Abstract:
Low cardiac output syndrome and the systemic inflammatory response are consequences of the cardiac surgical perioperative course. The mechanisms responsible are multifactorial, but recent studies have shown that nitric oxide (NO) may be a key component in mitigating some of these processes. Following on from literature reports detailing the use of inhaled NO added to the gas phase of the extracorporeal circuit, we set about developing a technique to perform this addition safely and efficiently. In the setting of cardiopulmonary bypass, the technique was validated in a randomized prospective trial looking at 198 children. The benefits observed in this trial then stimulated the incorporation of NO into all extracorporeal life support (ECLS) circuits. This required additional hardware modifications all of which were able to be performed safely. Initial results from the first series of ECLS patients using NO also appear promising.
Keywords: cardiopulmonary bypass, inflammatory response, SIRS, low cardiac output syndrome, LCOS, nitric oxide, ECLS, ECMO
OVERVIEW
Mortality rates for children undergoing cardiac surgery have steadily decreased over the past 20 years, with literature now quoting a value around 4% for all patients (1). Despite this reduction, the morbidity associated with cardiopulmonary bypass (CPB) remains significant with little change in length of postoperative stay and up to 25% of patients developing low cardiac output syndrome (LCOS) (2). CPB is required for most cardiac surgery procedures and has been identified as being a major contributor to much of this morbidity. The large artificial surface area of the extracorporeal circuit has been shown to activate a systemic inflammatory response (SIRS) reaction which leads to capillary leak and myocardial dysfunction (3). It is this process which contributes to the development of LCOS.
LCOS is the transient decrease in systemic perfusion secondary to myocardial dysfunction and results in a shift in the balance between oxygen supply and demand (4). Its impact can be devastating, resulting in multi-organ dysfunction, cerebral ischemia, cardiac arrest, and death. The long-term effects include neurodevelopmental disabilities and renal impairment with a subsequent negative impact on health-care costs and quality of life (5).
Procedure-specific or modifiable parameters have been identified which contribute to morbidity (6). In particular, CPB flow, temperature, blood gas management, surface modifications, and the use of modified ultrafiltration have all been highlighted as possible mediators of the inflammatory response. Despite this, there has been no clear strategy to reduce the incidence of LCOS.
It had been our groups thought that nitric oxide (NO) may possess properties that could mediate the inflammatory response. We had conducted some preliminary work examining the logistics of adding NO to our CPB circuit. As part of this earlier investigation, Straub (7) examined the reactivity of platelets in pediatric patients during CPB. The premise of this study was to show that if platelet activation did occur (which the study was able to confirm) then the mediation of this process by NO supplementation would result in a decrease in the incidence of CPB-induced coagulation disturbances.
Recently, two studies have been released showing that the addition of gaseous NO into the CPB circuit resulted in myocardial protection, a reduction in the length of mechanical ventilation, and postulated that this effect may be due to the anti-inflammatory properties of NO (8,9). Researchers hypothesized that NO replacement therapy could blunt the consequences of ischemic reperfusion damage, as a reduction in NO release was a proven early marker of reperfusion injury (8).
Conventionally, gaseous NO is administered to a patient via the ventilator circuit with its point of action being the pulmonary vasculature where it exhibits a dilatory effect, thus improving arterial oxygenation and reducing pulmonary artery pressures. It is postulated that the addition of NO directly into the gas phase of the oxygenator allows NO to be delivered systemically and mediate the whole body inflammatory response induced by the extracorporeal circuit.
Encouraged by our earlier work and the results of these small trials, we sought to reproduce these techniques and then validate the efficacy in our institution with a larger cohort of patients undergoing cardiac surgery using CPB. Following this, we looked to expand the delivery technique to the extracorporeal life support (ECLS) setting which presented additional technical challenges.
DESCRIPTION
The Ikaria INOmax DSIR (Delivery System, Infrared) Plus delivery system (Mallinckrodt, Ikaria, Victoria, Australia) is a standard device used to administer NO via the inspiratory limb of a patient breathing circuit in both the operating theater and pediatric intensive care unit. We took this device and successfully modified our standard circuit to allow NO to be added to the gas line of the oxygenator. In both CPB and ECLS settings, NO was added to the gas sweep line at a dose of 20 parts per million (ppm).
CPB Setting
The standard CPB setup consisted of a Maquet HL-30 heart–lung machine and HCU-30 heater–cooler (Maquet, Hirrlingen, Germany); an electronic gas blender, EGB30-250 (Em-tec, Finning, Germany); and a Terumo Fx05 or Fx15 oxygenator (Terumo Corporation, Tokyo, Japan), depending on patient weight.
