Abstract
Tamoxifen is a lifesaving treatment for millions of breast cancer patients worldwide. Yet taking tamoxifen may be challenging for some patients due to issues of compliance, drug interactions, and surgical considerations. Educating patients with a one-page teaching sheet, "Precautions for Patients Taking Tamoxifen," may improve tamoxifen’s effectiveness and prevent complications. Advanced practitioners are in a position to prescribe tamoxifen, review medication interactions, educate patients, impact patients’ quality of life, improve patients’ sense of control, and increase patients’ partnerships with their oncology providers.
Tamoxifen is the most widely prescribed breast cancer therapy; millions of patients take tamoxifen worldwide. Tamoxifen is an oral medication that treats hormone-sensitive breast cancers. A hormone-sensitive breast cancer is estrogen receptor–positive (ER+), progesterone receptor–positive (PR+), or both. Approximately 70% of breast cancers are ER+ (Allred et al., 2012). Research has demonstrated that tamoxifen therapy reduces the annual breast cancer death rate by 31% (Early Breast Cancer Trialists’ Collaborative Group, 2005) and reduces the risk of developing a contralateral breast cancer by as much as 50% (Early Breast Cancer Trialists’ Collaborative Group, 1998).
RESEARCH
Tamoxifen is a selective estrogen receptor modulator that blocks the transcriptional activity of estrogen receptors by directly binding to them, producing a nuclear complex that decreases DNA synthesis and inhibits estrogen effects (Ali, Rasool, Cahaoudry, & Jamal, 2016). Tamoxifen has been studied since the early 1970s after it failed as a postcoital contraceptive (Jordan, 2008). It is prescribed in multiple patient diagnoses. Tamoxifen is widely used as an adjuvant therapy in early-stage invasive breast cancer and ductal carcinoma in situ (Fisher et al., 1989). It has long been shown to work in advanced breast cancer disease. It is also the most effective treatment for men with breast cancer (Fentiman, Fourquet, & Hortobagyi, 2006). In the National Surgical Adjuvant Breast and Bowel Project’s Breast Cancer Prevention Trial, tamoxifen reduced the incidence of breast cancer in women who are at high risk for breast cancer (Fischer et al., 1998). It has long been known to be effective in postmenopausal women who are not able to tolerate aromatase inhibitors. Ongoing trials report that 10 years of tamoxifen is better than 5 years (Davises et al., 2013). Both the Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) trial, which enrolled 6,846 women, and the adjuvant Tamoxifen—To offer more? (aTTom) trial, which enrolled 6,953 women, reinforce the evidence that more than 5 years of tamoxifen is beneficial (Azvolinsky, 2013). The combined data showed an additional 25% reduction in breast cancer mortality 10 years and beyond the diagnosis of breast cancer. In fact, reduced mortality from cancer was seen 10 to 14 years after a breast cancer diagnosis. Ongoing tamoxifen studies will continue to report findings with follow-up of thousands of women for many years to come.
Tamoxifen is the backbone for breast cancer treatment; however, it poses challenges for patients and providers. A daily intake of tamoxifen is necessary for improved long-term survival, but noncompliance compromises survival.
