Abstract
Mechanical loading of the intervertebral disc (IVD) initiates cell‐mediated remodeling events that contribute to disc degeneration. Cells of the IVD, nucleus pulposus (NP) and anulus fibrosus (AF), will exhibit various responses to different mechanical stimuli which appear to be highly dependent on loading type, magnitude, duration, and anatomic zone of cell origin. Cells of the NP, the innermost region of the disc, exhibit an anabolic response to low‐moderate magnitudes of static compression, osmotic pressure, or hydrostatic pressure, while higher magnitudes promote a catabolic response marked by increased protease expression and activity. Cells of the outer AF are responsive to physical forces in a manner that depends on frequency and magnitude, as are cells of the NP, though they experience different forces, deformations, pressure, and osmotic pressure in vivo. Much remains to be understood of the mechanotransduction pathways that regulate IVD cell responses to loading, including responses to specific stimuli and also differences among cell types. There is evidence that cytoskeletal remodeling and receptor‐mediated signaling are important mechanotransduction events that can regulate downstream effects like gene expression and posttranslational biosynthesis, all of which may influence phenotype and bioactivity. These and other mechanotransduction events will be regulated by known and to‐be‐discovered cell‐matrix and cell‐cell interactions, and depend on composition of extracellular matrix ligands for cell interaction, matrix stiffness, and the phenotype of the cells themselves. Here, we present a review of the current knowledge of the role of mechanical stimuli and the impact upon the cellular response to loading and changes that occur with aging and degeneration of the IVD.
Keywords: biomechanics, degeneration, extracellular matrix, mechanobiology
1. INTRODUCTION
The intervertebral disc (IVD) is a heterogeneous structure that, together with two facet joints, functions as a “three‐joint complex” to provide for load support and flexibility of the spine1. The IVD is subject to substantial motions due to spinal flexion, extension, and torsion, and consequently physical forces of joint loading and muscle activation. The IVD is well‐adapted to sustain these motions and loads with a composite of sub‐structures that consists of confining end plates (EPs) on the superior and inferior faces, the highly fibrous anulus fibrosus (AF) on the outer periphery, and the highly hydrated nucleus pulposus (NP) at the center. Each substructure contains large amounts of water in a dense and negatively charged extracellular matrix (ECM), but with a unique morphology and compositional differences that contribute to variable magnitudes of interstitial osmotic and fluid pressures, fluid‐flow, compressive, tensile and shear stresses, and strains. The mechanical demands of IVD loading and motion may contribute to magnitudes of each and all of these factors that could facilitate tissue failure. As an avascular, alymphatic tissue with the lowest cell density of any tissue or organ in the body2, the IVD has limited mechanisms by which to heal and repair so that spinal loading can promote and contribute to IVD degeneration. Some hallmarks of IVD degeneration include decreased disc height and loss of hydration that may be accompanied by loss of IVD mobility, loss of fluid pressurization, a decreased T2 MRI signal, and changes to the vertebral EPs, all of which are believed to contribute to altered IVD function.
Increasingly, evidence suggests that the cell‐mediated and biological remodeling responses to mechanical stimuli generated by spinal motions are a large contributor to degenerative and pathological changes3. These cell‐level responses to loading environment occur decades before disc degeneration manifests4, 5. For these reasons, studies of mechanobiology and the interactions between mechanical stimuli and biological processes are performed to understand the development of disc degeneration. Many studies have documented the biological responses of IVD cells to mechanical stimuli, but only recently have studies begun to elucidate mechanisms governing these observed mechanobiologic responses in the IVD. In this article, we present a summary of the current knowledge of IVD mechanobiology and its relevance to disc degeneration, and identify several signaling events and mechanisms that govern cellular responses to mechanical stimuli.
2. STRUCTURE, MORPHOLOGY, AND BIOLOGY OF IVD CELLS
The disc consists of structurally distinct, anatomical regions, which dictate the range of IVD cell phenotypic characteristics, including unique physiological and biological responses to mechanical stimuli6, 7. The innermost NP is a highly hydrated, gelatinous tissue containing large quantities of proteoglycans, collagens, and noncollagenous proteins8, 9. Due to the large amounts of the proteoglycan aggrecan, which carries a net negative and “fixed” charge through its compositionally major sulfated‐glycosaminoglycans, the NP is subject to high interstitial swelling and osmotic pressures during joint loading10, 11. The notochordal‐derived cells of the NP can be present with large vacuoles12, 13, 14, 15 and young, immature NP cells display clustered, tightly packed cells with smaller compacted nuclei, and surrounded by dense pockets of ECM (Figure 1A). The ratio of large vacuolated notochordal to small nonvacuolated cells in the NP region declines with maturity of the human IVD15. With age, these phenotypically unique cells are rarely seen and instead an increased presence of chondrocyte‐like or fibroblast‐like cells is observed16. (Notochordal cells in different animal models are further discussed in Sections 3.3 and 4.1) Indeed, there is increasing evidence demonstrating that differentiation of large vacuolated notochordal cells into mature NP cells may be mechanically regulated. Recent work has shown that periods of ex vivo organ culture for porcine17 and mouse18 IVD under hydrostatic pressure or hyperosmotic overloading conditions can promote the transition and differentiation of notochordal cells into NP cells. The differences in vacuolated notochordal cell population with age should be considered when addressing different studies, as cells from younger specimens may be a more appropriate fit for addressing early events that can lead to degeneration, while older samples may give more accurate information on the degenerate or aged cellular response to further environmental challenges.
Figure 1.
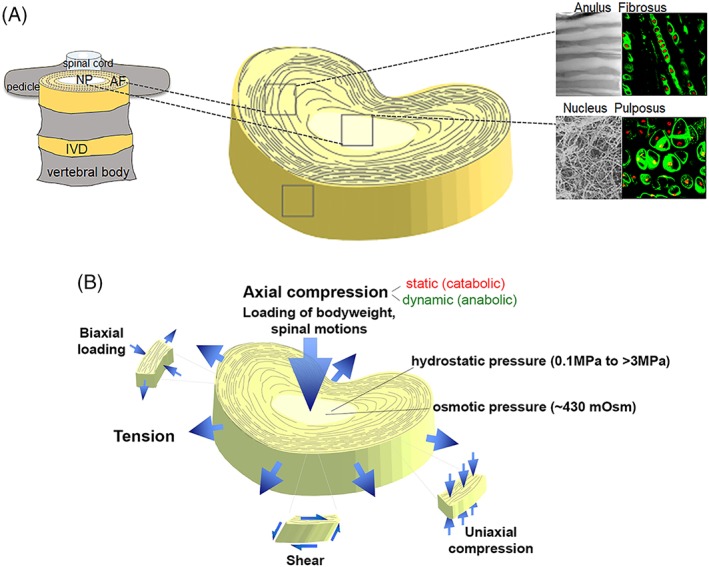
(A) Schematic representation of spinal anatomy (left) and the heterogeneous tissue composition of the intervertebral disc (IVD) (right). Insets (right) show matrix organization (scanning electron microscopy, gray) and cell organization (fluorescence) for anulus fibrosus (AF) and nucleus pulposus (NP) regions. AF matrix is composed of aligned collagen I fiber bundles, while a much random distribution of collagen II bundles and proteoglycan is observed in the NP. Fluorescence insets are rat IVD cells stained for collagen VI (green) and nucleus (red) revealing the different cellular arrangement in each tissue area. Adapted from Cao et al174. (B) Mechanical deformation of the IVD. Axial compression can be either catabolic or anabolic depending on mode (static, dynamic), magnitude, frequency, and duration. Loading of the hydrated NP matrix results in hydrostatic and osmotic pressures. When the disc is axially loaded, the NP region becomes compressed and the AF undergoes radial and circumferential tension to limit overall disc expansion in the transverse plane. Adapted from Setton et al195
Surrounding and constraining the NP is the AF, a lamellar and fibrocartilaginous structure containing highly organized, distinct lamellae19, 20 and largely aligned collagen fiber bundles21, 22, 23. Specifically, AF is constructed of concentric lamellae of alternating alignment, with each lamella composed of parallel collagen fibril bundles24 (Figure 1A). Sheaths of elastic fibers, formed by elastin, fibrillin, and other ECM proteins, enclose these collagen fibril bundles25, resembling what has been described for tendon and muscle. Elastin appears to maintain the integrity of the collagen lamellae, allowing its recoiling after deformation and to contribute to the anisotropy of the AF stiffness in response to shear26, 27. Cellular interactions with ECM of the AF and other regions can be quite complex, however, with evidence of cellular projections into lamella of the AF that can contribute to stellate‐like cells and some documented evidence of cellular “gliding” and lamellar cross bridges forming during simulated motion28, 29. Containing bundles of collagen I, the outer regions of the AF help resist tensile loads arising from physiological joint motions and swelling effects that give rise to annular bulging and deformation (Figure 1B)30. Cells of the AF, which are derived from the mesenchyme, display a more ellipsoidal morphology with many characteristics of fibroblasts and chondrocytes8, 9, 31. The inner region of the AF transitions to a more collagen II‐rich niche with increased proteoglycan content, allowing it to contribute to the adjacent NP's ability to support compressive loads. Similar to NP, cells of the inner AF (IAF) are more rounded and sparsely distributed as compared to cells of the outer AF (OAF; Figure 2). Containing bundles of collagen I, the OAF help resist tensile loads arising from physiological joint motions and swelling effects that give rise to annular bulging and deformation (Figure 1B). Cells residing in the OAF exhibit a flatter morphology, with major cell radii closely aligned with collagen fibers of the organized lamellae (Figure 2). Therefore, IAF cells appear to have greater similarity with NP over OAF, in terms of morphology and gene expression32. The elastic fiber network also differs between NP, IAF, and OAF. In OAF, microfibrils colocalize more with elastin fibrils when compared to IAF. In NPs, microfibrils surround the cell while elastin preferentially localizes in the interterritorial ECM. Moreover, the elastin content increases with the degree of degeneration and with age in NP, OAF, and IAF, with a higher increase in the latter33.
Figure 2.
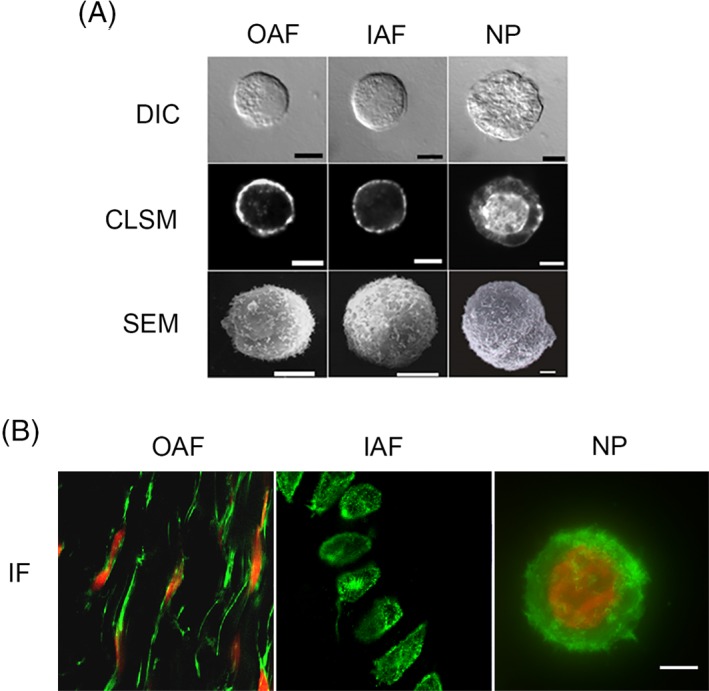
Representative images of isolated porcine cells from outer AF (OAF), inner AF (IAF), and nucleus pulposus (NP) regions. (A) Differential interference contrast (DIC, top), confocal laser scanning microscopy (CLSM, middle) depicting F‐actin (phalloidin) staining and scanning electron microscopy (SEM, bottom). Porcine NP cells are larger than OAF and IAF cells, and have a vacuolated morphology. F‐actin is localized to the cell cortex in OAF and IAF cells, but was found in abundance throughout NP cells, including the perinuclear region. SEM showed the presence of significant membrane ruffles and folds. Scale bar = 5 μm. Adapted from Guilak et al196. (B) Immunofluorescence of actin microfilaments (green) and the cell nucleus (red). OAF cells in situ are elongated and distributed in concentric layers mimicking the collagen arrangement. IAF cells in situ appear rounder than OAFs. Bovine NP cells (in alginate) have a dense network of punctuated cortical actin microfilaments and lack stress fibers, while OAF and IAF cells have a cortical actin distribution. Scale bar = 5 μm. Adapted from Setton et al195
The avascular nature of the IVD largely dictates the metabolism of disc cells. The primary means by which nutrients and metabolites reach the disc is diffusion through the vertebral EP and AF, which causes relatively low oxygen tension within the disc and cell metabolism largely relies on anaerobic glycolysis34, 35. This further contributes to high concentrations of lactate, the end product of glycolysis, and low pH conditions. When oxygen tension is low, cells will rely completely on glycolysis for ATP production and energy generation36. Disc cells are highly adapted to such a hypoxic environment and maintain maximum cell survival when oxygen tension is below 5%37, 38. The hypoxic demands of the disc thus require high amounts of glucose for glycolysis‐dependent ATP production and cell viability becomes compromised if glucose levels fall below physiologic levels (0.2 mM)39, 40, 41. The resulting lactate accumulation can lead to a reduced pH, if lactate is not properly cleared, and can be detrimental to cell survival. Acidic pH levels as low as 6.1 have been predicted,42 that is exacerbated during degeneration because the disc has diminished diffusion abilities to remove metabolic waste products. Degeneration‐induced calcified EPs block not only nutrient supply, but also clearance of metabolites, leading to lactic acid buildup and an increasingly acidic environment43. Few studies have compared metabolism of NP and AF in response to loading, though AF and NP cells exhibit different metabolic pathways. NP cells have higher adenosine triphosphate content, while AF cells have higher lactate production and glucose consumption44. The same study also showed that dynamic loading affects energy metabolism, with the effect being greater in AF.
Pronounced changes occur with aging in the IVD, where the already low cell density decreases further, along with marked changes in the ECM composition45. A reduction in aggrecan within the NP results in a more dehydrated tissue with a diminished ability to properly distribute and transmit mechanical loads46, 47. This in turn leads to altered AF mechanical properties as well, due to these forces being unnaturally transferred to the adjoining AF. An important characteristic of IVD degeneration is the pathological infiltration of other cell types, namely, nerve fibers, immune cells including macrophages, lymphoid‐derived T cells, Schwann cells, endothelial cells, and fibroblasts, the latter of which may be associated with increased vasculature4, 48, 49, 50, 51. Along with this aberrant vasculature and subsequent blood supply, there is also a reduction in nutrient diffusion52, 53, all of which are believed to support a particularly adverse cellular environment where matrix‐degrading metalloproteinases (MMPs) and pro‐inflammatory cytokines such as interleukin(IL)‐1, IL‐8, and tumor necrosis factor (TNF)‐α, exacerbate and further the progression of degeneration4, 48, 54, 55.