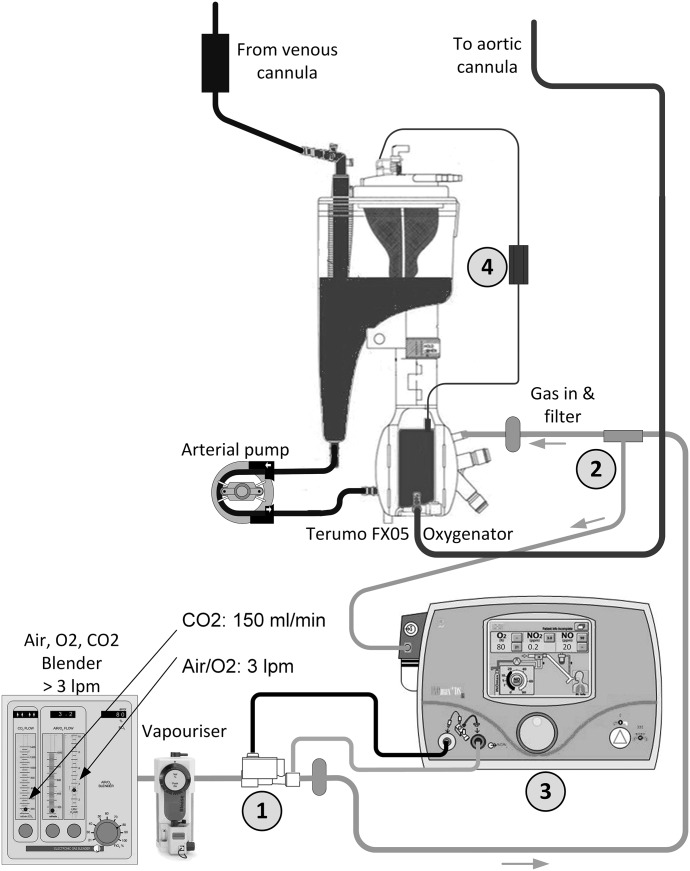
Figure 1 describes the CPB circuit configuration. Ten centimeters post electronic gas blender and volatile anesthetic vaporizer, an NO injector module was added to the gas line (point 1). This was the point of NO addition to the circuit by the INOmax DSIR. Then, 5 cm before the gas line filter and oxygenator inlet site, a ¼″ × ¼″ Luer lock connector with three-way tap was cut into the gas line (point 2). To the top of the three way tap, the sampling line of the INOmax DSIR was connected. This line returns the concentration of NO in the gas line back to the delivery device (point 3). Then by way of a feedback loop, the INOmax DSIR repeatedly adjusts the delivered concentration of NO according to the dose set at the device. The return line is continually sampled at a rate of 250 mL/min. When CPB is instituted, the gas sweep rate is set to a minimum of 3 L/min and the INOmax DSIR is set to 20 ppm. This high gas sweep rate is necessary as the INOmax DSIR must see a minimum gas flow through the injector module to accurately activate its automatic concentration titration system (in our clinical experience, 3 L/min was necessary to achieve a stable NO concentration). As a result of the high sweep gas flow rate, it was necessary to blend CO2 back into the gas line via the electronic flowmeter, with its rate guided by an inline CO2 analyzer (point 4) CDI 500 (Terumo Cardiovascular, Ann Arbor, MI).
Figure 1.
NO delivery system for CPB. 1) DSIR Plus injector module. 2) DSIR Plus sampling site. 3) INOmax DSIR Plus. 4) CDI shunt sensor.
ECLS Setting
The standard ECLS setup consisted of a Medos Deltastream MDC console and Heater Cooler Unit (Xenios, Heilbronn, Germany) and Medos DP3 pump head with Medos Hilite 2400 LT or 7000 LT oxygenator, depending on patient weight.
In contrast to the CPB setup, there existed no capability to blend CO2 back into the gas sweep line and thus compensate for the extra gas sweep flow necessary for accurate operation of the INOmax DSIR. To overcome these limitations, we developed a system which used a pressure regulator and high flow gas blow-off valve.