PATIENT COMPLIANCE
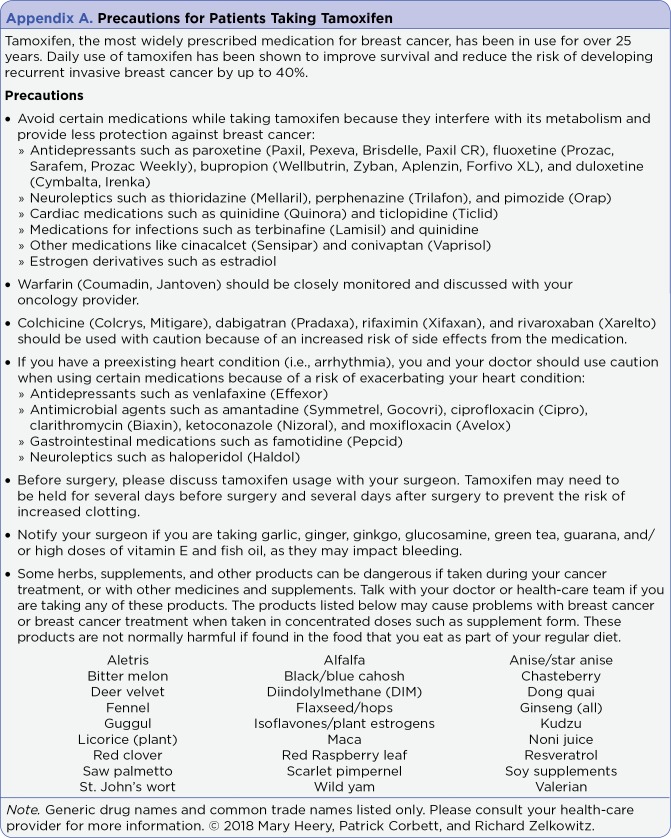
Tamoxifen’s effectiveness is well documented, but it is not without its concerns. There are safety and efficacy issues when taking tamoxifen with other medications. Patient education reduces complications and improves tamoxifen’s effectiveness, resulting in improved long-term survival. The patient teaching sheet, "Precautions for Patients Taking Tamoxifen" (see Appendix A), serves as a reference for patients and providers on which medications and supplements to avoid while taking tamoxifen.
Patient education is mandatory for medication adherence and the improvement of long-term survival. In a thorough literature review, Chlebowski, Kim, and Haque (2014) report that 30% to 80% of women do not take their daily tamoxifen. Multiple strategies may be implemented to increase compliance, including follow-up phone calls, pill containers, reviewing when prescriptions were filled, encouraging taking medication at the same time every day, changing the time of day (morning to night) to reduce side effects, and e-mail or texting reminders. Meeting with oncology providers on a regular basis makes patients accountable, encourages addressing patient concerns, educates patients on new research reports, and supports healthy behaviors.
ADVERSE EFFECTS
A major factor in adherence to tamoxifen is the adverse effects that patients experience. Patients who felt poorly informed regarding adverse effects with any adjuvant hormonal breast cancer therapy were more likely to stop therapy prematurely (Kahn, Schneider, Malin, Adams, & Epstein, 2007). Hot flashes are the most commonly reported adverse reaction, which is thought to be related to the central nervous system antiestrogenic effects causing thermoregulatory dysfunction (Stearns et al., 2002). Tamoxifen has been shown to be associated with an increased rate of venous thromboembolic events (Early Breast Cancer Trialists’ Collaborative Group, 1998). There is an increased risk of both endometrial cancer and uterine sarcoma with tamoxifen. These results are of statistical significance, but it is noted that 10 years of tamoxifen prevents 30 times as many breast cancer deaths (Azvolinsky, 2013). Also, with early detection, endometrial and uterine cancers can be treated. Endometrial hyperplasia, endometriosis, polyps, and uterine fibroids have occurred, resulting in reports of abnormal bleeding. Liver abnormalities such as fatty liver, cholestasis, and hepatic necrosis have occurred in rare instances. Reports of visual acuity, corneal changes and color perception changes should be promptly investigated. Tamoxifen is associated with other adverse events including nausea, vomiting, weight loss, weight gain, sexual dysfunction, arthralgia hypertension, and lymphedema in premenopausal women. There is a noted decline in bone mineral density, which may be associated with an increased risk of fractures (Watson Laboratories, 2011). Although all of these concerns are valid and documented, appropriate screening and education can reduce complications and patient concerns.
INTERACTIONS
Many patients are on medications that may interfere with the effectiveness of tamoxifen. Antidepressants such as paroxetine, fluoxetine, bupropion, and duloxetine may reduce the effectiveness of tamoxifen by inhibiting the conversion of tamoxifen to its active metabolites by inhibition of the cytochrome P450 2D6 (Jin et al., 2005; Kelly et al., 2010; Sideras et al., 2010). Neuroleptics such as thioridazine, perphenazine, and pimozide; certain anti-antimicrobials such as terbinafine and quinidine; and other medications like cinacalcet may also reduce the effectiveness of tamoxifen by inhibiting the conversion of tamoxifen to its active metabolites by the inhibition of the cytochrome P450 2D6 (Sideras et al., 2010). Other antimicrobials such as moxifloxacin and ciprofloxacin may impact cardiac function, especially with a preexisting condition such as an arrhythmia (Slovacek, Ansorgova, Macingova, Haman, & Petera, 2008). Cardiac medications should be reviewed with cardiologists, as certain medications may exacerbate heart conditions (Crewe, Ellis, Lennard, & Tucker, 1997; Iusuf et al., 2011; Mizutani et al., 2008; Slovacek et al., 2008; Tenni, Lalich, & Byrne, 1989). Whenever patients start new medications, it is important to remind them to review their current medications with their oncology provider.
