Abstract
Spot blotch (SB) caused by Bipolaris sorokiniana, is one of the most important diseases of wheat in the eastern part of south Asia causing considerable yield loss to the wheat crop. There is an urgent need to identify genetic loci closely associated with resistance to this pathogen for developing resistant cultivars. Hence, genomic regions responsible for SB resistance were searched using a wheat association mapping initiative (WAMI) panel involving 287 spring wheat genotypes of different origin. Genome-wide association mapping (GWAM) was performed using single nucleotide polymorphism (SNP) markers from a custom 90 K wheat SNP array. A mixed linear model (MLM) was used for assessing the association of SNP markers with spot blotch resistance in three consecutive years. Three traits were measured: incubation period, lesion number and area under the disease progress curve (AUDPC). Significant SNP markers were found linked to five, six and four quantitative trait loci (QTLs) for incubation period, lesion number and AUDPC respectively. They were detected on 11 different chromosomes: 1A, 1B, 1D, 4A, 5A, 5B, 6A, 6B, 6D, 7A, 7B with marker R2 range of 0.083 to 0.11. The greatest number of significant SNP-markers was found for lesion number and AUDPC on chromosome 6B and 5B, respectively, representing a better coverage of B-genome by SNPs. On the other hand, the most significant and largest SNP markers for incubation period were detected on 6A and 4A chromosomes indicating that this trait is associated with the A-genome of wheat. Although, QTLs for spot blotch resistance have been reported in wheat on these same chromosomes, the association of incubation period and lesion number with SB resistance has not been reported in previous studies. The panel exhibits considerable variation for SB resistance and also provides a good scope of marker-assisted selection using the identified SNP markers linked to resistant QTLs.
Introduction
Wheat (Triticum aestivum L.), one of the most widely adapted cereal crops [1], provides around one-fifth of the total calories to the human population. In addition, it also provides 20% of the protein to more than 4.5 billion people in 94 developing countries [2]. In south Asia, wheat is cultivated in over 40 million hectare area, out of which around 25% falls under Eastern Gangetic Plains (EGP) which contributes only 16% to the total wheat production. The demand of wheat in this densely populated region can only be met if annual production is increased by 1.5–2.0% [3, 4]. The EGP of south Asia is characterized as the mega environment 5A due to prevalence of high temperature and humidity during the wheat growing season [5, 6].In this region, wheat production is significantly impacted due to spot blotch disease [6, 7, 8] which may cause yield loss up to 30% [9]. The causal organism of spot blotch (SB) is Bipolaris sorokiniana, which is an anamorph (teleomorph Cochliobolus sativus).
Reduction in disease progress is contributed by few components of resistance i.e., latent period and lesion size [10] and epidemic development can be slow down by using genotypes with longer latent period and fewer lesions [11]. Hence molecular mapping for different components of resistance in plant populations to determine robust markers and their subsequent use is imperative for varietal improvement programs through an effective use of appropriate genetic resources [12, 13].Due to various limitations of the conventional bi-parental linkage mapping, there has been a greater preference for linkage disequilibrium (LD) based association mapping (AM) [6]. In AM, a collection of variable lines is scanned to understand marker-trait associations using linkage disequilibrium (LD)which is non-equilibrium association between different alleles at various loci [14] and varies across wheat chromosomes [12, 15]. Although wheat has a large homeologous genome that shows weak marker coverage compared to other cereal crops [13], AM can identify superior alleles with detailed marker data in large populations which can be utilized immediately in breeding [16].
Due to significant yield losses caused by spot blotch in EGP, a considerable effort has been invested by wheat breeders to develop spot blotch resistant cultivars in this part of South Asia. To continue this effort further, this study was undertaken to identify novel marker-trait associations and to detect the loci conferring spot blotch resistance through GWAM in a WAMI Panel.
Materials and methods
Experimental material
The experimental material comprised of 287 genetically diverse, elite spring wheat lines of the wheat association mapping initiative (WAMI) population assembled by CIMMYT in 2009 was used [17].
Experimental design and trait evaluation
The WAMI population was evaluated for spot blotch in three consecutive crop seasons during 2012–13, 2013–14 and 2014–15 at the Agricultural Research Farm of Banaras Hindu University (BHU), Varanasi, India. The experiment was conducted using Alfa lattice design with sowing done between 1st to 10th December in the three years. Two replications were planted and each genotype was sown in two rows of two meters maintaining row-to-row and plant-to-plant distance of 20 cm and 5 cm respectively. The lines were allocated randomly to each replication using the Fisher and Yates Random Table [18]. Fertilizer per hectare was applied as 120 kg N: 60 kg P2O5: 40 kg K2O. The full amount of K2O and P2O5 were applied at sowing whereas nitrogen was applied in three split doses, half at sowing, one fourth at first irrigation (21 days after sowing-DAS) and the remaining one fourth at second irrigation (45 DAS). Data were recorded on three traits: incubation period (days), lesion number and spot blotch AUDPC.
Inoculation of pathogen
For creating artificial epiphytotic, a pure culture of Bipolaris sorokiniana (NABM MAT1; NCBIJN128877, BHU, Varanasi, India) known to be highly aggressive was used [19]. The isolate was obtained from the Department of Plant Pathology and Mycology, BHU and multiplied on sorghum grain. An adjusted spore suspension (10−4 spores per ml) in water was applied in the evening and irrigated immediately after inoculation [20]. Frequent irrigations were provided to ensure environmental conditions conducive to spot blotch development in the field.
