Abstract
Calcium is thought to play an important role in regulating mitochondrial function. Evidence suggests that an increase in mitochondrial calcium can augment ATP production by altering the activity of calcium-sensitive mitochondrial matrix enzymes. In contrast, the entry of large amounts of mitochondrial calcium in the setting of ischemia-reperfusion injury is believed to be a critical event in triggering cellular necrosis. For many decades, the details of how calcium entered the mitochondria remained a biological mystery. In the last few years, significant progress has been made in identifying the molecular components of the mitochondrial calcium uniporter complex. Here, we review how calcium enters and leaves the mitochondria, the growing insight into the topology, stoichiometry and function of the uniporter complex and the early lessons learned from some initial mouse models that genetically perturb mitochondrial calcium homeostasis.
Introduction
Those involved in biological or medical research often have a certain degree of envy for colleagues who labor in other scientific disciplines. Take high energy physics as an example. In 1964, a group of six physicists that included Peter Higgs postulated the existence of a certain elementary particle that helped resolve a longstanding riddle in cosmology. For the next forty years, teams of physicists sought experimental evidence of this theoretical Higgs boson. The challenge, although arduous, was clearly defined. Although laudable, this degree of focus and determination is dwarfed by what can happen in mathematics. In 1637, the French mathematician Pierre de Fermat made a relatively simple mathematical conjecture in the margin of a book. For the next 358 years, mathematicians sought the elusive proof that Fermat was indeed correct. Again, with the framework of the problem clearly outlined, Fermat’s Last Theorem engaged nearly four centuries of mathematicians. In contrast, most biological and medical discoveries happen either by accident (think penicillin) or are the result of a relatively short-lived race (think the structure of DNA). That is not to say the biology does not have certain enduring challenges. One such example revolves around the relatively simple question as to how calcium enters the mitochondria. While perhaps not rising to the level of the search for a mysterious sub-atomic particle or deriving an elusive and fundamental mathematical proof, it has been clear since the 1960’s that energized mitochondria could somehow selectively allow calcium to enter1. Over the next five decades, pharmacological, biophysical, biochemical and electrophysiological evidence gathered for the existence of an inner mitochondrial protein termed the mitochondrial calcium uniporter (MCU). Yet, though clearly defined, for over fifty years, the molecular identity of the uniporter remained unsolved. Like all good mysteries, along the way there were some promising leads that suggested a solution had been found2. Then, when some had begun to question the very existence of the uniporter, two groups using different in silico approaches both identified a previously uncharacterized mitochondrial protein (CCDC109A) as the long sought inner membrane calcium pore3, 4. It soon became clear that the uniporter does not act alone but rather exists in a large multimeric complex, the details which are only slowly being understood. Here, we review the evidence that calcium plays a regulatory role in mitochondria-from modulating bioenergetics, to determining the threshold for cell death. We further describe what is known about the influx and efflux of calcium into the mitochondria with particular emphasis on the inner mitochondrial uniporter complex and its increasingly complex regulation. Finally, we describe the small but growing number of in vivo models in which mitochondrial calcium is perturbed and the sometimes surprising phenotypes that have emerged.
Mitochondrial calcium uptake
The first clear description of the ability of mitochondria to rapidly uptake calcium came in the early 1960’s, with the work of Vasington, Murphy, De Luca and Engstrom (reviewed in5). Over the intervening 50 years, pharmacological-based approaches played a significant role, especially after the discovery that mitochondrial calcium uptake was inhibited by the compound ruthenium red6, 7 and its more specific form Ru3608. Mitochondrial calcium uptake was reported to have a low affinity (high Km) of ~5–10 μM5. High extramitochondrial calcium and calmodulin antagonists were also shown to inhibit calcium uptake into the mitochondria9. Varying forms or modes of calcium uptake were described, although it was unclear whether these were mediated by the same transporter (with different regulatory factors) or multiple different transporters. For example, when rat liver mitochondria are pulsed for a few seconds with nanomolar levels of calcium, they exhibit calcium uptake with a rapid kinetics which was termed “rapid mode” calcium uptake10, 11. To explain these and other results, a number of different molecules have been implicated as potential mediators of mitochondrial calcium uptake. For instance, the skeletal ryanodine receptor (RyR1), the primary calcium release channel in skeletal sarcoplasmic reticulum, was reported to be present in mitochondria and ryanodine, an inhibitor of RyR1, was shown to block mitochondrial calcium uptake12. LETM1 (leucine zipper-EF-hand-containing transmembrane protein 1) was also proposed to function as a mitochondrial Ca2+/H+ antiporter, thereby mediating mitochondrial calcium influx13–15, although others have suggested that it is a mitochondrial K+/H+ exchanger16, 17. The mitochondrial Na+/Ca2+ exchanger, which normally functions as a calcium efflux pathway (see Figure 1) has also been suggested to be able to function in reverse, leading to mitochondrial calcium entry when the mitochondrial membrane potential is dissipated and the Na+ gradient is favorable18 (see section below on NCLX exchanger).
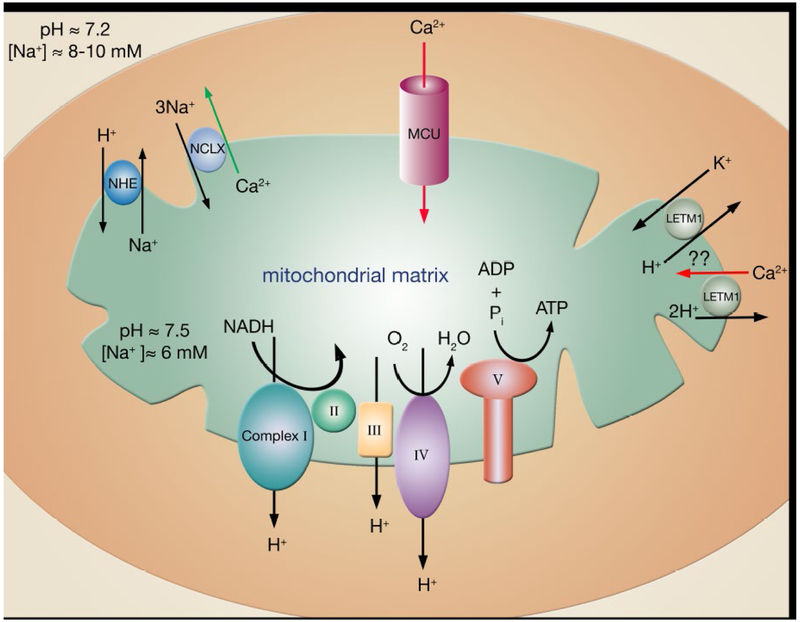
Figure 1:
Mitochondrial ion transport mechanisms involved in regulating calcium entry (red arrows) and efflux (green arrows). Calcium enters the mitochondria primarily through the mitochondria calcium uniporter (MCU). Although MCU appears to be the main calcium influx pathway, other influx mechanisms such as RyR1 (not shown) or LETM1 have also been proposed13–15. Questions remain however, as others have suggested that LETM1 is, in fact, a mitochondrial K+/H+ exchanger16, 17. Calcium efflux is driven by NCLX using the influx of sodium down its electrochemical gradient. The intracellular sodium is set below the cytosolic sodium by the sodium-proton exchanger (NHE), which uses the energy of the inwardly directed proton gradient to maintain mitochondrial sodium below the concentration of sodium in the cytosol.
