants were further classified as having global impairment if they had a total average deficit score ≥0.5 across all domains (Blackstone et al., 2012; Carey et al., 2004; Hinkin et al., 2004).
The use of the deficit score approach reflects our focus on abilities that may have been affected by central nervous system injury or disease. It has been used in many previous studies of HIV-associated neurocognitive disorders and, similar to clinical ratings of NP results (which does not allow for good/normal performances on some tests or domains obscure problematic results on others), purposely gives less weight to high test scores in the normal range (Blackstone et al., 2012).
Statistical Analysis
Descriptive statistics are reported as means and SDs or frequencies. We carried out regression analyses with the following dependent variables, one at a time: NP test performance measured in seven domains with results presented as T scores and domain and global deficit scores. In these analyses, years of education, age, and their interaction were included as continuous covariates. Results are presented as estimated means with associated standard errors for ages 35 and 47 years (lower and upper quartile in our data set), and for 9 years and 12 years of education years, which are the education levels at which major examinations are taken in Zambia to qualify for high school and tertiary education, respectively (UNESCO, 2011). This approach uses all the available data, focuses on conceptually relevant age and education contrasts, and allows for testing significant effects for age, education, and their interaction. Also, these education lengths are the lower and upper quartile in our data set. Education and age were analyzed as continuous covariates in the current study instead of dichotomizing these for two main reasons: first, dichotomizing implicitly means that the effect is assumed constant within each side of the cutpoint, and makes a discrete “jump” at the cutpoint. This is practically never realistic. Second, dichotomizing implies loss of statistical power compared with using the variable as it is.
Here, p values <0.05 are considered significant. However, due to multiple hypotheses, p values between 0.01 and 0.05 should be interpreted with caution. Analyses were carried out in SPSS Version 22.
Results
Demographics and Disease Characteristics
Demographics and disease characteristics are shown in Table 1. The HIV+ sample (n = 286) had a mean age of 41.35 (SD =8.56), and a mean number of years of education of 10.16 (SD =2.18). There were 37.1% males and 62.9% females. In our sample, 56 persons (19.6%) had less than 9 years of education, 128(44.8%) had 9 to 11 years, and 102 (35.7%) had 12 or more years of education.
Table 1.
Demographic and Disease/Treatment Characteristics of HIV+ Sample
| Demographic and disease characteristics | N | Mean/percentage | SD |
|---|---|---|---|
| Age | 286 | 41.35 | 8.56 |
| Education | 286 | 10.16 | 2.18 |
| Female gender | 286 | 62.9% | |
| Nadir CD4 | 207 | 212.89 | 156.31 |
| Current CD4 | 261 | 515.04 | 255.89 |
| Undetectable viral load | 261 | 78.3% | |
| Duration on ART | 198 | 57.57 | 30.83 |
| With AIDS | 224 | 37.4% | |
| BMI | 188 | 23.48 | 5.01 |
Note. The sample sizes (n) for disease characteristics vary due to missing data. Neuropsychological performance based on age and education in the HIV+ sample. ART = antiretroviral therapy; BMI = body mass index.
The “normal” effects of age, education, and sex on the NP test performance were controlled for by using demographically corrected standard scores (T scores) generated with a previously collected normative sample of 324 HIV-negative participants (Hestad et al., 2016).
Table 2 shows the estimated means of NP performance across the seven domains using T score means, Results are illustrated for the lower and upper quartiles of age and education: for younger and older age levels estimated for ages 35 and 47 years, respectively, and for low and high education levels, 9 and 12 years of education, respectively. In these analyses, years of education, age, and their interaction were included as continuous covariates.
Table 2.