SURGICAL CONSIDERATIONS
Advanced practitioners should discuss holding tamoxifen for several days before and possibly up to 2 weeks following surgery. The combination of surgery and tamoxifen increases the risk of venous thromboembolism. Tamoxifen plasma levels decline with an elimination half-life of 7 to 14 days (Hussain & Kneeshaw, 2012). Additionally, tamoxifen and its metabolite, 4-hydroxytamoxifen, significantly inhibited the ability of platelet aggregation (Johnson et al., 2017). In certain surgical situations, tamoxifen may be held to prevent such complications. Input from both the surgeon and the medical oncologist regarding the risks and benefits should be discussed and an appropriate plan shared with the patient.
Supplements of garlic, ginger, ginkgo, glucosamine, green tea, guarana and/or high doses of vitamin E and fish oil should also be stopped before and after surgery, as they may interfere with platelet function (Ang-Lee, Moss, & Yuan, 2001; Backon, 1991; Benjamin, Muir, Briggs, & Pentland, 2001; Bydlowski, D’Amico, & Chamone, 1991; Bydlowski, Yunker, & Subbiah, 1988; Carden, Good, Carden, & Good, 2002; Chamone et al., 1987; Knudsen & Sokol, 2008; Liede, Haukka, Saxen, & Heinonen, 1998; McMahon & Vargas, 1993; Pizzorno & Murray, 1999; Rosenblatt & Mindel, 1997; Rowin & Lewis, 1996; Sotaniemi, Haapakoski, & Rautio, 1995; Srivastava, 1989; Thomson et al., 2002; Vanschoonbeek et al., 2004; Vuksan et al., 2000).
Some herbs, supplements, and other products can be dangerous for patients taking tamoxifen. Although certain products (see Appendix A) are safe when taken as part of a regular diet, they may cause problems when they are taken as supplements in a concentrated form (Pizzorno & Murray, 1999).
EDUCATION
Patient education provides patients with some control over their tamoxifen management. Medication management is challenging for patients who don’t have the pharmacologic knowledge to understand drug interaction. Patients trust and rely on their providers to educate, guide and support them through their tamoxifen tenure. Reinforcing verbal education with a resource such as the "Precautions for Patients Taking Tamoxifen" handout provides a quick reference for patients and staff.
Appendix A.
Precautions for Patients Taking Tamoxifen
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Ali Shazia, Rasool Mahmood, Chaoudhry Hani, N Pushparaj Peter, Jha Prakash, Hafiz Abdul, Mahfooz Maryam, Abdus Sami Ghufrana, Azhar Kamal Mohammad, Bashir Sania, Ali Ashraf, Sarwar Jamal Mohammad. Molecular mechanisms and mode of tamoxifen resistance in breast cancer. Bioinformation. 2016;12:135–139. doi: 10.6026/97320630012135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allred D Craig, Anderson Stewart J, Paik Soonmyung, Wickerham D Lawrence, Nagtegaal Iris D, Swain Sandra M, Mamounas Elefetherios P, Julian Thomas B, Geyer Charles E, Costantino Joseph P, Land Stephanie R, Wolmark Norman. Adjuvant tamoxifen reduces subsequent breast cancer in women with estrogen receptor-positive ductal carcinoma in situ: a study based on NSABP protocol B-24. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:1268–1273. doi: 10.1200/JCO.2010.34.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ang-Lee M K, Moss J, Yuan C S. Herbal medicines and perioperative care. JAMA. 2001;286:208–216. doi: 10.1001/jama.286.2.208. [DOI] [PubMed] [Google Scholar]
- 4.Azvolinsky A. ASCO: Long-term tamoxifen benefit for breast cancer confirmed. 2013 Retrieved from http://www.cancernetwork.com/breast-cancer/asco-long-term-tamoxifen-benefit-breast-cancer-confirmed.