Recording for incubation period, lesion number and disease score
Incubation period (days) was recorded from the inoculation to first appearance of visible symptoms on five randomly tagged plants in each plot [10]. For number of lesions, five leaves from each plot were selected randomly. Total numbers of lesions present on flag leaves were counted. The leaf was divided into four parts with a marker pen and the number of lesions on each part was counted. Total number of spots of each part were added and recorded as lesions per leaf [21]. For AUDPC, disease severity (%) was recorded in three different growth stages (GS), GS 63 (beginning of anthesis to half complete), GS 69 (anthesis complete) and GS 77 (late milking). Disease severity was assessed by the formula (D1/9× D2/9) × 100 using the double digit scale (DD, 00–99) [7]. The first digit (D1) refers to vertical disease progress on the plant whereas the second digit (D2) was the disease severity score in the affected leaves. Thus, disease severity was used to estimate the AUDPC by following formula [22].
Where, Yi = disease severity at time ti, (ti+1−ti) = Time (days) between two disease scores, n = number of dates at which spot blotch was recorded.
DNA extraction and SNP Genotyping
The DNA was extracted from fresh leaves of each line following the CTAB procedure [23] and genotyped at CIMMYT, Mexico using the Illumina iSelect beadchip assay [17]for wheat having 26814 SNPs. To avoid monomorphic and low-quality SNPs, markers were removed with a minor allele frequency (MAF) <0.05. Nearly 21132polymorphic SNPs were selected and used for AM.
Phenotypic and STRUCTURE analysis
Analysis of variance (ANOVA) was completed using SAS 9.4 to determine genotypic, year and genotypic × year variances among the traits measured over the years. Population structure () was analyzed for filtered marker panel through a clustering approach based on the admixture model implemented in STRUCTURE [24]. Ten iterations of STRUCTURE runs were employed for each subpopulations K that ranged from 2 to 10 with additional parameters of 10,000 burn-in length and 10,000 Markov chain Monte Carlo (MCMC) iterations. Clusters with the greatest likelihood of occurrence were identified through the ΔK method [25]. The web-based tool for population structure analysis “Structure Harvester” [26] was done which maximizes ΔK and thus examines the likelihood distribution of K. Further, fixation index (Fst) for subpopulations was estimated by outputs generated from various STRUCTURE runs. Population Matrix Q was also obtained for further analysis. TASSEL 5.0 was used to calculate population Kinship matrix, which is based on scaled IBS (identity by state) method that uses marker data which has passed quality filtering.
Genome-wide association analysis
Marker–trait associations (MTAs) were detected by Mixed Linear Model (MLM) in TASSEL 5.0 (http://www.maizegenetics.net/) [27]. The general equation used for MLM analysis was as follows:
Where, y = phenotype vector, β = vector of marker fixed effects; v = vector of fixed effects; u = vector of random effects (the kinship matrix); e = vector of residuals; X = genotype of a marker; Q = Kinship Matrix (obtained using STRUCTURE software) and Z = Identity matrix which considers the familial relatedness between accessions.
The Q matrix was used as a covariate to improve the precision of results by avoiding false positives. The efficient mixed model analysis [28] was used to save computing time, while other MLM parameters were kept as default. MLM, also called Q + K model, takes into account the population structure as well as familial relatedness. Significant marker-trait associations were identified based on P-value and R2 used for estimating the magnitude of the QTL effects. Significant marker-trait associations were selected on basis of P-values ≤ 1e-06. In addition, pair-wise LD for pairs of markers was calculated using TASSEL 5.0 considering observed and expected allele frequencies of the markers.
Results
The results of the analysis of variance (ANOVA) are presented in Table 1. The traits-incubation period, lesion number and AUDPC revealed significant (P<0.01) differences among genotype, years and genotype × year (Table 1).
Table 1. Analysis of variance for incubation period (IP) lesion number (LN) and Area under disease progress curve (AUDPC).
| Source | d.f. | IP | LN | AUDPC | |||
|---|---|---|---|---|---|---|---|
| Mean square | F value | Mean square | F value | Mean square | F value | ||
| Genotype | 287 | 14.69* | 17.55 | 1732.66* | 42.64 | 102967.85* | 30.95 |
| Year | 2 | 190.83* | 227.98 | 787826.96* | 19386.40 | 553399.58* | 166.35 |
| Replication | 1 | 7.13* | 8.52 | 114.66 | 2.82 | 10928.33 | 3.28 |
| Genotype×year | 574 | 2.19* | 2.62 | 1076.66* | 26.49 | 18934.13* | 5.69 |
*Significant at the p-value (P<0.01) probability level; d.f., degrees of freedom.
