ABSTRACT
Maize, Zea mays, the second-most-widely-grown crop, yields 20 % of all consumed calories worldwide.1 Despite its agronomic importance, research progress is limited by costly transformation. We recently described the Trojan horse method as a useful tool to study maize proteins in situ that circumvents time- and space-consuming whole plant transformation. The Trojan horse approach uses the protein-folding and secretory properties of the corn smut fungus Ustilago maydis to secrete maize proteins from fungal cells into the maize apoplast. Here, we discuss the timing and location of U. maydis during infection and the protein secretion site in relation to anther anatomy. This spatiotemporal analysis enables the study of apoplastic anther proteins in various premeiotic anther developmental stages, and could be adapted for larger screens.
KEYWORDS: Maize, anther, development, cell fate, U. maydis, Trojan horse (TH), plant-pathogen interaction
In the 2017–2018 harvest, 1,033 million tons of corn were produced worldwide,2 making corn the most-grown cereal crop. People are highly dependent on this renewable food and energy source, motivating researchers to investigate new ways to increase yield.
Recent advances in maize genetic-editing using CRISPR-Cas9 technology3 make modern reverse genetics much easier than classical transposon insertion lines. Another milestone in stable maize transformation was achieved more recently with precise expression of maize BABYBOOM and WUSCHEL2 proteins enabling genotype-independent embryo transformation without time-consuming callus regeneration.4,5 Even so, the processes of vector construction and transformation are laborious, and consume greenhouse space. To overcome these drawbacks, we developed an alternative, the Trojan horse method.6,7
The biotrophic corn smut fungus U. maydis infects all aerial plant organs. During infection the hyphae never contact the plant cytoplasm; instead the plant plasma membrane invaginates resulting in a narrow apoplastic space between hyphae and plant cells. All interaction between the plant and fungus must traverse this tiny space, the biotrophic interaction zone.8,9 Upon infection U. maydis secretes diverse effector proteins to suppress the plant´s immune response. Sequences encoding signal peptides for classical secretion of these effectors have been defined.10 Unconventional secretion of proteins has been reported and can be hijacked by researchers to secrete non-post-translationally modified proteins in U. maydis liquid culture.11–13 The underlying mechanism is still poorly understood and has not yet been tested in a biotechnology setting in planta.13–15 Compared to its host, U. maydis has a small genome and is readily transformed.10 After decades of work with this genetic model organism, an array of cloning vectors with a variety of epitope tags and promoters is available. U. maydis has also become a protein expression tool in biotechnology because of its folding and post-transcriptional modification abilities.13,15,16 Collectively, these features facilitate exploiting U. maydis to express and secrete maize proteins in situ. This approach permits single-cell resolution by tracking the Trojan horse hyphae during plant infection and allows comparison of maize cells receiving secreted protein to non-receiving cells in the same tissue.7 Expression of foreign proteins by Trojan horse strains is driven by a strong in planta active promoter.6,7,17 This allows protein delivery to all infected tissue layers. To permit expression at specific time points or locations, alternative promoters may be used in the future. Even though U. maydis is known for its protein folding and secretion capabilities, secretion of foreign proteins of interest needs to be carefully monitored.17 Also, the protein size limit for secretion is not yet known. The U. maydis effector Cmu1 (Chorismate mutase1) at nearly 300 amino acids long plus an mCherry-tag successfully complemented the cmu1 deletion mutant.18
To implement the Trojan horse method and achieve the highest possible spatiotemporal resolution, detailed growth timelines for both the host and the pathogen are required. For maize, detailed analyses of premeiotic anther development have been performed.19–22 Premeiotic anthers double in length approximately once per day. During early stages, at least one new lobe tissue layer is formed each day over the course of four days of growth (Figure 1). Correlations of tassel size and anther stages in different zones of the tassel (for maize inbred W23) showed that a tassel of 5–7 cm contains all stages of premeiotic anthers.25 Consequently, the impact of infection by a Trojan horse strain can be assessed in all anther lobe cell types with just one tassel infection.
Figure 1.
