Abstract:
Intravascular hemolysis with elevated plasma-free hemoglobin (PFH) complicates extracorporeal membrane oxygenation (ECMO). In 50 consecutive pediatric cardiac patients requiring ECMO, we sought to describe the relationship between PFH and clinical outcomes; primary outcomes were acute kidney injury (AKI) and prolonged (>14 days) renal replacement therapy (RRT). Median age was 35 days, median weight 3.9 kg, and median ECMO duration 4.2 days. Seventy-eight percent (39/50) weaned off ECMO; survival to discharge was 50% (25/50). Seventy percent (35/50) had AKI on ECMO. Seventy-seven percent (30/39) required RRT post-ECMO; median duration was 5.2 days (0, 14.2). Prolonged RRT was associated with higher daily PFH (67.5 mg/dL [54.1, 102.5] vs. 46.7 mg/dL [40, 72.6], p = .025) and higher peak PFH (120 mg/dL [90, 200] vs. 60 mg/dL [40, 135], p = .016). After adjusting for ECMO duration and oliguria/elevated creatinine on ECMO day 0, peak PFH >90 mg/dL was associated with prolonged RRT (operating room [OR] = 18, confidence interval [CI] 1.9–167.8). Patients who died had higher daily PFH (65 mg/dL [51.6, 111.7] vs. 42.5 mg/dL [37.5, 60], p = .0040). Adjusting for ECMO duration and blood product administration, daily PFH >53 mg/dL was associated with mortality (OR 4.8, CI 1.01–23.3). Elevated PFH during pediatric cardiac ECMO is associated with prolonged RRT and non-survival to discharge. Initiatives to decrease PFH burden may improve clinical outcomes.
Keywords: hemolysis, acute kidney injury, renal replacement therapy, children, cardiac extracorporeal membrane oxygenation
Acute kidney injury (AKI) complicates up to 74% of pediatric patients supported with extracorporeal membrane oxygenation (ECMO) (1,2). The etiology is multifactorial and may include low cardiac output, inflammation, blood product exposure, drug-mediated nephrotoxicity, and intravascular hemolysis (3). Severe AKI that requires renal replacement therapy (RRT) during ECMO support is associated with increased mortality (1,2,4,5).
Hemolysis and plasma-free hemoglobin (PFH) generation on ECMO can occur from a number of patient and technical factors (6)—and might be worse with high ECMO flows, thrombus and/or excess negative pressures within the circuit, and blood transfusions (7). Elevated PFH is associated with multi-organ injury, including severe AKI (7,8). Although existing data show association among elevated PFH, RRT, and mortality while on ECMO (9), the impact of elevated PFH on patient morbidity following successful ECMO decannulation is less clear.
The objective of our study was to confirm the association of intravascular hemolysis with severe AKI (requiring RRT) and investigate the impact of hemolysis on outcomes both during and after ECMO in pediatric cardiac patients. We hypothesized that higher levels of PFH would have a persistent deleterious impact on outcomes after ECMO decannulation.
MATERIALS AND METHODS
Patients
Retrospective review of 50 consecutive pediatric cardiac patients receiving ECMO in the cardiac intensive care unit (CICU) from October 2012 to February 2016. Four patients received two ECMO runs; however, only the first ECMO episode for each patient was included in analysis. The Institutional Review Board at University of Alabama at Birmingham approved the study on November 4, 2014, and informed consent was waived. All data were collected retrospectively from our CICU clinical database and patient electronic medical records.
Plasma-Free Hemoglobin
PFH was quantified via Hemocue AB (Angelholm, Switzerland) every morning while on ECMO per protocol; additional levels were obtained per clinician discretion. Only morning levels were used for analysis to avoid sampling bias. Daily PFH was defined as the morning PFH for each patient. Peak PFH level was defined as the highest value during the entire ECMO course for each patient.
Definitions
AKI on ECMO was defined as doubling of the lowest pre-ECMO serum creatinine (SCr). RRT on ECMO was defined as patients receiving peritoneal dialysis (PD) (10), hemodiafiltration (HDF), or hemodialysis (HD) via hemofilter. Slow continuous ultrafiltration (SCUF) in isolation was not defined as RRT, as all patients receive prophylactic SCUF for fluid removal (see the following text). RRT after weaning from ECMO was performed either via PD or continuous RRT (CRRT) (HDF or HD). The duration of RRT included days both on/off ECMO. Prolonged RRT was defined as ≥14 days of RRT (>75th percentile). All patients who died while receiving active RRT were assigned to the prolonged RRT group, even if they died before 14 days. Ventilator-free days (VFDs) was defined as 28 days from ECMO cannulation minus total duration of mechanical ventilation. Death in first 28 days or duration of ventilation ≥28 days equals zero VFDs.