Estimated Mean of NP Performance From Linear Regression With Age, Education, and Their Interaction as Continuous Covariates (Mean T Scores)
| Domain | Age 35 years | Age 47 years | p value | ||
|---|---|---|---|---|---|
| 9 years edu. EM (SE) | 12 years edu. EM (SE) | 9 years edu. EM (SE) | 12 years edu. EM (SE) | ||
| Executive | 46.05 (.56) | 47.38 (.66) | 47.11 (.53) | 47.23 (.57) | .211 |
| Fluency | 46.39 (.65) | 46.59 (.76) | 47.53 (.61) | 47.12 (.65) | .483 |
| Working memory | 44.80 (.72) | 44.09 (.84) | 45.46 (.68) | 44.68 (.73) | .580 |
| Learning | 43.05 (.66) | 45.59 (.77) | 45.60 (.62) | 45.76 (.66) | .002 |
| Recall | 44.02 (.69) | 46.02 (.80) | 45.99 (.65) | 47.00 (.69) | .014 |
| Motor | 54.04 (.90) | 51.69 (1.05) | 52.05 (.85) | 49.86 (.91) | .008 |
| Speed of information processing | 45.83 (.67) | 46.53 (.72) | 47.17 (.58) | 46.81 (.62) | .292 |
| Global mean T | 46.18 (.48) | 46.80 (.56) | 47.27 (.45) | 46.98 (.48) | .249 |
Note. p value for the combined effect of these covariates (F test with 3 degrees of freedom). Bold values are meant to highlight the statistically significant results. Results are presented as estimated means with associated standard errors for ages 35 and 47 years (lower and upper quartile in our data set), and for 9 years and 12 years of education years. See the online supplemental materials for tables showing beta estimates and confidence intervals. NP = neuropsychological; EM = estimated mean; SE = standard error.
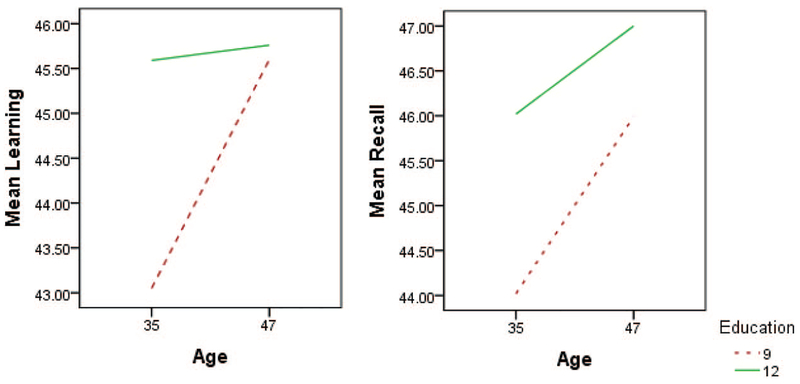
As seen in the table, there is a statistically significant effect of age and/or education for learning, recall, and motor functions. This is illustrated in Figure 1. In what follows, we report p values for age and education for these.
Figure 1.
Estimated mean T scores for learning, recall, and motor functions, from linear regression with age, education, and their interaction as covariates. See the online article for the color version of this figure.
For learning, young but not old age had a significant (p = .003) effect of education, with higher education showing relatively preserved/normal abilities. There is only a negligible and nonsignificant effect of education.
For recall, at the young age there is again a significant (p = .026) effect of education, with low education being associated with poorer performance. At an older age level, again higher education was marginally with better performance associated with higher education.
For motor functions, all mean estimated scores were high and normal, regardless of age and education with lower education actually being associated with better motor performance.
To further investigate the effects of age and education on NP performance across the seven domains, we conducted analyses adjusted for the following potential confounders simultaneously: nadir CD4, current CD4, AIDS status, duration on ART, viral load detection, BMI, and gender. These results remained substantially the same when adjusting for the potential confounders; data not shown.
Table 3 shows the estimated means of NP deficit scores across the seven domains and globally for ages 35 and 47, and 9 and 12 years of education. In these analyses, years of education, age, and their interaction were included as continuous covariates.
Table 3.
