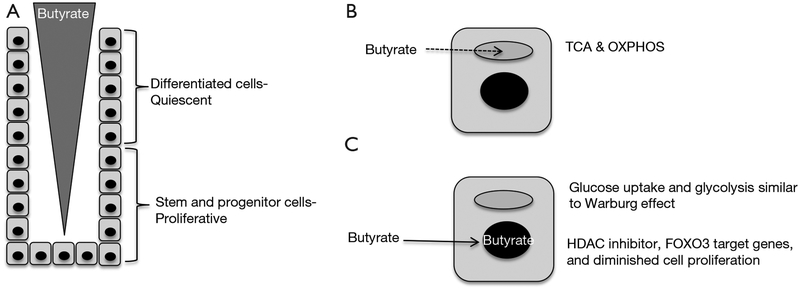
The mammalian intestinal tract epithelium is renewed every ~4–6 days, which is arguably the most rapidly turned over tissue in the body. Cellular turnover is facilitated by the intestinal epithelium being organized as crypts with highly proliferative stem cells and progenitor/transit-amplifying cells located near the crypt base and quiescent, terminally-differentiated cell types such as enterocytes, goblet cells, and enteroendocrine cells located above that undergo apoptosis and exfoliate into the lumen (Figure 1A). In recent years, tremendous progress has been made identifying Lgr5+ stem cells and crucial factors within the stem-cell niche such as Wnts, R-spondins, BMPs, Noggin, and the Notch pathway (1). This knowledge has been exploited to create 3D organoid culture systems that recapitulate the crypt with respect to stem cell renewal and their ability to give rise to descendants that represent each differentiated cell type.
Figure 1.
A model depicting the effect that cellular energy metabolism has on a butyrate gradient that regulates cell proliferation in the colonic crypt. (A) Butyrate is believed to be present in a gradient with higher levels near the lumen and lower levels near the crypt base. This gradient is due, in part, to bacterial fermentation of fiber being most prominent in the lumen. The position of quiescent differentiated cells and proliferative stem and progenitor cells is indicated; (B) differentiated cells in the upper crypt undergo oxidative metabolism (TCA cycle and OXPHOS), which results in butyrate being readily consumed (dashed line) in mitochondria (gray oval). This normal energy metabolism contributes to the butyrate gradient because less butyrate is available to diffuse down toward the bottom of the crypt; (C) stem and progenitor cells in the lower crypt utilize oxidative metabolism to a lesser extent, possibly due to their proliferative nature and increased glucose uptake and glycolysis similar to the Warburg effect. In cases where there is higher than normal levels of butyrate near the crypt base, such as if the upper crypt is damaged (resulting in less butyrate consumbed), the butyrate can accumulate in the nucleus (black circle) and function as an HDAC inhibitor to epigenetically regulate gene expression and decrease cell proliferation in a FOXO3-dependent manner. OXPHOS, oxidative phosphorylation; HDAC, histone deacetylase.
It is generally believed that bioactive food components and gut microbiota produce metabolites that influence crypt homeostasis for either better or worse. However, there has been relatively little evidence to support this idea, which makes a recent paper by Kaiko et al. noteworthy (2). Their study began by screening a library of 92 metabolites derived from bioactive food components and/or microbiota on 3D colonoid cultures carrying a fluorescent reporter of cell-cycle progression. Out of eight candidate metabolites that affected cell-cycle progression in the initial screen, one showed significant and reproducible effects at a physiologically relevant concentration. This particular molecule, butyrate, is a short-chain fatty acid produced by bacterial fermentation of dietary fiber in the colon where it is present at very high (mM) concentrations. Butyrate was an ideal candidate to follow-up on in more detail because it has potent energetic and epigenetic functions in colonocytes and is known to inhibit the proliferation of colorectal cancer cell lines.