In addition to the confusion as to what protein might mediate mitochondrial calcium uptake, there was for a long period of time, considerable confusion regarding how this process could occur. Measurements of basal cytosolic calcium were thought to be in the range of 100 nM and peak calcium transients were less than 1 μM19, 20. Given the measured low affinity (~5–10 μM) for calcium uptake, it initially appeared that under physiological conditions, cytosolic calcium would never rise to levels high enough to activate the MCU. However, it was later demonstrated that microdomains of high calcium can exist near the junction of mitochondria and the site of calcium release (the ER/SR) and that the concentration of calcium in these microdomains is in fact high enough to allow calcium uptake via the uniporter21.
Mitochondrial calcium efflux
In steady state, mitochondrial efflux must equal mitochondrial uptake. Mitochondrial efflux is primarily attributed to the function of the Na+-Ca2+-Li+ exchanger (NCLX22), which can be inhibited pharmacologically by CGP3715723. It was known for some time that calcium efflux from heart mitochondria was dependent on the Na+ gradient; although it was also shown that Li+ can stimulate Na+ efflux24. Matrix Na+ is reported to be lower than cytosolic Na+ due to Na+ efflux from the mitochondria involving the mitochondria Na+-H+ exchanger. Matrix Na+ is primarily regulated by the mitochondrial Na+-H+ exchanger, which is thought to operate close to equilibrium with the pH gradient25. Although the pH gradient in isolated mitochondria is typically measured at ~0.7 pH units (often in non-physiological buffers), it appears to be lower for mitochondria in situ. Studies in permeabilized cardiac myocytes also suggest that mitochondrial Na+ is lower than cytosolic Na+. This Na+ gradient (lower in the matrix) is used as a driving force to extrude calcium from the matrix via the NCLX. Although it is still not proven, there is some evidence suggesting that the NCLX is electrogenic, exchanging 3 Na+ ions for 1 Ca2+ ion26. The large negative mitochondrial membrane potential (−150 to −180 mV), coupled with the inwardly directed Na+ gradients, provide a large driving force for extruding calcium from the mitochondrial matrix. If the NCLX is electrogenic, it could only potentially function in reverse to transport calcium into the matrix under conditions where the membrane potential was dissipated. It was long known that extra-mitochondrial Na+ (or Li+)24 could stimulate calcium efflux from mitochondria and that ruthenium red did not inhibit the efflux, but rather stimulated it as would be expected for an efflux pathway24, 27. The NCLX was recently identified22 facilitating targeted genetic and biochemical studies of its function and characteristics that could potentially address many of the issues mentioned above. In this regard, recent studies have shown that overexpressing the NCLX protein increased mitochondrial calcium efflux and reducing expression using siRNA-mediated approaches resulted in diminished mitochondrial calcium efflux22, 26.
In isolated mitochondria, matrix calcium can vary widely depending on the extramitochondrial calcium level. Measurement of in situ matrix calcium in cardiomyocytes is reported in the range of 100–200 nM at low pacing rates and increases to 500 to 800 nM with β-adrenergic stimulation. It is controversial as to whether mitochondrial matrix calcium transients actually track the changes in cytosolic calcium transients on a beat-to-beat basis, or if matrix calcium somehow integrates the levels of cytosolic calcium28. One argument against matrix calcium transients is that the NCLX is not capable of rapidly removing calcium on this beat-to-beat time scale. It has also been proposed that a transient opening of the mitochondrial calcium permeability transition pore might, under certain conditions, serve as an additional calcium release valve29, 30, although recent data question this hypothesis31.
Buffering of matrix calcium:
Mitochondrial calcium buffering can also affect the level of matrix free calcium. As shown by several groups32–34 at levels below ~10 nmol Ca2+/mg of mitochondrial protein, uptake of mitochondrial calcium leads to an increase in free matrix calcium. Thus below 10 nmol Ca2+/mg there is a linear relationship between total and free calcium. In this range uptake of calcium into the mitochondria will change the free calcium. However, as total calcium rises above 10 nmol/mg matrix free calcium remains stable in the range of 1–5 μM due to buffering by calcium phosphate. In fact, Nicholls showed that with total mitochondrial calcium levels above ~10 nmol/mg that uptake of calcium buffers extramitochondrial calcium at a “set point”. Thus if in situ mitochondrial calcium operates in the range in which calcium is buffered by phosphate (~above ~10 nmol Ca2+/mg protein) then calcium uptake by MCU may not be a key factor for the bioenergetic signaling system. However, as changes in cytosolic calcium have been reported to lead to changes in free matrix calcium28 it is not clear whether in situ mitochondria operate in the range where matrix calcium is buffered.
Physiological role of mitochondrial calcium
Studies in the mid-1970s suggested that mitochondrial calcium might serve to regulate or modulate cytosolic calcium or act as a source for calcium release by a physiological agonist35. However, the latter hypothesis became unlikely when it became apparent that the endoplasmic reticulum/sarcoplasmic reticulum is the primary source of agonist-induced calcium release. A decade later, in the 1980’s, Denton and McCormick and others showed that calcium could regulate the activity of 3 mitochondrial dehydrogenases: pyruvate dehydrogenase, isocitrate dehydrogenase and α-ketoglutarate dehydrogenase36. This led to the suggestion that the role of mitochondrial calcium uptake was not to regulate cytosolic calcium, but rather to regulate mitochondrial matrix calcium and hence the activity of these calcium sensitive mitochondrial dehydrogenases. Thus, in a cardiomyocyte, under conditions of increased work, an increase in cytosolic calcium leads to an increase in matrix calcium and serves to couple the increase in work to the increase in ATP production that is needed to sustain the work. With an increase in work, matrix calcium would increase, and this in turn would activate the mitochondrial dehydrogenases to increase mitochondrial NADH, fueling electron transport and increasing the output of ATP37. More recently, Balaban and coworkers demonstrated that calcium also leads to activation of complex V, the F1F0 ATPase38, an enzyme that converts the mitochondrial protomotive force into ATP. Their observations were that in larger species with slower heart rates (e.g. pigs and dogs), raising matrix calcium lead to activation of complex V and that this activation was retained by the complex even after isolation39. It is worth noting that the baseline turnover numbers (nmoles Pi/nmole Complex) in the mouse heart were already equivalent to the maximum calcium-stimulated levels observed in the larger dog and pig species. Thus, in the mouse, there might be less calcium regulation as Complex V may be wired to be at or near maximal stimulation under basal conditions.
Pathophysiological role of mitochondrial calcium
There is a wealth of data suggesting that an increase in cytosolic calcium and the resultant mitochondrial calcium overload are primary factors leading to cell death following ischemia and reperfusion40–44. The exact target of calcium overload that triggers cell death has been debated, but recent data has converged to suggest that mitochondrial calcium overload is a primary trigger of necrotic cell death45. In the late 1970s, studies in isolated mitochondria showed that accumulation of large amounts of matrix calcium led to the opening of a large channel in the mitochondria inner membrane that subsequently results in the release of proteins and solutes less than 1.5 kD; this channel is referred to as the mitochondrial permeability transition pore (mPTP)46. Calcium uptake via the MCU is thought to be the primary mechanism for calcium uptake to activate the mPTP, and inhibitors of MCU have been reported to protect cells from chemical hypoxia47 and reduce cerebral and cardiac ischemia-reperfusion injury in vivo48. Chalmers and Nicholls also reported that total mitochondrial calcium rather than free matrix calcium was the trigger for opening the mPTP33. Later studies showed that cyclosporin A could inhibit the mPTP, which reinforced the concept that mPTP opening was not due to just non-specific membrane rupture49, 50. Mitochondrial cyclophilin D was soon identified as the target of cyclosporine A50. The identification of an inhibitor of the mPTP allowed studies to show that addition of cyclosporine A reduced cardiac ischemia-reperfusion injury51. Confirmation of the role of cyclophilin D in mediating calcium activation of the mPTP came from studies with genetic ablation of cyclophilin D. Indeed, isolated mitochondria from mice lacking cyclophilin D were resistant to calcium activated mPTP opening and hearts from these mice showed reduced cell death following ischemia and reperfusion52. A small proof of concept clinical trial has also reported that administration of cyclosporine A to patients with myocardial infarction is beneficial53.