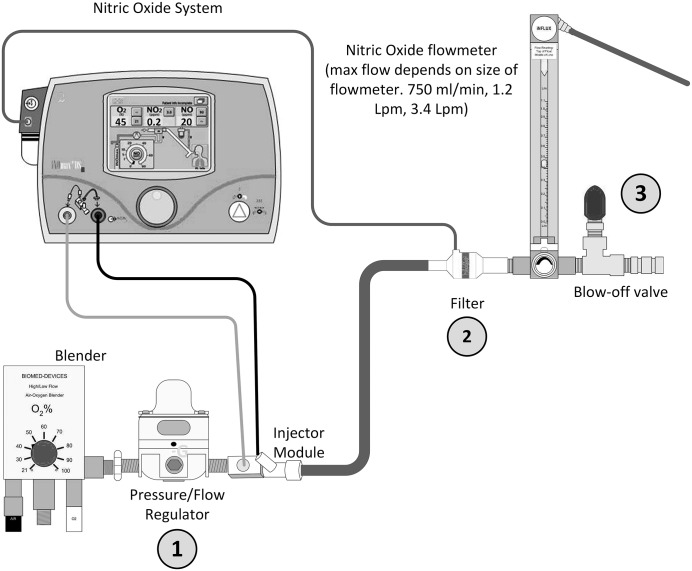
An air/oxygen blender (2001; Bio-Med Devices, Guilford, CT) is connected to the wall pendent and to this is connected a pressure regulator (point 1) (SMC IR2000-02B, Tokyo, Japan). Connected to the pressure regulator is the flow injector module (the site of nitric delivery) which is then connected to a bacterial gas filter where the NO is sampled (point 2). As in the CPB setting, for accurate operation of the INOmax DSIR, a gas flow greater than 3 L/min must be seen at this point. The filter is then connected to a flowmeter (SV1SS Uniflux; Duff & Macintosh, NSW, Australia) which is then joined to a high flow gas blow-off valve (point 3) (SS-1RS4; Hy-Lok, Houston, TX) (Figure 2).
Figure 2.
NO delivery system for ECLS incorporating pressure/flow regulator and flowmeter/blow-off valve. 1) Pressure regulator. 2) Gas filter/NO sampling point. 3) Flow meter/blow-off valve.
The difficulty we faced in developing this system occurs because of the need for a continuous 3 L/min gas flow to be delivered at the sampling point, while at the same time deliver gas sweep flows low enough to maintain a pCO2 within normal ranges. Initial prototypes we developed were prone to developing high gas line pressure due to the high gas flow rates required by the INOmax DSIR, but low flow rates required by the patient. The combination flowmeter/blow-off valve and pressure regulator enables safe delivery of patient gas as follows:
A minimum of 3 L/min gas flow is always presented to the gas filter and NO sampling site.
The patient gas flow is set to the desired rate at the gas flowmeter.
The difference between the patient gas flow and the INOmax DSIR required flow is purged via the blow off valve. E.g. patient flow .5 L/min (3 to .5) results in 2.5 L/min purged.
The pressure regulator provides a stable pressure and flow to avoid fluctuations at the patient flowmeter.
DISCUSSION
CPB
The technique described for the addition of NO into the bypass circuit was validated in our unit as being safe and easily controlled during a randomized control trial in 198 patients and is described by James et al. (10). In this study, children were randomized to receive either NO at 20 ppm via the gas phase of the CPB oxygenator or the standard air/oxygen mix. Using this technique, we observed no adverse events which could be attributed to the circuit modification or the addition of NO. The median methemoglobin (metHb) level observed in the NO group was .6% (.4–.8) compared with the control group value of .3% (.2–.4) p < .001.
NO oxidizes hemoglobin to methemoglobin, and in this form it is unable to release oxygen to the tissues. Low levels of metHb exist intrinsically in humans at less than 2% (11) with a value greater than 10% considered toxic (12). Although a difference in metHb values was detected, this study demonstrated that the exposure to NO as delivered through the gas phase of the oxygenator did not result in methemoglobinemia occurring.
The primary outcome examined in this study was the incidence of LCOS within the first 48 hours after surgery. Children who received NO were seen to have a lower incidence of LCOS (15 vs. 31%, p = .007) with the effect even more pronounced in children aged less than 2 years.