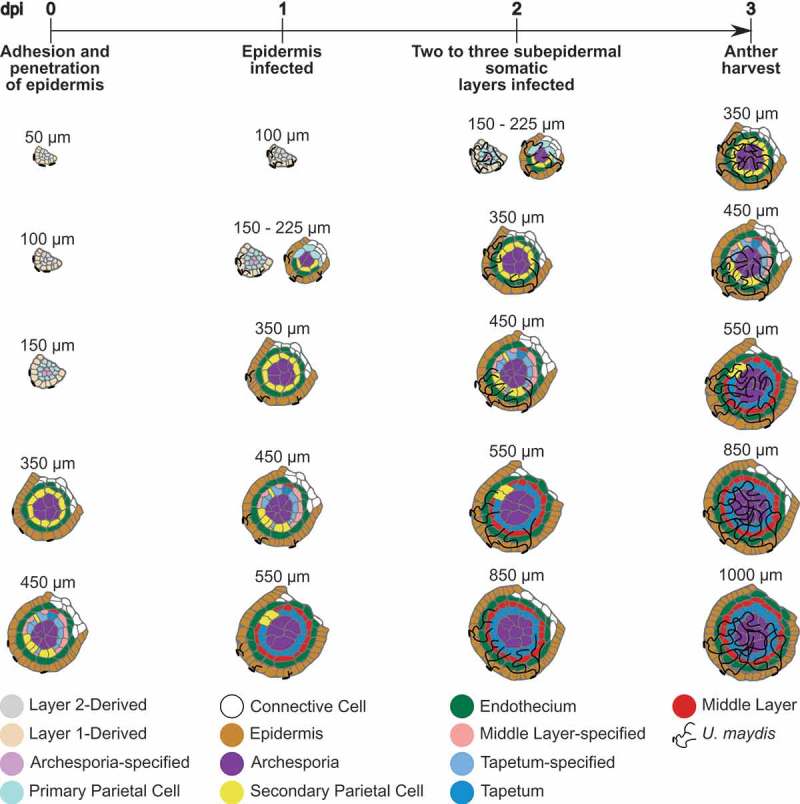
Timeline for the Trojan horse approach in premeiotic maize anthers.
Schematic illustration of different developmental stages of premeiotic anther lobes. Maize anther lobe development is strongly linked to anther size, as exemplified here for maize cultivar W23. New tissue layers form within the lobe, approximately one day apart.19,20,23 Anther development and cell fate specification is correlated to days post infection (dpi) with U. maydis based on previous studies.7–10,24
U. maydis infections were analyzed by various labs indicating reproducible patterns and timelines in all examined maize tissues.8–10,24 This is true even though U. maydis deploys divergent effectors for infection of different host tissues and host tissue responses vary in turn.26 An infectious hypha with an appressorium forms, and epidermal cell penetration starts within 12 h after contact with the plant. In the following 12 h infected epidermal cells are fully colonized (Figure 1). Two days post infection (dpi) two subepidermal layers are infected. After 3 dpi the entire organ is colonized.7–10,24 In seedling leaves, U. maydis strain SG200-induced cell proliferation occurs 4–5 dpi.27 In SG200-infected anthers, no significant formation of additional cells was observed 3 dpi. Subsequently, developmental studies using the Trojan horse approach were performed 3–4 dpi.7
To analyze the impact of the small secreted maize protein ZmMAC1 on Layer2-derived and archesporial cells present in 120–300 µm anthers, tassels with 50–125 µm anthers were infected. Three dpi, anthers were now 400–700 µm and were harvested for confocal imaging.7 By combining existing observations, a timeline for anther Trojan horse experiments can be estimated (Figure 1). Presumably, infection of 50 µm anthers should allow protein secretion to epidermal cells, primary parietal cells, secondary parietal cells, and the endothecium before observation of cellular responses is performed 3 dpi. At this time point, epidermal cells should have received the secreted protein for two days, while primary parietal cells, secondary parietal cells, and the endothecium should have been treated for 1 day. To analyze the response of middle layer cells to a secreted protein of interest, anthers 350–450 µm long have to be infected.
In addition to developmental studies, the Trojan horse approach is a tool for analysis of proteins in plant-pathogen interactions.6 Here tumor formation is used as an easy measure of plant susceptibility or resistance to the fungus after receiving a secreted protein of interest. The study of the function of apoplastic maize peptide ZIP1 resulting from proteolytic cleavage of PROZIP1 is one example of this. During compatible maize-U. maydis interactions, release of ZIP1 is blocked. Secretion of ZIP1 by U. maydis reduces tumor formation, and hence increases resistance to U. maydis.6 Thus, proving that not only proteins of interest but also individual protein domains can be functionally analyzed by this approach.