3. MECHANICAL LOADING EFFECTS ON IVD CELLS
During loading, cells of the IVD experience compressive, tensile and shearing deformation, fluid flows, pressures, and electrokinetic effects, all of which represent important regulators of cell metabolism in the IVD (Figure 1B). Cells of the separate anatomical regions are exposed to a range of mechanical loads and the biological response to a particular stimulus may depend on tissue location, magnitude, and frequency of loading3, 56, 57.
3.1. Compression
The differential response of IVD cells to loading has been most studied for compressive stimuli. Evidence consistently suggests that there is a threshold effect observed in IVD compression studies in both NP and AF that is dependent upon magnitude, duration, and frequency. Static compressive loading has been shown to induce changes in cell biosynthesis and gene expression for collagens and proteoglycans, and protease activation, as well as cell death when applied in vivo and to explants or isolated cells58, 59, 60, 61, 62, 63, 64, 65. Static compressive loading is thought to inhibit nutrient transport and necessary gas exchange that may be essential for promoting cellular biosynthesis and maintaining cell survival, whereas dynamic compression promotes convection that increases large molecular weight nutrient uptake into the disc42, 66. Interestingly, transport of small molecules such as oxygen and glucose are predominantly governed by diffusion rather than convection that are modulated under dynamic loading42, 67, 68, 69, 70. In terms of gene expression, one study showed 72 hours of immobilization followed by 2 hours of dynamic loading can produce damaging effects, measured by a decrease in anabolic gene expression and an upregulation of catabolic gene expression, that were not rescued by the brief period of dynamic loading71. In contrast, shorter periods of static loading can lead to an anabolic response in IVD cells, shown through increases in proteoglycan and collagen synthesis in a bovine explant culture system (5‐10 kg, ~0.2‐0.4 MPa)65. A similar effect was observed for AF cells isolated from tissue and embedded in alginate matrix, demonstrating elevated gene expression of collagens I and II, aggrecan, decorin, and biglycan for short periods of loading64. As has been shown for other mechanosensitive tissues and associated cell types, long periods of matrix compression give rise to altered physical signals to IVD cells and also disrupt gas and nutrient transport that is essential for maintaining metabolism. Transport properties of human AF are dependent on the magnitude of applied compressive strain, where transport decreased with increasing deformation, likely due to fluid exudation and reduction in pore size with compression72, 73. AF diffusivity is also anisotropic, with lower diffusivity in the radial direction than in the axial or circumferential directions, indicating that nutrient transport in human AF is anisotropic. This behavior is likely a consequence of the layered structure, unique collagen architecture, elastic fibrils arrangement, and high complexity of the ECM of AF tissue73, 74.
Dynamic compressive loads change the cell‐specific expression of crucial matrix genes (eg, collagens I and II, aggrecan) and catabolic genes (MMP‐1, ‐3, ‐13, ADAMTS‐4), but the degree of response is conditional upon frequency and magnitude71, 75, 76. Overall, an anabolic response is more likely elicited from dynamic loading, whereas static loading tends to produce a catabolic response. In a bovine IVD organ culture study, it was found that dynamic compression (0.2‐1 MPa) increased metabolic rates and biosynthesis of ECM molecules, without any structural changes or impaired mechanical properties, indicating there is some degree of healthy loading conditions required for all regions of the IVD77. Lower loading rates to cells in IVD matrix and as isolated cells (<0.5 Hz) have been shown to maintain proteoglycan content75, 78 and support normal disc metabolism79, whereas increased loading frequencies result in higher amounts of apoptotic cells80. Cell death has also been noted in cells in the NP and AF when the dynamic compressive magnitude reaches levels greater than 1.3 MPa76, although low frequency of loading and long periods of duration (>24 hours) also modulate this effect59, 60, 63, 71, 76. One study used a rat tail dynamic compression model to demonstrate that discs loaded for 2 weeks at 8 hours/day expressed higher levels of anabolic and anti‐catabolic genes compared to static compression81. However, when discs were loaded at the same magnitude for 8 hours/day out to 8 weeks, early signs of degeneration were noted through decreased TIMP‐3 and increased ADAMTS‐4 expression, which can promote matrix degradation. Thus, dynamic compression leads to an anabolic response when applied daily, but initial signs of degeneration may begin with extended dynamic loading duration.
Compressive loading also regulates the synthesis and consumption of glucose and ATP in the IVD42, 44, 65, 82, 83. Both static and dynamic loading conditions have been shown to significantly increase lactate accumulation in the AF and NP regions, likely a consequence of lactate production, occurring contemporaneously with increased ATP content and decreased pH84. It was also noted that lactate accumulation was higher in the 1 Hz compression group compared to the 0.1 Hz compression group, suggesting frequency‐dependent differences. Isolated porcine NP and AF cells in a three‐dimensional (3D) agarose culture model that were subjected to various mechanical loads (which had previously shown the greatest changes in biosynthetic activity by mesenchymal stem cells [MSCs]) demonstrated increased glucose consumption and lactate production and were further shown to produce higher levels of ATP compared to cells exposed to decreased loading conditions82. Given the fact that proteoglycan synthesis is a highly ATP‐demanding process in which it participates as an energy source and as a building block, it seems likely the increased ATP found under certain conditions may interact with proteoglycan synthesis85, 86. ATP regulates various cellular activities through purinergic signaling pathways, specifically by way of ATP hydrolysis to produce ADP and adenosine, which directly modulate cellular activity through purinergic P2Y and P1 receptors87, 88, 89, 90. Isolated porcine NP and AF cells have been shown to express P2X4 purinergic receptors and when this receptor is inhibited, disc cells lose the ATP‐induced membrane potential response observed when stimulated with exogenous ATP91, 92. Thus, influence of mechanical loading on disc cell energy metabolism and bioactivity may affect mechanotransduction pathways.
Dynamic loading has been shown to modulate cellular responses and mechanotransduction for many cell‐laden 3D tissues such as cartilage and IVD. For the IVD, finite element modeling of dynamic compression is associated with increased oxygen concentration throughout the tissue42 that will be associated with elevated metabolism, confirming many of the experimental observations described. This elevated transport of oxygen to cells in the IVD is associated with increased glucose consumption and lactate production (ie, energy conversion) that may be associated with elevated proteoglycan and collagen synthesis93. While oxygen transport has been reported to be sensitive to loading frequency and amplitude, the effect of dynamic loading on transport is dependent on the molecular weight of the solute. In dense hydrated tissues, small molecules are less sensitive to dynamic loading and associated changes in pore size, whereas larger molecular weight solutes, such as growth factors which dramatically influence anabolism (eg, IGF‐1, TGF‐β, FGF, PDGF), undergo enhanced nutrient transport under dynamic loading that is mediated by enhanced convection94, 95, 96, 97, 98. Thus, many of the reported effects for dynamic compressive loading on metabolism may in fact derive from dynamic compression‐related regulation of nutrient transport and associated receptor signaling99.
The distinct anatomical regions of the IVD are known to respond differently to compression, as will isolate cells from their respective tissue in terms of health and age. Many responses are similar for the IAF and NP tissues; however both short‐ and long‐term studies show that the more fibrocartilaginous OAF is not equally responsive to low to moderate loading magnitudes61, 62, 65. Rodent tail models have shown increased protease activation (MMP‐2) and decreased protein gene expression (collagen II and aggrecan) after 4 to 7 days of an in vivo loading protocol that was observed in the IAF and NP (Figure 3A)58, 79. The NP exhibited a frequency‐dependent response while the AF had a magnitude‐dependent response in collagen synthesis, which is believed to be due to the separate regions experiencing differences in hydrostatic and deformation‐related stimuli79, 100. The observed cell‐specific effects likely apply to duration of loading as well, where longer periods of loading (8 hours/day) can be beneficial for NP but damaging for AF81. Distinctions between the biological responses to load are related to the different magnitudes of deformation, fluid‐flow, and associated electrokinetic effects for cells in different tissue regions24, 42, 44, 70, 101. Age is also known to play a role in the response of NP and AF cells to dynamic compression, where mature cells lose the ability to maintain homeostasis to the degree that young cells do83. Overall, little is known of the mechanisms that govern these responses but could in part be attributed to transport of chemokines like growth factors, activation of mechanoreceptors, related ion channel activation, and cytoskeletal reorganization. These specific signaling mechanisms that contribute to the observed anabolic and catabolic mechanobiological responses of NP and AF cells have been little studied in the context of healthy, and more so in degenerated IVDs. A summary of known and proposed mechanisms regulating IVD mechanobiology is presented in Section 4.
Figure 3.
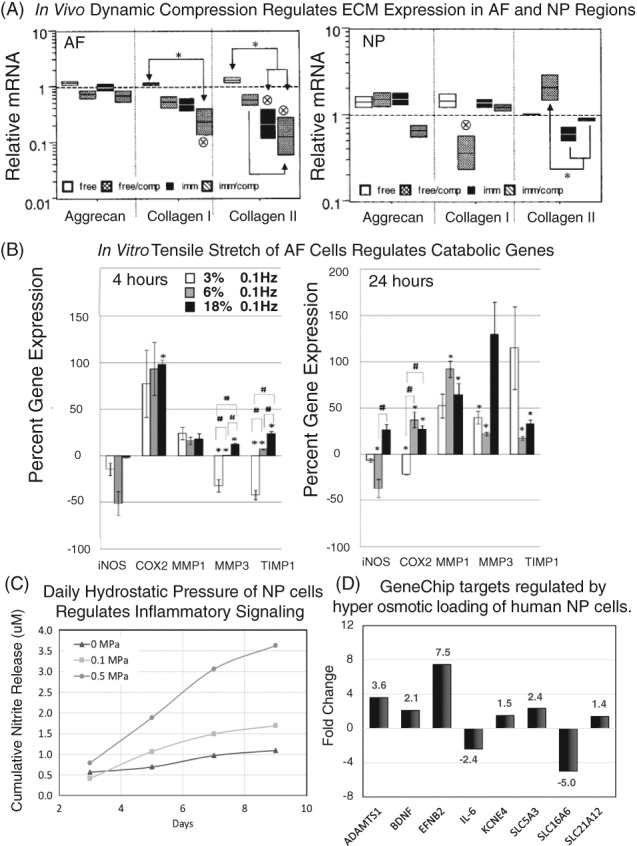
During loading, cells of the intervertebral disc (IVD) experience compressive and tensile deformation, as well as hydrostatic and osmotic pressures, among other physical effects. Mechanical signals are important regulators of cell biological responses in the IVD. (A) Dynamic compression applied to rat tail IVDs in vivo differentially regulates extracellular matrix (ECM) expression (aggrecan, collagen I, collagen II) in the anulus fibrosus (AF) and nucleus pulposus (NP) regions to the same magnitude of loading (adapted from Maclean et al71). (B) Applied stretch to AF cells in vitro results in a magnitude and duration dependent changes in inflammatory genes (iNOS, Cox2) and protease expression and regulation (MMP1, MMP3, TIMP1, adapted from Sowa et al109). (C) Daily applied hydrostatic pressure induces inflammatory signaling in NP cells in vitro in a magnitude and duration dependent manner. Adapted from Shah and Chahine 121. (D) Hyperosmotic loading of human NP cells induced decreased cell volume and cytoskeletal reorganization, as well as regulate genes include neurotrophins (BDNF), pro‐inflammatory cytokines (IL‐6), proteases (ADAMTS1), and multiple solute transporters and ion channels (adapted from Boyd et al135)
3.2. Tension
Most mechanical loading conditions present tensile strains in the AF region of the IVD, though the magnitude and response of these stimuli likely varies depending on the anatomic zone. in vivo102, 103 and explant studies have previously demonstrated tension will lead to a decrease in proteoglycan and collagen synthesis in the IVD; however, this effect may be magnitude dependent with modest inhibitory effects of physiological magnitudes of tension on isolated AF cell biosynthesis104. The OAF region has been shown to experience biaxial stretch during functional loading, with magnitudes between 4% and 6% during flexion and extension with minimal AF biologic response105, 106. More studies have shown that isolated AF cells at low magnitudes (1%) and physiologic frequencies (1 Hz) of tensile strain maintain proteoglycan production, consistent with a modest response to strain107. Loading frequencies outside of this physiologic range, however, can cause a catabolic response and upon exposure to frequencies greater or less than 1 Hz, AF cells lose the ability to maintain important matrix production108, 109. Increasing strains (5%‐20%) as well can lead to a catabolic response as shown through increased MMP‐3, COX2, nitric oxide (NO), TNFα, and decreased proteoglycan production (Figure 3B)107, 109, 110. It has been suggested that this transition from anabolic to catabolic response occurs during periods of continuous strain and may be explained by the cells' loss of energy production necessary to maintain biologic processes.
In contrast, the NP is not believed to experience high tensile strains under physiological loading, with no major changes in the NP observed (with the exception of ADAMTS‐4, which was also confirmed in a separate study111). Under hyperphysiologic stretch of 10% (applied at 0.5 Hz) or 20% (applied at 0.05 Hz), NP cells respond with increasing cell proliferation and collagen synthesis112. Short‐term hyperphysiologic stretch (20%) of NP cells also activates innate immune receptors, such as Toll‐like receptors (TLRs), in vitro110. It is believed that these differential responses of NP and AF cells, likely reflect cytoskeletal adaptation to their respective in situ mechanical demands, with NP cells experiencing hydrostatic and/or compressive pressure and AF cells subjected to tensile strains.
3.3. Hydrostatic pressure
The NP is highly hydrated, and therefore cells within the NP experience hydrostatic pressure when loaded. Hydrostatic pressure is distinct from other loading exposures due to the presumed conservation of cell and pericellular matrix volumes. Hydrostatic pressure magnitude also varies diurnally in a manner that depends on spinal alignment and physical activity113, 114. Both experimental and computational methods have been used to estimate the magnitude of hydrostatic pressure that disc cells are exposed to during activity. Hydrostatic pressure magnitudes range from 0.1to over 3 MPa, with baseline magnitude of around 0.1 MPa applied at all times regardless of posture or activity115, 116. With aging, hydrostatic pressure magnitude is likely reduced due to lower proteoglycan and water content of the disc17, 117. Exposure of disc cells to hydrostatic pressure is known to influence disc cell biologic responses in duration, magnitude, and frequency‐dependent manner, with some species‐specific variations observed. In general, higher magnitudes of hydrostatic pressure (>3 MPa) can lead to degenerative responses in IVD cells mediated by an increase in MMPs and reduction in ECM expression and synthesis when applied to NP cells seeded within a 3D culture system or within tissue explants113, 118. Hydrostatic pressure overloading of 3 to 10 MPa applied at frequency greater than 1 Hz on cells from bovine or porcine NP119, 120, 121, porcine AF122, as well as human IVD cells120 appear to decrease anabolic gene expression and increase catabolic gene expression. Whereas hydrostatic pressure (>3 MPa) applied at 20 Hz to rabbit IVD cells showed a higher ratio of collagen synthesis to degradation123. When disc cells are maintained under a physiological pressure range (0.3‐1 MPa), increased matrix synthesis occurs56, 124, 125, 126, 127. However, within this range, differing responses have been observed. Some studies have shown an upregulation in ECM proteoglycan synthesis, while others show a downregulation in ECM and upregulation of catabolic signaling (Figure 3C)124, 125, 126, 127, 128. Similarly, while some studies find that collagen synthesis to be upregulated in this range of loading magnitudes, others show decreased expression of collagens I and II at the gene level120, 121, 124, 127, 128. Some of these changes may be attributed to volumetric or cell morphological changes. Moreover, hydrostatic pressure effects appear to be dependent on cell density121 of tested samples, with higher cell density leading to higher cell stress under hydrostatic pressure.