Estimated Mean of NP Deficit Scores From Linear Regression With Age, Education, and Their Interaction as Continuous Covariates (Domain Deficit Scores)
| Domain | Age 35 years | Age 47 years | p value | ||
|---|---|---|---|---|---|
| 9 years edu. EM (SE) | 12 years edu. EM (SE) | 9 years edu. EM (SE) | 12 years edu. EM (SE) | ||
| Executive | .44 (.04) | .28 (.05) | .36 (.04) | .34 (.04) | .032 |
| Fluency | .52 (.06) | .45 (.07) | .43 (.05) | .44 (.06) | .589 |
| Working memory | .67 (.07) | .59 (.08) | .60 (.07) | .63 (.07) | .679 |
| Learning | .66 (.06) | .46 (.07) | .40 (.06) | .46 (.06) | .001 |
| Recall | .56 (.06) | .43 (.07) | .46 (.06) | .37 (.06) | .133 |
| Motor | .14 (.05) | .17 (.06) | .25 (.05) | .35 (.05) | .009 |
| Speed of information processing | .49 (.05) | .39 (.06) | .37 (.05) | .43 (.05) | .056 |
| Global deficit score | .49 (.04) | .39 (.04) | .40 (.04) | .43 (.04) | .035 |
Note. p value for the combined effect of these covariates (F test with 3 degrees of freedom). Bold entries meant to highlight the statistically significant results. Results are presented as estimated means with associated standard errors for ages 35 and 47 years (lower and upper quartile in our data set), and for 9 years and 12 years of education years. See the online supplemental materials for tables showing beta estimates and confidence intervals. NP = neuropsychological; EM = estimated mean; SE = standard error.
As seen in the table, there is a statistically significant effect of age and/or education for learning, executive function, motor functions, and the global deficit score and a trend for processing speed. This is illustrated in Figure 2. Although the overall model for motor functions was statistically significant, age and education on their own had no statistically significant effect in this domain. In what follows, we report p values for age and education for these.
Figure 2.
Estimated mean deficit scores for executive functions domain deficit score (DDS), Learning DDS, global deficit scores and speed of information processing DDS from linear regression with age, education and their interaction as covariates. See the online article for the color version of this figure.
For learning, young but not old age had a significant (p = .01) effect on education. The younger, less educated had poorer performance compared with the younger, more educated group.
For executive functions, at the younger age level there is a significant (p = .05) effect of education. The younger, less educated had poorer performance compared with the younger, more educated group. At the older age there is only a negligible and nonsignificant effect of less age or less education.
For speed of information processing, the younger, less educated had poorer performance (p = .03) compared with the younger, more educated group. The older age groups showed nonsignificant effects of both age and education.
For global deficit scores, the young age and less educated group had poorer performance (p = .03) compared with the younger more educated group. The older age group exhibited negligible and nonsignificant effect of age and education effects.
To further investigate the effects of age and education on NP performance across the seven domains, we conducted analyses adjusted for the following potential confounders’ simultaneously: nadir CD4, current CD4, AIDS status, duration on ART, viral load detection, BMI, and gender. The results remained substantially the same when adjusting for the potential confounders; data not shown.
Discussion
Age and Neurocognitive Functioning in the HIV+ Sample
We hypothesized that the older HIV+ participants would have poorer cognitive performance on the NP tests compared with the younger HIV+ participants. Advancing age is typically reported to be associated with a decline in cognitive functioning (Hardy & Vance, 2009; Kissel, Pukay-Martin, & Bornstein, 2005; Milanini & Valcour, 2017; Vance, 2009; Vance et al., 2014). In the current study, the effects of normal aging and education levels in healthy individuals were controlled by the use of demographically corrected NP test norms. Thus our explanation was that HIV infection would exacerbate the process of normal cognitive aging.
Contrary our hypothesis, advancing age in our HIV+ sample did not negatively affect cognitive performance on the NP tests. The statistically significant effect of age yielded from the current study showed that the younger HIV+ group with less education performed worse on learning and recall on the mean T scores than the younger HIV+ group with more education. A similar trend was observed on the impairment scores in which the younger, less educated performed more poorly on the learning domain scores and global deficit scores than the younger, more educated group. The results obtained in the current study are counterintuitive although similar to those reported by Wilkie et al., 2003, who reported that certain cognitive processes may be more impaired in the younger than the older HIV-1 infected adults. They reported that learning and delayed recall were more impaired in the younger HIV+ compared with the older HIV+ group. Caveat the NP performance differences observed in the current study we assume, were not due to age effects but rather due to education effects/cognitive reserve. Although the current study findings may appear to be counterintuitive, we postulates that, age effects (young vs. old) were likely masked because the sample in the current study was relatively young compared with the previous studies that reveal that individuals over the age of 60 typically demonstrate negative cognitive results owing to age on NP evaluation (Ardila, 1998; Becker, Lopez, Dew, & Aizenstein, 2004; De Ronchi et al., 2002; Milanini & Valcour, 2017; Wilkie et al., 2003).