The Trojan horse approach can be used to analyze protein or protein-domain functions with high spatiotemporal resolution in situ without stable host transformation. Successful implementation relies on detailed knowledge of host developmental timelines and pathogen infection strategies. Easy transformation, understanding of secretion mechanisms and good protein folding capacity on the pathogen side are indispensable. Fortunately, many plant- and animal-pathosystems offer these features making the Trojan horse approach widely generalizable in the future for systems that lack easy host transformation technologies.
Funding Statement
This work was supported by the National Science Foundation [IOS13-39229]; Estonian Ministry of Education and Research [IUT19- 3]; Deutsche Forschungsgemeinschaft (DFG) [SFB924].
Acknowledgments
Funding was provided by SFB924 (project A14) of the DFG, NSF IOS13-39229, and IUT19- 3 from the Estonian Ministry of Education and Research.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.FAO (2018). The Food and Agriculture Organization of the United Nations, http://www.fao.org/docrep/u8480e/u8480e07.htm.
- 2.USDA (2018). Grain: world markets and trade, apps.fas.usda.gov/psdonline/circulars/grain-corn-coarsegrains.pdf .
- 3.Char SN, Neelakandan AK, Nahampun H, Frame B, Main M, Spalding MH, Becraft PW, Meyers BC, Walbot V, Wang K, et al. An Agrobacterium-delivered CRISPR/Cas9 system for high-frequency targeted mutagenesis in maize. Plant Biotechnol J. 2017;15:257–268. doi: 10.1111/pbi.12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowe K, Wu E, Wang N, Hoerster G, Hastings C, Cho M-J, Scelonge C, Lenderts B, Chamberlin M, Cushatt J, et al. Morphogenic regulators Baby boom and Wuschel improve monocot transformation. Plant Cell. 2016;28:1998–2015. doi: 10.1105/tpc.16.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lowe K, La Rota M, Hoerster G, Hastings C, Wang N, Chamberlin M, Wu E, Jones T, Gordon-Kamm W.. Rapid genotype “independent” Zea mays L. (maize) transformation via direct somatic embryogenesis In Vitro Cell Dev Biol Plant. 2018;54:240–252. doi: 10.1007/s11627-018-9905-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ziemann S, van der Linde K, Lahrmann U, Acar B, Kaschani F, Colby T, Kaiser M, Ding Y, Schmelz E, Huffaker A, et al. An apoplastic peptide activates salicylic acid signalling in maize. Nat Plants. 2018;4:172–180. doi: 10.1038/s41477-018-0116-y. [DOI] [PubMed] [Google Scholar]
- 7.van der Linde K, Timofejeva L, Egger RL, Ilau B, Hammond R, Teng C, Meyers BC, Doehlemann G, Walbot V. Pathogen Trojan horse delivers bioactive host protein to alter maize (Zea mays) anther cell behavior in situ. Plant Cell. 2018;30:528–542. doi: 10.1105/tpc.17.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doehlemann G, Wahl R, Horst RJ, Voll LM, Usadel B, Poree F, Stitt M, Pons-Kühnemann J, Sonnewald U, Kahmann R, et al. Reprogramming a maize plant: transcriptional and metabolic changes induced by the fungal biotroph Ustilago maydis. Plant J. 2008b;56:181–195. doi: 10.1111/j.1365-313X.2008.03590.x. [DOI] [PubMed] [Google Scholar]
- 9.Doehlemann G, Wahl R, Vraneš M, de Vries RP, Kämper J, Kahmann R. Establishment of compatibility in the Ustilago maydis/maize pathosystem. J Plant Physiol. 2008a;165:29–40. doi: 10.1016/j.jplph.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Kämper J, Kahmann R, Bölker M, Ma LJ, Brefort T, Saville BJ, Banuett F, Kronstad JW, Gold SE, Müller O, et al. Insights from the genome of the biotrophic fungal plant pathogen Ustilago maydis. Nature. 2006;444:97–101. doi: 10.1038/nature05248. [DOI] [PubMed] [Google Scholar]