ECLS
Based on the encouraging results observed in the CPB setting, there was impetus to examine the logistics of adding NO to the ECLS circuit. The major issue faced was the need to present 3 L/min to the INOmax DSIR, without removing excessive CO2 from the patient. Initial concepts examined replicating the CPB set-up with additional CO2 blended back into the system, the use of an inline CO2 monitor, and the use of oxygenator exhaust gas analysis. The overriding desire was to develop a system which was simple to use and required minimal changes or additions to our existing circuit design and did not incur additional consumables and costs. This development process was not without challenges. An initial prototype did not contain the pressure regulator but instead included an adjustable blow-off valve. The concept was to adjust the amount of gas purged via the system in unison with any gas flow changes made at the patient flowmeter. In the laboratory, this system was manageable, but once introduced to the bedside, additional problems were encountered as over time the back pressure in the line between the flowmeter and oxygenator would increase because of condensation within the oxygenator. This resulted in more gas exiting through the blow-off valve and a resultant fluctuation in patient gas flow. In addition, it appeared that the pressure provided via the wall pendant was subject to small, transient changes. When this was coupled with a very low flow, flowmeter (0–250 mL/min), these variations were amplified and observed as instability of the bobbin within the flowmeter. This scenario was then difficult for the bed-side nurse to accurately control gas flow with frequent observations and adjustments required, which over the course of a shift would prove problematic. After a number of modifications, the issues were rectified with the addition of the pressure regulator. This presents a constant pressure (50 mmHg) to the flowmeter regardless of changes in flow at the flowmeter. Before a complete system induction, the flowmeter, blow-off valve, and pressure regulator are calibrated as a complete unit.
Over time (months), we have observed that the bobbin within the flowmeter assembly can tend to stick slightly against the side wall of the glass tube. This has necessitated periodic cleaning of the internal tube with alcohol and a cotton swab stick which always corrects the issue. It appears that this is due to NO having a slightly oily quality which builds up on the internal glass and, although not visible, causes adhesion.
The results of the first 30 patients supported with NO while on ECLS in our unit were examined in the article by Chiletti et al. (13). This analysis again found the addition of NO to be safe in terms of metHb levels. Across the 30 patients who had a median support duration of 96.7 hours, the median metHb level was observed to be 1.2% (.4–2.6). This was an important indicator of the safety of the system and NO administration, because of the duration of administration relative to the CPB setting (132 minutes) and reports of NO toxicity in neonates who received NO via the ventilation circuit, although this was associated with higher doses than 20 ppm (14,15). Chiletti then retrospectively compared the results of the first 30 patients supported with NO against the previous 101 who had not received NO into their ECLS circuit. Although only an observational report, the demographics of the two groups was the same and there was an increase in survival to discharge in the NO group (83% vs. 67%). Anecdotally, there was also a trend to a decrease in the use of blood products, length of ECLS support, and improved outcomes in patients receiving NO and going onto ECLS after cardiac arrest.
The reduction in blood product usage is a point of particular interest. Our group routinely administers Epoprostenol (Epo) as an infusion to all ECLS patient’s for its anticoagulant effects. The combination of NO and Epo has been reported to have a synergistic effect which may contribute to the improved anticoagulation management detected (16). Epo was introduced routinely in December 2015 and since the introduction of NO in April 2016, we have observed a notable decrease in the requirement to carry out a circuit change because of significant circuit thrombus. Table 1 outlines this reduction, which shows a decrease in circuit changes to only 6% of all runs or less than one circuit change for every 1,000 cumulative hours of support. This compares with the pre Epo/NO era, where one in five circuits were changed. This observation warrants further investigation, most suitably in the form of a randomized trial, but is supportive of the proposed inflammatory modulation effect offered by NO in addition to its overall effect on LCOS.
Table 1.
Circuit changes per 1,000 cumulative hours of ECMO support.
| Year | Number of ECMO Runs | Number of Circuits Changed | % of Runs Requiring Circuit Change | Circuits Changed/1,000 Hours of ECMO Support |
|---|---|---|---|---|
| 2011 | 51 | 10 | 20 | 1.5 |
| 2012 | 42 | 11 | 26 | 2.3 |
| 2013 | 48 | 11 | 23 | 2 |
| 2014 | 50 | 13 | 26 | 2.3 |
| 2015 | 56 | 9 | 16 | 1.7 |
| 2016 | 49 | 6 | 12 | 1 |
| 2017 | 69 | 4 | 6 | .5 |
ECMO, extracorporeal membrane oxygenation.
The novel method of delivery into the oxygenator sweep gas extends beyond the standard indications for use of the INOmax DSIR (17). Following on from the ethics-approved trial (10) and study (13), and when used in conjunction with the Terumo Fx and Medos Hiltite oxygenators, the absence of any identified adverse effects gives us reassurance as to the safety of the system. Individual institutions will need to validate other oxygenator combinations as currently oxygenator manufactures are unable to comment on the efficacy of NO addition to their devices.