Alterations in mitochondrial calcium have also been suggested to play a role in altered metabolism in heart failure. Heart failure has been shown to lead to an increase in cytosolic sodium which is suggested to reduce mitochondrial matrix calcium levels by enhancing efflux of matrix calcium via the NCLX54. The reduction in matrix calcium is reported to decrease oxidative phosphorylation and ATP production and oxidation of NAD(P)H resulting in reduced antioxidant capacity. Indeed inhibition of the NCLX with CGP37157 has been reported to be beneficial in a guinea pig model of heart failure54. Although reduced matrix calcium associated with increased efflux or reduced calcium uptake can have detrimental effects, it is likely that overexpression of MCU or total loss of NCLX and the resultant unrestrained accumulation of matrix calcium would also be detrimental.
MCU and its regulators MICU1 and MICU2
The past 5 years have resulted in an explosion of studies identifying mitochondrial transporter proteins. As mentioned previously, the identification of the long elusive uniporter was reported simultaneously by two groups3, 4. Both identified an approximate 40 kD inner mitochondrial membrane protein with two predicted transmembrane domains. Mutation of a single amino acid (serine 259) resulted in a uniporter that loses the ability to be inactivated by the classical inhibitor ruthenium red3. Moreover, mutations in the acidic linker domain, between the two transmembrane domains, resulted in markedly diminished calcium uptake, with the appearance of a protein having a dominant negative phenotype3, 4. Reconstitution of purified MCU in lipid bilayers demonstrated channel activity arguing that MCU, by itself, may be all that is required for at least some uniporter activity4. Nonetheless, this conclusion is not universally accepted55. There is also some disagreement on the orientation and topology of MCU that in vivo appears to exist as a tetramer56. Nonetheless, it would appear that the most likely configuration has the linker region of MCU facing into the inter-membrane space, while the N-terminal and C-terminal portions of the protein are in the matrix3, 57. This topology was more firmly established by a novel technique that targets a genetically engineered peroxidase called APEX to a specific subcellular domain (in this case the inner-membrane space) in order to allow for live-cell proteomics58. This strategy, if more widely adopted, should allow for refinement of the topology of other components of the uniporter complex, as well as other large protein complexes within the mitochondria. While electrophysiological studies in MCU knockout mitoplasts have not been performed, there appears little doubt that the 40 kD protein identified as MCU is indeed the uniporter, a fact further bolstered by observations that mitochondria from MCU knockout mice lack any appreciable mechanism for the rapid uptake of calcium59.
The first component of the uniporter complex identified was actually not the pore itself, but rather another inner mitochondrial membrane protein termed MICU1 (mitochondrial calcium uptake 1)60. MICU1 is a 54 kD protein containing two calcium-sensing EF hand domains. The identification of MICU1 was achieved through an ingenious targeted screen that employed the MitoCarta data base, a proteomic inventory of approximately 1000 known proteins that localize to the mitochondria61. To identify MICU1, the authors asked for proteins that existed within MitoCarta, further localized to the inner mitochondrial membrane (the presumptive localization of the uniporter) and were not present in S. Cerevisiae mitochondria (an organism known to lack the uniporter). Of the thousand plus genes whose protein products are found in MitoCarta, only 18 fulfilled these criteria. Then, using an RNAi-based approach, the authors asked whether knockdown of any of these eighteen candidates altered mitochondrial calcium uptake. One candidate did, a poorly characterized gene that was subsequently renamed MICU1. The fact that silencing MICU1 appears to inhibit mitochondrial calcium uptake must now, however, be viewed with certain degree of reservation. This is because it is now clear that the stability and expression of many of the components of the uniporter complex can be affected by knockdown of their interacting partners62. Hence, MICU1 knockdown can, for instance, influence MCU protein stability, thereby complicating interpretation.
In their initial description of MICU1, the authors suggested that the EF hands of this protein could potentially serve as a calcium sensing mechanism, making MICU1 an ideal candidate to act as a regulator of the uniporter60. Such regulation would seem essential based on electrophysiological studies of the pore that have indicated the open probability of the uniporter is near unity when the mitochondria are at their resting, hyperpolarized state63. Nonetheless, a uniporter that was open under basal conditions would rapidly dissipate the mitochondrial membrane potential leading to the inability to generate ATP. Several subsequent studies have suggested that knockdown of MICU1 actually increases mitochondrial calcium uptake, especially at low levels of calcium57, 64. These studies support the notion that MICU1 binds to and inhibits MCU opening at low cytosolic calcium levels, thereby acting as a molecular gatekeeper. These reports, however, do differ in what role they place on the EF hands of MICU1. In one study, mutation of the EF hand domain abrogated the ability of MICU1 to act in its gatekeeper role64. The authors suggested that the high affinity EF hands sensed matrix calcium levels and thereby inhibit MCU when the EF hands of MICU1 are complexed with calcium. In contrast, the other study proposed a model in which MICU1 senses inter-membrane levels of calcium (not matrix calcium levels) and inhibits MCU channel activity when the EF hands of MICU1 are in the apo or calcium-free state57. These authors, in contrast, proposed that calcium binding to the EF hands of MICU1 actually results in augmentation of MCU-dependent calcium uptake. As such, in this model, MICU1 has two opposing functions based on mitochondrial inter-membrane calcium levels. With low calcium levels MICU1 inhibits MCU, while at higher calcium levels MICU1 functions to activate the channel. This property could potentially explain the sigmoidal shape curve that is seen when calcium uptake is assessed as a function of calcium concentrations over the nanomolar to micromolar range57. The notion that the calcium-free form of MICU1 acts as negative regulator of MCU was also supported by recent structural studies of the calcium-bound and calcium-free MICU1 crystal structures65. These studies suggested that in the absence of calcium, MICU1 adopts a hexamer configuration that can interact with MCU and presumably inhibit channel activity. Binding of calcium to the EF hands of MICU1 results in a large conformational change in the protein, thereby converting the hexamer into a mixture of activating oligomers. Furthermore, this study measured the affinity of the EF hands of MICU1 for calcium at approximately 15–20 μM. Given that resting cytosolic calcium is significantly less than 1 μM, under basal conditions, if MICU1 senses the inter-membrane space (that is essentially in equilibrium with cytosolic calcium levels), one would predict it would indeed be in the apo state under non-stimulated, basal conditions.