Equipment
ECMO circuits were composed of centrifugal pumps (Sorin Revolution, Arvada, CO), oxygenators (Quadrox iD Pediatric Oxygenator; MAQUET, Hirrlingen, Germany), and in-line hemoconcentrators (Minntech Hemocor® HPH Mini, Minneapolis, MN). Alaris intravenous (Carefusion, San Diego, CA) pumps were used to regulate dialyzate flow and fluid removal rate. All volumes, infused and removed, were closely monitored and measured hourly using a SmartSite® Burette Infusion Set (Carefusion), a volumetric cylinder with accuracy measurements to 1 mL. For additional safety, desired hourly volumes were entered into Alaris pumps so devices would stop and require hourly clinician intervention. HDF/HD was performed through hemoconcentrator when increased solute clearance desired. Normosol (Hospira, Inc., Lake Forest, IL) was used as dialyzate.
PD used “Dialy-Nate” disposable PD delivery systems (Utah Medical Products, Midvale, UT). For surgical patients, PD was initiated in the early postoperative period, as detailed elsewhere (11). CRRT following ECMO decannulation was performed using a Gambro Prismaflex machine (Baxter Healthcare Corporation, Deerfield, IL) or Aquadex (Sunshine Heart, Inc., Eden Prairie, MN).
SCUF, RRT, and Fluid Management on ECMO
Fluid management while on ECMO is protocolized at our institution. Fluid removal was initiated following resolution of shock. All patients received SCUF during ECMO for fluid removal with a goal to prevent fluid overload and achieve net even fluid balance by the time of ECMO weaning trials. PD was added for fluid removal (if a drain was already in place) if SCUF and urine output (UOP) were inadequate. Diuretics were scarcely used during ECMO support. During fluid removal, surrogates of intravascular volume status such as physical examination, vital signs, weight, UOP, central venous pressure, and serum blood urea nitrogen/SCr were used to prevent hypovolemia and renal hypoperfusion. Fluid management was transitioned from SCUF to HDF/HD via the same hemofilter, per the nephrologist, for the development of AKI with metabolic disturbances to improve solute clearance and mitigate acidosis as indicated. The hemoconcentrator used on our ECMO circuit is capable of clearing PFH more efficiently with convection and dialyzate with HDF/HD. RRT was continued after ECMO decannulation until the child was producing enough urine to mitigate fluid overload.
Statistics
Continuous variables not normally distributed summarized as median with interquartile range, with group comparison performed using Mann–Whitney U test. Continuous variables with normal distribution were summarized as means with SD and compared using unpaired t test. Related samples Wilcoxon rank test was used to compare PFH on day 1 vs. subsequent days. Categorical data were compared using Chi square or Fisher’s exact test as indicated. Bivariate correlations were analyzed using Pearson for parametric variables and Spearman for non-parametric variables. Receiver operating characteristic (ROC) curves were used to find cutoff points of PFH associated with prolonged RRT. Survival analyses were presented as Kaplan–Meier curves and compared using log-rank test. Patients were censored if they expired before coming off RRT for the duration of RRT analysis and censored if lost to follow-up for survival to 180-day analysis. Multiple logistic regression was used to explore the relationship of PFH with prolonged RRT and mortality. All variables with p value < .1 were included in the model. Backward stepwise likelihood ratio method was used. Variables with Wald statistic > .1 were removed from the model with exception of doubling of baseline SCr and UOP on the day of cannulation which were a priori decided to be included in prolonged RRT analysis. p values < .05 were considered statistically significant. All statistical tests were two-tailed. SPSS® version 22 (IBM®, Chicago, IL) was used for all statistical analyses.
RESULTS
Table 1 shows pre-ECMO demographics. The median duration of ECMO was 4.2 days (2.2, 10.3). Seventy-eight percent (39/50) of subjects successfully weaned off ECMO; the overall survival to discharge was 50% (25/50). All patients who survived to discharge were still alive at 180-day follow-up (one patient was lost to follow-up). See Supplemental Figure 1 (online only) for the flowchart of patients.