Species‐specific variations may be due to phenotypical and morphological differences in NP cells which are derived from the notochord. In some species (eg, rodents and porcine), the NP cells maintain a notochordal morphology into adulthood, while others (human and bovine discs) have small nonvacuolated single NP cells surrounded by a dense ECM129. While the factors that drive this cellular transformation or transition are yet to be fully understood, hydrostatic pressurization may be a regulator of such transformation. One study found that dynamic pressurization can induce a transition of notochordal cells to a mature single cell phenotype in a porcine explant model17; however, hydrostatic pressure did not alter the expression of certain NP phenotypic markers (cytokeratin 18 or brachyury) in vacuolated notochordal NP cells. On the other hand, hydrostatic pressure was found to be a modulator of NP phenotypic markers (cytokeratin 19 and N‐cadherin) in nonvacuolated NP cells121. The presence of vacuoles has been postulated to function as a regulator of cell volume and tonicity during rapid osmotic stress, protecting the cells from potentially damaging swelling pressures130. Therefore, species variation is likely occurring due to differences in biomechanical function and biological uniqueness. Results from hydrostatic pressure studies continue to challenge our understanding of mechanisms that contribute or regulate IVD responses, and more work is needed to verify specific cell‐ and loading‐dependent responses.
3.4. Osmotic pressure
The osmotic environment of the IVD, resulting from a combination of mechanical deformations and fluid redistribution, can vary locally depending on biochemical composition and local volumetric change7. Osmotically induced swelling due to hypotonic conditions draws water into the disc tissue to maintain hydration, and causes volumetric gain50. Urban7 has demonstrated that the interstitial osmotic pressure is considerably higher in the disc (~430 mOsm) compared to plasma and the changes that occur on IVD loading following compression are closely associated with changes in the density of proteoglycans and extracellular hydration. An important component in maintaining and modulating osmotic forces, the presence of large, aggregating proteoglycans in the NP, particularly aggrecan, creates a high negative fixed charge density that allows tissue swelling and mechanically driven fluid flow with associated electrokinetic effects11, 101, 131, 132. This contributes to the primary biomechanical function of the NP where osmotic pressurization of the NP serves to balance loads endured by the spine through its distinct osmotic and water‐binding properties 50. Proteoglycan production is greatest under physiologic osmolality (~430 mOsm), but decreases if higher or lower osmotic pressures are reached7, 133, 134. Osmotic stimuli may exert its effects at the transcriptional level as altered (hypo‐ or hyper‐) osmolality has been shown to broadly influence mRNA expression of genes related to cytoskeletal remodeling, osmolyte transport, pro‐inflammatory cytokines, and neurotrophins (Figure 3D)135. Although it is known that the response to altered osmolality is regulated at the transcriptional level, exact mechanisms by which osmotic pressure modulates IVD cell metabolism are not known. Increased osmolality can also decrease DNA synthesis or even induce DNA damage, which has been shown to push human IVD cells into low or ceased proliferative states where cell cycles can be delayed at the G2/M and G0/1 phases136. This hypertonicity‐induced cell cycle arrest is partly mediated by MAPK/p53 activation and reduced ERK/Akt stimulation136, 137, 138.
Due to alterations in proteoglycan and hydration content in the aging and degenerate matrix, these osmotic pressure changes in the IVD are some of the most drastic changes known. Distinguished by its hypoxic, avascular environment with limited nutrient supply, the normal healthy disc maintains a relatively high osmotic pressure of the proteoglycan‐rich ECM7. These characteristics are markedly reduced, likely initiated by a loss in proteoglycans that can lead to decreased osmotic pressure and impaired water binding ability of the disc ECM. One study investigated the effect of long‐term osmotic loading on structural and functional properties of juvenile and adult bovine NP and EP tissues found that culture under osmotic conditions that recapitulate the healthy disc environment in situ (400 mOsm/kg) promoted matrix production and mechanical function of engineered tissues139. The persistence of hypoosmotic conditions in degenerate discs may not only allow for increased IVD cell proliferation137, 138, but may also accelerate senescence137, 140.
4. SUBCELLULAR MECHANOTRANSDUCTION
4.1. Cytoskeleton of the IVD
The response of cells to loading, and the specific mechanotransduction pathways mediating responses to load, is dependent on cell morphology, cell‐cell interactions, and cell‐ECM interactions. Loading of cells can act directly on cytoskeletal networks by promoting F‐actin reorganization, which can act as a mechanism for transducing mechanical stimuli to the nucleus64, 106. In healthy, rounded NP cells, F‐actin is distributed cortically around periphery of the cell and is distributed as short punctate fibers throughout the cytoplasm (Figure 2). NP cells in vivo and in situ lack stereotypical F‐actin stress fibers13, 141. In OAF cells, F‐actin is distributed throughout the cytoplasm extending into the cell processes, reflecting a fibroblast‐like morphology. Actin expression levels are higher in OAF compared to NP141. F‐Actin is the major cytoskeletal element contributing to stiffness of NP and AF cells142, with actin integrity playing a key contribution to cellular viscoelastic properties.
NP and AF cells also rely heavily on the presence of additional cytoskeletal components, such as intermediate filaments (IFs) and tubulin, the latter of which forms an extensive meshwork throughout the cytoplasm of NP and OAF cells, where expression level is higher in NP than OAF cells, but no appreciable differences in organization by zone have been observed141. IF proteins are classified into five main groups, with the following present in cells from the IVD: keratin 18 and 19 (Type I), keratin 8 (Type II), vimentin (Type III), and nuclear lamins (Type V). These specific forms of IF proteins have come to be important NP and AF cell‐specific molecular markers143. IFs can withstand large deformations and at the same time they can be highly sensitive to small forces in the context of cell signaling144. Vimentin IF spans the cytoplasm from the plasma to the nuclear membrane in NP cells, whereas in OAF cells, vimentin filaments also extend into the cellular processes. Interestingly, AF cells only express vimentin IF, whereas NP cells coexpress cytokeratins and vimentin. This broad IF coexpression in NP cells is shared by cells of other tissues exposed to fluid or semifluid environments, and with high concentrations of hyaluronic acid (ie, ovaries, endometrium, kidney, epithelial cells of the thyroid tissue, Sertoli cells, endoderm of embryo and notochord)145. The expression of cytokeratin is higher in NP compared to AF and chondrocytes145. While keratin IFs are an essential component of cells that experience shear loading146, vimentin has been more associated with protection against compressive forces (eg, fibrocartilage cells) and during development147. Vimentin IFs contribute little to cortical stiffness, but they appear to regulate intracellular dynamics by stabilizing organelles in the cell148.
During development as the notochord narrows to form the spine, compressive forces are exerted on the IAF while tensile forces are exerted on the OAF149. Correspondingly, AF cells induce formation of actin stress fibers and trigger the deposition of a fibrous ECM149. During this process, cells form stress fibers that mirror the alignment of fibronectin and collagen bundles indicating that internal cellular structure drives ECM organization149. While actin is essential for this process, vimentin seems more related to a later differentiation stage of AF cells when levels of compressive forces increase. After birth, there is reorganization of AF cytoskeletal elements and cells seem to rely more on the already deposited ECM149. In the NP, actin distribution changes with development; however, the changes depend on the presence of a vacuolated (notochordal) compared to nonvacuolated NP cells in a species. Notochordal NP cells form interconnected clusters of large vacuoles that have dense cortical actin with an actin filled cytoplasm12, 13, 14, 15. With postnatal development, vacuolated NP cells and their cytoskeleton persist in most species (mouse, rat, rabbit, and pig). In a few species (sheep, mongrel dogs, and beagles), vacuolated NP morphology and cytoskeleton change to a nonvacuolated type. Nonvacuolated NP cells appear unconnected and have robust but distributed staining for F‐actin, vimentin, and cytokeratins. In nonnotochordal species, no major differences have been observed in actin, tubulin, or vimentin organization with skeletal maturity141. Actin and tubulin expression do not appreciably change with age; however, vimentin expression decreases with age in OAF, suggestive of a transcriptional shutdown in a bovine model141. In human NP cells, the expression of cytokeratins and vimentin decrease with development and postnatal maturity145. Normal mature human discs have more heterogeneity in cellular cytoskeleton than is observed in animal models. For example, stellate cells with multiple cellular processes that are positive for F‐actin and vimentin have been observed in the IAF and NP of both normal and pathological human discs, which may reflect a response to abnormal loads or microenvironments.
Actin cytoskeletal remodeling is largely mediated by the RhoA/ROCK signaling pathway, which is involved in many responses such as cell migration, polarization, stress fiber, and adhesion formation150. RhoA/ROCK signaling is also altered in response to mechanical stimuli, which creates complex interactions between morphological and biomechanical changes. Porcine NP cells normally form cell‐cell interactions and cell clusters; however, this function is lost when cells are treated with ROCK inhibitors (Figure 4A), underscoring the role of ROCK‐dependent RhoA GTPase activation in regulating cell clustering in young NP cells151. Under these conditions, treated cells also have decreased ECM synthesis and expression of NP markers brachyury, and N‐cadherin (Figure 4B,C), suggesting that ROCK pathway modulates cell phenotype and morphology. These observed coincident changes reported for cytoskeletal organization and cell biosynthesis in IVD cell mechanobiology may be related to the ROCK signaling pathway.
Figure 4.
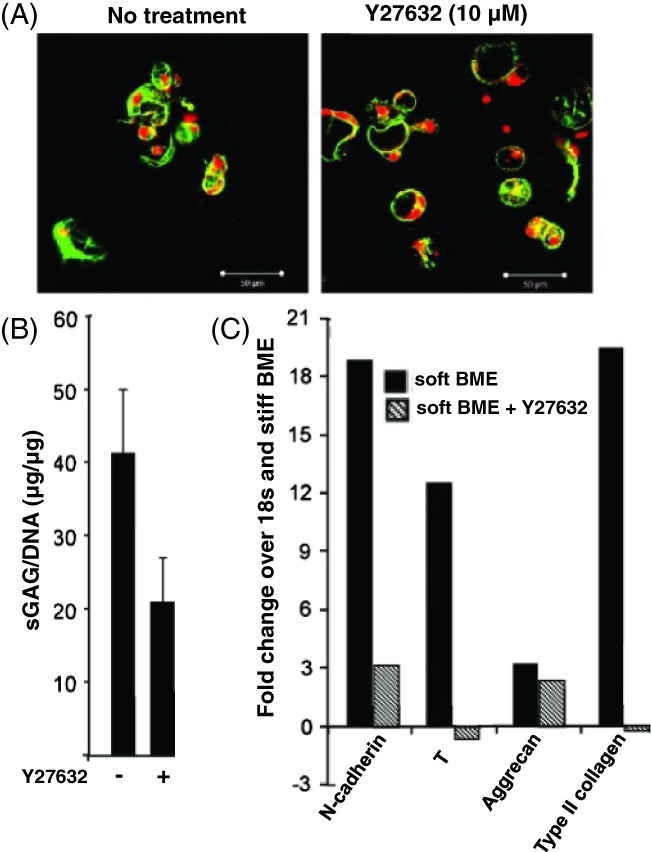
(A) When porcine nucleus pulposus (NP) cells are treated with the ROCK inhibitor Y27632, multicell cluster morphology is lost, and single cells are observed with little cell–cell interactions. (B) ROCK inhibition also promotes decreased biosynthetic s‐GAG activity and (C) expression of the NP markers n‐cadherin, T (brachyury), aggrecan, and collagen II (adapted from Hwang et al151)
4.2. Cytoskeletal responses in mechanical loading of IVD cells
Studies of isolated IVD cell to specific physical stimuli reveal broad changes in cell morphology and suggest that cytoskeletal reorganization as one mechanism by which IVD cells respond to physical stimuli. For example, cell volume changes occurring in response to hypoosmotic or hyperosmotic stress can occur only with associated F‐actin remodeling to accommodate the change in cell volume. Early studies showed that NP cell response to hypoosmotic stress is much slower than that for the AF cell, due to the dense cytoskeleton of the juvenile NP cells142, 152. Cellular differences in cytoskeleton appear to be the principal difference regulating differential responses of AF and NP cells to osmotic loading with associated effects on other signaling mechanisms (eg, calcium signaling). As NP or AF cells undergo changes in conformation during osmotic swelling, there may be an associated activation of mechanosensitive channels, allowing calcium and associated cellular signaling to mediate cell growth, differentiation, and motility134, 142, 153. These intracellular calcium transients were shown to be different for NP and AF cells and are, in part, modulated by the actin cytoskeleton's stability. Indeed, NP cells appear to be more sensitive to actin disruption as demonstrated by increased calcium spikes/responses under osmotic stress compared to AF cells142. Interestingly, a limited number of studies have investigated ion channels, including mechanosensitive channels, and their role in IVD mechanotransduction. One study found that osmolarity changes associated with proteoglycan degradation in IVD organ cultures can alter TRPV4, an osmosensing calcium channel154. A proteomic analysis155 identified candidate ion channels that are consistently and highly expressed in IVD tissue (Table 1), with zonal differences in NP and AF. Despite a current lack of knowledge of mechanosensitive ion channels in IVD cells, they are likely an important regulator given the presence of osmotic stimuli and membrane stretch in cell volume regulation, which may activate mechanosensitive ion channels.
Table 1.
Gene array data of ion channels found to be differentially expressed in AF or NP regions of the IVD (results from a rat Affymetrix data set, adapted from Tang et al155)
| Higher in AF than NP | SCN1A | Sodium voltage‐gated channel alpha subunit 1 |
|---|---|---|
| KCNK5 | Potassium channel subfamily K member 5 | |
| KCNC3 | Potassium voltage‐gated channel subfamily C member 3 | |
| TRPV1 | Transient receptor potential cation channel subfamily V member 1 | |
| KCNMA1 | Potassium calcium‐activated channel subfamily M alpha 1 | |
| Higher in NP than AF | CACNA1B | Calcium voltage‐gated channel subunit Alpha1 B |
| CACNA2D1 | Calcium voltage‐gated channel auxiliary subunit alpha2delta 1 |
Abbreviations: AF, anulus fibrosus; IVD, intervertebral disc; NP, nucleus pulposus.