As mentioned previously, the interpretation of MICU1 knockdown experiments needs to be interpreted with caution. This was made abundantly clear with the discovery of a paralog of MICU1, now termed MICU262. MICU2 is similar to MICU1 (approximately 40% sequence similarity) containing two EF hands and thought to be located facing the matrix side of the inner mitochondrialmembrane. MICU1 and MICU2 interact in the presence of calcium or EGTA, and knockdown of MICU1 dramatically reduces MICU2 expression, at least in some cell lines. This has led to concerns that the positive or negative actions on calcium uptake ascribed to MICU1 might in fact be a function of an inadvertent reduction in MICU2. Indeed, one recent paper has proposed that MICU2 not MICU1 is actually the inhibitor of MCU current at low calcium concentrations, while MICU1 acts to stimulate MCU activity at higher calcium concentrations66. Thus, these authors argue, the sigmoidal shape of calcium uptake across the inner mitochondrial membrane is due to an inhibitory effect of MICU2 at low calcium levels, and a stimulatory effect of MICU1 at high calcium concentrations. These authors also suggested that MICU1-MICU2 interaction occurs through a disulfide bond (cysteine 464 in MICU1 and cysteine 410 in MICU2). In contrast to this model that ascribes separate functions to MICU1 and MICU2, others have suggested that these two paralogs act cooperatively55. This cooperative model is based on a number of observations using cell-based knockout strategies. For instance, a zinc finger nuclease strategy to knockout MICU1 in 293T cells eliminated MICU1 expression and correspondingly reduced MICU2 levels by more than 80%. In contrast, knockout of MICU2 eliminated MICU2 expression without significantly altering MICU1 levels. Nonetheless, both knockout cell lines had augmented calcium uptake at low calcium levels (suggesting a loss of gatekeeper function), while expression of EF hand mutants of either MICU1 or MICU2 dramatically inhibited calcium uptake at high calcium levels. These data therefore supports a model in which a rise in inter-membrane calcium allows calcium binding to the EF hands of MICU1 and MICU2, which presumably induces a structural change in the complex that is required to ultimately open the pore at elevated calcium levels (Figure 2). It should also be considered that mutation or deletion of the MCU regulatory subunits could have indirect effects on calcium uptake for example by altering mitochondrial membrane potential or matrix pH. Interestingly, there has been a recent description of loss-of-function recessive mutations occurring at the MICU1 locus in humans67. These individuals develop both neurological and skeletal muscle abnormalities. Primary fibroblasts from subjects with this condition appear to show elevated basal levels of calcium, again consistent with a potential gatekeeping function of MICU1. Nonetheless, it is unclear whether this increase in mitochondrial calcium is responsible for the significant clinical findings observed.
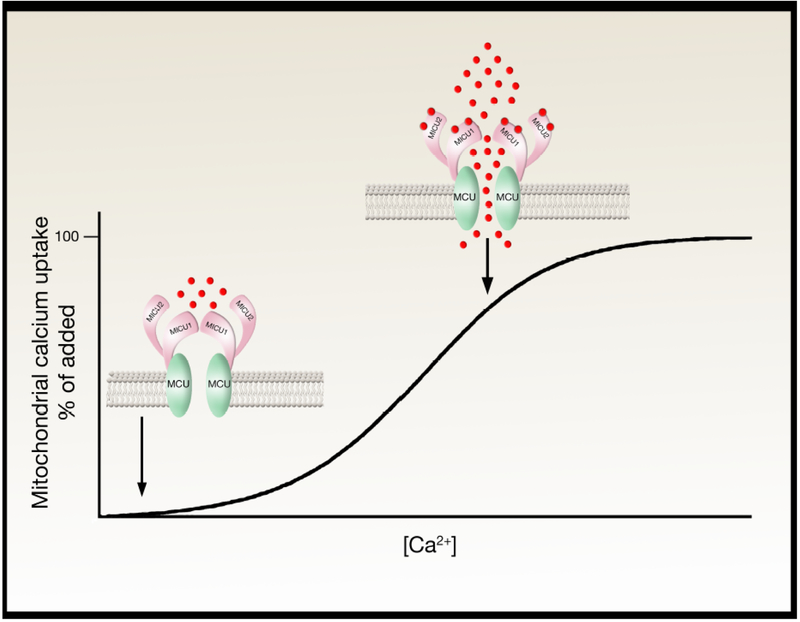
Figure 2:
Potential model to explain the observed sigmoidal calcium response curve. In this model, at low calcium concentrations (calcium ions are indicated as red circles), MICU1 and MICU2 act as a gatekeeper, preventing calcium entry. At higher calcium levels, calcium binds to the EF hands of MICU1/MICU2, resulting in a conformational change in these proteins, opening the pore and stimulating calcium entry. This is one potential model, however, additional models exist including where MICU1 and MICU2 have independent and opposite functions. See text for details.
The uniporter complex grows in complexity
As noted above, significant questions remain on the precise regulatory role of MICU1 and MICU2. Furthermore, there are unresolved issues regarding the exact topology and stoichiometry of the uniporter complex. On blue native gels, MCU is detected in a protein complex of approximately 480 kD68. Quantitative mass spectrometry revealed that this complex contained MCU, MICU1, MICU2 as well as an additional 10 kD protein named EMRE (essential MCU regulator). EMRE is a transmembrane, inner mitochondrial membrane protein found in most metazoan species which when knocked-down in 293T cells, appears to abrogate mitochondrial calcium uptake. In the absence of EMRE, MCU no longer appeared associated with MICU1 or MICU2, although MCU was still present and appeared to form oligomers68. This led the authors to conclude that EMRE is essential for uniporter activity and to propose a topology where MCU and EMRE interact in the inner mitochondrial membrane (and potentially in the matrix) while EMRE was essential for MICU1 binding in the inner membrane space (Figure 3). Reconstitution of uniporter activity has been recently described in yeast, an organism that does not have an electrogenic mechanism for mitochondrial calcium uptake69. Interestingly, expression of human MCU and EMRE is required to endow yeast with uniporter activity, while expression of either component individually is insufficient. Nonetheless, the precise role that EMRE plays is unclear and lower organisms such as Dictyostelium appear to have a uniporter that does not require this component69.
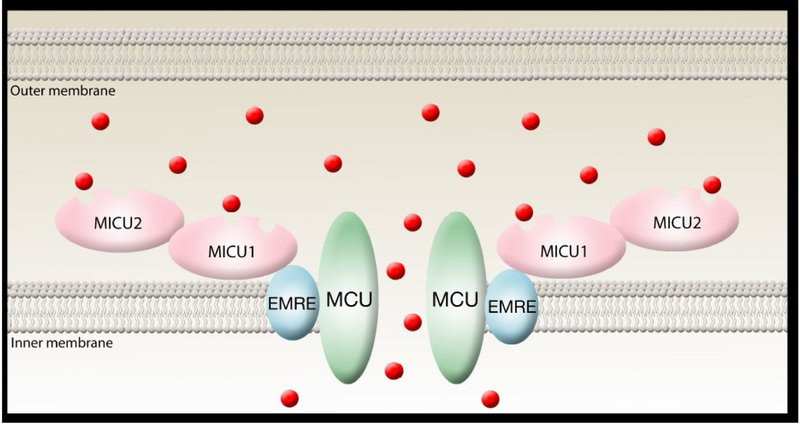
Figure 3:
Molecular components of the uniporter complex. Data strongly suggest that MCU is the pore. Most but not all experimental evidence suggests that MICU1 and MICU2 are located within the inner mitochondrial space. EMRE appears to link MCU with MICU1 and to be necessary for MCU to open and allow calcium entry (red circles) into the mitochondrial matrix. Not pictured here is MCUb, the dominant negative form of MCU that exists in various ratios with MCU. See text for further details.
