Abstract
Sustained control of group A Streptococcus (GAS) infections in settings of poverty has proven to be challenging, and an effective vaccine may be the most practical long-term strategy to reduce GAS-related disease burden. Candidate GAS vaccines based on the J8 peptide have demonstrated promising immunogenicity in mice, however, less is known about the role of J8 antibodies in the human immune response to GAS infection. We analysed the stimulation of J8 antibodies in response to infection, and the role of existing J8 antibodies in protection against subsequent infection, using data collected in the Fijian population: 1) cross sectional population serosurvey; 2) paired serum collection for assessment of M-specific and J8 antibody responses; and 3) longitudinal assessment of GAS infection and immunity. Median J8 antibody concentrations peaked in the 5–14 year age group, but there was no sustained increase with age. J8 antibody concentration was neither a significant predictor of time to next infection, nor did it show any relationship to the time since last recorded skin infection. Similarly, J8 antibody fold changes over a defined period were associated neither with the time since last skin infection, nor the number of intervening skin infections. While strong M-specific antibody responses were observed for skin infection, similarly strong J8 antibody responses were not observed. There is no indication that antibodies to the J8 antigen would be useful as either a marker of GAS infection or a measure of population immunity, with J8 antibody responses to infection fleeting, if existent at all.
Keywords: group A Streptococcus, J8, impetigo, immunity, vaccine antigen
Introduction
Superficial group A Streptococcus (GAS) infections, precursors of both invasive and immune-mediated disease sequelae, are ubiquitous with 111 million prevalent cases of GAS impetigo in children at any time and more than 600 million new cases of GAS pharyngitis per year [1]. Sustained control of GAS infections in settings of poverty has proven to be challenging, and an effective vaccine may be the most practical long-term strategy to reduce the burden of GAS-related disease such as rheumatic heart disease and post-streptococcal glomerulonephritis [2].
Research into GAS vaccines has been ongoing for many decades, and there are a number of vaccine candidates currently at various stages of development. Those based on the M-protein, the major virulence factor of GAS, include multivalent vaccines targeting the variable N-terminal of the M-protein, and vaccines that contain antigens from the conserved C-repeat region [2]. All vaccine antigen candidates aim to be immunogenic, but without cross-reacting with human tissue [3]. Vaccines based on a minimal non-cross-reactive B cell epitope, identified within the M-protein J8 peptide, have been shown to be immunogenic in mice and to protect against GAS challenge [3, 4]. While a number of vaccine studies have explored immune responses to J8 vaccination in animals [4, 5], less is known about the human response to the J8 epitope. The purpose of this study was to develop a deeper understanding of the role of the J8 epitope in the human immune system’s response to GAS. Specifically, we considered the stimulation of J8 antibodies in response to infection, and the role of existing J8 antibodies in protection against subsequent acquisition of infection.
Methods
Ethical approval.
This study was approved by the Fiji National Research Ethics Review Committee, the Fiji National Health Research Committee, the University of Melbourne Human Research Ethics Committee, and the Queensland Institute of Medical Research Human Research Ethics Committee. Written informed consent was required from participants or a parent or guardian prior to collection of information.
Objective.
Two distinct study protocols were undertaken to explore aspects of the human immune response to the J8 epitope. Data were collected in 2006 from a crosss-ectional survey in the major hospital of Fiji (“Study 1”) and from a prospective cohort study of primary school children in the Central Division of Fiji (“Study 2”).
Participants. Study 1: Cross-sectional seroprevalence survey.
Two hundred and eighty volunteers attending the Colonial War Memorial Hospital, Suva, Viti Levu, Fiji as patients or blood donors were enrolled and had blood collected, as previously described [6]. Briefly, participants were enrolled with an even distribution of ages from birth to 65 years, and not enrolled if any of the following conditions were met: sore throat or skin sores within the previous 14 days; any history of acute rheumatic fever, rheumatic heart disease, invasive GAS disease or acute post-streptococcal glomerulonephritis; or temperature >38.0°C on the day of enrolment.
Study 2: Prospective longitudinal cohort study of infection and immunity.
