ABSTRACT
In non-cyanogenic plants, cyanide is produced during ethylene biosynthesis and is mainly detoxified by the ß-cyanoalanine synthase CAS-C1. Arabidopsis plants lacking CAS-C1 show abnormal root hairs, which stop growing at early stages. Root hair elongates by polarized cell expansion at the tip, and we have observed that CAS-C1-driven GFP fluorescence locates in mitochondria and accumulates in root hair tips during root hair elongation. Genetic crosses have been performed between cas-c1 plants and scn1-1 mutants, defective in the SCN1 protein that regulates the NADPH oxidase RHD2/AtrbohC, and between cas-c1 and rhd2-1, defective in the NADPH oxidase necessary for the generation of ROS and the Ca2+ gradient necessary for root hair elongation. The phenotypic and molecular analysis of these crosses indicates that cas-c1 is hypostatic to scn1-1 and epistatic to rhd2-1. Furthermore, the action of cyanide in root hair development is independent of ROS and of direct NADPH oxidase inhibition by cyanide.
KEYWORDS: Cyanide, mitochondria, CAS-C1, cyanide, root hair, NADPH oxidase, SCN1, cell signaling
Mitochondria are double-membrane-bound organelle found in most eukaryotic organisms including plants. Mitochondria oxidize carbon fuels to form cellular energy through the oxidative phosphorylation, which requires electron transfer through several large protein complexes, some of which pump protons, forming a proton gradient that powers the synthesis of ATP. The generation of ATP through mitochondrial respiration also produces reactive oxygen species (ROS). In excess, ROS can damage the cell, but they are also an important signal produced in response to varied stresses.1 In addition to the classical electron transport chain (ETC), plant mitochondria possess an alternative ETC that modulates mitochondrial ROS production.2 Cyanide is a disruptor of the ETC because it binds to the heme iron of cytochrome c oxidase, thereby impairing vital functions.3
Despite its toxicity, cyanide has been proposed to act as a regulator of several biological processes such as seed dor-mancy and germination;4–8 plant immunity9–12 and root hair development.13 Our previous work has shown that Arabidopsis null mutants in the mitochodrial ß-cyanoalanine synthase, CAS-C1, accumulate cyanide to non-toxic level because the plant is completely viable but shows a root hairless phenotype suggesting a signaling role of CAS-C1 and cyanide in root development.7,13 cas-c1 root hair bulges start growing correctly but they do not elongate and form malformed protuberances. The root hairless phenotype is phenocopied when wild type plants grow in the presence of cyanide and is reverted in cas-c1 in the presence of the antidote hydroxocobalamin.13
Plant root hairs are tubular protrusions of root epidermal cells that increase the root surface to absorb nutrients and water, establish relationships with soil microbiota and contribute to anchoring the plant to the soil. An epidermal cell is differentiated to a root hair following a well-studied process involving position-dependent signaling and molecular feedback loops.14 Once differentiated, the root hair elongates unidirectionally through polarized cell expansion, similarly to other polarized cell growth such as pollen tube or filamentous fungi growth. Root hair can reach growth rates of more than 1 µm min−1,15 which implies, that an extremely efficient mechanism for the delivery and modification of cell wall at the tip of the root hair and not along the shanks is taking place. The polar growth has been extensively studied and it is well known that polysaccharides, proteins and other molecules necessary for the cell wall formation are enclosed in vesicles that are directed to the cell tip by cytoplasmic streamings.14,16–20 pH, ROS and calcium gradient signaling are involved in the establishment and organization of this traffic.14,18,20–22 For this, the endoplasmic reticulum and the Golgi apparatus are important for the formation of vesicles and mitochondria provide the high amounts of energy required for the active transport and are calcium stores that move along the cytoskeleton to provide Ca2+ to the cell tip.23,24 Therefore, the subapical zone of the root hair is rich in these organelles including mitochondria.17,18,25
To deepen in the function of CAS-C1 in the root hair growth and to delimit the position of the action of this protein in this process, several cellular and genetic strategies have been used. First of all, total ATP levels of wild type and cas-c1 plants have been measured, and no differences have been found, suggesting that cas-c1 mutants, despite they accumulate high levels of cyanide, have an appropriate energy homeostasis. Plants expressing a fusion protein CAS-C1-GFP under the control of the cas-c1 promoter have been constructed, showing that CAS-C1 locates in mitochondria and accumulates in the subapical region of the root hair tip, suggesting that it could have a function in maintaining the appropriate levels of cyanide for root tip elongation.