The growing complexity of the uniporter complex is further exemplified by the description of MCUb, a protein that shares approximately 50% homology with MCU and can physically interact with the pore forming unit56. Silencing of MCUb increases calcium uptake while overexpression results in a reduction of MCU activity. This suggests that MCUb acts in a dominant negative fashion. The expression of MCUb (which is confined to vertebrate species) varies widely from tissue to tissue and may help at least partially explain the tissue-specific variation in MCU activity that has been observed70. In this context, when MCUb and MCU are expressed at similar levels, the pore that forms is non-functional due to the dominant negative action of MCUb, while when MCUb is not expressed at high levels, MCU complexes largely with itself to form an active uniporter. As such, it seems possible that MCUb expression may be a mechanism to reduce MCU activity stably and over a long period of time, while MICU1 and or MICU2 appear responsible for acute gatekeeping functions. Finally, a number of additional proteins may associate with the uniporter complex. For instance, two separate mitochondrial proteins, MCUR164 and SLC25A2371, have both been suggested to modulate calcium uptake through the uniporter. Their exact function however have not been completely discerned, and for the case of MCUR1, recent evidence suggests that the previously observed effects may in fact be due to indirect modulation of the mitochondrial membrane potential and not the uniporter itself72.
Lessons from the MCU-KO mice
MCU-KO mitochondria do not take up calcium
The identification of the MCU allowed for studies to test the regulation and role of mitochondrial calcium in vivo. MCU knockout (MCU-KO) mice were recently developed and characterized59. Mitochondria isolated from heart or liver of MCU-KO mice showed no mitochondrial calcium uptake following addition of extra-mitochondrial calcium. These studies were performed by measuring calcium accumulation in the matrix, using mitochondria loaded with a calcium sensitive fluorescent indicator, as well as by following the loss of calcium from the extra-mitochondrial medium with a fluorescent calcium indicator (Ca Green) in the extracellular space. Similar experiments were performed in permeabilized cells to probe mitochondrial calcium uptake in situ (thapsigargin was added to inhibit SR calcium uptake). Together, these studies confirmed the apparent complete lack of rapid mitochondrial calcium uptake in the MCU-KO mice. Similar results were recently obtained in permeabilized cardiomyocytes isolated from a mouse with cardiac specific expression of a dominant negative MCU (DN-MCU), containing a mutation in the pore domain73. Again, there was no observed calcium uptake into mitochondria obtained from permeabilized myocytes generated from these DN-MCU mice73. Taken together, these data suggest that in the absence of MCU activity there is no measureable calcium uptake, implying that if other mitochondrial calcium uptake pathways exist, they either have a very slow rate of uptake or they are not operating under these experimental conditions. However, isolated mitochondria from the MCU-KO hearts contained significant measurable calcium (~25% of that present in the WT mitochondria), possibly suggesting that alternative mechanism for mitochondrial calcium accumulation (albeit reduced) do exist in the absence of MCU expression. It is therefore quite possible that additional slow calcium uptake mechanisms exist. It is also possible that calcium can enter the mitochondria, particularly in de-energized isolated mitochondria, via the “reverse mode” of the NCLX. Future studies will be needed to address this issue.
Mitochondrial calcium and metabolism
Alterations in mitochondrial calcium uptake and mitochondrial calcium levels would be expected to alter the activity of the mitochondrial dehydrogenases and thereby alter metabolism. However, no differences in basal oxygen consumption were seen between WT and MCU-KO mouse embryonic fibroblasts (MEFs) or with isolated mitochondria59, although this may not be surprising since an increase in mitochondria calcium is likely only needed to coordinate an increase in work with increased ATP. In addition, there were no differences in total body basal oxygen consumption between the WT and MCU−/− mice. These observations are consistent with initial MCU knockdown experiments that noted no changes in mitochondrial respiration when MCU expression was reduced3. Thus, it would seem that basal metabolism is not markedly altered in the absence of MCU expression. In contrast to basal oxygen consumption, the loss of MCU largely blocked the calcium stimulated increase in oxygen consumption in isolated skeletal muscle mitochondria59. However, only in skeletal muscle and under conditions of maximum work, was there a modest, but discernable physiological difference between WT and MCU-KO mice. In this specific setting, compared to the WT mice, the MCU knockout mice had reduced ability to generate maximal power59.
Role of MCU in the cardiovascular system
Isoproterenol stimulation of heart rate and MCU
It was also expected that beta-adrenergic stimulation of work might be compromised in the MCU-KO hearts because they would not be able to appropriately stimulate ATP production due to lack of an increase in mitochondrial calcium uptake by MCU. Consistent with this expectation, a recent report noted a blunted increase in heart rate in response to isoproterenol in hearts expressing the DN-MCU73. Echocardiography measurements showed that basal heart rates were similar between wild-type and DN-MCU hearts. However, addition of isoproterenol increased heart rate to ~650 bpm in wild type hearts, but only to ~500 bpm in DN-MCU hearts. This blunted response to heart rate was attributed to an inability of mitochondria to increase ATP production when MCU was inactivated. This relatively modest effect of MCU on heart rate after isoproterenol stimulation could possibly be explained by the reported low abundance of MCU dependent calcium current in the heart compared to other tissues70. The authors also reported a significant decrease in basal ATP in freshly isolated atrial tissue from DN-MCU mice; ATP in WT atria was 250 nmol/mg whereas ATP in atria from DN-MCU was only 75 nmol/mg protein.
Mitochondrial calcium and cell death
Because high levels of mitochondrial calcium uptake via the MCU leads to opening of the mPTP, it was hypothesized that mitochondria from MCU-KO mice, which do not accumulate matrix calcium, would not undergo calcium activated mPTP. Consistent with this hypothesis, exogenous calcium levels that activated mPTP in WT mitochondria did not activate mPTP in mitochondria isolated from MCU-KO mice59.
CaMKII phosphorylation sites on MCU have been identified and activation of CaMKII, by addition of a constitutively active CaMKII mutant, is reported to increase MCU current in mitoplast. It was further demonstrated that cardiac specific expression of a mitochondrial and membrane targeted CaMKII inhibitory protein reduced mPTP and I/R cell death74. However others have reported a much lower current for MCU and did not find data supporting a role for CaMKII regulation of MCU75. Additional studies will be needed to resolve this issue. Inhibition of CaMKII could have other targets in addition to MCU that could contribute to cardioprotection.