Table 1.
Patient characteristics with and without prolonged RRT before ECMO.
| Variable | Total Cohort, n = 50 | Prolonged RRT, n = 17 | No Prolonged RRT, n = 33 | p Value |
|---|---|---|---|---|
| Age at cannulation, days | 35 (7.5, 261) | 32.6 (8, 282) | 36.5 (7.1, 268) | .9 |
| Admit weight, kg | 3.9 (2.8, 8.9) | 3.3 (2.5, 6.7) | 4.2 (3.1, 9.8) | .1 |
| Post-surgical ECMO | 31 (62) | 11 (64.7) | 20 (60.6) | .7 |
| OR cannulation | 13 (26) | 3 (17.6) | 10 (30.3) | .4 |
| ECPR | 21 (42) | 7 (41.2) | 14 (42.4) | .9 |
| VV ECMO | 6 (12) | 1 (5.9) | 5 (15.2) | .6 |
| Single ventricle | 27 (54) | 9 (52.9) | 18 (54.5) | .9 |
| Serum lactate at cannulation, mmol/L | 9.2 (4.2, 13.5) | 8.8 (4.2, 16.5) | 9.4 (4.4, 13.5) | .9 |
| AKI pre-ECMO | 10 (20) | 5 (31.3) | 5 (15.6) | .2 |
| UOP, day of cannulation, mL/kg/h | 1.1 (.4, 2.9) | .65 (.2, 1.7) | 1.44 (.7, 3.2) | .06 |
Data presented as median (interquartile range) and frequency (%) as indicated.
VV, veno-venous.
AKI and ECMO
Frequency of pre-ECMO AKI for entire cohort was 20% (10/50) (Table 1). Seventy percent (35/50) of patients developed AKI on ECMO, with 52% (26/50) requiring RRT. The median time to diagnosis of AKI on ECMO was 2 days (1,3) and the median time to RRT on ECMO was 2 days (1,4). Thirty-nine patients survived ECMO decannulation and 61.5% (24/39) required RRT post-ECMO. The median duration of RRT was 5.2 days (0, 14.2). Patients receiving RRT on ECMO showed a trend toward higher mortality (61% [16/26] vs. 38% [9/24], p = .09).
Risk Factors and Outcomes for Prolonged RRT
For remaining analyses, group comparisons were made between those with/without prolonged RRT (Tables 1 and 2). There were no differences in pre-ECMO variables between groups; the prolonged RRT group had double the frequency of AKI before ECMO (31% vs. 16%), although not significant. AKI frequency and peak SCr while on ECMO were also similar. Twelve of twenty-six (46.2%) patients who required RRT on ECMO developed the need for prolonged RRT after ECMO decannulation. Patients requiring prolonged RRT had lower UOP (.4 vs. 1.1 mL/kg/h, p = .06) during the first three days of ECMO. Groups were similar in blood product and inotrope utilization while on ECMO. Patients with prolonged RRT had longer ECMO duration (7.9 vs. 3.4 days, p = .006) and higher mortality (76.5% [13/17] vs. 36.4% [12/33], p = .007).
Table 2.
Comparison between patients with and without prolonged RRT while on ECMO.
| Variable | Cohort, n = 50 | Prolonged RRT, n = 17 | No Prolonged RRT, n = 33 | p Value |
|---|---|---|---|---|
| RRT during ECMO* | 26 (52) | 14 (82.4) | 12 (36.4) | <.01 |
| AKI on ECMO | 35 (70) | 14 (82.4) | 21 (65.6) | .3 |
| Peak SCr, mg/dL | 1 (.7, 1.5) | 1.2 (.6, 1.3) | 1.2 (.8, 1.8) | .1 |
| UOP, first 72 hours, mL/kg/h* | 72.3 (35.2, 149.6) | .38 (.3, 1.2) | 1.08 (.7, 2.6) | .06 |
| FB, first 72 hours, mL | 50.9 (70.8, 144.2) | 50.8 (−22.3, 138.8) | 51.1 (−71.3, 160.3) | .8 |
| PRBC use, first 72 hours, mL | 75.3 (32.5, 148.9) | 83.5 (18.8, 159) | 68.9 (39.8, 141.4) | .6 |
| Patients on inotropes at 24 hours post-cannulation | 28 (56) | 9 (56.3) | 19 (57.6) | .6 |
Data presented as median (interquartile range) and frequency (%) as indicated.