Four hundred and fifty nine children attending three schools located in the Central Division of Fiji were prospectively screened for skin sores at two-monthly intervals for ten months, with six visits occurring over the observation period, as previously described [7]. Twice-weekly surveillance for sore throat was undertaken over the same period, as previously described [8]. Two of the schools were rural (“rural 1” and “rural 2”) with an exclusively iTauekei (Indigenous Fijian) population and the remaining school was a larger, mostly Indo-Fijian school in Suva, Fiji’s capital (“urban”). Blood was collected at visits 1, 4 and 6, corresponding to 0, 6 and 10 months follow up time.
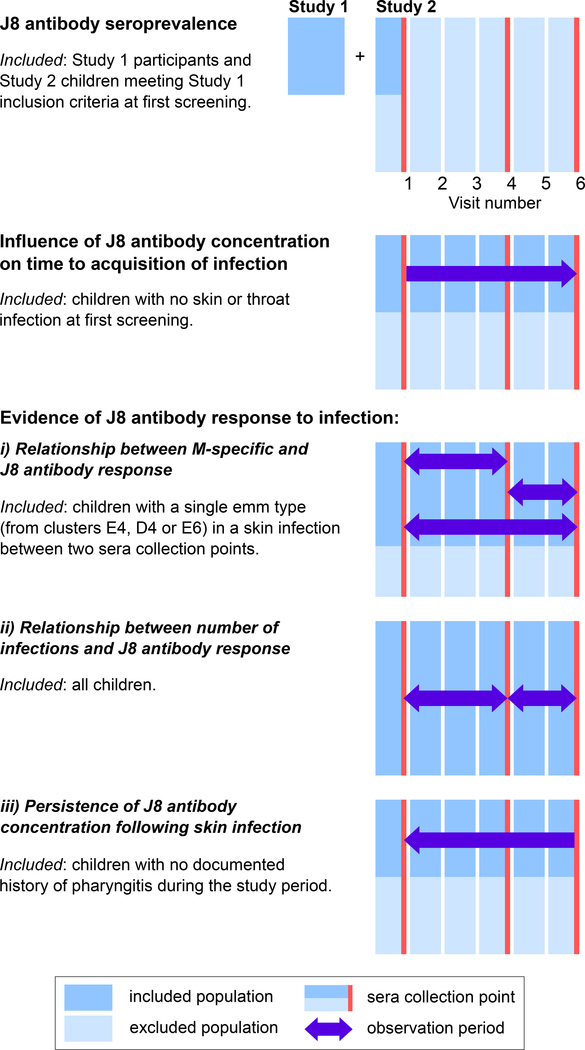
Participants from each of these studies were combined in five separate analyses described below. Each analysis had different inclusion criteria and observation periods, explained in Figure 1.
Figure 1:
Participants for the five analyses were drawn from a cross-sectional study (“Study 1”) and a prospective longitudinal cohort study (“Study 2”). Study 1 participants were only included in the seroprevalence analysis and Study 2 participants were included in all analyses, with the infographic showing the sub-populations included in each analysis. There were six screening visits in Study 2 (blue bars) with blood collections occurring at three of these (red bars). For each analysis, at each visit, the excluded population is shown in light blue and the included population in dark blue. Single colour bars represent the inclusion of all participants (dark blue) or exclusion of all participants (light blue), with the combined light/dark blue bars representing the inclusion of some participants. Arrows represent the direction of observation, with participants followed either prospectively or retrospectively until infection occurred. Double-headed arrows represent the inclusion of data for the entire period spanned by the arrows.
Laboratory methods.
Children identified with impetigo or pharyngitis had skin or throat swabs taken respectively, and infection with GAS confirmed as previously described [7, 8]. Emm-typing and emm-cluster identification were obtained for each GAS isolate as previously described [7–9]. Blood specimens were immediately cooled before transport to the laboratory in Fiji, where samples were centrifuged, divided into three aliquots and stored at −80°C. For each participant, one frozen aliquot was thawed and J8 IgG concentrations measured. Standard Enzyme-Linked Immunosorbent Assay (ELISA) techniques were used to determine anti-J8 and anti-M peptide antibody concentrations [10, 11]. A standard curve was included on all plates to quantitate the antigen specific antibodies in serum samples and results are reported in ELISA Units (EU). Positive controls include anti-diphtheria toxin responses and IVIG. For children with confirmed GAS skin infection to three major emm-clusters (E4, D4, and E6), antibody responses to the peptide deduced from the infecting emm type (M-specific antibodies) were calculated from the standard curve of that peptide, for the pre- and post-infection sera, as previously described [11].