On the contrary, similar to the current study findings, previous studies have indicated that there is a minimal interference and sometimes no evidence of age and HIV infection on neurocogni tive functions (Hardy et al., 1999; Van Gorp et al., 1994; Wilkie et al., 2003).
Education and Neurocognitive Functioning in the HIV+ Sample
We had postulated that within the context of HIV infection, participants with higher levels of education would show less cognitive impairment than those with lower levels of education. This was confirmed in the current study but only at the lower age range (see Tables 2 and 3). The younger and less educated in the current study were found to perform more poorly on learning, executive function, processing speed, and on the global deficit scores. The results yielded in the present study are in agreement with previous studies (Satz et al., 1993; De Ronchi et al., 2002), which show that level of education has an effect on cognitive functioning in the HIV+. This result shows that the effect of cognitive reserve was more apparent in the young age level than in the older age level participants with more years of education. Previous studies have shown that due to cognitive reserve, higher education groups tend to have a slower decline on cognitive tests than those with lower educational levels (Ardila, 1998; Ardila et al., 2000; Bornstein & Suga, 1988; Le Carret et al., 2003; Lezak, 1995; Ostrosky-Solis et al., 1998; Stern, 2002; Tombaugh, 2004; Welch et al., 1996).
In addition, the current study yielded peculiar results regarding education and performance on motor functions. Interestingly, although the scores for all age and education groups regarding motor functions were within the normal range; the older, more educated group did not perform as well as the less educated, younger group. This result is consistent with results obtained by Robertson et al. (2007) in Uganda and Heaton et al. (2011), who report that grooved pegboard tasks were less affected in the HIV+ in Combination antiretroviral therapy (CART) era. This result could be an indication that motor functions are not adversely affected in HIV+ individuals on ART in Zambia.
On the contrary, this result could possibly be an indicator that the younger sample with less education might be more involved in manual work that demands motor dexterity as opposed to the older sample. Presumably, since the older participants in the current study are engaged in cognitively demanding jobs, their performance on neuropsychology tests is better and more homogeneous due to the positive neuroplasticity resulting from the cognitively demanding tasks they perform. Furthermore, the assumption would be that the younger group in our sample is presumably engaged in different work conditions where the younger, less educated are involved in manual work and the younger, more educated in cognitively demanding jobs, which could account for the differences observed between the two groups. The younger, more educated benefit from the cognitive reserve as a result of education and continued use of cognitive skills and thus perform better on the NP tests (Mahncke, Bronstone, & Merzenich, 2006; Vance & Burrage, 2006).
The implication of this is that cognitive performance is worse for HIV+ people who are younger and less educated in Zambia. There is, however, a need to carry out further research to ascertain why this could be the case. It could also be necessary to carry out a longitudinal study that would better take into account the differences that could account for the effects of age and education on neurocognitive impairment in HIV+ adults in Zambia.
Conclusion
The findings obtained from the current study show that there was an effect of level of education on neurocognition in the HIV+ younger sample. The cognitive reserve properties associated with higher education enabled the younger, more educated participants to perform better than the younger, less educated group on neuro-cognitive tests. The current study also revealed that motor functions are preserved in the HIV+ on CART in Zambia. This could be an indication that the younger, less educated need to be empowered in disease management.