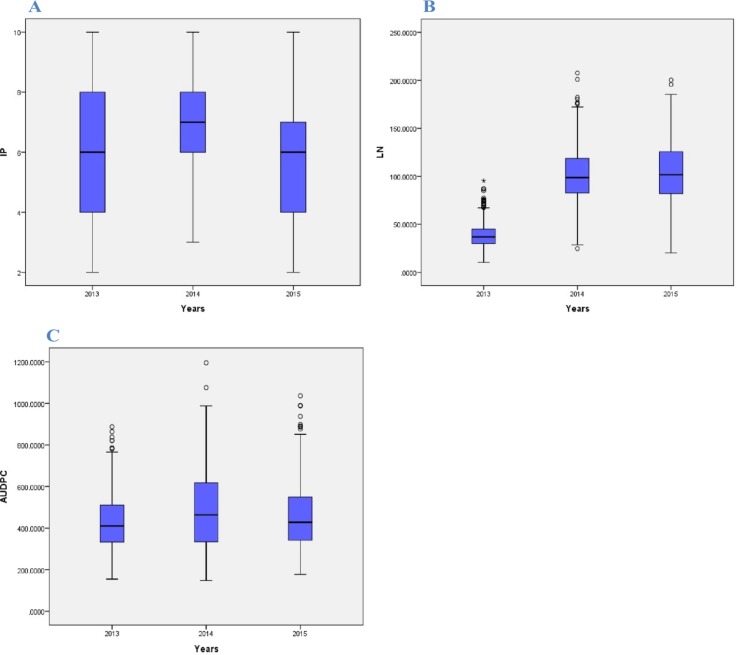
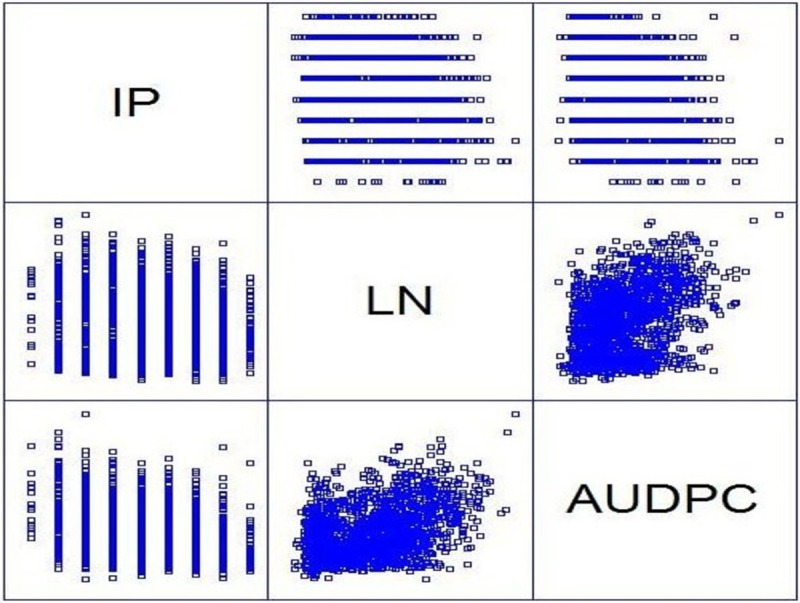
Distribution of phenotype under study has been generated which shows how performance of phenotype varies over the years (Fig 1A, 1B and 1C). More over to get the better description of phenotypes, correlation among traits under study has also been investigated (Fig 2).
Fig 1. Boxplots of the three different traits showing variation for three different years in 2012–13, 2013–14 and 2014–15.
(A) IP (Incubation Period) (B) LN (Lesion Number) (C) AUDPC (Area under disease progressive curve).
Fig 2. Graphical representation of phenotype correlation among three traits (IP, LN & AUDPC) in WAMI panel.
Population structure and marker distribution
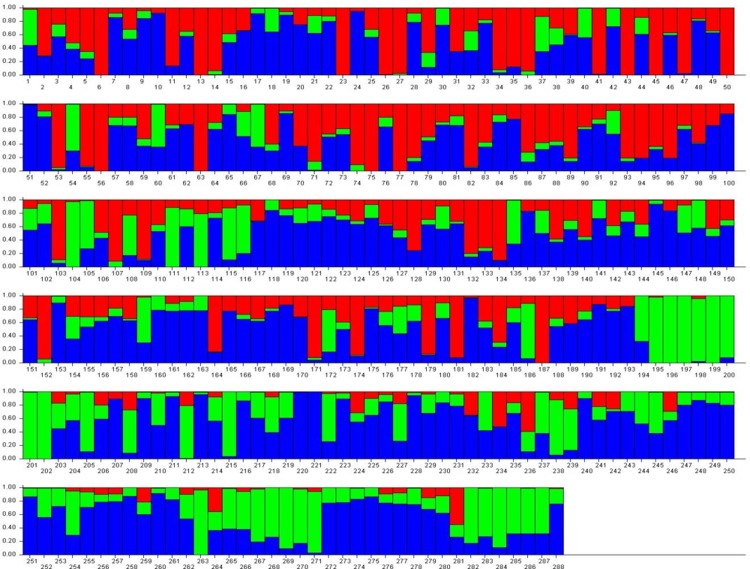
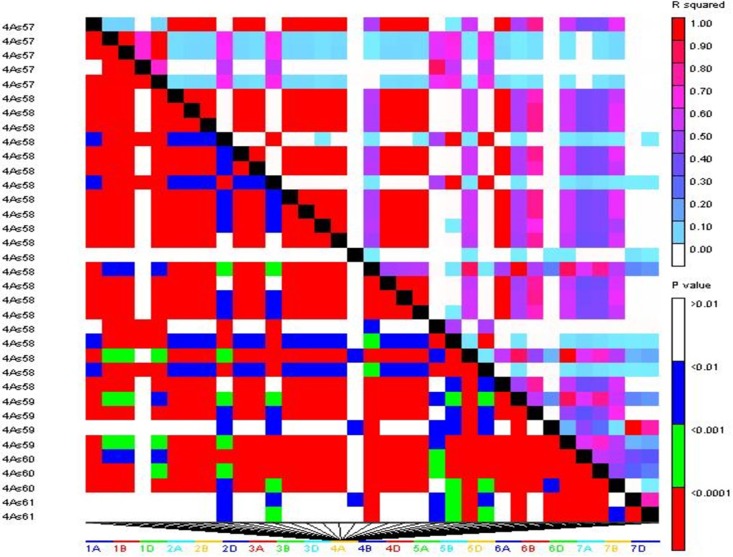
Optimal number of cluster i.e. K was determined to be 3 based on delta K approach implemented in STRUCTURE [24]. A bar graph representing population structure is also generated (Fig 3). A total 21132 marker which has passed quality filtering were used for the purpose of mapping. Linkage Disequilibrium (LD) plot based on association among markers has also been generated (Fig 4). It is clear from the figure that most of the markers are tightly linked as large chunks of red blocks observed below the diagonal of figure. It suggests that there has been limited scope of recombination between the markers, which facilitates association mapping of all three traits and minimum number of markers required to effectively cover the entire genome. More detailed distribution of marker over chromosome is presented in Table 2.
Fig 3. Bar graphs for three subpopulations (estimated following the approach of Evanno, 2005) are indicated by different colors.