AF cells isolated from the ECM undergo cell morphology changes and exhibit a tremendous capacity to reorganize their cytoskeleton in response to compression or tension. Studies with immature bovine IVDs showed that AF cells respond to applied cyclic tensile strain (10%, 1 Hz) with increases in the expression of β‐actin and β‐tubulin at both the transcriptional and protein level, while vimentin expression was decreased106. Together with this, actin in the AF cells formed a large number of stress fibers in response to tensile stretch accompanied by a marked cell elongation; in contrast, microtubules and vimentin filaments were less affected106. It was also observed that widespread remodeling of cytoskeletal components is primarily restricted to OAF cells in response to cyclic tensile strain, with increased F‐actin turnover and increased β‐actin, β‐tubulin, and collagen I expression. Supporting these findings, experiments show that human AF cells reorient themselves perpendicular to the direction of applied uniaxial stretch in a fashion dependent on actin and focal adhesions, and undergo apoptosis if this reorientation is restricted using micropatterned membranes that define cell orientation under loading156.
Under compressive loading, changes in cytoskeletal responses have been observed that vary with the zonal origin of the IVD cells. AF cells subjected to unconfined compression (25% strain) responded to mechanical deformation, with increasing ECM expression, increased vimentin expression and increased vimentin polymerization. In contrast, NP cells were not responsive to the same mechanical loading conditions. These distinctions are likely attributed to differences in strain magnitudes experienced in situ, but may also be due to the presence of a more diffuse and stiff cytoskeleton in the notochordal NP cells, which may restrict deformations or cell shape changes with loading.64
4.3. Cell‐matrix interactions
Integrins are transmembrane glycosylated proteins that mediate interactions of the cell surface with the extracellular environment. Structurally, integrins are heterodimers comprising two noncovalently associated subunits, referred to as the α and β subunits, which combine to form 24 distinct integrin receptors. Integrins are grouped into subfamilies based on ligand interactions. NP cells express integrin subunits α1, α2, α3, α5, α6, αv, β1, β3, β5, β6, and β8 57, 157, 158, 159, 160, while AF cells express α1, α5, αv, β1, β3, β5, and β6 integrin subunits160. In the context of the disc, the family of RGD integrins and laminin binding integrins has been best studied. RGD (arginine‐glycine‐aspartic acid) is a ligand motif that certain integrins recognize, including the fibronectin receptor α5β1, which is present in the ECM. Integrins also mediate interactions with other important constituents of the ECM, including multiple isoforms of collagens, laminins, and small glycoproteins158, 159, 161, 162, 163. Integrins acts as force sensors and are essential in cytoskeletal mechanotransduction with focal adhesions.
In evaluation of regional variations of integrins, the expression of α5β1 was particularly prominent in the cells from the human NP and IAF, with significantly lower numbers of immunopositive cells in the OAF57. A recognizable difference between NP and AF ECM was the finding of the uniquely expressed laminin isoforms in NP tissue, LM‐111, LM‐511, and LM‐332, compared to AF163. Immature NP cells also show expression of integrins that can mediate interactions with these laminins (α6β4, α3β1, and α6β1)163 to promote cell attachment, as well as upregulated proteoglycan synthesis and elevated mRNA for a subset of NP ECM molecules. It has further been shown that certain laminin‐mimetic peptides induce higher levels of biosynthetic activity compared to others (Figure 5B) and can even express statistically higher amounts of NP markers despite a stiff substrate presentation (Figure 5C). The staining of laminin seems to decrease with age in several species164 and their appearance on young individuals may be related to its role as component of basement membrane that surrounds the notochord during its formation and differentiation165.
Figure 5.
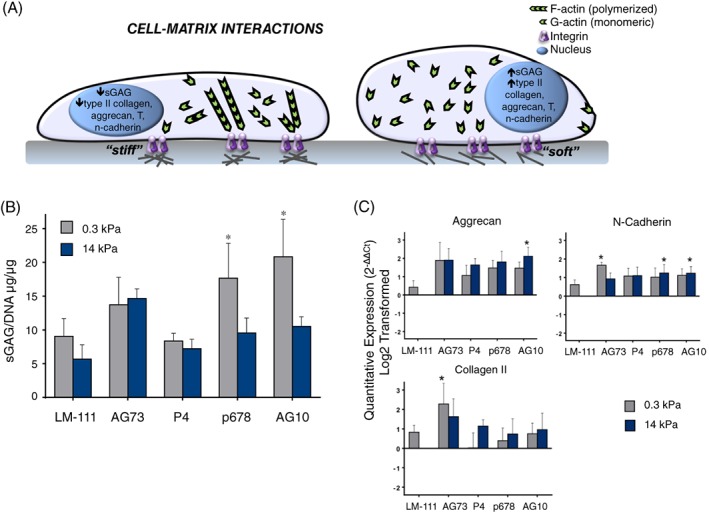
(A) Cell‐matrix interactions can regulate disc cell phenotype and bioactivity through stiffness‐ and ligand‐mediated effects, where rounded cells observed on “soft” matrices have increased production of nucleus pulposus (NP) markers and sGAG but flattened cells on “stiff” substrates can have decreased sGAG production. (B) Ligand presentation can overcome some stiffness‐mediated effects, as shown by Bridgen et al161 with primary human NP cells that only exhibit increased biosynthetic activity on soft substrates with select laminin‐mimetic peptides. NP cells were cultured on surfaces conjugated with LM‐111 (full length laminin), AG73 (syndecan peptide), P4 or P678 (α3 integrin receptor peptides), or AG10 (α2, α5, α6, β1 integrin‐recognizing peptide). (C) These select ligands can also produce differential NP marker expression independent of substrate stiffness
Many studies have revealed that cell interactions with matrix proteins through integrin binding contribute to activation of ERK and MAPK pathways that may amplify or attenuate signaling through other transduction pathways such as growth factor binding166. Indeed, ERK phosphorylation can be confirmed for NP cell binding to laminin in vitro but has not been specifically linked to a downstream biosynthetic event through ERK inhibition117. Additional studies are required to verify the precise role of ligand interactions with integrins on NP and AF cells, and to confirm a role for ligand density (eg, collagen or laminin density, RGD or other peptide density)161 and ligand specificity in activating cell signaling through ERK, MAPK, or other pathways.
An additional role for integrins is associated with the release and activation of latent TGF‐β that is stored in the ECM, bound to a peptide167. Some integrin subunits have been shown to participate as docking points to allow proteolytic cleavage of immobilized TGF‐β from the docking peptide, and to enable generation of cell traction that will mechanically separate TGF‐β to enable its activation167. TGF‐β is critical for the disc development at embryonic stage and for its postnatal maintenance168, 169 and regulates connective tissue growth factor CCN2, implicated in synthesis of aggrecan170. Both excess and deficiency of TGF‐β activation can be detrimental for IVD157, 169, 171, 172. In this manner, TGF‐β‐receptor‐mediated signaling could be regulated by integrin engagement with the ECM; however, investigations on changes in TGF‐β activation with disc aging and changes in ECM stiffness may provide further insight into disease related changes.
4.4. Cell‐cell interactions and the cadherin‐catenin pathway
Cells of the NP and AF interact with neighboring cells in a manner that varies with age, and that is distinctly different between these two regions. Some cells of the AF form elongated cell “arrays” or may spread into flattened cells as in the case of the stellate interlamellar AF cell. These cells may interact with adjacent cells through E‐cadherin mediated junctions that are known to be key regulators of cell signaling in multiple cell types. AF expression of E‐cadherin appears to be independent of age and matrix stiffness173, and little is known about the relationship between E‐cadherin expression and cellular responses to physical stimuli.
In contrast, cells of the juvenile NP cell are rounded and may exist in close proximity to other cells in rounded “cell‐matrix units.”174 These cells may interact through N‐cadherin‐mediated interactions that are shown to be essential for promoting mRNA expression of key markers of the NP phenotype, including brachyury, aggrecan, and laminin isoforms (Figure 6B‐D)117. These cadherin‐mediated interactions appear to promote β‐catenin phosphorylation (Figure 6E) and decreased nuclear lamin expression in human NP cells, which may be key signaling events responsible for the downstream upregulation in biosynthesis. The loss of N‐cadherin with aging could be related to a decrease in cell density with age173 and may affect both dedifferentiation of the NP cell phenotype and its subsequent capacity for injury‐induced regeneration or repair.
Figure 6.
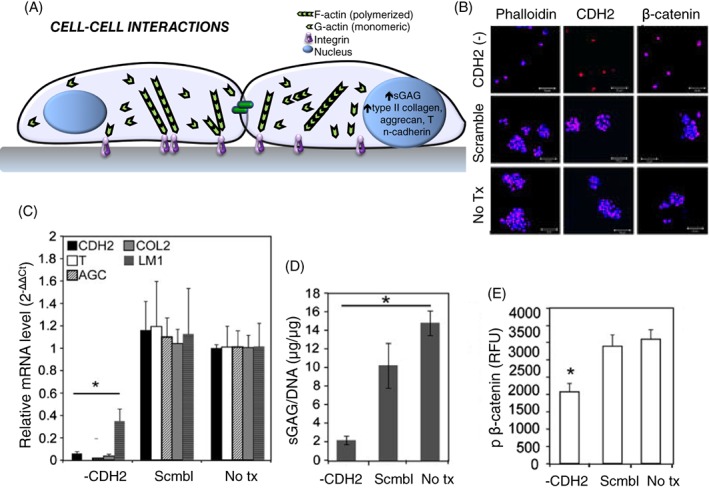
(A) Effects of cell‐cell interactions of the intervertebral disc have been observed in primary human nucleus pulposus (NP) cells where promotion of cell‐cell interactions via soft substrates or N‐cadherin (CDH2) signaling leads to enhanced biosynthesis and NP marker expression. (B) Hwang et al 117 demonstrated that the ability to form rounded multi‐cell clusters is lost when CDH2 is depleted. (C) When NP cells lose the ability to form cell‐cell interactions in the absence of CDH2, there is a significant loss of expression of NP markers and (D) decreased biosynthesis, (E) which is believed to be mediated by loss of β‐catenin
An important observation is that cells of the IVD may interact with adjacent cells while adopting rounded morphologies, as for NP cells or AF cells when isolated in vitro, or while adopting spindle and spread morphologies as for AF cells (or NP cells upon stiff substrates). Given the adaptive nature of the cytoskeleton and its important role in activating ion channels and RhoA kinases, we find that cell signaling through morphological confirmation is inextricably linked to cell signaling through cell‐cell interactions such as β‐catenin signaling. More work will be needed to isolate factors of cell morphology as distinct from N‐ or E‐cadherin mediated signaling in order to isolate the specific signaling events and their relationship to IVD cell phenotype.
4.5. Substrate stiffness effects
We and others have found an effect of substrate stiffness on mechanobiology in IVD cells, as shown through mRNA expression and ECM biosynthesis117, 151, 158, 161, 162, 173, 175, 176, but the degree of response seems to depend largely on cell type. This is also true for chondrogenesis in MSCs, where changing substrate stiffness can impact chondrogenic events. It has been shown that lower stiffness hydrogels promote increased aggrecan, collagen, and sGAG synthesis in MSCs, indicating enhanced chondrogenic ability, while nuclear stiffening induced by stiff substrates sensitizes the transcriptional responses in MSCs as a priming effect for mechanical perturbations177, 178. The mechanism by which substrate stiffness information is transduced in IVD cells is not completely understood, but it likely relates to cytoskeletal organization and related transcriptional activators such as YAP/TAZ and SRF (Figure 7A)179, 180, 181, 182, 183. The cytoskeletal regulation of primary human NP and AF cells has been observed through YAP/TAZ and MRTF signaling, cofactors of known mechanically regulated transcription factors TEAD and SRF, respectively (Fearing et al, unpublished data). When isolated cells are cultured upon stiff substrates (29 kPa), transactivation of TEAD and SRF occur at significantly higher levels compared to soft substrates (0.3 kPa), where they remain predominantly quiescent and their respective cofactors stay sequestered in the cytosol. These cells cultured on stiff substrates with diminished TEAD and SRF signaling also express significantly lower amounts of aggrecan, GLUT‐1, and collagen II (Figure 7B,C) and are less biosynthetically active. Additional work has shown when F‐actin is disrupted with the small molecule inhibitor Latrunculin B, both SRF and TEAD exhibit decreased activation along with higher expression of NP markers, even when cells are cultured on stiff substrates. The presence and activity of these cytoskeletal‐regulated transcription factors in primary human disc cells indicates IVD cells rely on mechanically regulated signaling pathways.
Figure 7.
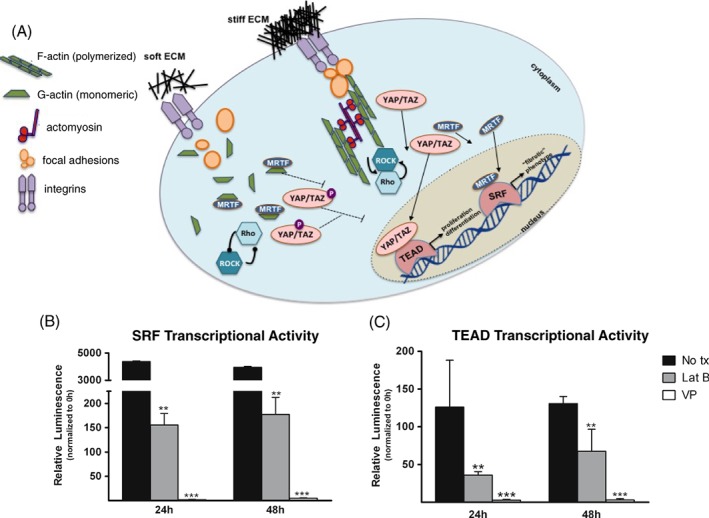
(A) Work in diverse fields has identified the critical role of actin as a direct regulator of transcription factors. SRF and TEAD are known to be active and present in primary nucleus pulposus (NP) and anulus fibrosus (AF) cells, where they are believed to be regulated by F‐actin turnover and Rho/ROCK signaling. On stiff substrates, increased polymerized F‐actin form contractile complexes with focal adhesions and actomyosin to sustain Rho/ROCK signaling to allow nuclear translocation of coactivator YAP/TAZ to bind TEAD and drive transcription of proliferation‐ and differentiation‐related genes. Similarly, coactivator MRTF is able to localize to the nucleus due to the decreased presence of monomeric actin (G‐actin) where it will bind and activate SRF to transcribe fibrotic response‐related genes. Soft substrates allow for liberation of G‐actin which sequesters MRTF in the cytosol and decreased Rho/ROCK signaling, due to the loss of the contractile actomyosin/F‐actin complex, prevents YAP/TAZ nuclear localization, thus keeping both SRF and TEAD transcriptionally silent. (B) Verteporfin (VP), a known inhibitor of YAP/TAZ, and Latrunculin B (Lat. B), which disrupts polymerized F‐actin, were cultured with SRF transcriptional reporter primary human NP cells, which resulted in significantly decreased transactivation of SRF with both Lat. B and VP treatment. (C) When TEAD transcriptional reporter NP cells were treated with Lat. B and VP, a similar decrease in transcriptional activity was observed, suggesting inhibition of a contractile cytoskeleton can prevent expression of fibrotic and aberrant cell cycle genes
5. RESPONSE TO ABNORMAL MECHANOBIOLOGY AND DEGENERATION
5.1. Degenerative microenvironment
Physical signals in the presence of chemical stimuli have the potential to alter cytoskeletal and biomechanical response to loading. Inflammatory stimulation of NP cells has been shown to disrupt F‐actin structure and related cell volume regulation under osmotic stress. Bovine NP cells stimulated with TNFα, a pro‐inflammatory cytokine implicated in disc degeneration, show a loss of intracellular F‐actin that is redistributed to the cell cortex184. Consequently, TNFα‐treated cells have an increased baseline volume and an increased hydraulic permeability184. Interestingly, these morphological and cell biophysical properties appear to be irreversible in vitro, even when inflammatory stimulus is returned to normal culture conditions184. The downstream signaling responses and associated catabolic upregulation in inflammatory environments are thought to mimic the degenerative milieu of the IVD. Evidence suggests that the response of cells from degenerate tissue to applied loading differs from cells isolated from normal IVD tissue. AF cells from degenerative tissues demonstrated a more pro‐inflammatory response to applied stretch (6% elongation, 0.1 Hz) compared to cells from nondegenerate discs, suggesting that degenerate AF cells are more reactive to exogenous stimuli. Moreover, the short‐lived beneficial effects of mechanical stretch seen in normal AF cells do not appear when degenerate AF cells are similarly stretched. Indeed, increasing evidence suggests that interactive effects of tensile stretch and inflammatory signaling in AF cells109, 185, 186. This may be mediated by altered cytoskeletal mechanotransduction. In AF cells, TNFα treatment increased F‐actin stress fibers α‐tubulin, which potentially sensitized AF cells to mechanical strain185. Short‐term hyperphysiological stretch of disc cells also activates innate immune receptors, such as TLRs, in vitro110. Activation of TLRs by the canonical agonist, LPS, induces cytoskeletal remodeling of NP cells, and altered biomechanical properties of NP cells, similar to the altered mechanotransduction observed in the presence of TNFα activation184. All these studies point to a positive feedback of degenerative microenvironments, mediated primarily by pro‐inflammatory cytokines, which alter cytoskeletal remodeling and related mechanotransduction.