Footnotes
Supplemental materials: http://dx.doi.org/10.1037/neu0000438.supp
References
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, … Wojna VE (2007). Updated research nosology for HIV- associated neurocognitive disorders. Neurology, 69, 1789–1799. http://dx.doi.org/10.1212/01.WNL.0000287431.88658.8b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardila A (1998). A note of caution: Normative neuropsychological test performance: Effects of age, education, gender and ethnicity: A comment on Saykin et al. (1995). Applied Neuropsychology, 5, 51–53. [DOI] [PubMed] [Google Scholar]
- Ardila A, Ostrosky-Solis F, Rosselli M, & Gómez C (2000). Age-related cognitive decline during normal aging: The complex effect of education. Archives of Clinical Neuropsychology, 15, 495–513. [PubMed] [Google Scholar]
- Becker JT, Lopez OL, Dew MA, & Aizenstein HJ (2004). Prevalence of cognitive disorders differs as a function of age in HIV virus infection. AIDS, 18, S11–S18. http://dx.doi.org/10.1097/00002030-200401001-00003 [PubMed] [Google Scholar]
- Blackstone K, Moore DJ, Franklin DR, Clifford DB, Collier AC, Marra CM, … Heaton RK (2012). Defining neurocognitive impairment in HIV: Deficit scores versus clinical ratings. The Clinical Neuropsychologist, 26, 894–908. http://dx.doi.org/10.1080/13854046.2012.694479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bociąga-Jasik M, Lickiewicz B, Cieśla AKNA, Mach T, & Garlicki A (2010). Neurocognitive disorders in HIV infected patients. HIV & AIDS Review, 9, 33–36. http://dx.doi.org/10.1016/S1730-1270(10)60066-5 [Google Scholar]
- Bornstein RA, & Suga LJ (1988). Educational level and neuropsychological performance in healthy elderly subjects. Developmental Neuropsychology, 4, 17–22. http://dx.doi.org/10.1080/87565648809540386 [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, … the HNRC Group. (2004). Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. Journal of Clinical and Experimental Neuropsychology, 26, 307–319. http://dx.doi.org/10.1080/13803390490510031 [DOI] [PubMed] [Google Scholar]
- Childs EA, Lyles RH, Selnes OA, Chen B, Miller EN, Cohen BA, … McArthur JC (1999). Plasma viral load and CD4 lymphocytes predict HIV-associated dementia and sensory neuropathy. Neurology, 52, 607–613. http://dx.doi.org/10.1212/WNL.52.3.607 [DOI] [PubMed] [Google Scholar]
- Cholewińska G, & Szymańska B (2009). Mental impairment and neurocognitive symptoms associated with HIV infection. HIV & AIDS Review, 8, 9–14. http://dx.doi.org/10.1016/S1730-1270(10)60030-6 [Google Scholar]
- Deeks SG, & Phillips AN (2009). HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. British Medical Journal, 338, 288–292. http://dx.doi.org/10.1136/bmj.a3172 [DOI] [PubMed] [Google Scholar]
- De Ronchi D, Faranca I, Berardi D, Scudellari P, Borderi M, Manfredi R, & Fratiglioni L (2002). Risk factors for cognitive impairment in HIV-1-infected persons with different risk behaviors. Archives of Neurology, 59, 812–818. http://dx.doi.org/10.1001/archneur.59.5.812 [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Hsia K, Spector SA, Nelson JA, Heaton RK, Wallace MR, … the HIV Neurobehavioral Research Center Group. (1997). Cerebrospinal fluid human immunodeficiency virus type 1 RNA levels are elevated in neurocognitively impaired individuals with acquired immunodeficiency syndrome. Annals of Neurology, 42, 679–688. http://dx.doi.org/10.1002/ana.410420503 [DOI] [PubMed] [Google Scholar]
- Farooqui T, & Farooqui AA (2009). Aging: An important factor for the pathogenesis of neurodegenerative diseases. Mechanisms of Ageing and Development, 130, 203–215. http://dx.doi.org/10.1016/j.mad.2008.11.006 [DOI] [PubMed] [Google Scholar]
- Floyd S, Marston M, Baisley K, Wringe A, Herbst K, Chihana M,… Zaba B (2012). The effect of antiretroviral therapy provision on all-cause, AIDS and non-AIDS mortality at the population level -a comparative analysis of data from four settings in Southern and East Africa. Tropical Medicine & International Health, 17(8), e84–e93. http://dx.doi.org/10.1111/j.1365-3156.2012.03032.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebo KA (2008). Epidemiology of HIV and response to antiretroviral therapy in the middle aged and elderly. Aging Health, 4, 615–627. http://dx.doi.org/10.2217/1745509X.4.6.615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy DJ, Satz P, Stefaniak M, van Gorp WG, Hinkin CH, & Stenquist PK (1999). Cognition in older adults with AIDS. Poster presented at the 4th Annual UCLA Research Conference on Aging, Los Angeles, CA. [Google Scholar]