Statistical methods. J8 Antibody Seroprevalence.
To establish J8 antibody age-specific reference ranges for “normal” individuals, we used the data from Study 1, and from the first time point in Study 2, applying the same exclusion criteria as in Study 1. We used a published technique [12], previously utilised for other GAS antibodies [6]. Data were log transformed, regressed using fractional polynomials, and age-specific reference ranges calculated using the xriml package in STATA [13].
Influence of J8 antibody concentration on time to acquisition of infection.
We used data from Study 2, excluding participants with a skin or throat infection at first screening. We also excluded participants who developed a symptomatic throat infection in the seven days following initial screening, as they may also have been infected at first screening. J8 antibody concentration at the first time point and the time to next infection (either skin or throat) were extracted. Seven missing J8 antibody concentrations were replaced with half the minimum value in the dataset. Cox proportional-hazards (Cox PH) methods were used to calculate hazard ratios (HR) and 95% confidence intervals (CIs) for the risk of infection (either skin or throat). Univariate log-rank tests of equality were performed for categorical variables, followed by univariate Cox PH regression of significantly associated variables. Proportional hazards assumptions were tested using Schoenfeld residuals, with the model stratified by variables not meeting this assumption. Variables with univariate P-values < 0.2 were included in the multivariate model and considered significant in the final model at P < 0.05.
Evidence of J8 antibody response to infection.
i). Relationship between M-specific and J8 antibody responses.
The first analysis involved participants from Study 2 with a single emm type identified in a GAS skin infection from the E4, D4, and E6 emm clusters between two sera collection points. Fold-change was calculated for each child as the ratio of the J8 antibody concentration in the post-infection sample (either timepoint 4 or 6) to that in the pre-infection sample (either timepoint 1 or 4). Thus comparisons were across timepoints 1 and 4; 4 and 6; and 1 and 6, depending on the availability of blood samples. Fold-changes were log transformed from a positively skewed distribution to a more normal distribution. Linear correlation between log-transformed fold-changes for J8 and M-specific antibodies was calculated using Pearson’s correlation coefficient.
ii). Relationship between J8 antibody response and number of infections.
A second analysis considered whether observed changes in J8 antibody concentrations over time across the total cohort were related to the number of documented episodes of either skin or throat infection. Study epochs were divided by the second blood collection (visit 4) into epochs 1 and 2. Fold changes were categorised for comparison across epochs as increased (>2), decreased (<0.5) or unchanged if between these values. For each epoch, the number of infections was tabulated against fold-change status. A Chi-squared test of independence was used to test whether the number of infections was independent of the J8 antibody fold change.
iii). Persistence of J8 antibody concentrations following skin infection.
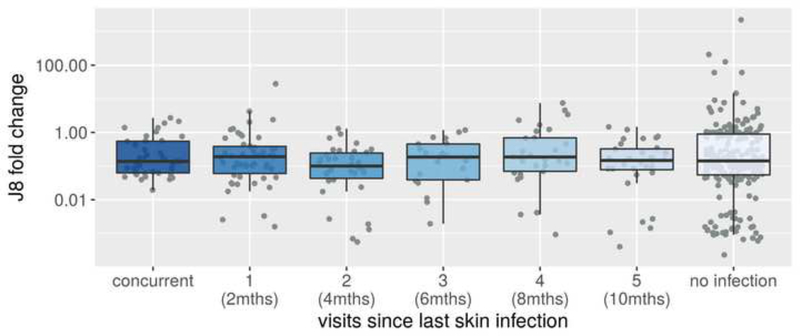
A third analysis investigated persistence of J8 antibodies following documented GAS skin infection, with J8 fold changes between visits 1 and 6, and log J8 antibody concentrations at visit 6 stratified and plotted by the number of visits since the most recent skin infection. Only children with no documented history of pharyngitis during the study period were included, to ensure it was the J8 response to skin infection, and not throat infection, which was analysed.