5.2. Cell‐ECM interactions
Although one study found that physiological level of dynamic compression induced integrin α5β1 expression in rat disc explants187, other studies find that degenerative static compressive loading in vivo upregulate β1 expression in NP cells, and activates downstream signaling188. Moreover, disruption of β1 integrin signaling may underlie disc cell apoptosis induced by mechanical stress189 in disc degeneration models. However, in human samples, cells derived from both degenerated and nondegenerated IVDs have similar expression of integrins subunits, despite major changes in ECM content and levels with degeneration57, 158, 159, 160. No significant differences were observed in the number of immunopositive cells for the α5, β1, or α5β1 heterodimer identified in the NP or IAF of nondegenerated discs as compared with IVDs with moderate or severe histologic degeneration57. However, the response of cells to integrin‐mediated mechanotransduction is altered in degeneration. For example, when dynamic compressive load (sinusoidal 0.45‐0.95 MPa, 1 Hz) was applied to human NP cells, both cells from degenerated and nondegenerated sources exhibited similar reduction in gene expression of aggrecan. However, blocking of integrin binding with an RGD peptide prevented this response only in nondegenerated cells57. This suggests that mechanotransduction signaling is altered in degenerated tissue, in that integrins may not be directing response of degenerate cells to loading or that there are changes in the mechanosensitivity of integrins to mechanical loading in degenerate cells57. Studies of human AF cells derived from degenerated and nondegenerated tissue and exposed to 10% strain, 1 Hz for 20 minutes also respond differently. Specifically, there was a decrease in expression of ADAMTS4 for nondegenerated cells and a decrease in expression of collagen I for degenerated cells. Blocking integrin binding transiently prevented a response to loading in nondegenerated cells that was mediated by focal adhesion kinase; however, it failed to affect the mechanotransduction in degenerated AF cells. These results suggest that integrins participate in mechanotransduction of AF cells from nondegenerated tissue, and that alternative mechanotransduction pathways mediate responses of degenerate cells190.
5.3. ER stress
Cells also protect themselves from stresses through cellular stress responses. In one study, Chooi and Chan191 found differential gene expression patterns based on compression loading regimens that suggest an increase in ER stress pathway genes as a form of cell survival. In a 3D collagen model with bovine NP cells, compressive loading was applied for various durations and assessed the heat shock protein response (HSR) and the unfolded protein response (UPR). Although an increase in both UPR and HSR stress response genes were observed in the highest group of static compression, the authors note that upon load removal, HSR genes remain upregulated while UPR genes become downregulated, which may suggest HSR genes' continual expression is a protective mechanism to allow cells to recover and avoid apoptosis. This was further confirmed by showing HSR gene‐expressing cells do not become apoptotic and those that did undergo apoptosis did not express these genes postloading. This points to a master regulator of cell survival and biosynthesis following periods of static compressive loading. Similarly, hyperphysiologic cyclic stretch has been shown to increase the production of ER stress markers CHOP, GRP78, and caspase‐12 in AF cells106, 192, 193. In one study, the addition of caspase inhibitors was able to partially suppress the stretch‐induced apoptosis as well as NO overproduction192. It has been suggested that cyclic stretch‐induced apoptosis may occur through mitochondrial pathways, as cyclic stretch has been noted to cause reduction of the mitochondrial membrane potential, though other cell death pathways are likely involved.
6. CONCLUSION
In conclusion, cells of the NP and AF have been observed to respond to compression, tension, osmotic stimuli, and hydrostatic pressure with changes in ECM, and regulators of ECM synthesis, in a manner that differs between these two cell types. Cellular studies reveal a role for differential regulation of cytoskeletal remodeling between AF and NP cells in contributing to these differential responses, with the remodeling being specific to actin, vimentin or IF reorganization and/or expression specific to the stimuli. Cytoskeletal reorganization can activate downstream effects not only through ROCK signaling and coactivators such as YAP/TAZ, but also through volumetric regulation that may affect mechanosensitive ion channels as observed through calcium signaling. Whether the cell is permitted to interact with adjacent cells or with ECM ligands will affect cell morphology and cytoskeletal organization, but may also activate β‐catenin and ERK phosphorylation events that are known regulators of gene expression and downstream biosynthesis. While cell morphology, cytoskeletal reorganization, ion channel activation, and cell‐ligand mediated events are linked in many cell types, the compelling differences shown between NP and AF cells within a single IVD structure motivate studies to reveal the precise mechanisms that govern mechanobiology in these systems.
In the process of performing a comprehensive review of the literature, we have identified some gaps in knowledge regarding topics related to mechanobiology of the IVD. Here, we briefly summarize key gaps, with the hope that future studies will inform some of the open questions in the field:
6.1. Development and aging
Mechanical factors that drive phenotypic changes are not well understood in the developing, adult and aging IVD. There is a need for in vitro and ex vivo studies that identify relationships between magnitudes of spinal motion and loading conditions in vivo with long‐term changes in cellular morphology, phenotype, and metabolism.
Tracing of cell fate through the continuum of notochord to chondrocyte‐like phenotype is needed to confirm standing hypotheses about phenotypic transition and/or transformation with aging. Nevertheless, the use of cells from younger specimens are better suited for studying early events that can lead to degeneration, whereas cells from older specimens give insight into how aged or degenerate cells react to further damage and inflammation.
6.2. Response of cells to loading and microenvironmental changes
Overall, little is known about the mechanisms that govern response of cells to loading and changes in physiochemical microenvironments.
While cell response to loading could in part be attributed to transport of chemokines like growth factors, activation of mechanoreceptors, related ion channel activation, and cytoskeletal reorganization, detailed mechanistic studies are needed to assess first‐order mechanisms. These specific signaling mechanisms that contribute to the observed anabolic and catabolic mechanobiological responses of NP and AF cells have been little studied in the context of degenerated IVDs.
The response to altered osmolality, pH, and oxygen tension is regulated at the transcriptional level, yet the exact mechanisms by which microenvironmental changes (alone and in combinations) modulate IVD cell fate, metabolism, and interactions with immune cell infiltrates are not known.
Knowledge of the effects of dynamic and static loading upon nutrient transport to varying compartments and substructures of the IVD. Modern approaches to monitoring tracers and contrast agents could be employed to better track transport and fluid flow to varying substructures and thus to varying cell types.
6.3. Cell‐ECM interactions and cell‐cell signaling
Future studies are required to verify the precise role of ligand interactions with integrins on NP and AF cells, and to confirm a role for ligand density and specificity in activating cell signaling through known and to be discovered pathways. Emphasis on changes in cell‐ECM interactions with growth factor and chemokine signaling is also warranted.
Cellular interactions with ECM, both visualization of and knowledge of the biochemical nature of these interactions, is needed. The role of integrin and nonintegrin matrix protein receptors, aquaporins, ion channels, and mechanosensitive channels in interacting with ECM are only limitedly studied.
Investigations on changes in growth factor (eg, TGF‐β) activation with disc aging and changes in ECM stiffness may provide further insight into disease‐related changes.
The mechanism by which substrate stiffness information is transduced in IVD cells is not completely understood, but it likely relates to cytoskeletal organization and related transcriptional activators.
Given the close relationship between morphological confirmation and cell signaling through cell‐cell interactions, more work will be needed to isolate factors of cell morphology from cell‐cell mediated signaling in relationship to IVD cell phenotype.
There is a need for in vitro culture environments that can reproduce the anaerobic metabolism and cell‐ECM interactions of the native tissue to address some of these gaps.
6.4. Mechanotransduction
Only a limited number of studies have investigated ion channels, including mechanosensitive channels, and their role in IVD mechanotransduction. Investigations into the link between mechanosensitive channels, cell mechanics, and downstream transduction pathways will provide insight into physiological disc cell function.
Evidence suggests that alternative mechanotransduction pathways mediate responses of degenerate cells to loading. Comprehensive approaches are needed to characterize and delineate alternative mechanisms of mechanotransduction with degeneration.
Cell stress response including role of mitochondrial function in recovery from or protection against overloading are also needed.
6.5. Organ‐level interactions
Knowledge of the cross talk between IVD substructures is not well‐described, particularly cross talk between the vertebral body, EP, and AF or NP. Aging, or a lifetime of spinal motion and loading, is often associated with vertebral body changes that can include EP calcification, EP thinning, or evidence of bony microdamage. Factors that drive osteoblast and osteoclast remodeling in the vertebral body, and the formation of osteophytes where relevant, would be important to determine.
The mechanism(s) underlying the biochemical changes of the cartilage EP in disc degeneration are currently unknown, though some recent studies are beginning to address these questions175, 194. Further investigations are needed to assess related mechanism and to unveil effect of cartilage EP changes on cell mechanobiology, mediated through the cytoskeleton and other pathways. Changes in structure and cell mechanics could help in understanding the induction of mineralization, reduction in diffusion and onset of tissue damage that could result in herniation through the EP.
Other connective tissues in the spine are also mechanosensitive and warrant consideration, including cartilage EP, vertebrae‐IVD interactions, and spinal ligaments.
To conclude, it is expected that mechanistic studies of mechanobiology of IVD cells in healthy and disease conditions may lead to novel concepts, and potential targets, for mitigating or reversing the effects of altered biomechanics on biological functions of IVD cells. Such findings will expand the basic understanding and drive the development of therapeutic strategies for recovering “physiological” mechanotransduction of the disc.
ACKNOWLEDGEMENTS
The authors would like to thank their funding sources (NIH F32 AR070579, R01 AR069588, R01 AR070975, R01 AR047442, R01 AR069668, NSF CAREER 1763281), Ms. Julie Speer for data mining on existing cDNA datasets, and Dr. Bhranti Shah for assistance with hydrostatic pressure data set.
Author contributions
All authors contributed equally to the data research, discussion, writing, and revising of the manuscript.
Conflict of interest
The authors declare no potential conflict of interests.
Fearing BV, Hernandez PA, Setton LA, Chahine NO. Mechanotransduction and cell biomechanics of the intervertebral disc. JOR Spine. 2018;1:e1026. 10.1002/jsp2.1026
Funding information Division of Civil, Mechanical and Manufacturing Innovation, Grant/Award Number: NSF CAREER 1763281; National Institute of Arthritis and Musculoskeletal and Skin Diseases, Grant/Award Number: F32AR070579 R01AR047442 R01AR069588 R01AR069668 R01AR070975
Contributor Information
Lori A. Setton, Email: setton@wustl.edu
Nadeen O. Chahine, Email: noc7@columbia.edu.