The vertical coordinates of each subpopulation indicates the membership coefficient for each individual. The horizontal axis shows genotypes under study. In each sub population, each vertical bar represents one genotype.
Fig 4. Linkage disequilibrium plots of significant SNP markers associated with spot blotch AUDPC.
R square (R2) and P-value of pair-wise analyses are indicated by color in the right-side bars.
Table 2. Distribution of 21,132 SNPs in 21chromosomes identified in 287 wheat genotypes.
| Chr. | Size (Mb) | No. of SNP | Average number of SNPs per Mb |
Chr. LD |
|---|---|---|---|---|
| 1A | 594.1 | 1346 | 2 | 0.289 |
| 1B | 689.85 | 1953 | 3 | 0.325 |
| 1D | 495.45 | 540 | 1 | 0.399 |
| 2A | 780.8 | 1176 | 2 | 0.305 |
| 2B | 801.26 | 2064 | 3 | 0.306 |
| 2D | 651.85 | 679 | 1 | 0.417 |
| 3A | 750.84 | 970 | 1 | 0.207 |
| 3B | 830.83 | 1403 | 2 | 0.252 |
| 3D | 615.55 | 284 | 1 | 0.159 |
| 4A | 744.59 | 925 | 1 | 0.249 |
| 4B | 673.62 | 589 | 1 | 0.206 |
| 4D | 509.86 | 74 | 1 | 0.111 |
| 5A | 709.77 | 1151 | 2 | 0.275 |
| 5B | 713.15 | 1995 | 3 | 0.303 |
| 5D | 566.08 | 197 | 1 | 0.123 |
| 6A | 618.08 | 1308 | 2 | 0.287 |
| 6B | 720.99 | 1509 | 2 | 0.298 |
| 6D | 473.59 | 199 | 1 | 0.115 |
| 7A | 736.71 | 1281 | 2 | 0.184 |
| 7B | 750.62 | 1298 | 2 | 0.247 |
| 7D | 638.69 | 191 | 1 | 0.140 |
Genome-wide Marker-traits association with SNP-markers
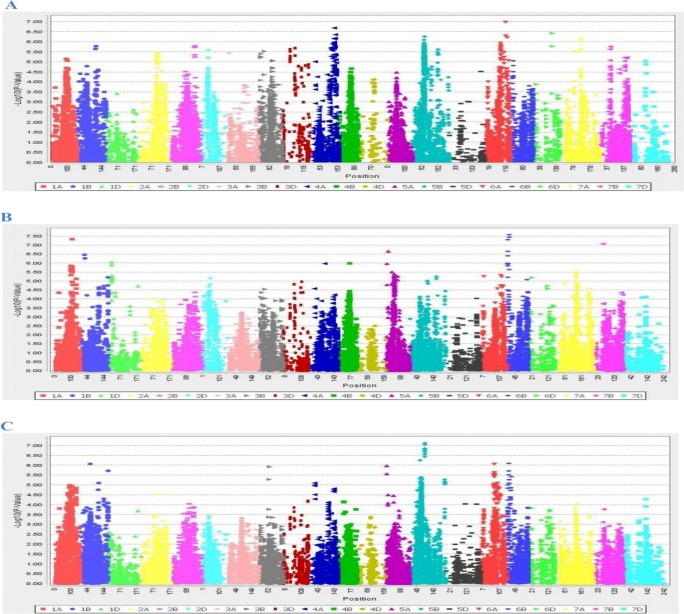
In the present study, several markers associated with attributes like incubation period, lesion number and AUDPC were identified. The number of SNPs found significant at P-value threshold 1e-06 in the marker-trait association for the three traits, incubation period, lesion number and AUDPC were 13, 8 and 14, respectively (Table 3 and Fig 5A, 5B and 5C). A total of 35 SNPs markers were detected on eleven chromosomes (1A, 1B, 1D, 4A, 5A, 5B, 6A, 6B, 6D, 7A and 7B) with coefficient of determination (R2) ranging between 0.083 and 0.11 (Table 3).