- Hardy DJ, & Vance DE (2009). The neuropsychology of HIV/AIDS in older adults. Neuropsychology Review, 19, 263–272. http://dx.doi.org/10.1007/s11065-009-9087-0 [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR Jr., Woods SP, Ake C, Vaida F, … the CHARTER Group. (2010). HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology, 75, 2087–2096. http://dx.doi.org/10.1212/WNL.0b013e318200d727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, … the CHARTER Group, & the HNRC Group. (2011). HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: Differences in rates, nature, and predictors. Journal of Neurovirology, 17, 3–16. http://dx.doi.org/10.1007/s13365-010-0006-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, & Grant I (2004). Revised comprehensive norms for an expanded Halstead Reitan Battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults. Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Heaton R, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, … the HNRC Group. (2004). The impact of HIV-associated neuropsychological impairment on everyday functioning. Journal of the International Neuropsychological Society, 10, 317–331. http://dx.doi.org/10.1017/S1355617704102130 [DOI] [PubMed] [Google Scholar]
- Hestad KA, Menon JA, Serpell R, Kalungwana L, Mwaba SO, Kabuba N, … Heaton RK (2016). Do neuropsychological test norms from African Americans in the United States generalize to a Zambian population? Psychological Assessment, 28, 18–38. http://dx.doi.org/10.1037/pas0000147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestad KA, Menon JA, Silalukey-Ngoma M, Franklin DR Jr., Imasiku ML, Kalima K, … Heaton RK (2012). Sex differences in neuropsychological performance as an effect of human immunodeficiency virus infection: A pilot study in Zambia, Africa. Journal of Nervous and Mental Disease, 200, 336–342. http://dx.doi.org/10.1097/NMD.0b013e31824cc225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Durvasula RS, Lam MN, … Stefaniak M (2004). Medication adherence in HIV-infected adults. AIDS, 18, S19–S25. http://dx.doi.org/10.1097/00002030-200401001-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iudicello JE, Woods SP, Deutsch R, Grant I, & the HIV Neurobehavioral Research Program (HNRP) Group. (2012). Combined effects of aging and HIV infection on semantic verbal fluency: A view of the cortical hypothesis through the lens of clustering and switching. Journal of Clinical and Experimental Neuropsychology, 34, 476–488. http://dx.doi.org/10.1080/13803395.2011.651103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joska JA, Westgarth-Taylor J, Hoare J, Thomas KG, Paul R, Myer L, … Stein DJ (2012). Neuropsychological outcomes in adults commencing highly active anti-retroviral treatment in South Africa: A prospective study. BMC Infectious Diseases, 12, 39 http://dx.doi.org/10.1186/1471-2334-12-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joska JA, Westgarth-Taylor J, Myer L, Hoare J, Thomas KG, Combrinck M, … Flisher AJ (2011). Characterization of HIV-associated neurocognitive disorders among individuals starting antiretroviral therapy in South Africa. AIDS and Behavior, 15, 1197–1203. http://dx.doi.org/10.1007/s10461-010-9744-6 [DOI] [PubMed] [Google Scholar]
- Justice AC (2010). HIV and aging: Time for a new paradigm. Current HIV/AIDS Reports, 7, 69–76. http://dx.doi.org/10.1007/s11904-010-0041-9 [DOI] [PubMed] [Google Scholar]
- Kabuba N, Menon JA, Franklin DR Jr., Heaton RK, & Hestad KA (2017). Use of Western neuropsychological test battery in detecting HIV-associated neurocognitive disorders (HAND) in Zambia. AIDS and Behavior, 21, 1717–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabuba N, Menon JA, & Hestad K (2011). Moderate alcohol consumption and cognitive functioning in a Zambian population. Medical Journal of Zambia, 38, 8–14. [Google Scholar]
