Abstract
The isolation of polyclonal antibodies from the serum of immunized mammals has significantly contributed to scientific research and diagnosis. The fact that recent technologies allow the production of antibodies in the yolk of eggs laid by hens, has led to the development of an alternative method for antibody generation that is less stressful to animals. As hens are kept under almost all their natural conditions, antibodies are isolated from the collected eggs; this technology is expected to become an interesting alternative to the conventionally serum-based techniques that eventually require to sacrifice the animal.
Here we present a modified protocol for the isolation of IgY antibodies from immunized chickens and provide comparison between two chicken breeds in relative to IgY yield per egg. Our results show the possibility of generating large quantities of highly pure IgY from chicken eggs and also show large differences in the yield of IgY production between the two studied breeds. The results of this work indicate that IgY technology can be used for the production of primary antibodies for immunological work and disease diagnosis.
Keywords: IgY, Chicken egg yolk, Gel filtration chromatography, Salmonella typhimurium
1. Introduction
Immunoglobulins (Ig) are glycoproteins called antibodies, which are secreted by plasma cells in response to antigen exposures and are considered a product that majorly effects humoral immunity. The commercially available Igs play critical roles in diagnostic assays, therapy, and purification of specific target compounds. Hens egg yolk immunoglobulins IgY have been studied intensively due to their importance. Serum IgG of the hen is transferred from the mother hen to the offspring egg yolk to acquire immunity. Immunoglobulin is called IgY because it is present in the egg yolk and due to the differences in protein nature compared to that of the mammalian Ig [1], [2]. Immunized hens with a specific antigen such as Salmonella typhimurium can give a specific IgY produced in the egg yolk against the given antigen. Therefore, IgY has been applied successfully in scientific, diagnostic, prophylactic, therapeutic purposes, immunochemical reagents, and in food formulation or supplements due to the stability of IgY under food processing conditions [3], [4].
In the immunodiagnostic technologies, IgY is an excellent antibody for using in immunological assays involving mammalian sera, due to discriminative properties of IgY compared to mammalian IgG, as IgY does not react with the rheumatoid factor and human anti-mouse IgG antibodies do not activate the complement system and do not bind to Fc receptor [5]. Also, they have poor cross reactivity to mammalian IgG due to immunological differences. IgY is usually low-cost and can be generated through convenient production processes that make it an attractive antibody for research and diagnosis [6], [7]. The advantages can be concluded as; (1) IgY is produced in egg yolk; so there is no need to bleed animals, (2) considerable amounts of antibodies can be obtained in a fairly low cost, (3) usually rapid production process, (4) IgY can be stored in eggs at 4 °C for at least one year, (5) it is achievable to produce a specific antibody to small amounts of antigen that is poorly immunogenic in mammals [6], [8]. There are several IgY isolation methods available, but mostly based on using polyethylene glycol (such as PEG6000) for precipitation from the supernatant extracts, which usually yield protein impurities [9], [10]. The aim of this study was to optimize the production and purification of IgY antibodies from chicken egg yolk to achieve high yield of production for research and commercial uses.
2. Materials and methods
2.1. Immunization of chicken and egg collection
Three chickens of Single Comb White Leghorns that were 6 months old were inoculated orally with 0.1 ml Zoosaloral cultures (IDT Biologika, Lot #. 0551012). Three doses were given to the chickens via drinking water. The interval between the first and the second vaccination was two weeks, and one week between the second and the third vaccination. For the first dose, 3 mg of the Salmonella typhimurium vaccine antigen powder was dissolved in 30 ml distilled water and then given in 10 ml volumes for each chicken using a laboratory syringe. After two weeks, the second dose was prepared by adding 3 mg in 30 ml dH2O then given to the three chickens and the third dose was prepared similarly as above. During three weeks following immunization, the eggs were collected from the immunized chickens between specific time intervals of the three doses to detect the best quantities of IgY antibodies. Samples were then stored at 4 °C until the next step, which was the extraction of the IgY from the immunized egg yolk.