Table 3. Significant single nucleotide polymorphism (SNP) markers associated with incubation period (IP), lesion number (LN) and Area under disease progress curve (AUDPC) in 287 spring wheat lines in the WAMI panel.
| Trait | Markers | Chromosome | Position (cM) | p-value | Marker R2 |
|---|---|---|---|---|---|
| IP | BobWhite_c20306_111 | 4A | 137 | 2.08E-07 | 0.093 |
| BobWhite_c11327_185 | 4A | 137 | 6.81E-07 | 0.085 | |
| BobWhite_c20322_153 | 4A | 137 | 7.17E-07 | 0.084 | |
| BobWhite_c17524_242 | 4A | 144 | 4.47E-07 | 0.089 | |
| Tdurum_contig81450_90 | 4A | 142 | 6.81E-07 | 0.087 | |
| Excalibur_rep_c69981_75 | 6A | 119 | 1.06E-07 | 0.098 | |
| wsnp_Ra_c2270_4383252 | 6A | 119 | 5.07E-07 | 0.089 | |
| BS00004466_51 | 6A | 128 | 6.15E-07 | 0.089 | |
| Ku_c15750_761 | 7A | 126 | 6.97E-07 | 0.086 | |
| TA002294-0887 | 7A | 126 | 8.96E-07 | 0.086 | |
| Kukri_c23752_659 | 5B | 71 | 5.55E-07 | 0.089 | |
| Excalibur_c96334_93 | 5B | 68 | 8.10E-07 | 0.093 | |
| Tdurum_contig75700_411 | 6D | 117 | 3.78E-07 | 0.091 | |
| LN | RAC875_c32379_216 | 1A | 110 | 4.68E-08 | 0.110 |
| BobWhite_c23992_300 | 5A | 16 | 2.23E-07 | 0.102 | |
| BobWhite_c17559_105 | 1B | 28 | 3.45E-07 | 0.096 | |
| wsnp_Ex_c13421_21142520 | 1B | 31 | 5.51E-07 | 0.090 | |
| RAC875_c68978_126 | 6B | 9 | 2.62E-08 | 0.108 | |
| BS00092845_51 | 6B | 9 | 3.63E-08 | 0.104 | |
| wsnp_Ex_c2103_3947695 | 7B | 50 | 8.65E-08 | 0.107 | |
| Excalibur_rep_c105429_528 | 1D | 21 | 8.92E-07 | 0.088 | |
| AUDPC | BobWhite_c35961_80 | 6A | 79 | 8.71E-07 | 0.083 |
| BobWhite_c3661_88 | 1B | 64 | 8.63E-07 | 0.083 | |
| tplb0027f13_1493 | 5B | 90 | 7.64E-08 | 0.101 | |
| wsnp_Ku_c40334_48581010 | 5B | 90 | 8.99E-08 | 0.099 | |
| BobWhite_c48435_165 | 5B | 90 | 1.44E-07 | 0.096 | |
| Tdurum_contig25513_195 | 5B | 90 | 1.48E-07 | 0.095 | |
| Tdurum_contig12066_126 | 5B | 90 | 1.60E-07 | 0.093 | |
| Tdurum_contig25513_123 | 5B | 90 | 1.95E-07 | 0.093 | |
| tplb0027f13_1346 | 5B | 90 | 2.08E-07 | 0.093 | |
| BS00010590_51 | 5B | 90 | 2.72E-07 | 0.090 | |
| Tdurum_contig12066_247 | 5B | 90 | 2.76E-07 | 0.090 | |
| IACX9261 | 5B | 90 | 3.71E-07 | 0.090 | |
| BS00009311_51 | 5B | 62 | 5.62E-07 | 0.090 | |
| Excalibur_c96134_182 | 6B | 5 | 8.09E-07 | 0.087 |
Fig 5. Manhattan plots for the GWAM scan on different traits with p value threshold (-log10 (P-value)>6.0).
(A) Incubation period, (B) Lesion number, (C) AUDPC.
For incubation period, 13 SNP markers were detected on five chromosomes in variable numbers; 5 on 4A, 3 on 6A, 2 on 7A, 2 on 5B and 1 on 6D (Table 3)whereas no MTA was found on rest of the chromosomes (Fig 5A). These markers were significant at P values less than 1e-06 and R2 for significant markers ranged from 0.084 to 0.098 (Table 3). A prominent QTL was identified for incubation period on 6A Chromosome at 199 cM. Out of five markers detected on 4A chromosome, three markers (BobWhite_c20306_111, BobWhite_c11327_185 and BobWhite_c20322_153) were mapped at 0 cM and the other two (BobWhite_c17524_242 and Tdurum_contig81450_90) at 2 cM away. Two SNPs (Excalibur_rep_c69981_75 and wsnp_Ra_c2270_4383252) were mapped at 0 cM on the chromosome 6A while one SNP marker (BS00004466_51) was detected at 9 cM. Two regions (Ku_c15750_761 and TA002294-0887) were significantly associated with incubation period on 7A chromosome at 0 cM. The other three markers were significantly associated with incubation period on chromosome 5B (71 and 68 cM) and 6D (117 cM).