- 11.Koepke J, Kaffarnik F, Haag C, Zarnack K, Luscombe NM, König J, Ule J, Kellner R, Begerow D, Feldbrügge M. The RNA-binding protein Rrm4 is essential for efficient secretion of endochitinase Cts1. Mol Cell Proteomics. 2011;10:M111.011213. doi: 10.1074/mcp.M111.011213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krombach S, Reissmann S, Kreibich S, Bochen F, Kahmann R. Virulence function of the Ustilago maydis sterol carrier protein 2. New Phytol. 2018;220:553–566. doi: 10.1111/nph.15268. [DOI] [PubMed] [Google Scholar]
- 13.Stock J, Sarkari P, Kreibich S, Brefort T, Feldbrügge M, Schipper K. Applying unconventional secretion of the endochitinase Cts1 to export heterologous proteins in Ustilago maydis. J Biotechnol. 2012;161:80–91. doi: 10.1016/j.jbiotec.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Stock J, Terfrüchte M, Schipper K. A reporter system to study unconventional secretion of proteins avoiding N-glycosylation in Ustilago maydis. Methods Mol Biol. 2016;1459:149–160. doi: 10.1007/978-1-4939-3804-9_10. [DOI] [PubMed] [Google Scholar]
- 15.Terfrüchte M, Reindl M, Jankowski S, Sarkari P, Feldbrügge M, Schipper K. Applying unconventional secretion in Ustilago maydis for the export of functional nanobodies. Int J Mol Sci. 2017;18:937. doi: 10.3390/ijms18050937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarkari P, Feldbrügge M, Schipper K. The corn smut fungus Ustilago maydis as an alternative expression system for biopharmaceuticals In: Schmoll M, Dattenböck C editors. Gene expression systems in fungi: advancements and applications. Cham: Springer International Publishing; 2016. p. 183–200. [Google Scholar]
- 17.Fiedler I-C, Weiberg A, van der Linde K. Guidelines for using Ustilago maydis as a Trojan horse for in situ delivery of maize proteins. JoVE. accepted. [DOI] [PubMed] [Google Scholar]
- 18.Djamei A, Schipper K, Rabe F, Ghosh A, Vincon V, Kahnt J, Osorio S, Tohge T, Fernie AR, Feussner I, et al. Metabolic priming by a secreted fungal effector. Nature. 2011;478:395–398. doi: 10.1038/nature10454. [DOI] [PubMed] [Google Scholar]
- 19.Egger RL, Walbot V. A framework for evaluating developmental defects at the cellular level: an example from ten maize anther mutants using morphological and molecular data. Dev Biol. 2016;419:26–40. doi: 10.1016/j.ydbio.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Kelliher T, Walbot V. Emergence and patterning of the five cell types of the Zea mays anther locule. Dev Biol. 2011;350:32–49. doi: 10.1016/j.ydbio.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelliher T, Walbot V. Hypoxia triggers meiotic fate acquisition in maize. Science. 2012;337:345–348. doi: 10.1126/science.1220080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walbot V, Egger RL. Pre-meiotic anther development: cell fate specification and differentiation. Annu Rev Plant Biol. 2016;67:365–395. doi: 10.1146/annurev-arplant-043015-111804. [DOI] [PubMed] [Google Scholar]
- 23.Kelliher T, Egger RL, Zhang H, Walbot V. Unresolved issues in pre-meiotic anther development. Front Plant Sci. 2014;5. doi: 10.3389/fpls.2014.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao L, Kelliher T, Nguyen L, Walbot V. Ustilago maydis reprograms cell proliferation in maize anthers. Plant J. 2013;75:903–914. doi: 10.1111/tpj.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Egger RL, Walbot V. Quantifying Zea mays tassel development and correlation with anther developmental stages as a guide for experimental studies. Maydica. 2015;60:M34. [Google Scholar]
- 26.Skibbe DS, Doehlemann G, Fernandes J, Walbot V. Maize tumors caused by Ustilago maydis require organ-specific genes in host and pathogen. Science. 2010;328:89–92. doi: 10.1126/science.1185775. [DOI] [PubMed] [Google Scholar]
- 27.Redkar A, Hoser R, Schilling L, Zechmann B, Krzymowska M, Walbot V, Doehlemann G. A secreted effector protein of Ustilago maydis guides maize leaf cells to form tumors. Plant Cell. 2015;27:1332–1351. doi: 10.1105/tpc.114.131086. [DOI] [PMC free article] [PubMed] [Google Scholar]