We have successfully managed to incorporate NO into the gas line of both our CPB and ECLS circuits. Together, the ease of administration, safety of the system, and favorable outcomes have encouraged our group to pursue its use further. We are currently participating in a large multicenter randomized trial looking at the addition of NO in children undergoing CPB (18). It is hoped that the results of this trial will reveal if NO should be used routinely in this setting. Future advances will no doubt see closer consultation with the industry and the development of equipment specifically designed to be used with extracorporeal circuitry.
REFERENCES
- 1.Jacobs JP, He X, Mayer JE, et al. Mortality trends in pediatric and congenital heart surgery: An analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. Ann Thorac Surg. 2016;102:1345–52. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman TM, Wernovsky G, Atz AM, et al. Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation. 2003;107:996–1002. [DOI] [PubMed] [Google Scholar]
- 3.Landis RC, Brown JR, Fitzgerald D, et al. Attenuating the systemic inflammatory response to adult cardiopulmonary bypass: A critical review of the evidence base. J Extra Corpor Technol. 2014;46:197–211. [PMC free article] [PubMed] [Google Scholar]
- 4.Massé L, Antonacci M. Low cardiac output syndrome: Identification and management. Crit Care Nurs Clin N Am. 2005;17:375–83. [DOI] [PubMed] [Google Scholar]
- 5.Hovels-Gurich HH. Factors influencing neurodevelopment after cardiac surgery during infancy. Front Pediatr. 2016;4:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballweg JA, Wernovsky G, Gaynor JW. Neurodevelopmental outcomes following congenital heart surgery. Pediatr Cardiol. 2007;28:126–33. [DOI] [PubMed] [Google Scholar]
- 7.Straub A, Smolich J, d’Udekem Y, et al. Activation of platelets in young infants during cardiopulmonary bypass. Thromb Haemost. 2010;103:466–9. [DOI] [PubMed] [Google Scholar]
- 8.Gianetti J, Del Sarto P, Bevilacqua S, et al. Supplemental nitric oxide and its effect on myocardial injury and function in patients undergoing cardiac surgery with extracorporeal circulation. J Thorac Cardiovasc Surg. 2004;127:44–50. [DOI] [PubMed] [Google Scholar]
- 9.Checchia PA, Bronicki RA, Muenzer JT, et al. Nitric oxide delivery during cardiopulmonary bypass reduces postoperative morbidity in children—A randomized trial. J Thorac Cardiovasc Surg. 2013;146:530–6. [DOI] [PubMed] [Google Scholar]
- 10.James C, Millar J, Horton SB, et al. Nitric oxide administration during paediatric cardiopulmonary bypass: A randomised controlled trial. Intensive Care Med. 2016;42:1744–52. [DOI] [PubMed] [Google Scholar]
- 11.Raut MS, Maheshwari A. Inhaled nitric oxide, methemoglobinemia, and route of delivery. Saudi J Anaesth. 2017;11:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: Etiology, pharmacology, and clinical management. Ann Emerg Med. 1999;34:646–56. [DOI] [PubMed] [Google Scholar]
- 13.Chiletti R, Horton S, Bednarz A, et al. Safety of nitric oxide added to the ECMO circuit: A pilot study in children. Perfusion. 2018;33:74–6. [DOI] [PubMed] [Google Scholar]
- 14.The Neonatal Inhaled Nitric Oxide Study Group. Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure. N Engl J Med. 1997;336:597–604. [DOI] [PubMed] [Google Scholar]
- 15.Davidson D, Barefield ES, Kattwinkel J, et al. Inhaled nitric oxide for the early treatment of persistent pulmonary hypertension of the term newborn: Randomized, double-masked, placebo-controlled, dose-response, multicenter study. Pediatrics. 1998;101:325–34. [DOI] [PubMed] [Google Scholar]
- 16.Hermon M, Golej J, Burda G, et al. Intravenous prostacyclin mitigates inhaled nitric oxide rebound effect: A case control study. Artif Organs. 2001;23:975–8. [DOI] [PubMed] [Google Scholar]
- 17.INOmax DSIR Plus operational manual. Mallinckrodt Pharmaceuticals. Rev-03, 2015-07.
- 18.Nitric oxide during cardio pulmonary bypass during surgery for congenital heart defects: A randomised controlled trial. ACTRN12617000821392.