REFERENCES
- 1. Bogduk N. Clinical and Radiological Anatomy of the Lumbar Spine. 5th ed. Churchill Livingstone Elsevier: London; 2012. [Google Scholar]
- 2. Roberts S, Evans H, Trivedi J, Menage J. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am. 2006;88(Suppl 2):10‐14. [DOI] [PubMed] [Google Scholar]
- 3. Setton LA, Chen J. Mechanobiology of the intervertebral disc and relevance to disc degeneration. J Bone Joint Surg Am. 2006;88(Suppl 2):52‐57. [DOI] [PubMed] [Google Scholar]
- 4. Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, Nerlich AG. Classification of age‐related changes in lumbar intervertebral discs: 2002 Volvo award in basic science. Spine (Phila Pa 1976). 2002;27(23):2631‐2644. [DOI] [PubMed] [Google Scholar]
- 5. Miller JA, Schmatz C, Schultz AB. Lumbar disc degeneration: correlation with age, sex, and spine level in 600 autopsy specimens. Spine (Phila Pa 1976). 1988;13(2):173‐178. [PubMed] [Google Scholar]
- 6. Lotz JC, Hsieh AH, Walsh AL, Palmer EI, Chin JR. Mechanobiology of the intervertebral disc. Biochem Soc Trans. 2002;30(Pt 6):853‐858. [DOI] [PubMed] [Google Scholar]
- 7. Urban JP. The role of the physicochemical environment in determining disc cell behaviour. Biochem Soc Trans. 2002;30(Pt 6):858‐864. [DOI] [PubMed] [Google Scholar]
- 8. Bayliss MT, Johnstone B. Biochemistry of the Intervertebral Disc In: Jayson MI, (ed), The lumbar spine and back pain. 4th ed Churchill Livingstone: Edinburgh, Scotland; 1992:111‐131. [Google Scholar]
- 9. Oegema TR Jr. Biochemistry of the intervertebral disc. Clin Sports Med. 1993;12(3):419‐439. [PubMed] [Google Scholar]
- 10. Nachemson A. Lumbar Intradiscal Pressure: Experimental Studies on Post‐Mortem Material. Acta Orthopaedica Scandinavica. 1960;31(suppl43):1‐104, 10.3109/ort.1960.31.suppl-43.01 [DOI] [PubMed] [Google Scholar]
- 11. Urban JP, Maroudas A. Swelling of the intervertebral disc in vitro. Connect Tissue Res. 1981;9(1):1‐10. [DOI] [PubMed] [Google Scholar]
- 12. Butler WF, Fujioka T. Fine structure of the anulus fibrosus of the intervertebral disc of the cat. Anat Anz. 1972;132(5):454‐464. [PubMed] [Google Scholar]
- 13. Hunter CJ, Matyas JR, Duncan NA. The three‐dimensional architecture of the notochordal nucleus pulposus: novel observations on cell structures in the canine intervertebral disc. J Anat. 2003;202(Pt 3:279‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meachim G, Cornah MS. Fine structure of juvenile human nucleus pulposus. J Anat. 1970;107(Pt 2):337‐350. [PMC free article] [PubMed] [Google Scholar]
- 15. Trout JJ, Buckwalter JA, Moore KC, Landas SK. Ultrastructure of the human intervertebral disc. I. Changes in notochordal cells with age. Tissue Cell. 1982;14(2):359‐369. [DOI] [PubMed] [Google Scholar]
- 16. Lawson L, Harfe BD. Notochord to nucleus pulposus transition. Curr Osteoporos Rep. 2015;13(5):336‐341. [DOI] [PubMed] [Google Scholar]
- 17. Purmessur D, Guterl CC, Cho SK, et al. Dynamic pressurization induces transition of notochordal cells to a mature phenotype while retaining production of important patterning ligands from development. Arthritis Res Ther. 2013;15(5):R122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Palacio‐Mancheno PE, Evashwick‐Rogler TW, Laudier DM, Purmessur D, Iatridis JC. Hyperosmolarity induces notochordal cell differentiation with aquaporin3 upregulation and reduced N‐cadherin expression. J Orthop Res. 2018;36(2):788‐798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coventry MB, Ghormley RK, Kernohan JW. The intervertebral disc ‐ its microscopic anatomy and pathology. 1 Anatomy, Development, and Physiology. J Bone Joint Surg. 1945;27(1):105‐112. [Google Scholar]
- 20. Marchand F, Ahmed AM. Investigation of the laminate structure of lumbar disc anulus fibrosus. Spine (Phila Pa 1976). 1990;15(5):402‐410. [DOI] [PubMed] [Google Scholar]
- 21. Cassidy JJ, Hiltner A, Baer E. Hierarchical structure of the intervertebral disc. Connect Tissue Res. 1989;23(1):75‐88. [DOI] [PubMed] [Google Scholar]
- 22. Hickey DS, Hukins DW. X‐ray diffraction studies of the arrangement of collagenous fibres in human fetal intervertebral disc. J Anat. 1980;131(Pt 1):81‐90. [PMC free article] [PubMed] [Google Scholar]
- 23. Horton WG. Further observations on the elastic mechanism of the intervertebral disc. J Bone Joint Surg Br. 1958;40(3):552‐557. [DOI] [PubMed] [Google Scholar]
- 24. Travascio F, Jackson AR, Brown MD, Gu WY. Relationship between solute transport properties and tissue morphology in human anulus fibrosus. J Orthop Res. 2009;27(12):1625‐1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yu J, Schollum ML, Wade KR, Broom ND, Urban JP. ISSLS prize winner: a detailed examination of the elastic network leads to a new understanding of anulus fibrosus organization. Spine (Phila Pa 1976). 2015;40(15):1149‐1157. [DOI] [PubMed] [Google Scholar]
- 26. Michalek AJ, Buckley MR, Bonassar LJ, Cohen I, Iatridis JC. Measurement of local strains in intervertebral disc anulus fibrosus tissue under dynamic shear: contributions of matrix fiber orientation and elastin content. J Biomech. 2009;42(14):2279‐2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yu J, Tirlapur U, Fairbank J, et al. Microfibrils, elastin fibres and collagen fibres in the human intervertebral disc and bovine tail disc. J Anat. 2007;210(4):460‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Han SK, Chen CW, Wierwille J, Chen Y, Hsieh AH. Three dimensional mesoscale analysis of translamellar cross‐bridge morphologies in the anulus fibrosus using optical coherence tomography. J Orthop Res. 2015;33(3):304‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bruehlmann SB, Matyas JR, Duncan NA. ISSLS prize winner: collagen fibril sliding governs cell mechanics in the anulus fibrosus: an in situ confocal microscopy study of bovine discs. Spine (Phila Pa 1976). 2004;29(23):2612‐2620. [DOI] [PubMed] [Google Scholar]
- 30. Nachemson A. Lumbar intradiscal pressure. Experimental studies on post‐mortem material. Acta Orthop Scand Suppl. 1960;43:1‐104. [DOI] [PubMed] [Google Scholar]
- 31. Higuchi M, Abe K. Postmortem changes in ultrastructures of the mouse intervertebral disc. Spine (Phila Pa 1976). 1987;12(1):48‐52. [DOI] [PubMed] [Google Scholar]
- 32. van den Akker GGH, Koenders MI, van de Loo FAJ, van Lent P, Blaney Davidson E, van der Kraan PM. Transcriptional profiling distinguishes inner and outer anulus fibrosus from nucleus pulposus in the bovine intervertebral disc. Eur Spine J. 2017;26(8):2053‐2062. [DOI] [PubMed] [Google Scholar]
- 33. Cloyd JM, Elliott DM. Elastin content correlates with human disc degeneration in the anulus fibrosus and nucleus pulposus. Spine (Phila Pa 1976). 2007;32(17):1826‐1831. [DOI] [PubMed] [Google Scholar]
- 34. Bartels EM, Fairbank JC, Winlove CP, Urban JP. Oxygen and lactate concentrations measured in vivo in the intervertebral discs of patients with scoliosis and back pain. Spine (Phila Pa 1976). 1998;23(1):1‐7. discussion 8. [DOI] [PubMed] [Google Scholar]
- 35. Urban JP, Holm S, Maroudas A, Nachemson A. Nutrition of the intervertebral disk. An in vivo study of solute transport . Clin Orthop Relat Res. 1977;129:101‐114. [PubMed] [Google Scholar]
- 36. Agrawal A, Guttapalli A, Narayan S, Albert TJ, Shapiro IM, Risbud MV. Normoxic stabilization of HIF‐1alpha drives glycolytic metabolism and regulates aggrecan gene expression in nucleus pulposus cells of the rat intervertebral disk. Am J Physiol Cell Physiol. 2007;293(2):C621‐C631. [DOI] [PubMed] [Google Scholar]
- 37. Risbud MV, Fertala J, Vresilovic EJ, Albert TJ, Shapiro IM. Nucleus pulposus cells upregulate PI3K/Akt and MEK/ERK signaling pathways under hypoxic conditions and resist apoptosis induced by serum withdrawal. Spine (Phila Pa 1976). 2005;30(8):882‐889. [DOI] [PubMed] [Google Scholar]
- 38. Risbud MV, Guttapalli A, Albert TJ, Shapiro IM. Hypoxia activates MAPK activity in rat nucleus pulposus cells: regulation of integrin expression and cell survival. Spine (Phila Pa 1976). 2005;30(22):2503‐2509. [DOI] [PubMed] [Google Scholar]
- 39. Bibby SR, Jones DA, Ripley RM, Urban JP. Metabolism of the intervertebral disc: effects of low levels of oxygen, glucose, and pH on rates of energy metabolism of bovine nucleus pulposus cells. Spine (Phila Pa 1976). 2005;30(5):487‐496. [DOI] [PubMed] [Google Scholar]
- 40. Bibby SR, Urban JP. Effect of nutrient deprivation on the viability of intervertebral disc cells, European spine journal : official publication of the European spine society, the European spinal deformity society, and the European section of the cervical. Spine Res Soc. 2004;13(8):695‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stairmand JW, Holm S, Urban JP. Factors influencing oxygen concentration gradients in the intervertebral disc. A theoretical analysis. Spine (Phila Pa 1976). 1991;16(4):444‐449. [DOI] [PubMed] [Google Scholar]
- 42. Huang CY, Gu WY. Effects of mechanical compression on metabolism and distribution of oxygen and lactate in intervertebral disc. J Biomech. 2008;41(6):1184‐1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nachemson A, Lewin T, Maroudas A, Freeman MA. In vitro diffusion of dye through the end‐plates and the anulus fibrosus of human lumbar inter‐vertebral discs. Acta Orthop Scand. 1970;41(6):589‐607. [DOI] [PubMed] [Google Scholar]
- 44. Salvatierra JC, Yuan TY, Fernando H, et al. Difference in energy metabolism of anulus fibrosus and nucleus pulposus cells of the intervertebral disc. Cell Mol Bioeng. 2011;4(2):302‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine (Phila Pa 1976). 2004;29(23):2691‐2699. [DOI] [PubMed] [Google Scholar]
- 46. Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine (Phila Pa 1976. 1995;20(11):1307‐1314. [DOI] [PubMed] [Google Scholar]
- 47. Heuer F, Schmidt H, Wilke HJ. Stepwise reduction of functional spinal structures increase disc bulge and surface strains. J Biomech. 2008;41(9):1953‐1960. [DOI] [PubMed] [Google Scholar]
- 48. Johnson WE, Evans H, Menage J, Eisenstein SM, El Haj A, Roberts S. Immunohistochemical detection of Schwann cells in innervated and vascularized human intervertebral discs. Spine (Phila Pa 1976). 2001;26(23):2550‐2557. [DOI] [PubMed] [Google Scholar]
- 49. Shamji MF, Setton LA, Jarvis W, et al. Proinflammatory cytokine expression profile in degenerated and herniated human intervertebral disc tissues. Arthritis Rheum. 2010;62(7):1974‐1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shapiro IM, Risbud MV. The Intervertebral Disc: Molecular and Structural Studies of the Disc in Health and Disease. Wien: Springer; 2014. [Google Scholar]
- 51. Weiler C, Nerlich AG, Zipperer J, Bachmeier BE, Boos N. 2002 SSE award competition in basic science: expression of major matrix metalloproteinases is associated with intervertebral disc degradation and resorption. Eur Spine J. 2002;11(4):308‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rajasekaran S, Babu JN, Arun R, Armstrong BR, Shetty AP, Murugan S. ISSLS prize winner: a study of diffusion in human lumbar discs: a serial magnetic resonance imaging study documenting the influence of the endplate on diffusion in normal and degenerate discs. Spine (Phila Pa 1976). 2004;29(23):2654‐2667. [DOI] [PubMed] [Google Scholar]
- 53. Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc, Spine (Phila Pa 1976. 2004;29(23):2700‐2709. [DOI] [PubMed] [Google Scholar]
- 54. Kang JD, StefanovicRacic M, McIntyre LA, Georgescu HI, Evans CH. Toward a biochemical understanding of human intervertebral disc degeneration and herniation—contributions of nitric oxide, interleukins, prostaglandin E‐2, and matrix metalloproteinases. Spine. 1997;22(10):1065‐1073. [DOI] [PubMed] [Google Scholar]
- 55. Le Maitre CL, Pockert A, Buttle DJ, Freemont AJ, Hoyland JA. Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem Soc Trans. 2007;35(Pt 4):652‐655. [DOI] [PubMed] [Google Scholar]
- 56. Le Maitre CL, Frain J, Fotheringham AP, Freemont AJ, Hoyland JA. Human cells derived from degenerate intervertebral discs respond differently to those derived from non‐degenerate intervertebral discs following application of dynamic hydrostatic pressure. Biorheology. 2008;45(5):563‐575. [PubMed] [Google Scholar]
- 57. Le Maitre CL, Frain J, Millward‐Sadler J, Fotheringham AP, Freemont AJ, Hoyland JA. Altered integrin mechanotransduction in human nucleus pulposus cells derived from degenerated discs. Arthritis Rheum. 2009;60(2):460‐469. [DOI] [PubMed] [Google Scholar]
- 58. Hsieh AH, Lotz JC. Prolonged spinal loading induces matrix metalloproteinase‐2 activation in intervertebral discs. Spine (Phila Pa 1976). 2003;28(16):1781‐1788. [DOI] [PubMed] [Google Scholar]
- 59. Lotz JC, Colliou OK, Chin JR, Duncan NA, Liebenberg E. Compression‐induced degeneration of the intervertebral disc: an in vivo mouse model and finite‐element study. Spine (Phila Pa 1976). 1998;23(23):2493‐2506. [DOI] [PubMed] [Google Scholar]
- 60. Lotz JC, Chin JR. Intervertebral disc cell death is dependent on the magnitude and duration of spinal loading. Spine (Phila Pa 1976). 2000;25(12):1477‐1483. [DOI] [PubMed] [Google Scholar]
- 61. Iatridis JC, Mente PL, Stokes IA, Aronsson DD, Alini M. Compression‐induced changes in intervertebral disc properties in a rat tail model. Spine (Phila Pa 1976). 1999;24(10):996‐1002. [DOI] [PubMed] [Google Scholar]
- 62. Hutton WC, Toribatake Y, Elmer WA, Ganey TM, Tomita K, Whitesides TE. The effect of compressive force applied to the intervertebral disc in vivo. A study of proteoglycans and collagen. Spine (Phila Pa 1976). 1998;23(23):2524‐2537. [DOI] [PubMed] [Google Scholar]
- 63. Ariga K, Yonenobu K, Nakase T, et al. Mechanical stress‐induced apoptosis of endplate chondrocytes in organ‐cultured mouse intervertebral discs: an ex vivo study. Spine (Phila Pa 1976). 2003;28(14):1528‐1533. [PubMed] [Google Scholar]
- 64. Chen J, Yan W, Setton LA. Static compression induces zonal‐specific changes in gene expression for extracellular matrix and cytoskeletal proteins in intervertebral disc cells in vitro. Matrix Biol. 2004;22(7):573‐583. [DOI] [PubMed] [Google Scholar]
- 65. Ohshima H, Urban JP, Bergel DH. Effect of static load on matrix synthesis rates in the intervertebral disc measured in vitro by a new perfusion technique. J Orthop Res. 1995;13(1):22‐29. [DOI] [PubMed] [Google Scholar]
- 66. Gullbrand SE, Peterson J, Mastropolo R, et al. Low rate loading‐induced convection enhances net transport into the intervertebral disc in vivo. Spine J. 2015;15(5):1028‐1033. [DOI] [PubMed] [Google Scholar]
- 67. Ferguson SJ, Ito K, Nolte LP. Fluid flow and convective transport of solutes within the intervertebral disc. J Biomech. 2004;37(2):213‐221. [DOI] [PubMed] [Google Scholar]