2.2. Extraction of chicken IgY
Extraction of chicken IgY antibodies from immunized Single Comb White Leghorns egg yolk was first carried out by using Polyethylene Glycol (PEG 6000) precipitation. For comparison between chicken breeds in relative to IgY yield per egg, we also isolated IgY from Rhode Island Red chicken eggs using similar protocols used for the Single Comb White Leghorns. The eggshell was cracked carefully and the egg yolk was transferred to a filter paper to remove the remaining egg white. The egg yolk skin membrane was cut before the yolk was poured into a 50 ml tube to measure its volume. Twice the egg yolk volume of PBS (Phosphate-buffered saline) was added to the yolk tube and mixed by vortexing. A 3.5% of PEG 6000 of the total volume was added to the yolk tube, vortexed and rolled for 10 min by a rolling mixer before the tube was centrifuged at 5000 rpm for 40 min at 4 °C. The supernatant was then poured through a folded filter paper and transferred to another tube before 8.5% PEG 6000 was added in relative to the new volume and then vortexed and centrifuged at 5000 rpm for 40 min at 4 °C. The supernatant discarded and the pellet dissolved in 1 ml PBS using a glass stick, mixed by vortexing before 9 ml of PBS were added to the tube to reach a final volume of 10 ml. The solution was then mixed with 12% PEG 6000 and the tube centrifuged at 5000 rpm for 40 min at 4 °C. The supernatant was discarded and the pellet dissolved in 800 µl PBS using a glass stick and vortex. Finally, the extract was transferred to a dialysis membrane (Cellu Sep H1, part #5050-28) to remove the salt. Dialysis tube was first soaked in dH2O for 15 min before the sample was injected through the opening end of the tube. The filled dialysis membrane immersed in the dialysis buffer solution (10 mM SP buffer) and the sample was stirred over night using magnetic stirrer. In the next day, the SP buffer was discarded and the sample was soaked in PBS for 3 h. Following the soak, the sample was pipetted from the dialysis bag in 2 ml Eppendorf tubes and stored at −20 °C.
Immunoglobulin Y (IgY) purification by low pressure chromatography system (Bio-Rad Biologic LP low pressure chromatography) was used to further purify chicken IgY antibodies by using 0.02 M Tris-HCl pH 8.0 and deionized water. The pure IgY fractions were collected using a fraction collector as described below. A standard Bradford assay was used for protein quantification.
The procedure was as follows: Buffer “A” contained Tris-HCl, and buffer “B” contained deionized water. The low pressure chromatography column contained 35 ml of gel filtration resin (Sepharose 6 fast Flow with 20% ethanol as preservative 17-0159-01, GE Healthcare, Lot 10223228). The pump was programmed according to the following protocol: buffer A was selected with a flow rate of 1 ml/min for 10 min followed by buffer B and then back to buffer A with similar conditions as the first step to pump 30 ml as a waste volume. After 30 min, the sample (2 ml of IgY) was injected and a gradient flow rate with 1 ml/min was followed for all steps which were collected in 3 ml glass tubes using an automated fraction collector. The method involved: 15 min for 100% buffer A, then with gradient starting from 0% to 100% buffer B over 60 min, then 60 min gradient flow from 100% to 0% buffer B followed by 15 min from buffer A pumped as a final wash.
2.3. IgY confirmation by SDS-PAGE and Western blot
SDS-PAGE gel electrophoreses was used to determine the molecular weight of the purified chicken IgY antibodies. Western blot was used to confirm the identity of the purified IgY. Briefly, SDS-PAGE gel with the desired samples was carefully washed with double distilled water. Then, the gel, nitrocellulose membrane (NC) (Amersham™ Protran™ 0.2 µm NC/GE Healthcare Life science/10600001) and blotting membrane (BioRad/1703932) were soaked in transfer buffer for 3 min. The protein bands on the gel were transferred to the NC using Trans-Blot®SD Semi-Dry Transfer Cell (BioRad) at 18 V for 1 h. The NC that contained the transferred proteins was blocked using TBST buffer Tris-buffered saline, 0.1% Tween 20 containing 3% (w/v) skim milk (Oxoid/LP0031) for overnight at 4 °C. The secondary antibody (Anti-chicken IgY (IgG) (whole molecule) alkaline phosphatase conjugate Sigma A9171) was applied to the membranes at a dilution of 1:16,000 in blocking buffer. The incubation with secondary Abs was for 2 h at room temperature. After that, the membranes were separately washed three times with TBST buffer (5 min for each). The membrane was washed three times with TBST buffer (5 min/each). The colorimetric staining was performed using alkaline phosphates substrate, which is BCIP®/NBT (Sigma/B5655) for 5 min, then the membranes were washed in water to stop the reaction.