Mutants affected in essential early steps of the root hair elongation have been crossed with cas-c1 in order to establish the epistatic relationships between them. SUPERCENTIPEDE (SCN1) is a Rho GTPase GDP dissociator inhibitor that acts as a negative regulator of Rho-related GTPases (ROPs). ROPs are essential for the regulation of the activity of the NADPH oxidase RHD2/AtbrohC, which produces ROS specifically at the root hair tip and therefore stimulates local Ca2+ influx. The appropriate localization of the RHD2 activity at the root hair tip guarantees the correct elongation of the root hair, thus mutants in SCN1 show supernumerary bulges that do not elongate,26 whereas the phenotype of rhd2 mutants is similar to cas-c1, i.e., it has abnormal short root hairs.27 Double mutants scn1-1 cas-c1 and rhd2-1 cas-c1 have been generated and their phenotypic analysis has established that cas-c1 mutation is hypostatic to scn1-1 and epistatic to rhd2-1, since the phenotype of the double scn1-1 cas-c1 mutant is identical to the single scn1-1 mutant and the phenotype of the double rhd2-1 cas-c1 is identical to the single cas-c1 mutant (Figure 1). Hydroxocobalamin treatment reverts the rhd2-1 cas-c1 phenotype to rhd2-1, whereas it has no effect in the double scn1-1 cas-c1 mutant or the scn1-1 single mutant. Molecular data support this epistatic relationship because several genes involved in the root hair development are not significantly affected by the cas-c1 mutation in a scn1-1 background while they show a further decrease compared to the single rhd2-1 mutation when combining the rhd2-1 and cas-c1 mutations.
Figure 1.
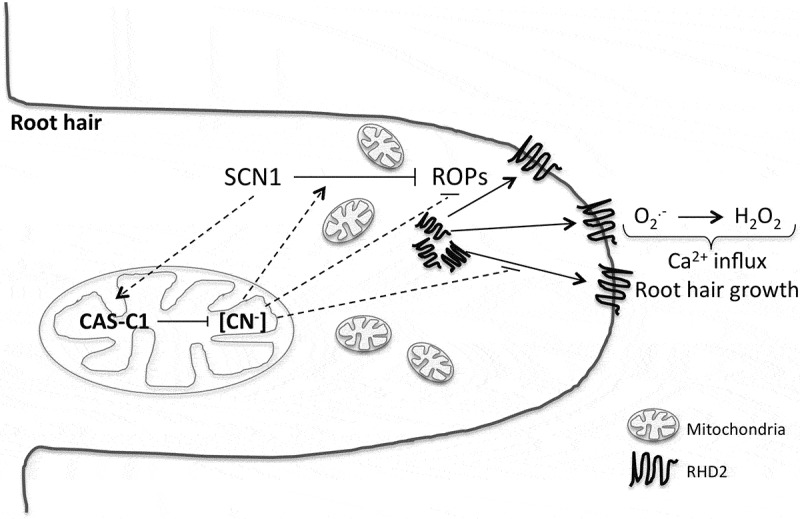
Mitochondria, which are located at the subapical zone of the elongating root hair, possess a mechanism for cyanide detoxification based in the CAS-C1 protein. Cyanide action in the root hair elongation process is exerted in a step between the SCN1 action, which inhibits ROP proteins, and the RHD2 NADPH oxidase action, which is regulated by ROPs and is essential for ROS production, Ca2+ influx and, ultimately, polar root hair growth. Full lines: established relationships; dashed lines: proposed relationships.