- 68. Katz MM, Hargens AR, Garfin SR. Intervertebral disc nutrition. Diffusion versus convection. Clin Orthop Relat Res. 1986;210:243‐245. [PubMed] [Google Scholar]
- 69. Urban JP, Holm S, Maroudas A, Nachemson A. Nutrition of the intervertebral disc: effect of fluid flow on solute transport. Clin Orthop Relat Res. 1982;170:296‐302. [PubMed] [Google Scholar]
- 70. Yao H, Gu WY. Physical signals and solute transport in human intervertebral disc during compressive stress relaxation: 3D finite element analysis. Biorheology. 2006;43(3, 4):323‐335. [PubMed] [Google Scholar]
- 71. MacLean JJ, Lee CR, Grad S, Ito K, Alini M, Iatridis JC. Effects of immobilization and dynamic compression on intervertebral disc cell gene expression in vivo. Spine (Phila Pa 1976). 2003;28(10):973‐981. [DOI] [PubMed] [Google Scholar]
- 72. Jackson AR, Huang CY, Brown MD, Gu WY. 3D finite element analysis of nutrient distributions and cell viability in the intervertebral disc: effects of deformation and degeneration. J Biomech Eng. 2011;133(9):091006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jackson AR, Yuan TY, Huang CY, Brown MD, Gu WY. Nutrient transport in human anulus fibrosus is affected by compressive strain and anisotropy. Ann Biomed Eng. 2012;40(12):2551‐2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Travascio F, Gu WY. Anisotropic diffusive transport in anulus fibrosus: experimental determination of the diffusion tensor by FRAP technique. Ann Biomed Eng. 2007;35(10):1739‐1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ching CT, Chow DH, Yao FY, Holmes AD. Changes in nuclear composition following cyclic compression of the intervertebral disc in an in vivo rat‐tail model. Med Eng Phys. 2004;26(7):587‐594. [DOI] [PubMed] [Google Scholar]
- 76. Walsh AJ, Lotz JC. Biological response of the intervertebral disc to dynamic loading. J Biomech. 2004;37(3):329‐337. [DOI] [PubMed] [Google Scholar]
- 77. Korecki CL, MacLean JJ, Iatridis JC. Dynamic compression effects on intervertebral disc mechanics and biology. Spine (Phila Pa 1976). 2008;33(13):1403‐1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Neidlinger‐Wilke C, Wurtz K, Liedert A, et al. A three‐dimensional collagen matrix as a suitable culture system for the comparison of cyclic strain and hydrostatic pressure effects on intervertebral disc cells. J Neurosurg Spine. 2005;2(4):457‐465. [DOI] [PubMed] [Google Scholar]
- 79. Maclean JJ, Lee CR, Alini M, Iatridis JC. Anabolic and catabolic mRNA levels of the intervertebral disc vary with the magnitude and frequency of in vivo dynamic compression. J Orthop Res. 2004;22(6):1193‐1200. [DOI] [PubMed] [Google Scholar]
- 80. Illien‐Junger S, Gantenbein‐Ritter B, Grad S, et al. The combined effects of limited nutrition and high‐frequency loading on intervertebral discs with endplates. Spine (Phila Pa 1976). 2010;35(19):1744‐1752. [DOI] [PubMed] [Google Scholar]
- 81. Wuertz K, Godburn K, MacLean JJ, et al. In vivo remodeling of intervertebral discs in response to short‐ and long‐term dynamic compression. J Orthop Res. 2009;27(9):1235‐1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Fernando HN, Czamanski J, Yuan TY, Gu W, Salahadin A, Huang CY. Mechanical loading affects the energy metabolism of intervertebral disc cells. J Orthop Res. 2011;29(11):1634‐1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Korecki CL, Kuo CK, Tuan RS, Iatridis JC. Intervertebral disc cell response to dynamic compression is age and frequency dependent. J Orthop Res. 2009;27(6):800‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wang C, Gonzales S, Levene H, Gu W, Huang CY. Energy metabolism of intervertebral disc under mechanical loading. J Orthop Res. 2013;31(11):1733‐1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hirschberg CB, Robbins PW, Abeijon C. Transporters of nucleotide sugars, ATP, and nucleotide sulfate in the endoplasmic reticulum and Golgi apparatus. Annu Rev Biochem. 1998;67:49‐69. [DOI] [PubMed] [Google Scholar]
- 86. Prydz K, Dalen KT. Synthesis and sorting of proteoglycans. J Cell Sci. 2000;113(Pt 2):193‐205. [DOI] [PubMed] [Google Scholar]
- 87. Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev. 2006;58(1):58‐86. [DOI] [PubMed] [Google Scholar]
- 88. Chowdhury TT, Knight MM. Purinergic pathway suppresses the release of .NO and stimulates proteoglycan synthesis in chondrocyte/agarose constructs subjected to dynamic compression. J Cell Physiol. 2006;209(3):845‐853. [DOI] [PubMed] [Google Scholar]
- 89. Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5(3):247‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Millward‐Sadler SJ, Wright MO, Flatman PW, Salter DM. ATP in the mechanotransduction pathway of normal human chondrocytes. Biorheology. 2004;41(3–4):567‐575. [PubMed] [Google Scholar]
- 91. Gonzales S, Rodriguez B, Barrera C, Huang CY. Measurement of ATP‐induced membrane potential changes in IVD cells. Cell Mol Bioeng. 2014;7(4):598‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wang C, Huang CY, Lin WC. Optical ATP biosensor for extracellular ATP measurement. Biosens Bioelectron. 2013;43:355‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Grunhagen T, Wilde G, Soukane DM, Shirazi‐Adl SA, Urban JP. Nutrient supply and intervertebral disc metabolism. J Bone Joint Surg Am. 2006;88(Suppl 2):30‐35. [DOI] [PubMed] [Google Scholar]
- 94. Mauck RL, Hung CT, Ateshian GA. Modeling of neutral solute transport in a dynamically loaded porous permeable gel: implications for articular cartilage biosynthesis and tissue engineering. J Biomech Eng. 2003;125(5):602‐614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Evans RC, Quinn TM. Dynamic compression augments interstitial transport of a glucose‐like solute in articular cartilage. Biophys J. 2006;91(4):1541‐1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Yao H, Gu WY. Convection and diffusion in charged hydrated soft tissues: a mixture theory approach. Biomech Model Mechanobiol. 2007;6(1–2):63‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zhang L, Gardiner BS, Smith DW, Pivonka P, Grodzinsky A. The effect of cyclic deformation and solute binding on solute transport in cartilage. Arch Biochem Biophys. 2007;457(1):47‐56. [DOI] [PubMed] [Google Scholar]
- 98. Chahine NO, Albro MB, Lima EG, et al. Effect of dynamic loading on the transport of solutes into agarose hydrogels. Biophys J. 2009;97(4):968‐975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Huang CY, Travascio F, Gu WY. Quantitative analysis of exogenous IGF‐1 administration of intervertebral disc through intradiscal injection. J Biomech. 2012;45(7):1149‐1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Baer AE, Laursen TA, Guilak F, Setton LA. The micromechanical environment of intervertebral disc cells determined by a finite deformation, anisotropic, and biphasic finite element model. J Biomech Eng. 2003;125(1):1‐11. [DOI] [PubMed] [Google Scholar]
- 101. Gu W, Zhu Q, Gao X, Brown MD. Simulation of the progression of intervertebral disc degeneration due to decreased nutritional supply. Spine (Phila Pa 1976). 2014;39(24):E1411‐E1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Court C, Colliou OK, Chin JR, Liebenberg E, Bradford DS, Lotz JC. The effect of static in vivo bending on the murine intervertebral disc. Spine J. 2001;1(4):239‐245. [DOI] [PubMed] [Google Scholar]
- 103. Hutton WC, Yoon ST, Elmer WA, et al. Effect of tail suspension (or simulated weightlessness) on the lumbar intervertebral disc: study of proteoglycans and collagen. Spine (Phila Pa 1976). 2002;27(12):1286‐1290. [DOI] [PubMed] [Google Scholar]
- 104. Setton LA, Chen J. Cell mechanics and mechanobiology in the intervertebral disc. Spine (Phila Pa 1976). 2004;29(23):2710‐2723. [DOI] [PubMed] [Google Scholar]
- 105. Stokes IA. Surface strain on human intervertebral discs. J Orthop Res. 1987;5(3):348‐355. [DOI] [PubMed] [Google Scholar]
- 106. Li SJ, Jai X, Duance VC, Blain EJ. The effects of cyclic tensile strain on the organisation and expression of cytoskeletal elements in bovine intervertebral disc cells: an in vitro study. Eur Cells Mater. 2011;21:508‐522. [DOI] [PubMed] [Google Scholar]
- 107. Rannou F, Richette P, Benallaoua M, et al. Cyclic tensile stretch modulates proteoglycan production by intervertebral disc anulus fibrosus cells through production of nitrite oxide. J Cell Biochem. 2003;90(1):148‐157. [DOI] [PubMed] [Google Scholar]
- 108. Gilbert HT, Hoyland JA, Millward‐Sadler SJ. The response of human anulus fibrosus cells to cyclic tensile strain is frequency‐dependent and altered with disc degeneration. Arthritis Rheum. 2010;62(11):3385‐3394. [DOI] [PubMed] [Google Scholar]
- 109. Sowa G, Coelho P, Vo N, et al. Determination of anulus fibrosus cell response to tensile strain as a function of duration, magnitude, and frequency. J Orthop Res. 2011;29(8):1275‐1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Gawri R, Rosenzweig DH, Krock E, et al. High mechanical strain of primary intervertebral disc cells promotes secretion of inflammatory factors associated with disc degeneration and pain. Arthritis Res Ther. 2014;16(1):R21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Barbir A, Godburn KE, Michalek AJ, Lai A, Monsey RD, Iatridis JC. Effects of torsion on intervertebral disc gene expression and biomechanics. using a rat tail model. Spine (Phila Pa 1976). 2011;36(8):607‐614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Matsumoto T, Kawakami M, Kuribayashi K, Takenaka T, Tamaki T. Cyclic mechanical stretch stress increases the growth rate and collagen synthesis of nucleus pulposus cells in vitro. Spine (Phila Pa 1976). 1999;24(4):315‐319. [DOI] [PubMed] [Google Scholar]
- 113. Chan SC, Ferguson SJ, Gantenbein‐Ritter B. The effects of dynamic loading on the intervertebral discEuropean spine journal : official publication of the European spine society, the European spinal deformity society, and the European section of the cervical. Spine Res Soc. 2011;20(11):1796‐1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Neidlinger‐Wilke C, Galbusera F, Pratsinis H, et al. Mechanical loading of the intervertebral disc: from the macroscopic to the cellular level. Eur Spine J. 2014;23(Suppl 3):S333‐S343. [DOI] [PubMed] [Google Scholar]
- 115. Wilke HJ, Rohlmann A, Neller S, Graichen F, Claes L, Bergmann G. ISSLS prize winner: a novel approach to determine trunk muscle forces during flexion and extension: a comparison of data from an in vitro experiment and in vivo measurements. Spine (Phila Pa 1976). 2003;28(23):2585‐2593. [DOI] [PubMed] [Google Scholar]
- 116. Sato K, Kikuchi S, Yonezawa T. In vivo intradiscal pressure measurement in healthy individuals and in patients with ongoing back problems. Spine (Phila Pa 1976). 1999;24(23):2468‐2474. [DOI] [PubMed] [Google Scholar]
- 117. Hwang PY, Jing L, Chen J, et al. N‐cadherin is key to expression of the nucleus pulposus cell phenotype under selective substrate culture conditions. Sci Rep. 2016;6:28038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kasra M, Merryman WD, Loveless KN, Goel VK, Martin JD, Buckwalter JA. Frequency response of pig intervertebral disc cells subjected to dynamic hydrostatic pressure. J Orthop Res. 2006;24(10):1967‐1973. [DOI] [PubMed] [Google Scholar]
- 119. Gokorsch S, Nehring D, Grottke C, Czermak P. Hydrodynamic stimulation and long term cultivation of nucleus pulposus cells: a new bioreactor system to induce extracellular matrix synthesis by nucleus pulposus cells dependent on intermittent hydrostatic pressure. Int J Artif Organs. 2004;27(11):962‐970. [DOI] [PubMed] [Google Scholar]
- 120. Neidlinger‐Wilke C, Wurtz K, Urban JP, et al. Regulation of gene expression in intervertebral disc cells by low and high hydrostatic pressure. Eur Spine J. 2006;15(Suppl 3):S372‐S378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Shah BS, Chahine NO. Dynamic hydrostatic pressure regulates nucleus pulposus phenotypic expression and metabolism in a cell density‐dependent manner. J Biomech Eng. 2018;140(2):021003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Wenger KH, Woods JA, Holecek A, Eckstein EC, Robertson JT, Hasty KA. Matrix remodeling expression in anulus cells subjected to increased compressive load. Spine (Phila Pa 1976). 2005;30(10):1122‐1126. [DOI] [PubMed] [Google Scholar]
- 123. Kasra M, Goel V, Martin J, Wang ST, Choi W, Buckwalter J. Effect of dynamic hydrostatic pressure on rabbit intervertebral disc cells. J Orthop Res. 2003;21(4):597‐603. [DOI] [PubMed] [Google Scholar]
- 124. Ishihara H, McNally DS, Urban JP, Hall AC. Effects of hydrostatic pressure on matrix synthesis in different regions of the intervertebral disk. J Appl Physiol (1985). 1996;80(3):839‐846. [DOI] [PubMed] [Google Scholar]
- 125. Hutton WC, Elmer WA, Bryce LM, Kozlowska EE, Boden SD, Kozlowski M. Do the intervertebral disc cells respond to different levels of hydrostatic pressure? Clin Biomech (Bristol, Avon). 2001;16(9):728‐734. [DOI] [PubMed] [Google Scholar]
- 126. Handa T, Ishihara H, Ohshima H, Osada R, Tsuji H, Obata K. Effects of hydrostatic pressure on matrix synthesis and matrix metalloproteinase production in the human lumbar intervertebral disc. Spine (Phila Pa 1976). 1997;22(10):1085‐1091. [DOI] [PubMed] [Google Scholar]
- 127. Hutton WC, Elmer WA, Boden SD, et al. The effect of hydrostatic pressure on intervertebral disc metabolism. Spine (Phila Pa 1976). 1999;24(15):1507‐1515. [DOI] [PubMed] [Google Scholar]
- 128. Gantenbein B, Grunhagen T, Lee CR, van Donkelaar CC, Alini M, Ito K. An in vitro organ culturing system for intervertebral disc explants with vertebral endplates: a feasibility study with ovine caudal discs. Spine (Phila Pa 1976). 2006;31(23):2665‐2673. [DOI] [PubMed] [Google Scholar]
- 129. Hunter CJ, Matyas JR, Duncan NA. Cytomorphology of notochordal and chondrocytic cells from the nucleus pulposus: a species comparison. J Anat. 2004;205(5):357‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Hunter CJ, Bianchi S, Cheng P, Muldrew K. Osmoregulatory function of large vacuoles found in notochordal cells of the intervertebral disc running title: an osmoregulatory vacuole. Mol Cell Biomech. 2007;4(4):227‐237. [PMC free article] [PubMed] [Google Scholar]
- 131. Urban JP, McMullin JF. Swelling pressure of the inervertebral disc: influence of proteoglycan and collagen contents. Biorheology. 1985;22(2):145‐157. [DOI] [PubMed] [Google Scholar]
- 132. Urban JP, McMullin JF. Swelling pressure of the lumbar intervertebral discs: influence of age, spinal level, composition and degeneration. Spine (Phila Pa 1976). 1988;13(2):179‐187. [DOI] [PubMed] [Google Scholar]
- 133. Bayliss MT, Urban JP, Johnstone B, Holm S. In vitro method for measuring synthesis rates in the intervertebral disc. J Orthop Res. 1986;4(1):10‐17. [DOI] [PubMed] [Google Scholar]