As MCU-KO mitochondria were found to be resistant to calcium activation of mPTP, it was further hypothesized that MCU-KO hearts would have reduced cell death following ischemia and reperfusion. Surprisingly, following ischemia and reperfusion, MCU-KO hearts had infarct size and recovery of contractile function that was indistinguishable from WT hearts. While there was no mPTP opening in MCU KO mitochondria exposed to large amounts of calcium, this did not result in measurable cardioprotection. Presumably, the absence of MCU expression does not preclude mPTP opening in a calcium-independent manner in MCU−/− hearts. This would be consistent with suggestions that ROS, rather than calcium, may act as the primary activator of mPTP in vivo76, 77. If mPTP activation actually does occur in MCU-KO heart, then one would expect that cyclosporine A, an inhibitor of the mPTP, should still provide protection in the MCU-KO hearts. This was indeed tested in the MCU knockout mice. Following ischemia and reperfusion, WT hearts were protected from necrosis by the administration of cyclosporine A, but MCU-KO hearts were not. These data suggest that in contrast to the WT hearts, inhibition of the mPTP is not protective in the MCU-KO hearts. There are several possible explanations for this surprising finding. One explanation is that in the absence of MCU expression, there are compensatory mechanisms that lead to an up-regulation of an alternative cell death pathway, one that does not involve mPTP opening. If there are compensatory mechanisms, it would be consistent with observations that acute inhibition of MCU results in cardioprotection78. Another possible explanation is that mPTP opening is occurring in the MCU-KO hearts, but it is not inhibited by cyclosporine A, perhaps because the stimulus for mPTP opening is somehow greater in MCU-KO hearts than in the WT hearts. It is well established that mPTP opening, while facilitated by cyclophilin D, can still occur in its absence or in the presence of cyclosporine A, as long as the stimulus is sufficiently great52, 79. Finally, the lack of protection against ischemia and reperfusion in the MCU-KO hearts might suggest that cytosolic calcium, rather than mitochondrial calcium is the key determinant of cell death.
Summary
For over five decades, the question of how calcium entered the mitochondria remained a biological mystery. Even in the absence of knowing precisely how calcium passed through the inner mitochondrial membrane, numerous lines of evidence still emerged demonstrating the important regulatory role of mitochondrial matrix calcium. Among these observations was the concept that in small amounts, mitochondrial calcium was essential to fine-tune cellular energetics, while in larger amounts, mitochondrial calcium was critical for initiating cell death. The last few years has resulted in remarkable progress in obtaining a more molecular understanding of these events. This progress has been catalyzed by the discovery of MCU and its accessory proteins. Numerous questions however remain. In particular, how is the uniporter complex regulated so that it only allows calcium entry under stimulated conditions? What is the topology/structure of the uniporter complex? What components are essential to its function and how is the observed tissue variation in uniporter activity achieved? Even more important, what is the physiological role of mitochondrial calcium in areas as diverse as bioenergetics to cell death? The characterization of additional mouse models in which MCU and its interacting partners are deleted in either a whole body or tissue-specific fashion will be critical in addressing these remaining questions. Given the overall pace of late, it’s unlikely we will have to wait another five decades for these answers.
Acknowledgements:
We are grateful to Ilsa I Rovira for help with the manuscript. This work was supported by NHLBI Intramural funds and a grant from the Leducq Foundation.
References:
- 1.Deluca HF, Engstrom GW. Calcium uptake by rat kidney mitochondria. Proceedings of the National Academy of Sciences of the United States of America. 1961;47:1744–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trenker M, Malli R, Fertschai I, Levak-Frank S, Graier WF. Uncoupling proteins 2 and 3 are fundamental for mitochondrial ca2+ uniport. Nature cell biology. 2007;9:445–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V, Mootha VK. Integrative genomics identifies mcu as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carafoli E The interplay of mitochondria with calcium: An historical appraisal. Cell calcium. 2012;52:1–8 [DOI] [PubMed] [Google Scholar]
- 6.Reed KC, Bygrave FL. The inhibition of mitochondrial calcium transport by lanthanides and ruthenium red. The Biochemical journal. 1974;140:143–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore CL. Specific inhibition of mitochondrial ca++ transport by ruthenium red. Biochemical and biophysical research communications. 1971;42:298–305 [DOI] [PubMed] [Google Scholar]
- 8.Matlib MA, Zhou Z, Knight S, Ahmed S, Choi KM, Krause-Bauer J, Phillips R, Altschuld R, Katsube Y, Sperelakis N, Bers DM. Oxygen-bridged dinuclear ruthenium amine complex specifically inhibits ca2+ uptake into mitochondria in vitro and in situ in single cardiac myocytes. The Journal of biological chemistry. 1998;273:10223–10231 [DOI] [PubMed] [Google Scholar]
- 9.Moreau B, Nelson C, Parekh AB. Biphasic regulation of mitochondrial ca2+ uptake by cytosolic ca2+ concentration. Current biology: CB. 2006;16:1672–1677 [DOI] [PubMed] [Google Scholar]
- 10.Sparagna GC, Gunter KK, Sheu SS, Gunter TE. Mitochondrial calcium uptake from physiological-type pulses of calcium. A description of the rapid uptake mode. The Journal of biological chemistry. 1995;270:27510–27515 [DOI] [PubMed] [Google Scholar]
- 11.Buntinas L, Gunter KK, Sparagna GC, Gunter TE. The rapid mode of calcium uptake into heart mitochondria (ram): Comparison to ram in liver mitochondria. Biochimica et biophysica acta. 2001;1504:248–261 [DOI] [PubMed] [Google Scholar]
- 12.Beutner G, Sharma VK, Lin L, Ryu SY, Dirksen RT, Sheu SS. Type 1 ryanodine receptor in cardiac mitochondria: Transducer of excitation-metabolism coupling. Biochimica et biophysica acta. 2005;1717:1–10 [DOI] [PubMed] [Google Scholar]
- 13.Jiang D, Zhao L, Clish CB, Clapham DE. Letm1, the mitochondrial ca2+/h+ antiporter, is essential for normal glucose metabolism and alters brain function in wolf-hirschhorn syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E2249–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai MF, Jiang D, Zhao L, Clapham D, Miller C. Functional reconstitution of the mitochondrial ca2+/h+ antiporter letm1. The Journal of general physiology. 2014;143:67–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doonan PJ, Chandramoorthy HC, Hoffman NE, Zhang X, Cardenas C, Shanmughapriya S, Rajan S, Vallem S, Chen X, Foskett JK, Cheung JY, Houser SR, Madesh M. Letm1-dependent mitochondrial ca2+ flux modulates cellular bioenergetics and proliferation. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2014;28:4936–4949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nowikovsky K, Bernardi P. Letm1 in mitochondrial cation transport. Frontiers in physiology. 2014;5:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Froschauer E, Nowikovsky K, Schweyen RJ. Electroneutral k+/h+ exchange in mitochondrial membrane vesicles involves yol027/letm1 proteins. Biochimica et biophysica acta. 2005;1711:41–48 [DOI] [PubMed] [Google Scholar]