FB, fluid balance; PRBC, packed red blood cells; FFP, fresh frozen plasma.
Indicates statistical significance.
PFH Levels
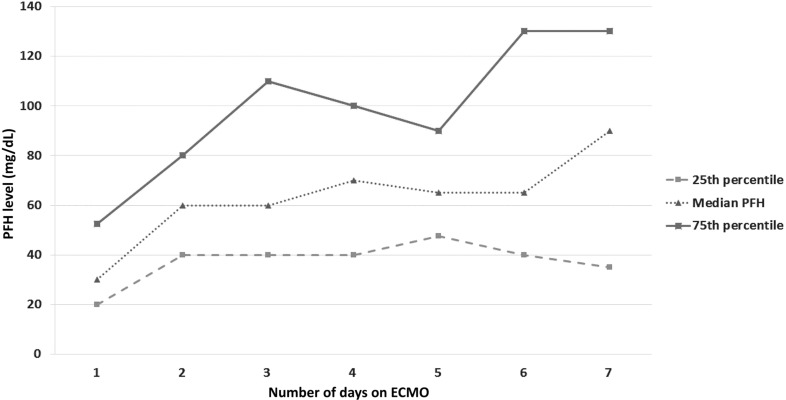
The median daily PFH was 56.2 mg/dL (40, 85.1) and the median peak PFH was 85 mg/dL (57, 152.5) for the entire cohort. Figure 1 displays daily PFH levels over first 7 days of ECMO. For the entire cohort, PFH levels significantly increased by ECMO day 2 and then remained relatively stable.
Figure 1.
Daily PFH trend on ECMO for all patients. Daily PFH was significantly higher on all days vs. ECMO day 1, but remained relatively stable after ECMO day 2.
PFH and Prolonged RRT
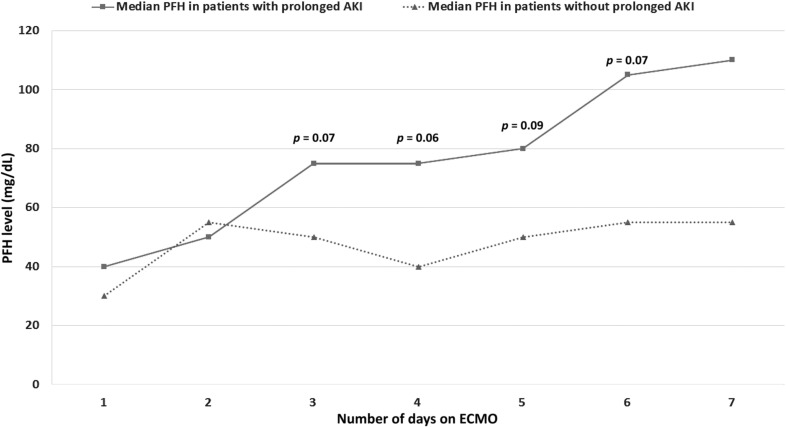
Figure 2 shows daily PFH in patients with/without prolonged RRT. Patients requiring prolonged RRT were exposed to significantly higher median daily PFH (67.5 mg/dL [54.1, 102.5] vs. 46.7 mg/dL [40, 72.6], p = .025) and higher peak PFH (120 mg/dL [90, 200] vs. 60 mg/dL [40, 135], p = .016).
Figure 2.
Median PFH trends for patients with AKI requiring prolonged RRT vs. patients with spontaneous renal recovery following ECMO.