Butyrate is believed to exist in a concentration gradient in the colon, with higher levels near the lumen and lower levels near stem and progenitor cells close to each crypt base (Figure 1A), due to bacterial fermentation of fiber occurring primarily in the lumen. Peristalsis and an upward flow of mucous are believed to sharpen the butyrate gradient. The current study demonstrates that cellular energy metabolism contributes to this concentration gradient with butyrate being metabolized efficiently by differentiated cells in the upper crypt, which decreases the amount that is available to diffuse down to the base (Figure 1B). This finding is consistent with previous reports that colonocytes utilize butyrate as their primary energy source instead of glucose. As a fatty acid, butyrate undergoes β-oxidation via the enzyme ACADS (acyl-CoA dehydrogenase) to acetyl-CoA in mitochondria, and this is followed by the TCA cycle and oxidative phosphorylation (OXPHOS) to generate ATP (Figure 1B). The butyrate-consuming properties of cells in the upper crypt apparently prevent too much butyrate from reaching the crypt base where stem and progenitor cells are vulnerable to butyrate-induced decreases in cell proliferation. Kaiko et al. demonstrate that, compared to differentiated colonocytes in the upper crypt, stem and progenitor cells have diminished mitochondrial oxidative metabolic capacity (Figure 1C), which could be due to their rapid proliferation and a process similar or identical to the Warburg effect. The Warburg effect is a metabolic reprogramming event that occurs in most tumor cells where glucose uptake and glycolysis are upregulated, while oxidative metabolism (TCA cycle and OXPHOS) is utilized to a lesser extent than normal, less proliferative cells. Although glycolysis is relatively inefficient for ATP production, it and the attendant metabolic pathways that are reprogrammed serve as a conduit to funnel carbon and nitrogen into biosynthetic pathways to replenish pools of dNTPs, amino acids, and fatty acids that are the limiting factors for the proliferation of rapidly dividing cells.
The current study increased butyrate levels near the crypt base using a combination of experimental approaches: treatment with dextran sodium sulfate (DSS) to damage butyrate-consuming cells in the upper crypt, analysis of crypts from Acads knockout mice which have impaired butyrate-consuming properties, and delivery of exogenous butyrate. Increased butyrate levels near the crypt base triggered decreased proliferation of stem and progenitor cells, which could have context-dependent detrimental or beneficial health effects in people. On the one hand, decreased proliferation might be expected to impede wound repair and exacerbate inflammatory bowel disease (IBD). On the other hand, decreased proliferation might be expected to diminish colorectal cancer initiation since the Lgr5+ stem cell is the cell-of-origin for colorectal cancer. Although most studies suggest that butyrate is tumor-suppressive, some studies suggest that it can be oncogenic (3,4).
Butyrate is a pleiotropic molecule that functions by multiple mechanisms, and Kaiko et al. demonstrate that butyrate inhibits stem and progenitor cell proliferation as a histone deacetylase (HDAC) inhibitor rather than as a ligand for G protein coupled receptors. Butyrate increased histone acetylation at H3K9 and H3K27 residues and altered the expression of >2,400 genes. Motif analysis of these butyrate-regulated genes revealed that direct targets of FOXO1 and FOXO3 transcription factors were enriched. To demonstrate a functional link, pharmacologic and genetic approaches demonstrated that butyrate inhibited stem and progenitor cell proliferation in a FOXO3-dependent manner (Figure 1C).
This study makes an important conceptual advance by demonstrating that a microbial metabolite is an important component of the stem cell niche. Although butyrate has been studied extensively for many years, there has been an overreliance on adding relatively high doses to colorectal cancer cell lines, which lack the crypt architecture and functionally heterogeneous cell types of 3D organoids that makes this study so insightful. A more general take-home message of equal importance is that the experimental approach merges two disciplines of GI research: (I) stem cells, stem cell niche, and organoid cultures; (II) microbiome and the function of microbial metabolites. The microbiome community is moving from metagenomic sequencing to functionally interrogating gut microbiota and their metabolites, and Kaiko et al. are at the vanguard of a new strategy that will co-culture 3D organoids with gut microbiota, bioactive food components, and microbial-derived metabolites.
Acknowledgements
Funding: This work was supported by the National Institutes of Health [RO1-OD-02057] and the United States Department of Agriculture [2015–055336].
Footnote
Provenance: This is an invited article commissioned by the Section Editor Xia Fang (Department of hematology, Shanghai Tongji Hospital, Tongji University School of Medicine, Shanghai, China).
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- 1.Barker N Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol 2014;15:19–33. [DOI] [PubMed] [Google Scholar]
- 2.Kaiko GE, Ryu SH, Koues OI, et al. The Colonic Crypt Protects Stem Cells from Microbiota-Derived Metabolites. Cell 2016;165:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bultman SJ, Jobin C. Microbial-derived butyrate: an oncometabolite or tumor-suppressive metabolite? Cell Host Microbe 2014;16:143–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donohoe DR, Holley D, Collins LB, et al. A gnotobiotic mouse model demonstrates that dietary fiber protects against colorectal tumorigenesis in a microbiota- and butyrate-dependent manner. Cancer Discov 2014;4:1387–97. [DOI] [PMC free article] [PubMed] [Google Scholar]