In all investigations, missing J8 antibody concentrations were replaced with half of the minimum recorded value in the dataset. Reasons for missing values were unknown, but may have included antibody levels below the limit of detection of the ELISA test, or lack of a suitable sample. Other than for the J8 antibody seroprevalence study, only children with confirmed GAS infections were included in analyses.
Analyses were undertaken in MATLAB, The MathWorks, Inc., STATA, StataCorp 2015, Release 14, College Station, Texas, and R version 3.4.3, The R Foundation for Statistical Computing.
Results
J8 Antibody Seroprevalence.
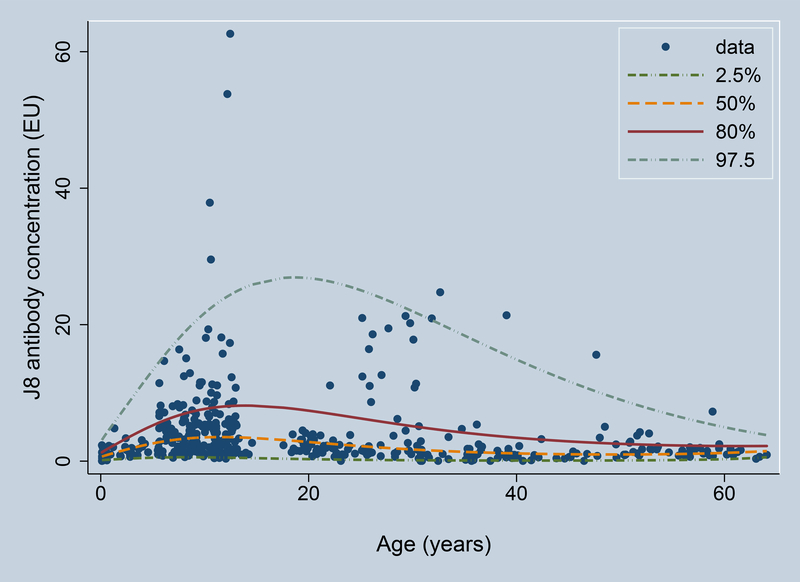
The combined number of participants meeting the inclusion criteria was 424, with 280 from Study 1 and 144 from Study 2. Median J8 antibody concentrations peaked in the 5–14 year age group, gradually decreasing with age thereafter (Figure 2). Some individuals in the 20–30 year age group were found to have elevated antibody concentrations, consistent with child/parent exposure, but there was no sustained increase with age, suggesting limited persistence of J8 antibodies. Median and 80% upper limit of normal values are provided in Table 1.
Figure 2:
J8 antibody concentration variation with age. Shown are the 2.5th centile (lower dash-dot), median (yellow dashed), 80th centile (upper limit of normal, red solid), and 97.5th centile (upper dash-dot). Antibody concentrations are provided in ELISA Units (EU).
Table 1:
Median and 80% upper limit of normal reference values for J8 antibody concentrations, by age group.
| Age group (years) | Number of subjects | Median | 80% upper limit of normal |
|---|---|---|---|
| 1–4 | 44 | 1.2 | 2.4 |
| 5–14 | 186 | 3.4 | 7.3 |
| 15–24 | 50 | 2.7 | 7.2 |
| 25–34 | 51 | 1.8 | 5.4 |
| 35–49 | 50 | 1.2 | 3.4 |
| >50 | 43 | 1.1 | 2.3 |
Influence of J8 antibody concentration on time to acquisition of infection.
After excluding participants who were infected at enrolment (or within the next 7 days), 346 participants remained in the analysis, of whom 146 experienced at least one episode of impetigo or pharyngitis in the following 10 months. While the log-rank test was significant between strata of ethnicity (P<0.0001), ethnicity did not meet the proportional hazards assumption and so the final model was stratified by ethnicity. Gender was not significantly associated with time to next infection using the log-rank test (P=0.35), and thus not considered in the models. Although baseline J8 antibody concentration was not significant in the univariate analyses (P=0.48), as it was the subject of our analysis it was retained in the multivariate model. Only age was significantly associated with time to next infection in the multivariate model (P=0.037), with a decreased risk of infection of 7% for each year of increasing age (Table 2).