- 134. Ishihara H, Warensjo K, Roberts S, Urban JP. Proteoglycan synthesis in the intervertebral disk nucleus: the role of extracellular osmolality. Am J Physiol. 1997;272(5 Pt 1):C1499‐C1506. [DOI] [PubMed] [Google Scholar]
- 135. Boyd LM, Richardson WJ, Chen J, Kraus VB, Tewari A, Setton LA. Osmolarity regulates gene expression in intervertebral disc cells determined by gene array and real‐time quantitative RT‐PCR. Ann Biomed Eng. 2005;33(8):1071‐1077. [DOI] [PubMed] [Google Scholar]
- 136. Mavrogonatou E, Kletsas D. High osmolality activates the G1 and G2 cell cycle checkpoints and affects the DNA integrity of nucleus pulposus intervertebral disc cells triggering an enhanced DNA repair response. DNA Repair (Amst). 2009;8(8):930‐943. [DOI] [PubMed] [Google Scholar]
- 137. Mavrogonatou E, Kletsas D. Effect of varying osmotic conditions on the response of bovine nucleus pulposus cells to growth factors and the activation of the ERK and Akt pathways. J Orthop Res. 2010;28(10):1276‐1282. [DOI] [PubMed] [Google Scholar]
- 138. Pratsinis H, Constantinou V, Pavlakis K, Sapkas G, Kletsas D. Exogenous and autocrine growth factors stimulate human intervertebral disc cell proliferation via the ERK and Akt pathways. J Orthop Res. 2012;30(6):958‐964. [DOI] [PubMed] [Google Scholar]
- 139. O'Connell GD, Newman IB, Carapezza MA. Effect of long‐term osmotic loading culture on matrix synthesis from intervertebral disc cells. Biores Open Access. 2014;3(5):242‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Vo NV, Hartman RA, Patil PR, et al. Molecular mechanisms of biological aging in intervertebral discs. J Orthop Res. 2016;34(8):1289‐1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Li S, Duance VC, Blain EJ. Zonal variations in cytoskeletal element organization, mRNA and protein expression in the intervertebral disc. J Anat. 2008;213(6):725‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Pritchard S, Guilak F. The role of F‐actin in hypo‐osmotically induced cell volume change and calcium signaling in anulus fibrosus cells. Ann Biomed Eng. 2004;32(1):103‐111. [DOI] [PubMed] [Google Scholar]
- 143. Risbud MV, Schoepflin ZR, Mwale F, et al. Defining the phenotype of young healthy nucleus pulposus cells: recommendations of the spine research interest group at the 2014 annual ORS meeting. J Orthop Res. 2015;33(3):283‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Bertaud J, Qin Z, Buehler MJ. Intermediate filament‐deficient cells are mechanically softer at large deformation: a multi‐scale simulation study. Acta Biomater. 2010;6(7):2457‐2466. [DOI] [PubMed] [Google Scholar]
- 145. Stosiek P, Kasper M, Karsten U. Expression of cytokeratin and vimentin in nucleus pulposus cells. Differentiation. 1988;39(1):78‐81. [DOI] [PubMed] [Google Scholar]
- 146. Sivaramakrishnan S, DeGiulio JV, Lorand L, Goldman RD, Ridge KM. Micromechanical properties of keratin intermediate filament networks. Proc Natl Acad Sci USA. 2008;105(3):889‐894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Mendez MG, Restle D, Janmey PA. Vimentin enhances cell elastic behavior and protects against compressive stress. Biophys J. 2014;107(2):314‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Guo M, Ehrlicher AJ, Mahammad S, et al. The role of vimentin intermediate filaments in cortical and cytoplasmic mechanics. Biophys J. 2013;105(7):1562‐1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Hayes AJ, Benjamin M, Ralphs JR. Role of actin stress fibres in the development of the intervertebral disc: cytoskeletal control of extracellular matrix assembly. Dev Dyn. 1999;215(3):179‐189. [DOI] [PubMed] [Google Scholar]
- 150. Amano M, Nakayama M, Kaibuchi K. Rho‐kinase/ROCK: a key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken). 2010;67(9):545‐554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Hwang PY, Chen J, Jing L, Hoffman BD, Setton LA. The role of extracellular matrix elasticity and composition in regulating the nucleus pulposus cell phenotype in the intervertebral disc: a narrative review. J Biomech Eng. 2014;136(2):021010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Elfervig MK, Minchew JT, Francke E, Tsuzaki M, Banes AJ. IL‐1beta sensitizes intervertebral disc anulus cells to fluid‐induced shear stress. J Cell Biochem. 2001;82(2):290‐298. [DOI] [PubMed] [Google Scholar]
- 153. Janmey PA, Kinnunen PK. Biophysical properties of lipids and dynamic membranes. Trends Cell Biol. 2006;16(10):538‐546. [DOI] [PubMed] [Google Scholar]
- 154. Walter BA, Purmessur D, Moon A, et al. Reduced tissue osmolarity increases TRPV4 expression and pro‐inflammatory cytokines in intervertebral disc cells. Eur Cell Mater. 2016;32:123‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Tang X, Jing L, Chen J. Changes in the molecular phenotype of nucleus pulposus cells with intervertebral disc aging. PloS One. 2012;7(12):e52020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Abbott RD, Howe AK, Langevin HM, Iatridis JC. Live free or die: stretch‐induced apoptosis occurs when adaptive reorientation of anulus fibrosus cells is restricted. Biochem Biophys Res Commun. 2012;421(2):361‐366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Bian Q, Ma L, Jain A, et al. Mechanosignaling activation of TGFbeta maintains intervertebral disc homeostasis. Bone Res. 2017;5:17008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Bridgen DT, Gilchrist CL, Richardson WJ, et al. Integrin‐mediated interactions with extracellular matrix proteins for nucleus pulposus cells of the human intervertebral disc. J Orthop Res. 2013;31(10):1661‐1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Gilchrist CL, Chen J, Richardson WJ, Loeser RF, Setton LA. Functional integrin subunits regulating cell‐matrix interactions in the intervertebral disc. J Orthop Res. 2007;25(6):829‐840. [DOI] [PubMed] [Google Scholar]
- 160. Nettles DL, Richardson WJ, Setton LA. Integrin expression in cells of the intervertebral disc. J Anat. 2004;204(6):515‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Bridgen DT, Fearing BV, Jing L, et al. Regulation of human nucleus pulposus cells by peptide‐coupled substrates. Acta Biomater. 2017;55:100‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Gilchrist CL, Darling EM, Chen J, Setton LA. Extracellular matrix ligand and stiffness modulate immature nucleus pulposus cell‐cell interactions. PloS One. 2011;6(11):e27170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Gilchrist CL, Francisco AT, Plopper GE, Chen J, Setton LA. Nucleus pulposus cell‐matrix interactions with laminins. Eur Cell Mater. 2011;21:523‐532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Chen J, Jing L, Gilchrist CL, Richardson WJ, Fitch RD, Setton LA. Expression of laminin isoforms, receptors and binding proteins unique to nucleus pulposus cells of immature intervertebral disc. Connect Tissue Res. 2009;50(5):294‐306. [PMC free article] [PubMed] [Google Scholar]
- 165. Parsons MJ, Pollard SM, Saude L, et al. Zebrafish mutants identify an essential role for laminins in notochord formation. Development. 2002;129(13):3137‐3146. [DOI] [PubMed] [Google Scholar]
- 166. Francisco AT, Mancino RJ, Bowles RD, et al. Injectable laminin‐functionalized hydrogel for nucleus pulposus regeneration. Biomaterials. 2013;34(30):7381‐7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Wipff PJ, Hinz B. Integrins and the activation of latent transforming growth factor beta1—an intimate relationship. Eur J Cell Biol. 2008;87(8–9):601‐615. [DOI] [PubMed] [Google Scholar]
- 168. Baffi MO, Slattery E, Sohn P, Moses HL, Chytil A, Serra R. Conditional deletion of the TGF‐beta type II receptor in Col2a expressing cells results in defects in the axial skeleton without alterations in chondrocyte differentiation or embryonic development of long bones. Dev Biol. 2004;276(1):124‐142. [DOI] [PubMed] [Google Scholar]
- 169. Jin H, Shen J, Wang B, Wang M, Shu B, Chen D. TGF‐beta signaling plays an essential role in the growth and maintenance of intervertebral disc tissue. FEBS Lett. 2011;585(8):1209‐1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Tran CM, Markova D, Smith HE, et al. Regulation of CCN2/connective tissue growth factor expression in the nucleus pulposus of the intervertebral disc: role of Smad and activator protein 1 signaling. Arthritis Rheum. 2010;62(7):1983‐1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Bian Q, Jain A, Xu X, et al. Excessive activation of TGFbeta by spinal instability causes vertebral endplate sclerosis. Sci Rep. 2016;6:27093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Gruber HE, Hoelscher GL, Ingram JA, Bethea S, Zinchenko N, Hanley EN Jr. Variations in aggrecan localization and gene expression patterns characterize increasing stages of human intervertebral disk degeneration. Exp Mol Pathol. 2011;91(2):534‐539. [DOI] [PubMed] [Google Scholar]
- 173. Hwang PY, Jing L, Michael KW, Richardson WJ, Chen J, Setton LA. N‐cadherin‐mediated signaling regulates cell phenotype for nucleus pulposus cells of the intervertebral disc. Cell Mol Bioeng. 2015;8(1):51‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Cao L, Guilak F, Setton LA. Three‐dimensional morphology of the pericellular matrix of intervertebral disc cells in the rat. J Anat. 2007;211(4):444‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Liu MH, Sun C, Yao Y, et al. Matrix stiffness promotes cartilage endplate chondrocyte calcification in disc degeneration via miR‐20a targeting ANKH expression. Sci Rep. 2016;6:25401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Zhang YH, Zhao CQ, Jiang LS, Dai LY. Substrate stiffness regulates apoptosis and the mRNA expression of extracellular matrix regulatory genes in the rat annular cells. Matrix Biol. 2011;30(2):135‐144. [DOI] [PubMed] [Google Scholar]
- 177. Bian L, Hou C, Tous E, Rai R, Mauck RL, Burdick JA. The influence of hyaluronic acid hydrogel crosslinking density and macromolecular diffusivity on human MSC chondrogenesis and hypertrophy. Biomaterials. 2013;34(2):413‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Heo SJ, Driscoll TP, Thorpe SD, et al. Differentiation alters stem cell nuclear architecture, mechanics, and mechano‐sensitivity. Elife. November 30, 2016;5:e18207 10.7554/eLife.18207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Cosgrove BD, Mui KL, Driscoll TP, et al. N‐cadherin adhesive interactions modulate matrix mechanosensing and fate commitment of mesenchymal stem cells. Nat Mater. 2016;15(12):1297‐1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Dupont S, Morsut L, Aragona M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179‐183. [DOI] [PubMed] [Google Scholar]
- 181. Low BC, Pan CQ, Shivashankar GV, Bershadsky A, Sudol M, Sheetz M. YAP/TAZ as mechanosensors and mechanotransducers in regulating organ size and tumor growth. FEBS Lett. 2014;588(16):2663‐2670. [DOI] [PubMed] [Google Scholar]
- 182. Trappmann B, Gautrot JE, Connelly JT, et al. Extracellular‐matrix tethering regulates stem‐cell fate. Nat Mater. 2012;11(7):642‐649. [DOI] [PubMed] [Google Scholar]
- 183. Wozniak MA, Chen CS. Mechanotransduction in development: a growing role for contractility. Nat Rev Mol Cell Biol. 2009;10(1):34‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Maidhof R, Jacobsen T, Papatheodorou A, Chahine NO. Inflammation induces irreversible biophysical changes in isolated nucleus pulposus cells. PloS One. 2014;9(6):e99621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Likhitpanichkul M, Torre OM, Gruen J, Walter BA, Hecht AC, Iatridis JC. Do mechanical strain and TNF‐alpha interact to amplify pro‐inflammatory cytokine production in human anulus fibrosus cells? J Biomech. 2016;49(7):1214‐1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Tisherman R, Coelho P, Phillibert D, et al. NF‐kappaB signaling pathway in controlling intervertebral disk cell response to inflammatory and mechanical stressors. Phys Ther. 2016;96(5):704‐711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Kurakawa T, Kakutani K, Morita Y, et al. Functional impact of integrin alpha5beta1 on the homeostasis of intervertebral discs: a study of mechanotransduction pathways using a novel dynamic loading organ culture system. Spine J. 2015;15(3):417‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Yan Z, Pan Y, Wang S, et al. Static compression induces ECM remodeling and integrin alpha2beta1 expression and signaling in a rat tail caudal intervertebral disc degeneration model. Spine (Phila Pa 1976). 2017;42(8):E448‐E458. [DOI] [PubMed] [Google Scholar]
- 189. Zhang K, Ding W, Sun W, et al. Beta1 integrin inhibits apoptosis induced by cyclic stretch in anulus fibrosus cells via ERK1/2 MAPK pathway. Apoptosis. 2016;21(1):13‐24. [DOI] [PubMed] [Google Scholar]
- 190. Gilbert HT, Nagra NS, Freemont AJ, Millward‐Sadler SJ, Hoyland JA. Integrin ‐ dependent mechanotransduction in mechanically stimulated human anulus fibrosus cells: evidence for an alternative mechanotransduction pathway operating with degeneration. PloS One. 2013;8(9):e72994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Chooi WH, Chan BP. Compression loading‐induced stress responses in intervertebral disc cells encapsulated in 3D collagen constructs. Sci Rep. 2016;6:26449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Zhang YH, Zhao CQ, Jiang LS, Dai LY. Cyclic stretch‐induced apoptosis in rat anulus fibrosus cells is mediated in part by endoplasmic reticulum stress through nitric oxide production. Eur Spine J. 2011;20(8):1233‐1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Zhang YH, Zhao CQ, Jiang LS, Dai LY. Lentiviral shRNA silencing of CHOP inhibits apoptosis induced by cyclic stretch in rat annular cells and attenuates disc degeneration in the rats. Apoptosis. 2011;16(6):594‐605. [DOI] [PubMed] [Google Scholar]
- 194. Grant MP, Epure LM, Bokhari R, Roughley P, Antoniou J, Mwale F. Human cartilaginous endplate degeneration is induced by calcium and the extracellular calcium‐sensing receptor in the intervertebral disc. Eur Cell Mater. 2016;32:137‐151. [DOI] [PubMed] [Google Scholar]
- 195. Bowles RD, Masuda K, Bonassar LA, Setton LA. Tissue Engineering for Regeneration and Replacement of the Intervertebral Disc In: Lanza R, Langer R, Vacanti J, eds. Principles of Tissue Engineering. 4th ed. London: Academic Press/Elsevier; 2014:1223‐1251. [Google Scholar]
- 196. Guilak F, Ting‐Beall HP, Baer AE, Trickey WR, Erickson GR, Setton LA. Viscoelastic properties of intervertebral disc cells. Identification of two biomechanically distinct cell populations. Spine (Phila Pa 1976). 1999;24(23):2475‐2483. [DOI] [PubMed] [Google Scholar]