- 5.Backon J. Ginger as an antiemetic: possible side effects due to its thromboxane synthetase activity. Anaesthesia. 1991;46:705–706. doi: 10.1111/j.1365-2044.1991.tb09754.x. [DOI] [PubMed] [Google Scholar]
- 6.Benjamin J, Muir T, Briggs K, Pentland B. A case of cerebral haemorrhage-can Ginkgo biloba be implicated? Postgraduate medical journal. 2001;77:112–113. doi: 10.1136/pmj.77.904.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bydlowski S P, D’Amico E A, Chamone D A. An aqueous extract of guaraná (Paullinia cupana) decreases platelet thromboxane synthesis. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas. 1991;24:421–424. [PubMed] [Google Scholar]
- 8.Bydlowski S P, Yunker R L, Subbiah M T. A novel property of an aqueous guaraná extract (Paullinia cupana): inhibition of platelet aggregation in vitro and in vivo. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas. 1988;21:535–538. [PubMed] [Google Scholar]
- 9.Carden Susan M, Good William V, Carden Patricia A, Good Robert M. Garlic and the strabismus surgeon. Clinical & experimental ophthalmology. 2002;30:303–304. doi: 10.1046/j.1442-9071.2002.00540.x. [DOI] [PubMed] [Google Scholar]
- 10.Chamone D A F, Ivanysilva M, Cassaro C, Bellotti G, Massumoto C M, Hoshikawafujimura A Y. Guaraná (Paullinia cupana) inhibits aggregation in whole blood. Thrombosis and Haemostasis. 1987;58(1):474. [Google Scholar]
- 11.Chlebowski Rowan T, Kim Jisang, Haque Reina. Adherence to endocrine therapy in breast cancer adjuvant and prevention settings. Cancer prevention research (Philadelphia, Pa.) 2014;7:378–387. doi: 10.1158/1940-6207.CAPR-13-0389. [DOI] [PubMed] [Google Scholar]
- 12.Crewe H K, Ellis S W, Lennard M S, Tucker G T. Variable contribution of cytochromes P450 2D6, 2C9 and 3A4 to the 4-hydroxylation of tamoxifen by human liver microsomes. Biochemical pharmacology. 1997;53:171–178. doi: 10.1016/s0006-2952(96)00650-8. [DOI] [PubMed] [Google Scholar]
- 13.Davies Christina, Pan Hongchao, Godwin Jon, Gray Richard, Arriagada Rodrigo, Raina Vinod, Abraham Mirta, Medeiros Alencar Victor Hugo, Badran Atef, Bonfill Xavier, Bradbury Joan, Clarke Michael, Collins Rory, Davis Susan R, Delmestri Antonella, Forbes John F, Haddad Peiman, Hou Ming-Feng, Inbar Moshe, Khaled Hussein, Kielanowska Joanna, Kwan Wing-Hong, Mathew Beela S, Mittra Indraneel, Müller Bettina, Nicolucci Antonio, Peralta Octavio, Pernas Fany, Petruzelka Lubos, Pienkowski Tadeusz, Radhika Ramachandran, Rajan Balakrishnan, Rubach Maryna T, Tort Sera, Urrútia Gerard, Valentini Miriam, Wang Yaochen, Peto Richard. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet (London, England) 2013;381:805–816. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet (London, England) 1998;351:1451–1467. [PubMed] [Google Scholar]
- 15.Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomized trials. Lancet. 2005;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 16.Fentiman Ian S, Fourquet Alain, Hortobagyi Gabriel N. Male breast cancer. Lancet (London, England) 2006;367:595–604. doi: 10.1016/S0140-6736(06)68226-3. [DOI] [PubMed] [Google Scholar]
- 17.Fisher B, Costantino J, Redmond C, Poisson R, Bowman D, Couture J, Dimitrov N V, Wolmark N, Wickerham D L, Fisher E R. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. The New England journal of medicine. 1989;320:479–484. doi: 10.1056/NEJM198902233200802. [DOI] [PubMed] [Google Scholar]