- Kanmogne GD, Kuate CT, Cysique LA, Fonsah JY, Eta S, Doh R, … Njamnshi AK (2010). HIV-associated neurocognitive disorders in sub-Saharan Africa: A pilot study in Cameroon. BMC Neurology, 10, 60 http://dx.doi.org/10.1186/1471-2377-10-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissel EC, Pukay-Martin ND, & Bornstein RA (2005). the relationship between age and cognitive functioning HIV-infected men. Journal of Neuropsychiatry and Clinical Neuroscience, 17, 180–184. [DOI] [PubMed] [Google Scholar]
- Kuhlmann I, Minihane AM, Huebbe P, Nebel A, & Rimbach G (2010). Apolipoprotein E genotype and hepatitis C, HIV and herpes simplex disease risk: A literature review. Lipids in Health and Disease, 9, 8 http://dx.doi.org/10.1186/1476-511X-9-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Carret N, Lafont S, Letenneur L, Dartigues J-F, Mayo W, & Fabrigoule C (2003). The effect of education on cognitive performances and its implication for the constitution of the cognitive reserve. Developmental Neuropsychology, 23, 317–337. http://dx.doi.org/10.1207/S15326942DN2303_1 [DOI] [PubMed] [Google Scholar]
- Lezak MD (1995). Neuropsychological assessment (3rd ed.). New York, NY: Oxford University Press. [Google Scholar]
- Mahncke HW, Bronstone A, & Merzenich MM (2006). Brain plasticity and functional losses in the aged: Scientific bases for a novel intervention. Progress in Brain Research, 157, 81–109. http://dx.doi.org/10.1016/S0079-6123(06)57006-2 [DOI] [PubMed] [Google Scholar]
- McArthur JC, McClernon DR, Cronin MF, Nance-Sproson TE, Saah AJ, St Clair M, … Lanier ER (1997). Relationship between human immunodeficiency virus-associated dementia and viral load in cerebrospinal fluid and brain. Annals of Neurology, 42, 689–698. http://dx.doi.org/10.1002/ana.410420504 [DOI] [PubMed] [Google Scholar]
- Milanini B, & Valcour V (2017). Differentiating HIV-associated neurocognitive disorders from Alzheimer’s disease: An emerging issue in geriatric neuroHIV. Current HIV/AIDS Reports, 14, 123–132. http://dx.doi.org/10.1007/s11904-017-0361-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negin J, Mills EJ, & Bärnighausen T (2012). Aging with HIV in Africa: The challenges of living longer. AIDS, 26, S1–S5. http://dx.doi.org/10.1097/QAD.0b013e3283560f54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negin N, & Cumming R (2010). HIV infection in older adults in sub-Saharan Africa: Exploring prevalence from existing data. Bulletin of the World Health Organization, 88, 847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrosky-Solis F, Ardila A, Rosselli M, Lopez-Arango G, & Uriel-Mendoza V (1998). Neuropsychological test performance in illiterate subjects. Archives of Clinical Neuropsychology, 13, 645–660. http://dx.doi.org/10.1093/arclin/13.7.645 [DOI] [PubMed] [Google Scholar]
- Pathai S, Bajillan H, Landay AL, & High KP (2014). Is HIV a model of accelerated or accentuated aging? The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 69, 833–842. http://dx.doi.org/10.1093/gerona/glt168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reniers G, Araya T, Davey G, Nagelkerke N, Berhane Y, Coutinho R, & Sanders EJ (2009). Steep declines in population-level AIDS mortality following the introduction of antiretroviral therapy in Addis Ababa, Ethiopia. AIDS (London, England), 23, 511–518. http://dx.doi.org/10.1097/qad.0b013e32832403d0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson K, Liner J, & Heaton R (2009). Neuropsychological assessment of HIV-infected populations in international settings. Neuropsychology Review, 19, 232–249. http://dx.doi.org/10.1007/s11065-009-9096-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KR, Nakasujja N, Wong M, Musisi S, Katabira E, Parsons TD, … Sacktor N (2007). Pattern of neuropsychological performance among HIV patients in Uganda. BMC Neurology, 7, 8 http://dx.doi.org/10.1186/1471-2377-7-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosca EC, Rosca O, Simu M, & Chirileanu RD (2012). HIV-associated neurocognitive disorders: A historical review. The Neurologist, 18, 64–67. http://dx.doi.org/10.1097/NRL.0b013e318247bc7a [DOI] [PubMed] [Google Scholar]