Finally, the effect of the cas-c1 mutation is independent of ROS production, since the double rhd2-1 cas-c1 mutant has a cas-c1 phenotype but it shows neither O2− nor H2O2 staining. Moreover, it has been confirmed that the NADPH oxidase activity is not inhibited by cyanide: root protein extracts of cas-c1, wild type and wild type extracts treated with 0,1 mM KCN show very similar NADPH oxidase activities.
In conclusion, we suggest that cyanide itself could have a signaling role. Indeed, cyanide has been proposed to act as a regulator of several biological processes4 and besides its possible role as a ROS producer for signaling purpose, a direct effect of cyanide as signaling molecule cannot be discarded.
Funding Statement
This work was supported by the Spanish Agencia Estatal de Investigación [BIO2016-76633-P].
Acknowledgments
We would like to thank the European Regional Development Fund and Agencia Estatal de Investigación (grant No. BIO2016-76633-P) for their financial support. L.A.-A. was supported by the Ministerio de Economía y Competitividad through the program of Formación de Personal Investigador.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Suzuki N, Miller G, Morales J, Shulaev V, Torres MA, Mittler R.. Respiratory burst oxidases: the engines of ROS signaling. Curr Opin Plant Biol. 2011;14:691–699. doi: 10.1016/j.pbi.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Albury MS, Elliott C, Moore AL. Towards a structural elucidation of the alternative oxidase in plants. Physiol Plant. 2009;137:316–327. doi: 10.1111/j.1399-3054.2009.01270.x. [DOI] [PubMed] [Google Scholar]
- 3.Donato DB, Nichols O, Possingham H, Moore M, Ricci PF, Noller BN. A critical review of the effects of gold cyanide-bearing tailings solutions on wildlife. Environ Int. 2007;33:974–984. S0160-4120(07)00081-5 [pii]. doi: 10.1016/j.envint.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Siegien I, Bogatek R. Cyanide action in plants - from toxic to regulatory. Acta Physiol Plant. 2006;28:483–497. doi: 10.1007/BF02706632. [DOI] [Google Scholar]
- 5.Bethke PC, Libourel LGL, Reinohl V, Jones RL. Sodium nitroprusside, cyanide, nitrite, and nitrate break Arabidopsis seed dormancy in a nitric oxide-dependent manner. Planta. 2006;223:805–812. doi: 10.1007/s00425-005-0116-9. [DOI] [PubMed] [Google Scholar]
- 6.Oracz K, El-Maarouf-Bouteau H, Bogatek R, Corbineau F, Bailly C. Release of sunflower seed dormancy by cyanide: cross-talk with ethylene signalling pathway. J Exp Bot. 2008;59:2241–2251. doi: 10.1093/jxb/ern089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia I, Gotor C, Romero LC. Beyond toxicity: A regulatory role for mitochondrial cyanide. Plant Signal Behav. 2014;9:e27612. doi: 10.4161/psb.27612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylorson RB, Hendricks SB. Promotion of seed germination by cyanide. Plant Physiol. 1973;52:23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alvarez C, Bermudez MA, Romero LC, Gotor C, Garcia I. Cysteine homeostasis plays an essential role in plant immunity. New Phytol. 2012;193:165–177. doi: 10.1111/j.1469-8137.2011.03889.x. [DOI] [PubMed] [Google Scholar]
- 10.Seo S, Mitsuhara I, Feng J, Iwai T, Hasegawa M, Ohashi Y. Cyanide, a coproduct of plant hormone ethylene biosynthesis, contributes to the resistance of rice to blast fungus. Plant Physiol. 2011;155:502–514. pp.110.162412 [pii]. doi: 10.1104/pp.110.162412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chivasa S, Carr JP. Cyanide restores N gene-mediated resistance to tobacco mosaic virus in transgenic tobacco expressing salicylic acid hydroxylase. Plant Cell. 1998;10:1489–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong CE, Carson RA, Carr JP. Chemically induced virus resistance in Arabidopsis thaliana is independent of pathogenesis-related protein expression and the NPR1 gene. Mol Plant Microbe Interact. 2002;15:75–81. doi: 10.1094/MPMI.2002.15.1.75. [DOI] [PubMed] [Google Scholar]