- 18.Fisher B, Costantino J P, Wickerham D L, Redmond C K, Kavanah M, Cronin W M, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. Journal of the National Cancer Institute. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 19.Hussain Tasadooq, Kneeshaw Peter J. Stopping tamoxifen peri-operatively for VTE risk reduction: a proposed management algorithm. International journal of surgery (London, England) 2012;10:313–316. doi: 10.1016/j.ijsu.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Iusuf Dilek, Teunissen Sebastiaan F, Wagenaar Els, Rosing Hilde, Beijnen Jos H, Schinkel Alfred H. P-glycoprotein (ABCB1) transports the primary active tamoxifen metabolites endoxifen and 4-hydroxytamoxifen and restricts their brain penetration. The Journal of pharmacology and experimental therapeutics. 2011;337:710–717. doi: 10.1124/jpet.110.178301. [DOI] [PubMed] [Google Scholar]
- 21.Johnson Kelly E, Forward Jodi A, Tippy Mason D, Ceglowski Julia R, El-Husayni Saleh, Kulenthirarajan Rajesh, Machlus Kellie R, Mayer Erica L, Italiano Joseph E, Battinelli Elisabeth M. Tamoxifen Directly Inhibits Platelet Angiogenic Potential and Platelet-Mediated Metastasis. Arteriosclerosis, thrombosis, and vascular biology. 2017;37:664–674. doi: 10.1161/ATVBAHA.116.308791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jordan V Craig. Tamoxifen: catalyst for the change to targeted therapy. European journal of cancer (Oxford, England : 1990) 2008;44:30–38. doi: 10.1016/j.ejca.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin Yan, Desta Zeruesenay, Stearns Vered, Ward Bryan, Ho Herbert, Lee Kyung-Hoon, Skaar Todd, Storniolo Anna Maria, Li Lang, Araba Adjei, Blanchard Rebecca, Nguyen Anne, Ullmer Lynda, Hayden Jill, Lemler Suzanne, Weinshilboum Richard M, Rae James M, Hayes Daniel F, Flockhart David A. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. Journal of the National Cancer Institute. 2005;97:30–39. doi: 10.1093/jnci/dji005. [DOI] [PubMed] [Google Scholar]
- 24.Kahn Katherine L, Schneider Eric C, Malin Jennifer L, Adams John L, Epstein Arnold M. Patient centered experiences in breast cancer: predicting long-term adherence to tamoxifen use. Medical care. 2007;45:431–439. doi: 10.1097/01.mlr.0000257193.10760.7f. [DOI] [PubMed] [Google Scholar]
- 25.Kelly C M, Juurlink D N, Gomes T, Duong-Hua M, Pritchard K I, Austin P C, Paszat L F. Selective serotonin reuptake inhibitors and breast cancer mortality in women receiving tamoxifen: A population based cohort study. British Medical Journal. 2010;340:c693. doi: 10.1136/bmj.c693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knudsen James F, Sokol Gerald H. Potential glucosamine-warfarin interaction resulting in increased international normalized ratio: case report and review of the literature and MedWatch database. Pharmacotherapy. 2008;28:540–548. doi: 10.1592/phco.28.4.540. [DOI] [PubMed] [Google Scholar]
- 27.Liede K E, Haukka J K, Saxén L M, Heinonen O P. Increased tendency towards gingival bleeding caused by joint effect of alpha-tocopherol supplementation and acetylsalicylic acid. Annals of medicine. 1998;30:542–546. [PubMed] [Google Scholar]
- 28.McMahon F G, Vargas R. Can garlic lower blood pressure? A pilot study. Pharmacotherapy. 1993;13(4):406–407. [PubMed] [Google Scholar]
- 29.Mizutani Takaharu, Masuda Masatoshi, Nakai Emi, Furumiya Kenji, Togawa Hiroshi, Nakamura Yutaka, Kawai Yuko, Nakahira Keiko, Shinkai Shigeko, Takahashi Kazuhiko. Genuine functions of P-glycoprotein (ABCB1). Current drug metabolism. 2008;9:167–174. doi: 10.2174/138920008783571756. [DOI] [PubMed] [Google Scholar]