Table 2:
Hazard Ratios for time to next infection among originally uninfected participants, Cox Proportional Hazards Regression. Results were stratified by ethnicity.
| Variable | Univariate HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value |
|---|---|---|---|---|
| age | 0.93 (0.87–0.99) | 0.026 | 0.93 (0.87–1.00) | 0.037 |
| J8 | 0.99 (0.96–1.02) | 0.480 | 1.00 (0.97–1.03) | 0.940 |
| school | ||||
| urban | reference | reference | ||
| rural 1 | 1.51 (0.86–2.65) | 0.150 | 1.47 (0.83–2.59) | 0.180 |
| rural 2 | 1.70 (1.03–2.82) | 0.038 | 1.64 (0.97–2.77) | 0.060 |
Evidence of J8 antibody response to infection.
i). Relationship between M-specific and J8 antibody responses.
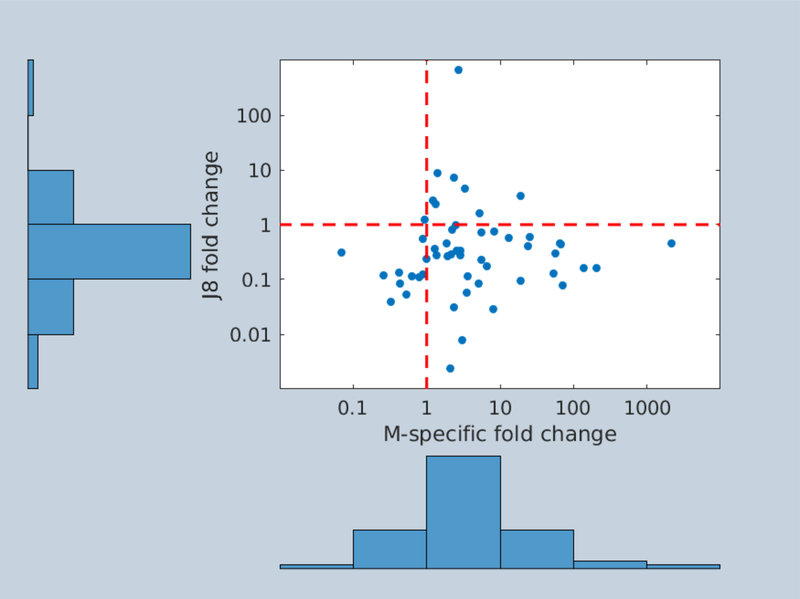
Fifty-three participants experienced infection with a single, identified emm type from the E4, D4 or E6 emm cluster between two sera collection points. The J8 fold change was lower than the M-specific fold change in 45 of the 53 participants. Most J8 fold changes were reductions, while most M-specific fold changes were increases (Figure 3). There was no association between the log-transformed fold-changes for J8 and M-specific antibodies, with a Pearson’s correlation coefficient of 0.03. For the nine individuals experiencing an increase in J8 antibody concentration, emm types (89, 109, 232, 52, 93, 11, and 65) belonging to each of theE4, D4, and E6 clusters were observed.
Figure 3:
Association between M-specific and J8 antibody responses in 53 children experiencing a single infection with an identified emm type from the E4, D4, or E6 cluster. Dashed lines represent fold changes of 1, meaning unchanged pre- and post-infection antibody concentrations.
ii). Relationship between J8 antibody response and number of infections.
Across the entire cohort of 459 children, there was no significant difference in the J8 antibody response by the number of infections experienced in either epoch 1(χ2 = 7.2, p = 0.51) or epoch 2 (χ2 = 0.65, p = 0.96) (Table 3).
Table 3:
J8 antibody responses in 459 children across consecutive sera collections, by number of infections (impetigo and pharyngitis)
| Epoch 1 (visits 1–4) | Epoch 2 (visits 4–6) | |||||
|---|---|---|---|---|---|---|
| Infections | Decrease | Stable | Increase | Decrease | Stable | Increase |
| 0 | 169 | 74 | 10 | 147 | 145 | 59 |
| 1 | 95 | 32 | 7 | 43 | 36 | 18 |
| 2 | 44 | 7 | 2 | 5 | 4 | 2 |
| 3 | 12 | 5 | 1 | 0 | 0 | 0 |
| 4 | 1 | 0 | 0 | 0 | 0 | 0 |
| Total | 321 | 118 | 20 | 195 | 185 | 79 |
iii). Persistence of J8 antibody concentrations following skin infection.