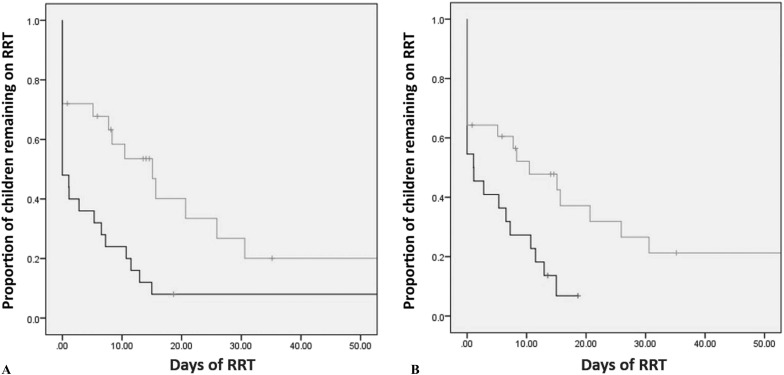
ROC analysis demonstrated daily PFH (area under the curve [AUC] = .74, p = .025) and peak PFH (AUC = .76, p = .017) predicted prolonged RRT. Daily PFH >53 mg/dL predicted prolonged RRT with 71% sensitivity and 69% specificity; peak PFH >90 mg/dL predicted prolonged RRT with 88% sensitivity and 58% specificity. After adjusting for ECMO duration and AKI and/or UOP ≤ .5 mL/kg/day on the day of cannulation, peak PFH >90 mg/dL remained associated with prolonged RRT, operating room (OR) = 18 (95% CI 1.9–167.8) (Table 3). Kaplan–Meier survival analysis demonstrates patients with peak PFH >90 mg/dL (p = .04) and daily PFH >53 mg/dL (p = .03) remained on RRT for a significantly longer duration (Figures 3A and 3B), respectively. PFH >90 mg/dL is associated with significantly worse 6-month survival, p = .01 (Supplemental Figure 2 [online only]).
Figure 3.
Kaplan–Meier cumulative (Cum) survival analysis for (A) peak plasma-free Hb (PFH) >90 mg/dL (light line) and <90 mg/dL (dark line) and (B) daily PFH >53 mg/dL (light line) and <53 mg/dL (dark line) and days on RRT. Both PFH cutoffs are associated with persistent AKI and longer time to wean from RRT. Vertical tick marks represent censored patients.
PFH and Other Outcomes
Univariate comparison of patient characteristics between those with/without hospital mortality is shown in Supplemental Table 1 (online only). Patients suffering mortality had higher median daily PFH (65 mg/dL [51.6, 111.7] vs. 42.5 mg/dL [37.5, 60], p = .004), higher peak PFH (130 mg/dL [75, 225] vs. 60 mg/dL [40, 90], p = .002), and longer duration of ECMO (10.1 days [3.8, 14.3] vs. 3.2 [1.8, 4.7], p = .001). The mortality rate was higher in patients with peak PFH >90 mg/dL (18/25 [78%] vs. 7/25 [28%], p = .004) and daily PFH >53 mg/dL (19/28 [76%] vs. 6/22 [24%], p = .01). After adjusting for ECMO duration and blood product administration in the first 72 hours post-ECMO, daily PFH >53 mg/dL remained associated with mortality (OR 4.8, 95% CI 1.01–23.3).
Cutoff points of peak PFH >90 mg/dL and daily PFH >53 mg/dL were not predictive of a difference in VFD; however, peak PFH >75 mg/dL was associated with decreased VFDs (median 14 days [0, 20.5] vs. 21 days [14.5, 22] p = .021).
PFH and ECMO Pump Parameters
There was no significant correlation between PFH levels and ECMO parameters, including pre-pump pressure, post-pump pressure, ECMO flow, or ECMO pump rotations per minute. No correlations were found between PFH and daily volume of blood product transfusions while on ECMO (data not shown).
DISCUSSION
AKI occurs frequently in pediatric ECMO patients and is associated with hospital mortality (1,2). Intravascular hemolysis is an important complication of ECMO and is associated with morbidity, including AKI (12). The etiology of ECMO-related AKI is multifactorial and may include intravascular hemolysis, but demonstration of a causal relationship between PFH and AKI on ECMO remains elusive. Given that PFH may represent a potentially modifiable risk factor for ECMO-related AKI, it is paramount to further investigate the relationship of PFH and AKI in this vulnerable population.
In this study, we confirm that a high proportion of pediatric cardiac patients develop AKI on ECMO, with approximately half requiring RRT (2). Persistent need for RRT represents the most severe form of functional AKI (76.5% mortality in this cohort). A significant novel finding of our study is that elevated PFH predicts prolonged RRT, which represents an important clinical burden after successful weaning from ECMO. We established cutoff points for peak and daily PFH that predict prolonged RRT, and demonstrate these cutoff values also have independent association with mortality. Initiatives aimed at decreasing exposure to PFH on ECMO should be investigated as a means to decrease AKI and associated morbidity/mortality.