- Ryan EL, Baird R, Mindt MR, Byrd D, Monzones J, & Bank SM, & Morgello S (2005). Neuropsychological impairment in racial/ethnic minorities with HIV infection and low literacy levels: Effects of education and reading level in participant characterization. Journal of the International Neuropsychological Society, 11, 889–898. http://dx.doi.org/10.1017/S1355617705051040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satz P, Morgenstern H, Miller EN, Selnes OA, McArthur JC, Cohen BA, … Visscher B (1993). Low education as a possible risk factor for cognitive abnormalities in HIV-1: Findings from the multi-center AIDS Cohort Study (MACS). Journal of Acquired Immune Deficiency Syndromes, 6, 503–511. http://dx.doi.org/10.1097/00126334-199305000-00011 [PubMed] [Google Scholar]
- Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, … McArthur JC (2016). HIV-associated neurocognitive disorder—Pathogenesis and prospects for treatment. Nature Reviews Neurology, 12, 234–248. http://dx.doi.org/10.1038/nrneurol.2016.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard DP, Iudicello JE, Bondi MW, Doyle KL, Morgan EE, Massman PJ, … Woods SP (2015). Elevated rates of mild cognitive impairment in HIV disease. Journal of Neurovirology, 21, 576–584. http://dx.doi.org/10.1007/s13365-015-0366-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y (2002). What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsycho-logical Society, 8, 448–460. http://dx.doi.org/10.1017/S1355617702813248 [PubMed] [Google Scholar]
- Stringer JS, Zulu I, Levy J, Stringer EM, Mwango A, Chi BH, & Saag MS (2006). Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. Jama, 296, 782–793. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN (2004). Trail Making Test A and B: Normative data stratified by age and education. Archives of Clinical Neuropsychology, 19, 203–214. http://dx.doi.org/10.1016/S0887-6177(03)00039-8 [DOI] [PubMed] [Google Scholar]
- UNAIDS. (2016). AIDS by the numbers ending the aids epidemic by 2030 as part of the sustainable development goals. Retrieved from http://www.unaids.org/sites/default/files/media_asset/AIDS-by-the-numbers-2016_en.pdf
- UNESCO. (2011). World data on education-Zambia. Retrieved from http://www.ibe.unesco.org/sites/default/files/Zambia.pdf [Google Scholar]
- Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes O,… Sacktor N (2004). Higher frequency of dementia in older HIV-1 individuals: The Hawaii Aging with HIV-1 Cohort. Neurology, 63, 822–827. http://dx.doi.org/10.1212/01.WNL.0000134665.58343.8D [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance DE (2009). Speed of processing in older adults: A cognitive overview for nursing. Journal of Neuroscience Nursing, 41, 290–297. [DOI] [PubMed] [Google Scholar]
- Vance DE, & Burrage JW Jr. (2006). Promoting successful cognitive aging in adults with HIV: Strategies for intervention. Journal of Gerontological Nursing, 32, 34–41. [DOI] [PubMed] [Google Scholar]
- Vance DE, Fazeli PL, Ross LA, Wadley VG, & Ball KK (2012). Speed of processing training with middle-age and older adults with HIV: A pilot study. Journal of the Association of Nurses in AIDS Care, 23, 500–510. http://dx.doi.org/10.1016/j.jana.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance DE, McDougall GJ Jr., Wilson N, Debiasi MO, & Cody SL (2014). Cognitive consequences of aging with HIV. Topics in Geriatric Rehabilitation, 30, 35–45. http://dx.doi.org/10.1097/TGR.0000000000000002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gorp WG, Miller EN, Marcotte TD, Dixon W, Paz D, Selnes O, … Stenquist PK (1994). The relationship between age and cognitive impairment in HIV-1 infection: Findings from the Multicenter AIDS Cohort Study and a clinical cohort. Neurology, 44, 929–935. http://dx.doi.org/10.1212/WNL.44.5.929 [DOI] [PubMed] [Google Scholar]
- Welch LW, Doineau D, Johnson S, & King D (1996). Educational and gender normative data for the Boston Naming Test in a group of older adults. Brain and Language, 53, 260–266. http://dx.doi.org/10.1006/brln.1996.0047 [DOI] [PubMed] [Google Scholar]
- Wilkie FL, Goodkin K, Khamis I, van Zuilen MH, Lee D, Lecusay R, … Eisdorfer C (2003). Cognitive functioning in younger and older HIV-1-infected adults. Journal of Acquired Immune Deficiency Syndromes, 33, S93–S105. http://dx.doi.org/10.1097/00126334-200306012-00006 [DOI] [PubMed] [Google Scholar]
- Zambia Ministry of Health. (2010). Adult and adolescent antiretroviral therapy protocols. Retrieved from http://www.who.int/hiv/pub/guidelines/zambia_art.pdf
- Zambia National Educational Profile. (2014). Retrieved from http://www.epdc.org/sites/default/files/documents/EPDC%20NEP_Zambia.pdf