Eight SNPs showed significant association with lesion number and were mapped on six chromosomes, 1A, 1B, ID, 5A, 6B and 7B (Table 3). The highest number and most significant (P = 2.62e-08, R2 = 0.11) SNP markers for lesion number were on chromosome 6B. The largest numbers of MTAs (-log10; P-value>6.0) were on chromosomes 1A, 1B, 5A, 6B, 7Bwhile no MTA was detected on the rest of the chromosomes(Fig 5B).Two SNPs (BobWhite_c17559_105 and wsnp_Ex_c13421_21142520) were mapped within 3 cM on chromosome 1B (P < 1e-06, R2 = 0.093). Two SNPs (RAC875_c68978_126 and BS00092845_51) significantly associated with lesion number on 6B chromosome were mapped at 0 cM(P < 1e-06, R2 = 0.11), suggesting that one QTL for lesion number was detected by these SNPs (Table 3).The four other single SNPs (RAC875_c32379_216,Excalibur_rep_c105429_528, BobWhite_c23992_300, wsnp_Ex_c2103_3947695) were significantly associated with lesion number on chromosomes 1A, 1D, 5A, 7B at 110, 21, 16 and 50 cM, respectively.
Fourteen SNP markers were found significantly associated with AUDPC and detected on chromosomes 1B, 5B, 6A, 6B(Table 3 and Fig 5C). The most significant associations were recorded for AUDPC on chromosome 5B (P = 7.64e-08, R2 = 0.10). The greatest number (11) of SNPs were mapped on chromosome 5B for resistance to spot blotch; of these 11 SNPs, 10were mapped at 0 cM suggesting that one QTL for resistance to spot blotch may underly these SNPs (Table 3).Three other SNPs (BobWhite_c3661_88, BobWhite_c35961_80, Excalibur_c96134_182) were significantly associated with spot blotch AUDPC on different chromosomes (1B, 6A, 6B) (Table 3).
Discussion
Spot blotch disease of wheat is a major concern in warm humid south Asia and also other countries where similar climatic parameters exist. This is despite the significant progress already made in understanding the biology of Bipolaris sorokiniana and also about the cultural practices that can be used to manage this disease. Although some resistance QTL/genes have been identified and resistant cultivars developed, none of these cultivars are completely resistant since resistance is quantitative in nature and controlled by several genes with relatively minor effect. Conventional breeding approaches have contributed immensely to wheat improvement but the progress in developing wheat cultivars resistant to spot blotch has not been satisfactory [29, 30] perhaps because of the complex and polygenic nature of resistance [8, 29].
Initial exploratory analysis of phenotypic data which shows differential performance of each traits across the years, encouraged the environments under investigation is suitable for further analysis. Correlations among the traits are not having much significance suggesting rare common QTL associated with traits under study. Significant differences among genotype and genotype × year observed for all the three traits i.e. incubation period, lesion number and AUDPC revealed the differential performance of all the selected genotypes among themselves as well as across the years for three important traits. The panel exhibited considerable phenotypic, possibly due to the diverse genetic background of the germplasm. Consequently, this result acclaim the selected lines under study are important for further GWAM analysis. In this study, we analyzed the association between spot blotch incubation period, lesion number and AUDPC with SNPs from 287 spring wheat lines in the WAMI panel. We identified 13SNP markers significantly associated with loci conferring resistance to spot blotch AUDPC. In addition, 8 SNPs were identified for incubation period, and 14 for lesion number. Applicability of SNPs for genome-wide association study were verified with already detected DArT markers [31, 32, 33]. GWAM was strengthened by the high-throughput SNP genotyping array and a high-density map to identify putative QTLs associated with spot blotch resistance with better resolution [34].Association mapping is considered superior over bi-parental mapping due to high minor allele frequency, low LD and limited population structure to detect QTLs [32, 35].
Using the WAMI panel in this study, we identified SNP markers linked to five and six QTLs for incubation period and spot blotch lesion number on chromosomes 4A, 6A, 7A, 5B, 6D and 1A, 1B, ID, 5A, 6B, 7B, respectively. Among them, SNPs markers closely linked to one QTL each on the chromosome 4A, 6A and 7A revealed that QTL on the A genome are strongly associated with the incubation period. Likewise, for lesion number, SNPs closely linked to one QTL on the chromosome 6Bwere significant. The associations of incubation period and lesion number with spot blotch resistance has not been reported in previous linkage or GWAM studies.