Intravascular hemolysis may lead to AKI on ECMO (8,9). Under physiologic conditions, PFH is removed by serum haptoglobin. Increased hemolysis overwhelms haptoglobin–Hb complexes, resulting in excessive PFH which may contribute to multi-organ dysfunction, including AKI. PFH may cause AKI via three general mechanisms: 1) generation of reactive oxygen species that damage renal tubular epithelium, 2) endothelial nitric oxide depletion causing renal ischemia via microcirculatory vasoconstriction, and 3) PFH precipitation and heme cast formation causing renal tubular luminal obstruction (13). A number of studies support the association between PFH and AKI in patients on extracorporeal support, including cardiopulmonary bypass (CPB) and ECMO (14–19), although no direct causative link between hemolysis and AKI has been established in this population. Our study adds to this literature by demonstrating that PFH levels have significant associations with prolonged RRT, hospital mortality, and 6-month survival—with cutoff levels that can be used to predict prolonged RRT with good sensitivity. Importantly, these associations are independent of ECMO duration—prolonged ECMO courses are often hallmarked by increased hemolysis/PFH from older circuits and persistent high ECMO flows in patients with worse cardiac function and/or multi-organ failure, including AKI.
With standardized measurement of daily PFH levels, we were able to demonstrate PFH trends on ECMO. We found that, starting ECMO day two, patients requiring prolonged RRT had higher levels of PFH compared with patients without prolonged RRT requirement. Not surprisingly, longer ECMO duration was associated with prolonged RRT and higher peak PFH (increased severity of illness leads to increased AKI). However, after controlling for ECMO duration, and peri-cannulation AKI (oliguria and/or double SCr on day of cannulation), PFH remained strongly associated with prolonged RRT. We cannot assign PFH causality to prolonged RRT, but it is feasible, given the well-described PFH pathophysiology that contributes to multi-organ dysfunction and AKI. If these findings are validated prospectively, daily monitoring of PFH could become essential to identify patients at a higher risk for morbidity/mortality, and to diagnose potentially modifiable patient and mechanical factors contributing to elevated PFH on cardiac ECMO (17,18,20).
Children supported with cardiac ECMO, especially those immediately postoperative, have already been exposed to multiple previously described AKI risk factors (i.e., young age, CPB, hemodynamic instability, acidosis, blood product exposure, etc.) (21). We surmise that exposure to excessive PFH after restoration of adequate cardiac output on ECMO may represent a “second hit” phenomenon, further exacerbating AKI injury. In this study, patients with oliguria on the day of ECMO cannulation (presumably functional AKI) were more likely to have prolonged RRT when exposed to high peak PFH; in addition, there was a suggestion that the presence of AKI pre-ECMO was associated with prolonged RRT. Previous studies have demonstrated that PFH is associated with AKI while on ECMO as diagnosed by a rise in SCr (8,9). However, in our study, PFH is not only associated with elevated SCr on ECMO but we are the first to show that PFH is also associated with persistent severe functional AKI and failure to wean from RRT after decannulation. Prolonged RRT, in turn, likely contributes to increased morbidity and late mortality seen in our cohort, as well as increased ICU exposure and resource utilization after weaning from ECMO.
Unlike many AKI etiologies, elevated PFH represents a potentially modifiable risk factor. Administration of haptoglobin, nitric oxide, nitrite donors, antioxidants, and alkalization has been used to various degrees of success to attenuate the deleterious effects of PFH in other clinical models of intravascular hemolysis (13,22) and may warrant study in a pediatric cardiac ECMO cohort. Successful reduction in PFH could lead to decreased rates of severe persistent AKI and improve other clinical outcomes in this population. At minimum, hypovolemia and acidosis should be avoided in the presence of elevated PFH, as they may exacerbate the deleterious effects of PFH on the often already injured kidney (9,13,23).