- 18.Griffiths EJ. Reversal of mitochondrial na/ca exchange during metabolic inhibition in rat cardiomyocytes. FEBS letters. 1999;453:400–404 [DOI] [PubMed] [Google Scholar]
- 19.Brinley FJ, Jr., Tiffert T, Scarpa A, Mullins LJ. Intracellular calcium buffering capacity in isolated squid axons. The Journal of general physiology. 1977;70:355–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allen DG, Blinks JR. Calcium transients in aequorin-injected frog cardiac muscle. Nature. 1978;273:509–513 [DOI] [PubMed] [Google Scholar]
- 21.Rizzuto R, Brini M, Murgia M, Pozzan T. Microdomains with high ca2+ close to ip3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–747 [DOI] [PubMed] [Google Scholar]
- 22.Palty R, Silverman WF, Hershfinkel M, Caporale T, Sensi SL, Parnis J, Nolte C, Fishman D, Shoshan-Barmatz V, Herrmann S, Khananshvili D, Sekler I. Nclx is an essential component of mitochondrial na+/ca2+ exchange. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:436–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cox DA, Conforti L, Sperelakis N, Matlib MA. Selectivity of inhibition of na(+)-ca2+ exchange of heart mitochondria by benzothiazepine cgp-37157. Journal of cardiovascular pharmacology. 1993;21:595–599 [DOI] [PubMed] [Google Scholar]
- 24.Carafoli E, Tiozzo R, Lugli G, Crovetti F, Kratzing C. The release of calcium from heart mitochondria by sodium. Journal of molecular and cellular cardiology. 1974;6:361–371 [DOI] [PubMed] [Google Scholar]
- 25.Murphy E, Eisner DA. Regulation of intracellular and mitochondrial sodium in health and disease. Circulation research. 2009;104:292–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyman L, Williams GS, Khananshvili D, Sekler I, Lederer WJ. Nclx: The mitochondrial sodium calcium exchanger. Journal of molecular and cellular cardiology. 2013;59:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicholls DG, Crompton M. Mitochondrial calcium transport. FEBS letters. 1980;111:261–268 [DOI] [PubMed] [Google Scholar]
- 28.O’Rourke B, Blatter LA. Mitochondrial ca2+ uptake: Tortoise or hare? Journal of molecular and cellular cardiology. 2009;46:767–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altschuld RA, Hohl CM, Castillo LC, Garleb AA, Starling RC, Brierley GP. Cyclosporin inhibits mitochondrial calcium efflux in isolated adult rat ventricular cardiomyocytes. The American journal of physiology. 1992;262:H1699–1704 [DOI] [PubMed] [Google Scholar]
- 30.Elrod JW, Wong R, Mishra S, Vagnozzi RJ, Sakthievel B, Goonasekera SA, Karch J, Gabel S, Farber J, Force T, Brown JH, Murphy E, Molkentin JD. Cyclophilin d controls mitochondrial pore-dependent ca(2+) exchange, metabolic flexibility, and propensity for heart failure in mice. The Journal of clinical investigation. 2010;120:3680–3687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Marchi E, Bonora M, Giorgi C, Pinton P. The mitochondrial permeability transition pore is a dispensable element for mitochondrial calcium efflux. Cell calcium. 2014;56:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hansford RG, Castro F. Intramitochondrial and extramitochondrial free calcium ion concentrations of suspensions of heart mitochondria with very low, plausibly physiological, contents of total calcium. Journal of bioenergetics and biomembranes. 1982;14:361–376 [DOI] [PubMed] [Google Scholar]
- 33.Chalmers S, Nicholls DG. The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. The Journal of biological chemistry. 2003;278:19062–19070 [DOI] [PubMed] [Google Scholar]
- 34.Wei AC, Liu T, Winslow RL, O’Rourke B. Dynamics of matrix-free ca2+ in cardiac mitochondria: Two components of ca2+ uptake and role of phosphate buffering. The Journal of general physiology. 2012;139:465–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicholls DG. The regulation of extramitochondrial free calcium ion concentration by rat liver mitochondria. The Biochemical journal. 1978;176:463–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Denton RM, McCormack JG. On the role of the calcium transport cycle in heart and other mammalian mitochondria. FEBS letters. 1980;119:1–8 [DOI] [PubMed] [Google Scholar]
- 37.Glancy B, Willis WT, Chess DJ, Balaban RS. Effect of calcium on the oxidative phosphorylation cascade in skeletal muscle mitochondria. Biochemistry. 2013;52:2793–2809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Territo PR, Mootha VK, French SA, Balaban RS. Ca(2+) activation of heart mitochondrial oxidative phosphorylation: Role of the f(0)/f(1)-atpase. American journal of physiology. Cell physiology. 2000;278:C423–435 [DOI] [PubMed] [Google Scholar]
- 39.Phillips D, Covian R, Aponte AM, Glancy B, Taylor JF, Chess D, Balaban RS. Regulation of oxidative phosphorylation complex activity: Effects of tissue-specific metabolic stress within an allometric series and acute changes in workload. American journal of physiology. Regulatory, integrative and comparative physiology. 2012;302:R1034–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Halestrap AP. Calcium, mitochondria and reperfusion injury: A pore way to die. Biochemical Society transactions. 2006;34:232–237 [DOI] [PubMed] [Google Scholar]
- 41.Steenbergen C, Murphy E, Levy L, London RE. Elevation in cytosolic free calcium concentration early in myocardial ischemia in perfused rat heart. Circulation research. 1987;60:700–707 [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Dorado D, Ruiz-Meana M, Inserte J, Rodriguez-Sinovas A, Piper HM. Calcium-mediated cell death during myocardial reperfusion. Cardiovascular research. 2012;94:168–180 [DOI] [PubMed] [Google Scholar]
- 43.Marban E, Koretsune Y, Corretti M, Chacko VP, Kusuoka H. Calcium and its role in myocardial cell injury during ischemia and reperfusion. Circulation. 1989;80:IV17–22 [PubMed] [Google Scholar]
- 44.Murphy E, Perlman M, London RE, Steenbergen C. Amiloride delays the ischemia-induced rise in cytosolic free calcium. Circulation research. 1991;68:1250–1258 [DOI] [PubMed] [Google Scholar]
- 45.Murphy E, Steenbergen C. Preconditioning: The mitochondrial connection. Annual review of physiology. 2007;69:51–67 [DOI] [PubMed] [Google Scholar]
- 46.Hunter DR, Haworth RA, Southard JH. Relationship between configuration, function, and permeability in calcium-treated mitochondria. The Journal of biological chemistry. 1976;251:5069–5077 [PubMed] [Google Scholar]
- 47.Schwartz J, Holmuhamedov E, Zhang X, Lovelace GL, Smith CD, Lemasters JJ. Minocycline and doxycycline, but not other tetracycline-derived compounds, protect liver cells from chemical hypoxia and ischemia/reperfusion injury by inhibition of the mitochondrial calcium uniporter. Toxicology and applied pharmacology. 2013;273:172–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao Q, Wang S, Li Y, Wang P, Li S, Guo Y, Yao R. The role of the mitochondrial calcium uniporter in cerebral ischemia/reperfusion injury in rats involves regulation of mitochondrial energy metabolism. Molecular medicine reports. 2013;7:1073–1080 [DOI] [PubMed] [Google Scholar]
- 49.Crompton M, Ellinger H, Costi A. Inhibition by cyclosporin a of a ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. The Biochemical journal. 1988;255:357–360 [PMC free article] [PubMed] [Google Scholar]
- 50.Griffiths EJ, Halestrap AP. Further evidence that cyclosporin a protects mitochondria from calcium overload by inhibiting a matrix peptidyl-prolyl cis-trans isomerase. Implications for the immunosuppressive and toxic effects of cyclosporin. The Biochemical journal. 1991;274 (Pt 2): 611–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Griffiths EJ, Halestrap AP. Protection by cyclosporin a of ischemia/reperfusion-induced damage in isolated rat hearts. Journal of molecular and cellular cardiology. 1993;25:1461–1469 [DOI] [PubMed] [Google Scholar]