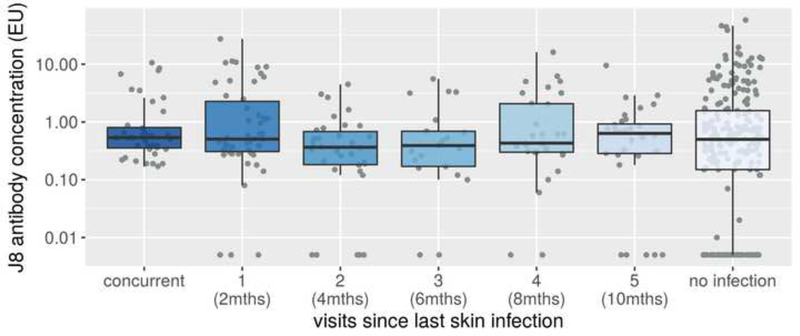
For the 427 children with no documented history of pharyngitis during the study period, J8 antibody concentration at the final visit did not vary by the time elapsed since their most recently recorded skin infection (Figure 4). Similarly, the J8 fold change comparing the final visit to the first visit in these children (Figure 5) did not vary by the time elapsed since their most recent recorded skin infection. However, all children with skin infection present at the final visit had detectable J8 antibody concentrations.
Figure 4:
J8 antibody concentration at the final visit, by number of visits (and months) since the last skin infection. Restricted to children not experiencing pharyngitis during the observation period. Boxes represent 25th–75th percentile, solid line the median and dots representing results for individuals. Note that all concurrently infected children had detectable J8 antibody concentrations. Antibody concentrations are provided in ELISA Units (EU).
Figure 5:
J8 fold change comparing the final visit to the first visit, by number of visits (and months) since the last skin infection. Restricted to children not experiencing pharyngitis during the observation period. Boxes represent 25th–75th percentile, solid line the median and dots representing results for individuals.
Discussion
Our analyses found no indication that antibodies to the J8 antigen would be useful as either a marker of GAS infection or a measure of population immunity. Although some individuals in the seroprevalence survey had elevated J8 antibody concentrations, consistent with recent exposure in this high GAS prevalence setting [7], the lack of a sustained increase with age revealed limited persistence following infection. Moreover, this failure to consolidate immunity over the life course in a high prevalence setting suggests that anamnestic responses to subsequent infection are unlikely. Neither the absolute J8 antibody concentration nor the change over a defined period showed any relationship to the time since last recorded skin infection or the number of intervening skin infections. While strong M-specific antibody responses in children confirmed that skin infections had triggered an immune response, similarly strong J8 antibody responses were not observed. Taken together, these results suggest that either J8 antibodies may not be stimulated by skin infection, or may wane considerably more rapidly than other GAS antibodies, limiting their use as a marker of infection. The lack of association between J8 antibody concentration and the time to next impetigo or pharyngitis infection raises the question of whether a protective threshold for J8 exists and whether naturally-acquired J8 antibodies can protect against GAS infection in humans.
We showed that there is considerable heterogeneity in both J8 antibody concentrations and J8 antibody responses to exposure. The use of prospectively collected, regularly sampled longitudinal data on infection status, serology, and infecting emm type is a key strength of our study. By using only immunologically-confirmed GAS skin and throat infections in our analyses, we excluded infections with similar symptoms which would not stimulate J8 antibodies, enabling us to more accurately identify the relationship between J8 antibodies and GAS infection.
The parent peptide of the J8 epitope is p145. Brandt et al [14] found that the concentrations of salivary IgA p145 specific antibodies in Indigenous Australians increased with age, across three broad age groups (1–10 years; 11–19 years; 20+ years). We measured serum IgG, not salivary IgA, making this and our study difficult to compare. Nonetheless, while we did observe a trend to increase over the first two decades of life, antibody concentrations declined steadily through adulthood. Brandt et al found an inverse relationship between salivary IgA and serum IgG in individuals over 11 years old, but only in participants with ARF and RHD and not in healthy controls [14]. As ARF and RHD are immune-mediated diseases, it may be that immune responses in affected participants are different to those in the general population. Furthermore, in our seroprevalence study, participants affected by GAS-related diseases were actively excluded.