In addition to incubation period and lesion number, we identified SNP markers linked to four QTLs for spot blotch AUDPC on the chromosomes 1B, 5B, 6A and 6B. Among them, several SNPs closely linked on chromosome 5B. In an earlier association mapping study [32]having an association panel of 566 spring wheat landraces and using 832 Diversity Array Technology (DArT) markers, several genomic regions associated with spot blotch resistance were also identified on four chromosomes (1A, 3B, 7B, 7D). In a follow-up GWAM study, the same group used 528 diverse spring wheat genotypes that were phenotyped for SB and were genotyped using a 9K wheat SNP chip [35], led to the identification of nine associated SNPs located on five different chromosomes (1B, 5A, 5B, 6B, 7B). We detected MTAs that closely corresponded to fourteen loci on four chromosomes that were previously identified and mapped at low resolution by bi-parental QTL analyses using SSRs [22, 29, 30]. The similar position of earlier mapped QTLs with the SNPs of the present study validated QTL for spot blotch resistance.
Conclusion
SNP markers and GWAS have been useful for QTL discovery in spring wheat. Considerable phenotypic and molecular variation was observed in the WAMI panel used suggesting the diverse genetic background of the germplasm. This study identified 15genomic regions to be associated with resistance to spot blotch disease of wheat. Of these, four regions were for AUDPC, while five and six were for incubation period and lesion number, respectively. A greater number of SNP markers were significantly associated with spot blotch AUDPC than for incubation period and lesion number. This study is the first time association of between markers and incubation period or lesion number were established and QTLs mapped through GWAM. Our data revealed that most of the SNPs were present on the B-genome of wheat. The identified SNP markers linked to resistant QTLs will be useful in augmenting breeding for spot blotch resistance in wheat.
Supporting information
(XLSX)
Acknowledgments
The authors gratefully acknowledge Matthew P. Reynolds, CIMMYT, Mexico, for providing WAMI population and the open access molecular data used in this study.
Data Availability
Genotypic data used in this experiment is available at http://hdl.handle.net/11529/10714.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Reynolds M, Bonnett D, Chapman SC, Furbank RT, Manes Y, Mather DE, et al. Raising yield potential of wheat. I. Overview of a consortium approach and breeding strategies. J Exp Bot. 2011; 62: 439–452. 10.1093/jxb/erq311 [DOI] [PubMed] [Google Scholar]
- 2.Braun HJ, Atlin G, Payne T. Multi-location testing as a tool to identify plant response to global climate change In: Reynolds MP, ed, Climate change and crop production. CABI, London, UK: 2010; pp.115–138. [Google Scholar]
- 3.Chatrath R, Mishra B, Ortz-Ferrara G, Singh SK, Joshi AK. Challenges to wheat production in south Asia. Euphytica. 2007; 157: 447–456. [Google Scholar]
- 4.Joshi AK, Azab M, Mosaad M, Moselhy M, Osmanzai M, Gelalcha S, et al. Delivering rust resistant wheat to farmers: a step towards increased food security. Euphytica. 2011; 179: 187–196. [Google Scholar]
- 5.Braun HJ, Pfeiffer WH, Pollmer WG. Environments for selecting widely adapted spring wheat. Crop Sci. 1992; 32: 1420–1427. [Google Scholar]
- 6.Goldstein DB, Weale ME. Population genetics: linkage disequilibrium holds the key. Curr Biol. 2001; 11: 576–579. [DOI] [PubMed] [Google Scholar]
- 7.Saari EE, Prescott JM. A scale for appraising the foliar intensity of wheat disease. Pl Dis Rep. 1975; 59: 337–380. [Google Scholar]
- 8.Singh PK, Zhang Y, He X, Singh RP, Chand R, Mishra VK, et al. Development and characterization of the 4th CSISA-spot blotch nursery of bread wheat. Euro. J. Pl. Pathol. 2015; 143: 595–605. [Google Scholar]
- 9.Duveiller E, Kandel YR, Sharma RC, Shrestha SM. Epidemiology of foliar blights (spot blotch and tan spot) of wheat in the plains bordering the Himalayas. Phytopathology. 2005; 95(3): 248–256. 10.1094/PHYTO-95-0248 [DOI] [PubMed] [Google Scholar]
- 10.Parlevliet JE. Components of resistance that reduce the rate of epidemic development. Annu Rev Phytopathol. 1979; 17: 203–222. [Google Scholar]
- 11.Wilcoxson RD. Genetics of slow rusting in cereals. Phytopathology 1981; 71:989–92. 10.1094/Phyto-71-989 [Google Scholar]
- 12.Chao SM, Zhang WJ, Dubcovsky J, Sorrells M. Evaluation of genetic diversity and genome-wide linkage disequilibrium among US wheat (Triticum aestivum L.) germplasm representing different market classes. Crop Sci. 2007; 47: 1018–1030. 10.2135/cropsci2006.06.0434 [Google Scholar]
- 13.Zhang D, Bai G, Zhu C, Yu J, Carver BF. Genetic diversity, population structure and linkage disequilibrium in U.S. elite winter wheat. Plant Gen. 2010; 3:117–127. doi: 10.3835/plant genome 2010.03.0004 [Google Scholar]