Risk factors for excessive intravascular hemolysis on ECMO are multifactorial and are most often related to mechanical shear stress within ECMO circuit, oxygenator or hemofilters, and pump thrombosis (20,24). Cannula position, high ECMO flows, hypovolemia, increased red blood cell transfusion, circuit thrombosis, and RRT may all increase PFH while on ECMO (7,9). We did not find a significant correlation between elevated PFH and ECMO circuit or flow variables, fluid balance, or with red cell transfusion. However, this result was limited by the retrospective nature of our data, as there was often only one PFH available per day—which was difficult to correlate with frequent changes or alterations in circuit pressures and flows while on ECMO. In addition, our institutional practice of SCUF for nearly all cardiac ECMO patients prevents the ability to ascertain if hemofilters (when initiating CRRT) were responsible for the increased production of PFH (9,25), and limits the applicability of our findings to centers with different fluid removal and RRT practices. Carefully designed prospective studies will be necessary to conclude what modifiable ECMO circuit factors and patient conditions lead to increased PFH generation.
This study was limited by concerns inherent in a retrospective, single-center, small study design. Our program has a fairly aggressive strategy for fluid removal on ECMO (see methods), which may have impacted the presence of PFH and incidence of AKI on ECMO. In addition, frequent use of PD and SCUF for fluid removal may have led to increased clearance of SCr with subsequent under-diagnosis of AKI. The impact of SCUF and RRT on the removal of PFH also cannot be determined from available data. Contrarily, a strength of this study is that the primary outcome (prolonged RRT) is an objective clinical outcome of functional AKI, unlike SCr-based AKI diagnosis, which is not always correlated with important outcomes or resource utilization. The causes of AKI and prolonged RRT are most certainly multifactorial and likely included variables not evaluated in this study. Finally, this study was not intended to delineate causes of PFH elevation. Although we checked PFH daily, we may not have identified the highest peak PFH in some patients.
In summary, persistent AKI with prolonged RRT is an important complication after decannulation from cardiac ECMO and is associated with mortality. PFH elevation is associated with prolonged RRT and mortality. Future prospective studies are needed to validate these findings and further delineate the impact of PFH on organ injury and survival to discharge. Attempts to identify and ameliorate sources of PFH could improve AKI-related outcomes in ECMO survivors.
Supplementary Material
ACKNOWLEDGMENT
Funding for this project was provided through the University of Alabama at Birmingham Pediatric Cardiology departmental funds.
REFERENCES
- 1.Askenazi DJ, Ambalavanan N, Hamilton K, et al. . Acute kidney injury and renal replacement therapy independently predict mortality in neonatal and pediatric noncardiac patients on extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2011;12:e1–6. [DOI] [PubMed] [Google Scholar]
- 2.Fleming GM, Sahay R, Zappitelli M, et al. . The incidence of acute kidney injury and its effect on neonatal and pediatric extracorporeal membrane oxygenation outcomes: A multicenter report from the kidney intervention during extracorporeal membrane oxygenation study group. Pediatr Crit Care Med. 2016;17:1157–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Askenazi DJ, Selewski DT, Paden ML, et al. . Renal replacement therapy in critically Ill patients receiving extracorporeal membrane oxygenation. Clin J Am Nephrol. 2012;7:1328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta P, Beam B, Schmitz ML. Outcomes associated with use of renal replacement therapy in children receiving extracorporeal membrane oxygenation after heart surgery: A multi-institutional analysis. Pediatr Nephrol. 2015;30:1019–26. [DOI] [PubMed] [Google Scholar]
- 5.Wolf MJ, Chanani NK, Heard ML, et al. . Early renal replacement therapy during pediatric cardiac extracorporeal support increases mortality. Ann Thorac Surg. 2013;28:2189–98. [DOI] [PubMed] [Google Scholar]