3. Results
3.1. Purification of IgY
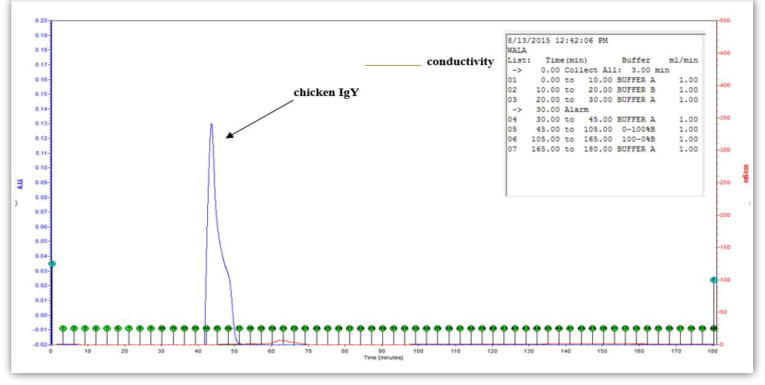
The purification of IgY using the low pressure chromatography device yielded fractions that corresponded to a protein with high purity that is thought to be the IgY (Fig. 1). It showed a large peak, which apparently represented the chicken IgY. As the purification was carried out under none denaturing conditions, the peak represented both chains of IgY (Fig. 1). The protein between the corresponding fractions was collected using an automated fraction collector for further analyses.
Fig. 1.
A highly pure IgY from immunized Single Comb White Leghorns egg yolk after the third dose of vaccine was obtained using gel filtration chromatography. Y-axis represents the absorbance units (AU), X-axis represents the time (minutes). Pure IgY from fractions No. 14–17 were collected for further quality testing and with 0.13 AU for the two chains of the IgY.
The purified IgY samples showed antibody concentrations ranging from (3.66 mg/ml for Single Comb White Leghorns egg yolk to 8.37 mg/ml for Rhode Island Red chicken egg yolk) as measured by Bradford assay, an indication that some chicken breeds may have a better immunological response to pathogens.
The purity and yield of chicken IgY can vary greatly from method to method and require extreme optimization for each experiment. Extraction of IgY from egg yolk by a precipitation method using PEG is cost-effective and involves two major steps: removal of the lipids and then precipitation of the total IgY from the supernatant of the first step [11]. Dialysis of the extracted IgY against PBS will give pure extracted IgY, which can be stored for more than one year at −20 °C. Our results showed that the purity of IgY preparation can be increased by a combination of PEG, dialysis and chromatographic methods including gel-filtration chromatography.
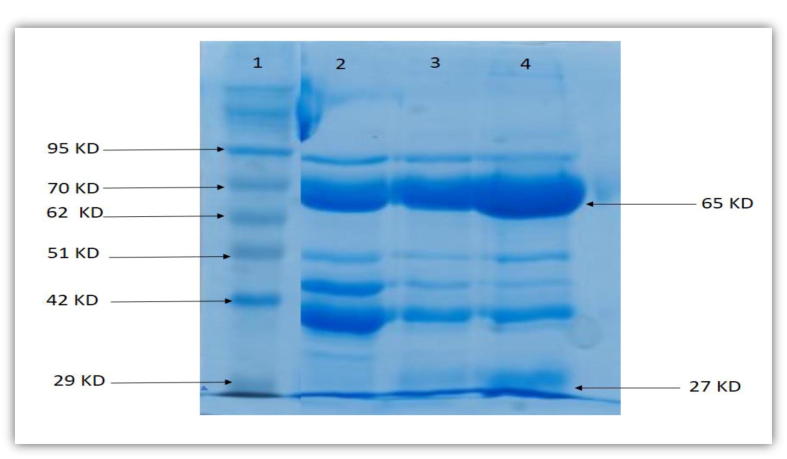
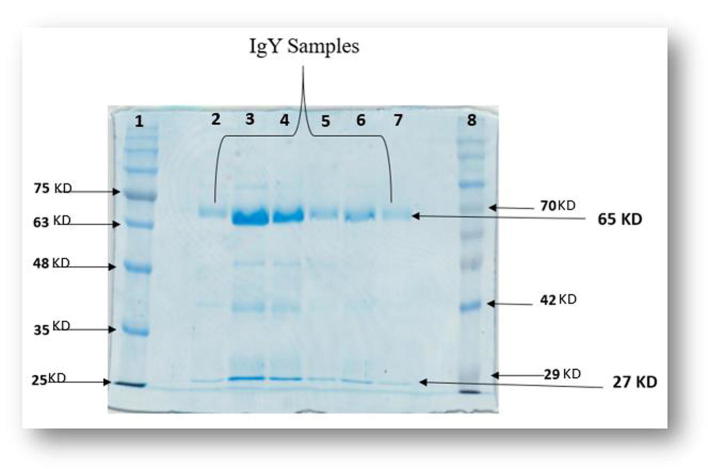
The result of the water-soluble fractions obtained during IgY extraction were analyzed by SDS-PAGE Sodium dodecyl sulfate polyacrylamide gel electrophoresis. The molecular weight patterns agreed with the expected molecular mass for the heavy and light chains, respectively. The sizes were 65 and 27 kDa of egg yolk antibody IgY according to the protein ladder (Fig. 2, Fig. 3). Using SDS-PAGE, we showed that the pure fraction shown in our chromatography results corresponded to the heavy and light chains expected sizes for IgY (Fig. 3). Consequently, the heavy chain with 65 kDa and the light chain with 27 kDa were confirmed through SDS PAGE.