- 14.Flint-Garcia SA, Thorns berry JM, Buckler ES. Structure linkage disequilibrium in plants. Annu Rev Plant Biol. 2003; 54: 357–374. 10.1146/annurev.arplant.54.031902.134907 [DOI] [PubMed] [Google Scholar]
- 15.Breseghello F, Sorrells ME. Association mapping of kernel size and milling quality in wheat (Triticum aestivum L.) cultivars. Genetics. 2006; 172: 1165–1177. 10.1534/genetics.105.044586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu J, Buckler ES. Genetic association mapping and genome organization of maize. CurrOpinBiotechnol. 2006; 17: 155–160. [DOI] [PubMed] [Google Scholar]
- 17.Sivakumar S, Crossa J, Jarquin D, Lopes M, Reynolds MP. Genomic and pedigree prediction with Genotype × Environment Interaction in spring Wheat Grown in South and Western Asia, North Africa, and Mexico. CIMMYT Research Data & software Repository Network; 2016; http://hdl.handle.net/11529/10714. WAMI PHENOTYPIC AND MARKER DATA. zip. Hdl/11529/10714. [Google Scholar]
- 18.Panse VG, Sukhatme PV. Statistical method for agricultural workers, 4th edition 1985; New Delhi. [Google Scholar]
- 19.Joshi AK, Ferrara O, Crossa J, Singh G, Sharma RC, Chand R, et al. Combining superior agronomic performance and terminal heat tolerance with resistance to spot blotch (Bipolarissorokiniana) in the warm humid Gangetic plains of south Asia. Field Crops Res. 2007; 103: 53–61. [Google Scholar]
- 20.Chaurasia S, Joshi AK, Dhari R, Chand R. Resistance to foliar blight of wheat; a research, Genet Resour Crop Evol. 1999; 46: 469–475. [Google Scholar]
- 21.Roumen EC. Effect of leaf age on components of partial resistance in rice to leaf blast. Euphytica. 1992; 63(3): 271–279. [Google Scholar]
- 22.Sharma RC, Duveiller E. Advancement toward new spot blotch resistant wheats in South Asia. Crop Sci. 2007; 47: 961–968. doi: 10.235/cropsci2006.03.0201 [Google Scholar]
- 23.Saghai-maroof MA, Solima KM, Jorgenson RA, Allard RW. Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location and population dynamics. Proc Natl Acad Sci. 1984; USA81: 8014–8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pritchard KJ, Stephens Matthew, Donnelly Peter.Inference of population structure using multilocus genotype data.Genetics. 2000; 155: 945–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol. 2005; 14: 2611–2620. 10.1111/j.1365-294X.2005.02553.x [DOI] [PubMed] [Google Scholar]
- 26.Earl Dent A. STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conservation Genetics Resources, Volume 4, Issue 2 2012; pp 359–361. [Google Scholar]
- 27.Bradbury PJ,Zhang Z, Kroon DE, Casstevens TM, Ramdoss Y, Buckler ES. TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics. 2007; 23: 2633–2635. 10.1093/bioinformatics/btm308 [DOI] [PubMed] [Google Scholar]
- 28.Kang HM, Zaitlen NA, Wade CM, Kirby A, Heckerman D, Daly MJ, et al. Efficient control of population structure in model organism association mapping. Genetics. 2008; 178: 1709–23. 10.1534/genetics.107.080101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joshi AK, Mishra B, Chatrath R, Ortiz Ferrara G, Singh RP. Wheat improvement in India: present status, emerging challenges and future prospects. Euphytica. 2007. a; 157: 431–446. [Google Scholar]
- 30.Kumar U, Joshi AK, Kumar S, Chand R, Röder MS. Quantitative trait loci for resistance to spot blotch caused by B. sorokiniana in wheat (T. aestivum L.) lines ‘Ning 8201’ and ‘Chirya 3’. Mol Breed. 2010; 26: 477–491. [Google Scholar]
- 31.Adhikari TB, Jacksond EW, Gurung S, Hansen JM, Bonman JM. Association mapping of quantitative resistance to Phaeosphaerianodorum in spring wheat landraces from the USDA National Small Grains Collection. Phytopathology. 2011; 101: 1301–1210. 10.1094/PHYTO-03-11-0076 [DOI] [PubMed] [Google Scholar]
- 32.Adhikari TB, Gurung S, Hansen JM, Jackson EW, Bonman JM. Association mapping of quantitative trait loci in spring wheat landraces conferring resistance to bacterial leaf streak and spot blotch. Plant Gen. 2012; 5: 1–16. [Google Scholar]
- 33.Gurung S, Mamidi S, Bonman JM, Jackson EW, del Río LE, Acevedo M, et al. Identification of novel genomic regions associated with resistance to Pyrenophoratritici-repentis races 1 and 5 in spring wheat landraces using association analysis. Theor Appl Genet. 2011; 123: 1029–1041. 10.1007/s00122-011-1645-1 [DOI] [PubMed] [Google Scholar]
- 34.Cavanagh CR, Chao S, Wang S, Huang BE, Stephen S, Kiani S, et al. Genome wide comparative diversity uncovers multiple targets of selection for improvement in hexaploid wheat landraces and cultivars. Proc Natl Acad Sci USA. 2013; 110: 8057–8062. 10.1073/pnas.1217133110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gurung S, Mamidi S, Bonman JM, Xiong M, Brown-Guedira GB, Adhikar TB. Genome-wide association Study Reveals Novel Quantitative Traits Loci Associated with Resistance to Multiple Leaf Spot disease of Spring Wheat. PLoS ONE. 2014; 9 (9): e108179 10.1371/journal.pone.0108179 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
Genotypic data used in this experiment is available at http://hdl.handle.net/11529/10714.