- 52.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin d reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662 [DOI] [PubMed] [Google Scholar]
- 53.Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant D, Macia C, Raczka F, Sportouch C, Gahide G, Finet G, Andre-Fouet X, Revel D, Kirkorian G, Monassier JP, Derumeaux G, Ovize M. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. The New England journal of medicine. 2008;359:473–481 [DOI] [PubMed] [Google Scholar]
- 54.Liu T, Takimoto E, Dimaano VL, DeMazumder D, Kettlewell S, Smith G, Sidor A, Abraham TP, O’Rourke B. Inhibiting mitochondrial na+/ca2+ exchange prevents sudden death in a guinea pig model of heart failure. Circulation research. 2014;115:44–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kamer KJ, Mootha VK. Micu1 and micu2 play nonredundant roles in the regulation of the mitochondrial calcium uniporter. EMBO reports. 2014;15:299–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raffaello A, De Stefani D, Sabbadin D, Teardo E, Merli G, Picard A, Checchetto V, Moro S, Szabo I, Rizzuto R. The mitochondrial calcium uniporter is a multimer that can include a dominant-negative pore-forming subunit. The EMBO journal. 2013;32:2362–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Csordas G, Golenar T, Seifert EL, Kamer KJ, Sancak Y, Perocchi F, Moffat C, Weaver D, de la Fuente Perez S, Bogorad R, Koteliansky V, Adijanto J, Mootha VK, Hajnoczky G. Micu1 controls both the threshold and cooperative activation of the mitochondrial ca(2)(+) uniporter. Cell metabolism. 2013;17:976–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lam SS, Martell JD, Kamer KJ, Deerinck TJ, Ellisman MH, Mootha VK, Ting AY. Directed evolution of apex2 for electron microscopy and proximity labeling. Nature methods. 2015;12:51–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pan X, Liu J, Nguyen T, Liu C, Sun J, Teng Y, Fergusson MM, Rovira II, Allen M, Springer DA, Aponte AM, Gucek M, Balaban RS, Murphy E, Finkel T. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nature cell biology. 2013;15:1464–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE, Mootha VK. Micu1 encodes a mitochondrial ef hand protein required for ca(2+) uptake. Nature. 2010;467:291–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong SE, Walford GA, Sugiana C, Boneh A, Chen WK, Hill DE, Vidal M, Evans JG, Thorburn DR, Carr SA, Mootha VK. A mitochondrial protein compendium elucidates complex i disease biology. Cell. 2008;134:112–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Plovanich M, Bogorad RL, Sancak Y, Kamer KJ, Strittmatter L, Li AA, Girgis HS, Kuchimanchi S, De Groot J, Speciner L, Taneja N, Oshea J, Koteliansky V, Mootha VK. Micu2, a paralog of micu1, resides within the mitochondrial uniporter complex to regulate calcium handling. PloS one. 2013;8:e55785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364 [DOI] [PubMed] [Google Scholar]
- 64.Mallilankaraman K, Cardenas C, Doonan PJ, Chandramoorthy HC, Irrinki KM, Golenar T, Csordas G, Madireddi P, Yang J, Muller M, Miller R, Kolesar JE, Molgo J, Kaufman B, Hajnoczky G, Foskett JK, Madesh M. Mcur1 is an essential component of mitochondrial ca2+ uptake that regulates cellular metabolism. Nature cell biology. 2012;14:1336–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang L, Yang X, Li S, Wang Z, Liu Y, Feng J, Zhu Y, Shen Y. Structural and mechanistic insights into micu1 regulation of mitochondrial calcium uptake. The EMBO journal. 2014;33:594–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patron M, Checchetto V, Raffaello A, Teardo E, Vecellio Reane D, Mantoan M, Granatiero V, Szabo I, De Stefani D, Rizzuto R. Micu1 and micu2 finely tune the mitochondrial ca2+ uniporter by exerting opposite effects on mcu activity. Molecular cell. 2014;53:726–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Logan CV, Szabadkai G, Sharpe JA, Parry DA, Torelli S, Childs AM, Kriek M, Phadke R, Johnson CA, Roberts NY, Bonthron DT, Pysden KA, Whyte T, Munteanu I, Foley AR, Wheway G, Szymanska K, Natarajan S, Abdelhamed ZA, Morgan JE, Roper H, Santen GW, Niks EH, van der Pol WL, Lindhout D, Raffaello A, De Stefani D, den Dunnen JT, Sun Y, Ginjaar I, Sewry CA, Hurles M, Rizzuto R, Consortium UK, Duchen MR, Muntoni F, Sheridan E. Loss-of-function mutations in micu1 cause a brain and muscle disorder linked to primary alterations in mitochondrial calcium signaling. Nature genetics. 2014;46:188–193 [DOI] [PubMed] [Google Scholar]
- 68.Sancak Y, Markhard AL, Kitami T, Kovacs-Bogdan E, Kamer KJ, Udeshi ND, Carr SA, Chaudhuri D, Clapham DE, Li AA, Calvo SE, Goldberger O, Mootha VK. Emre is an essential component of the mitochondrial calcium uniporter complex. Science. 2013;342:1379–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kovacs-Bogdan E, Sancak Y, Kamer KJ, Plovanich M, Jambhekar A, Huber RJ, Myre MA, Blower MD, Mootha VK. Reconstitution of the mitochondrial calcium uniporter in yeast. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:8985–8990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fieni F, Lee SB, Jan YN, Kirichok Y. Activity of the mitochondrial calcium uniporter varies greatly between tissues. Nature communications. 2012;3:1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hoffman NE, Chandramoorthy HC, Shanmughapriya S, Zhang XQ, Vallem S, Doonan PJ, Malliankaraman K, Guo S, Rajan S, Elrod JW, Koch WJ, Cheung JY, Madesh M. Slc25a23 augments mitochondrial ca(2)(+) uptake, interacts with mcu, and induces oxidative stress-mediated cell death. Molecular biology of the cell. 2014;25:936–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Paupe V, Prudent J, Dassa EP, Rendon OZ, Shoubridge EA. Ccdc90a (mcur1) is a cytochrome c oxidase assembly factor and not a regulator of the mitochondrial calcium uniporter. Cell metabolism. 2015;21:109–116 [DOI] [PubMed] [Google Scholar]
- 73.Wu Y, Rasmussen TP, Koval OM, Joiner ML, Hall DD, Chen B, Luczak ED, Wang Q, Rokita AG, Wehrens XH, Song LS, Anderson ME. The mitochondrial uniporter controls fight or flight heart rate increases. Nature communications. 2015;6:6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Joiner ML, Koval OM, Li J, He BJ, Allamargot C, Gao Z, Luczak ED, Hall DD, Fink BD, Chen B, Yang J, Moore SA, Scholz TD, Strack S, Mohler PJ, Sivitz WI, Song LS, Anderson ME. Camkii determines mitochondrial stress responses in heart. Nature. 2012;491:269–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fieni F, Johnson DE, Hudmon A, Kirichok Y. Mitochondrial ca2+ uniporter and camkii in heart. Nature. 2014;513:E1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochimica et biophysica acta. 2009;1787:1395–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Juhaszova M, Wang S, Zorov DB, Nuss HB, Gleichmann M, Mattson MP, Sollott SJ. The identity and regulation of the mitochondrial permeability transition pore: Where the known meets the unknown. Annals of the New York Academy of Sciences. 2008;1123:197–212 [DOI] [PubMed] [Google Scholar]
- 78.Garcia-Rivas Gde J, Carvajal K, Correa F, Zazueta C. Ru360, a specific mitochondrial calcium uptake inhibitor, improves cardiac post-ischaemic functional recovery in rats in vivo. British journal of pharmacology. 2006;149:829–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bernardi P, Krauskopf A, Basso E, Petronilli V, Blachly-Dyson E, Di Lisa F, Forte MA. The mitochondrial permeability transition from in vitro artifact to disease target. The FEBS journal. 2006;273:2077–2099 [DOI] [PubMed] [Google Scholar]