Studies in animals have found that while J8 vaccination is immunogenic in mice [3] and non-human primates [5], infection of immunologically naïve mice does not induce antibodies to J8 [15]. Protection against infection upon challenge has been found to be correlated with J8-specific antibodies in mice [3], and baboons previously vaccinated with J8-DT developed high titres following whole organism challenge [16]. Importantly, Sheel found that unlike their vaccinated counterparts, non-vaccinated baboons did not develop high J8 titres upon challenge [16], suggesting that natural exposure alone is insufficient to stimulate responses similar to that achieved after conjugate vaccine priming. Similarly, repeated infection of mice previously vaccinated with J8-DT boosted the J8 immune response [17]. In a longitudinal study of GAS pharyngeal infection in humans, an immune response to the J14 peptide (which spans the J8 region of the M-protein) was observed in only 1 of 51 episodes [18]. Our finding that there is no relationship between J8 antibody concentrations and infection is consistent with these observations in humans, mice and non-human primates. It is likely that conjugation of J8 with carrier proteins during vaccine preparation is responsible for the immunogenicity observed in vaccine studies in animals.
While our study focused on J8 antibody concentrations, other mechanisms may be responsible for protection against GAS infection. Pandey et al [19] showed a greater than 16 fold reduction in IgG titres in mice 14–16 weeks after immunization, but also found that it is not the J8 antibody concentration at the time of challenge that is important, rather it is the presence of memory B cells. Whether or not this finding also holds true for humans remains to be tested.
There are limitations to our study. While we excluded from our seroprevalence analysis children who had sore throat or skin sores in the previous 14 days, we will probably have included children who experienced quite recent infection, in whom a fleeting antiJ8 antibody response may not yet have subsided. Given the two-month sampling interval between skin screening visits, it is possible that there may have been GAS skin infections that arose and resolved between visits. Similarly, collection of sera at months 0, 6, and 10 may mean that J8 antibody concentrations rose and returned to baseline between sera collections. Our study suggests that J8 antibody responses to infection are fleeting — more frequent sera collection and shorter intervals between skin screening visits would have improved our ability to observe changes related to infection. Furthermore, all of the data available to infer dose-response were collected in schoolchildren, and so we are unable to comment on the relationship between J8 antibody and infection in older age groups.
In conclusion, this study has demonstrated that the presence of J8 antibodies in response to infection in humans is fleeting, if existent at all, and that naturally-acquired J8 antibodies may have a limited ability to protect against acquisition of infection. Whether vaccination of humans with J8-DT can produce a different result, replicating the protection observed in mice, remains an open question.
Highlights.
studied antibodies to J8, a potential group A Streptococcus vaccine antigen
presence of J8 antibodies following infection is fleeting, if existent at all
J8 antibodies would not be useful as a marker of population immunity
evidence will be useful for planning clinical trials of the J8 vaccine
Acknowledgements
The authors thank the participants of the studies, The Fijian Ministry of Health and the Fijian Group A Streptococcal Project Team.
Funding
This study was funded by the National Institute of Allergy and Infectious Diseases (grant U01AI60579); National Health and Medical Research Council (NHMRC) project grant (GNT1051297); NHMRC Centre of Research Excellence PRISM2 (GNT1058804); and the Murdoch Children’s Research Institute of Melbourne, Australia. JM is supported by an NHMRC Principal Research Fellowship (GNT 1117140).
Abbreviations
- CI
confidence interval
- Cox PH
Cox proportional hazards
- ELISA
Enzyme-Linked Immunosorbent Assay
- GAS
group A Streptococcus
- HR
hazard ratio
Footnotes
Conflict of Interest
The authors have no conflicts to disclose.