- 30.Pizzorno J E, Murray M T. Textbook of natural medicine. 2nd ed. New York, NY: Churchill Livingston; 1999. [Google Scholar]
- 31.Rosenblatt M, Mindel T. Spontaneous hyphema associated with ingestion of Ginkgo biloba extract. New England Journal of Medicine. 1997;336:1108. doi: 10.1056/NEJM199704103361518. [DOI] [PubMed] [Google Scholar]
- 32.Rowin J, Lewis S L. Spontaneous bilateral subdural hematomas associated with chronic Ginkgo biloba ingestion. Neurology. 1996;46:1775–1776. doi: 10.1212/wnl.46.6.1775. [DOI] [PubMed] [Google Scholar]
- 33.Sideras Kostandinos, Ingle James N, Ames Matthew M, Loprinzi Charles L, Mrazek David P, Black John L, Weinshilboum Richard M, Hawse John R, Spelsberg Thomas C, Goetz Matthew P. Coprescription of tamoxifen and medications that inhibit CYP2D6. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:2768–2776. doi: 10.1200/JCO.2009.23.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silagy C A, Neil H A. A meta-analysis of the effect of garlic on blood pressure. Journal of hypertension. 1994;12:463–468. [PubMed] [Google Scholar]
- 35.Slovacek L, Ansorgova V, Macingova Z, Haman L, Petera J. Tamoxifen-induced QT interval prolongation. Journal of clinical pharmacy and therapeutics. 2008;33:453–455. doi: 10.1111/j.1365-2710.2008.00928.x. [DOI] [PubMed] [Google Scholar]
- 36.Sotaniemi E A, Haapakoski E, Rautio A. Ginseng therapy in non-insulin-dependent diabetic patients. Diabetes care. 1995;18:1373–1375. doi: 10.2337/diacare.18.10.1373. [DOI] [PubMed] [Google Scholar]
- 37.Srivastava K C. Effect of onion and ginger consumption on platelet thromboxane production in humans. Prostaglandins, leukotrienes, and essential fatty acids. 1989;35:183–185. doi: 10.1016/0952-3278(89)90122-1. [DOI] [PubMed] [Google Scholar]
- 38.Stearns Vered, Ullmer Lynda, López Juan F, Smith Yolanda, Isaacs Claudine, Hayes DanielF. Hot flushes. Lancet (London, England) 2002;360:1851–1861. doi: 10.1016/s0140-6736(02)11774-0. [DOI] [PubMed] [Google Scholar]
- 39.Tenni P, Lalich D L, Byrne M J. Life threatening interaction between tamoxifen and warfarin. British Medical Journal. 1989;298(6666):93. doi: 10.1136/bmj.298.6666.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomson M, Al-Qattan K K, Al-Sawan S M, Alnaqeeb M A, Khan I, Ali M. The use of ginger (Zingiber officinale Rosc.) as a potential anti-inflammatory and antithrombotic agent. Prostaglandins, leukotrienes, and essential fatty acids. 2002;67:475–478. doi: 10.1054/plef.2002.0441. [DOI] [PubMed] [Google Scholar]
- 41.Vanschoonbeek Kristof, Feijge Marion A H, Paquay Martine, Rosing Jan, Saris Wim, Kluft Cornelis, Giesen Peter L A, de Maat Moniek P M, Heemskerk Johan W M. Variable hypocoagulant effect of fish oil intake in humans: modulation of fibrinogen level and thrombin generation. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:1734–1740. doi: 10.1161/01.ATV.0000137119.28893.0b. [DOI] [PubMed] [Google Scholar]
- 42.Vuksan V, Sievenpiper J L, Koo V Y, Francis T, Beljan-Zdravkovic U, Xu Z, Vidgen E. American ginseng (Panax quinquefolius L) reduces postprandial glycemia in nondiabetic subjects and subjects with type 2 diabetes mellitus. Archives of internal medicine. 2000;160:1009–1013. doi: 10.1001/archinte.160.7.1009. [DOI] [PubMed] [Google Scholar]
- 43.Watson Laboratories. Tamoxifen citrate package insert. 2011 Retrieved from https://medlibrary.org/lib/rx/meds/tamoxifen-citrate-5/