- 13.Garcia I, Castellano JM, Vioque B, Solano R, Gotor C, Romero LC. Mitochondrial beta-cyanoalanine synthase is essential for root hair formation in Arabidopsis thaliana. Plant Cell. 2010;22:3268–3279. doi: 10.1105/tpc.110.076828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grierson C, Nielsen E, Ketelaarc T, Schiefelbein J. Root hairs. Arabidopsis Book. 2014;12:e0172. doi: 10.1199/tab.0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ketelaar T, Faivre-Moskalenko C, Esseling JJ, de Ruijter NCA, Grierson CS, Dogterom M, Emons AMC. Positioning of nuclei in Arabidopsis root hairs: an actin-regulated process of tip growth. Plant Cell. 2002;14:2941–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ketelaar T. The actin cytoskeleton in root hairs: all is fine at the tip. Curr Opin Plant Biol. 2013;16:749–756. [DOI] [PubMed] [Google Scholar]
- 17.Carol RJ, Dolan L. Building a hair: tip growth in Arabidopsis thaliana root hairs. Philos Trans R Soc Lond B Biol Sci. 2002;357:815–821. doi: 10.1098/rstb.2002.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Datta S, Kim CM, Pernas M, Pires ND, Proust H, Tam T, Vijayakumar P, Dolan L. Root hairs: development, growth and evolution at the plant-soil interface. Plant Soil. 2011;346:1–14. doi: 10.1007/s11104-011-0845-4. [DOI] [Google Scholar]
- 19.Balcerowicz D, Schoenaers S, Vissenberg K. Cell fate determination and the switch from diffuse growth to planar polarity in Arabidopsis root epidermal cells. Front Plant Sci. 2015;6:1163. doi: 10.3389/fpls.2015.01163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salazar-Henao JE, Schmidt W. An inventory of nutrient-responsive genes in Arabidopsis root hairs. Front Plant Sci. 2016;7:237. doi: 10.3389/fpls.2016.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schiefelbein JW, Shipley A, Rowse P. Calcium influx at the tip of growing root-hair cells of Arabidopsis thaliana. Planta. 1992;187:455–459. doi: 10.1007/BF00199963. [DOI] [PubMed] [Google Scholar]
- 22.Ishida T, Kurata T, Okada K, Wada T. A genetic regulatory network in the development of trichomes and root hairs. Annu Rev Plant Biol. 2008;59:365–386. doi: 10.1146/annurev.arplant.59.032607.092949. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Zhu Y, Ling Y, Zhang H, Liu P, Baluška F, Šamaj J, Lin J, Wang Q. Disruption of actin filaments induces mitochondrial Ca2+ release to the cytoplasm and [Ca2+]c changes in Arabidopsis root hairs. BMC Plant Biol. 2010;10:53. doi: 10.1186/1471-2229-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Libault M, Brechenmacher L, Cheng J, Xu D, Stacey G. Root hair systems biology. Trends Plant Sci. 2010;15:641–650. doi: 10.1016/j.tplants.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Foreman J, Dolan L. Root hairs as a model system for studying plant cell growth. Ann Bot. 2001;88:1–7. doi: 10.1006/anbo.2001.1430. [DOI] [Google Scholar]
- 26.Carol RJ, Takeda S, Linstead P, Durrant MC, Kakesova H, Derbyshire P, Drea S, Zarsky V, Dolan L. A RhoGDP dissociation inhibitor spatially regulates growth in root hair cells. Nature. 2005;438:1013–1016. doi: 10.1038/nature04198. [DOI] [PubMed] [Google Scholar]
- 27.Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JDG, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–446. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]