- 6.Meyer AD, Wiles AA, Rivera O, et al. . Hemolytic and thrombocytopathic characteristics of extracorporeal membrane oxygenation systems at simulated flow rate for neonates. Pediatr Crit Care Med. 2012;13:e255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toomasian JM, Bartlett RH. Hemolysis and ECMO pumps in the 21st century. Perfusion. 2011;26:5–6. [DOI] [PubMed] [Google Scholar]
- 8.Rother RP, Bell L, Hillmen P, et al. . The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: A novel mechanism of human disease. J Am Med Assoc. 2005;293:1653–62. [DOI] [PubMed] [Google Scholar]
- 9.Gbadegesin R, Zhao S, Charpie J, et al. . Significance of hemolysis on extra-corporeal life support after cardiac surgery in children. Pediatr Nephrol. 2009;24:589–95. [DOI] [PubMed] [Google Scholar]
- 10.Sasser WC, Robert SM, Askenazi DJ, et al. . Peritoneal dialysis: An alternative modality of fluid removal in neonates requiring extracorporeal membrane oxygenation after cardiac surgery. J Extra Corpor Technol. 2014;46:157–61. [PMC free article] [PubMed] [Google Scholar]
- 11.Sasser WC, Dabal RJ, Askenazi DJ, et al. . Prophylactic peritoneal dialysis following cardiopulmonary bypass in children is associated with decreased inflammation and improved clinical outcomes. Congenit Heart Dis. 2014;9:106–15. [DOI] [PubMed] [Google Scholar]
- 12.Lou S, MacLaren G, Best D, et al. . Hemolysis in pediatric patients receiving centrifugal-pump extracorporeal membrane oxygenation: Prevalence, risk factors and outcomes. Crit Care Med. 2014;42:1213–20. [DOI] [PubMed] [Google Scholar]
- 13.Windsant IC, Hanssen SJ, Buurman WA, et al. . Cardiovascular surgery and organ damage: Time to reconsider the role of hemolysis. J Thorac Cardiovasc Surg. 2011;142:1–11. [DOI] [PubMed] [Google Scholar]
- 14.Omar HR, Mirsaeidi M, Socias S, et al. . Plasma free hemoglobin is an independent predictor of mortality among patients on extracorporeal membrane oxygenation support. PLoS One. 2015;10:e0124034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ricci Z, Pezzella C, Romagnoli S, et al. . High levels of free haemoglobin in neonates and infants undergoing surgery on cardiopulmonary bypass. Interact Cardiovasc Thorac Surg. 2014;19:183–7. [DOI] [PubMed] [Google Scholar]
- 16.Mamikonian LS, Mamo LB, Smith PB, et al. . Cardiopulmonary bypass is associated with hemolysis and acute kidney injury in neonates, infants, and children. Pediatr Crit Care Med. 2014;15:e111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyu L, Long C, Hei F, et al. . Plasma free hemoglobin is a predictor of acute renal failure during adult venous-arterial extracorporeal membrane oxygenation support. J Cardiothorac Vasc Anesth. 2016;30:891–5. [DOI] [PubMed] [Google Scholar]
- 18.Pan KC, McKenzie DP, Pellegrino V, et al. . The meaning of high plasma free haemoglobin: Retrospective review of prevalence of haemolysis and circuit thrombosis in an adult ECMO center over 5 years. Perfusion. 2016;31:223–31. [DOI] [PubMed] [Google Scholar]
- 19.Hanssen SJ, van de Poll MC, Houben AJ, et al. . Hemolysis compromises nitric oxide-dependent vasodilatory responses in patients undergoing major cardiovascular surgery. Thorac Cardiovasc Surg. 2012;60:255–61. [DOI] [PubMed] [Google Scholar]
- 20.Neal JR, Quintana E, Pike RB, et al. . Using daily plasma-free hemoglobin levels for diagnosis of critical pump thrombus in patients undergoing ECMO or VAD support. J Extra Corpor Technol. 2015;47:103–8. [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SE, Yu MY, Lee J, et al. . Risk factors for acute kidney injury and in-hospital mortality in patients receiving extracorporeal membrane oxygenation. PLoS One. 2015;10:e0140674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubota K, Egi M, Mizobuchi S. Haptoglobin administration in cardiovascular surgery patients: Its association with the risk of postoperative acute kidney injury. Anesth Analg. 2017;24:1771–6. [DOI] [PubMed] [Google Scholar]
- 23.Zager RA, Johnson AC, Lund S, et al. . Acute renal failure: Determinants and characteristics of the injury-induced hyperinflammatory response. Am J Physiol Renal Physiol. 2006;291:F546–56. [DOI] [PubMed] [Google Scholar]
- 24.Williams DC, Turi JL, Hornik CP, et al. . Circuit oxygenator contributes to extracorporeal membrane oxygenation-induced hemolysis. ASAIO J. 2015;61:190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luckraz H, Woods M, Large SR; Papworth VAD Group. And hemolysis goes on: Ventricular assist device in combination with veno-venous hemofiltration. Ann Thorac Surg. 2002;73:546–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.