Data availability. The data and computer code used in the study are available on request.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685–94. [DOI] [PubMed] [Google Scholar]
- [2].Steer AC, Dale JB, Carapetis JR. Progress toward a global group A streptococcal vaccine. The Pediatric Infectious Disease Journal. 2013;32:180–2. [DOI] [PubMed] [Google Scholar]
- [3].Batzloff MR, Hayman WA, Davies MR, Zeng M, Pruksakorn S, Brandt ER, et al. Protection against group A Streptococcus by immunization with J8-diphtheria toxoid: contribution of J8- and diphtheria toxoid-specific antibodies to protection. The Journal of Infectious Diseases. 2003;187:1598–608. [DOI] [PubMed] [Google Scholar]
- [4].Batzloff MR, Fane A, Gorton D, Pandey M, Rivera-Hernandez T, Calcutt A, et al. Preclinical immunogenicity and safety of a Group A streptococcal M protein-based vaccine candidate. Human Vaccines and Immunotherapeutics. 2016;12:3089–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Caro-Aguilar I, Ottinger E, Hepler RW, Nahas DD, Wu C, Good MF, et al. Immunogenicity in mice and non-human primates of the Group A Streptococcal J8 peptide vaccine candidate conjugated to CRM197. Human Vaccines and Immunotherapeutics. 2013;9:488–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Steer AC, Vidmar S, Ritika R, Kado J, Batzloff M, Jenney AWJ, et al. Normal ranges of streptococcal antibody titers are similar whether streptococci are endemic to the setting or not. Clin Vaccine Immunol. 2009;16:172–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Steer AC, Jenney AWJ, Kado J, Batzloff MR, La Vincente S, Waqatakirewa L, et al. High burden of impetigo and scabies in a tropical country. PLoS Negl Trop Dis. 2009;3:e467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Steer AC, Jenney AWJ, Kado J, Good MF, Batzloff M, Magor G, et al. Prospective surveillance of streptococcal sore throat in a tropical country. The Pediatric Infectious Disease Journal. 2009;28:477–82. [DOI] [PubMed] [Google Scholar]
- [9].Sanderson-Smith M, De Oliveira DMP, Guglielmini J, McMillan DJ, Vu T, Holien JK, et al. A systematic and functional classification of Streptococcus pyogenes that serves as a new tool for molecular typing and vaccine development. The Journal of Infectious Diseases. 2014;210:1325–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hnasko RE. ELISA Methods and Protocols. New York: Humana Press; 2015. [Google Scholar]
- [11].Frost HR, Laho D, Sanderson-Smith ML, Licciardi P, Donath S, Curtis N, et al. Immune cross-opsonization within emm clusters following group A Streptococcus skin infection: broadening the scope of type-specific immunity. Clin Infect Dis. 2017;65:1523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Royston P Constructing time-specific reference ranges. Stat Med. 1991;10:675–90. [DOI] [PubMed] [Google Scholar]
- [13].Wright E, Royston P. Age-specific reference intervals (“normal ranges”). STATA Technical Bulletin. 1996;34:24–33. [Google Scholar]
- [14].Brandt ER, Hayman WA, Currie B, Carapetis J, Jackson DC, Do K-A, et al. Functional analysis of IgA antibodies specific for a conserved epitope within the M protein of group A streptococci from Australian Aboriginal endemic communities. Int Immunol. 1999;11:569–76. [DOI] [PubMed] [Google Scholar]
- [15].Pandey M, Ozberk V, Calcutt A, Langshaw E, Powell J, Rivera-Hernandez T, et al. Streptococcal immunity is constrained by lack of immunological memory following a single episode of pyoderma. PLoS Pathog. 2016;12:e1006122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sheel M Immunogenicity and protective efficacy of an anti-Streptococcus pyogenes vaccine candidate in multiple animal species. Queensland: Queensland University of Technology; 2010. [Google Scholar]
- [17].Pandey M, Ozberk V, Langshaw EL, Calcutt A, Powell J, Batzloff MR, et al. Skin infection boosts memory B-cells specific for a cryptic vaccine epitope of group A streptococcus and broadens the immune response to enhance vaccine efficacy. npj Vaccines. 2018;3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hysmith ND, Kaplan EL, Cleary PP, Johnson DR, Penfound TA, Dale JB. Prospective longitudinal analysis of immune responses in pediatric subjects after pharyngeal acquisition of Group A Streptococci. Journal of the Pediatric Infectious Diseases Society. 2017;6:187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pandey M, Wykes MN, Hartas J, Good MF, Batzloff MR. Long-term antibody memory induced by synthetic peptide vaccination is protective against Streptococcus pyogenes infection and is independent of memory T cell help. J Immunol. 2013;190:2692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]