Fig. 2.
The SDS-PAGE for the purity at each stage in purification and precipitation steps from egg yolk. Lane 1: PiNK Plus Prestained Protein Ladder, lane 2: the sample after 3.5% PEG precipitation, lane 3: the sample after 8.5% PEG precipitation, lane 4: the sample after 12% PEG precipitation. The IgY HC ≈ 65 kDa, and the LC ≈ 27 kDa.
Fig. 3.
The SDS-PAGE profile of IgY. The two IgY chains appeared on the SDS PAGE by using 10% resolving SDS-PAGE gel. The HC heavy chain with 65 kDa, LC the light chain with 27 kDa. lane 1 = BLUeye Prestained Protein Ladder, lane 2–7 = purified IgY, lane 8 = PiNK Plus Prestained Protein Ladder.
Measurements of the purity and yield for each stage of purification from egg yolk are shown (Fig. 2). Following precipitation with 3.5% PEG, different bands, apparently with high concentrations, eluted, then started to dilute with increased concentration of PEG to 8.5%, then some of these bands totally disappeared when increasing the concentration to 12% PEG. Other bands that appeared between the heavy and light chains of IgY were minor impurities corresponding to molecular weights between 51 and 40 kDa, and have been removed by dialysis steps and the size exclusion low pressure chromatography purification as shown (Fig. 3). This indicates that the combination between PEG, dialysis and gel filtration methods is quite important to improve purity [12].
3.2. Confirmation of IgY identity
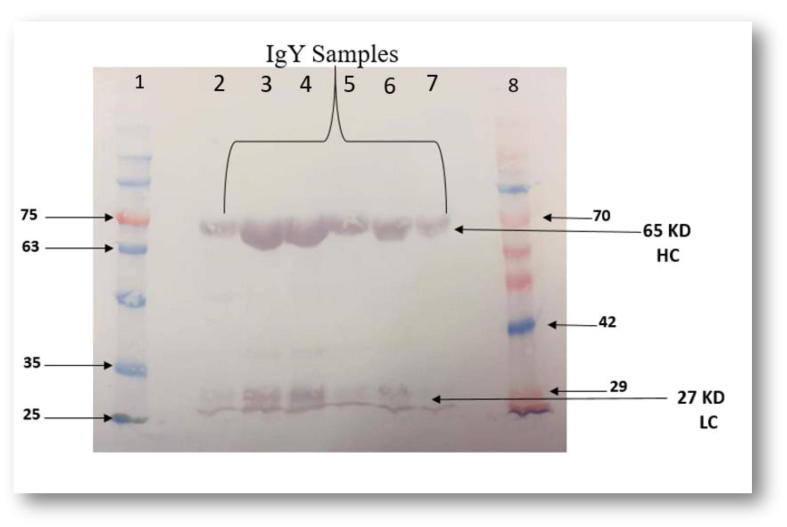
The results obtained from the Western blot showed two protein bands corresponding to the heavy and light chains of chicken IgY (Fig. 4). The two chains were identified using Anti-chicken IgY (IgG) (whole molecule) alkaline phosphatase-antibody produced in rabbit. The heavy chain with approximately 65 kDa and the light chain with approximately 27 kDa, which indicates that the purified protein from the egg yolk was the chicken IgY.
Fig. 4.
Identification of IgY by Western blot. Lane 1 = BLUeye Prestained Protein Ladder, lane 2–7 = purified IgY, lane 8 = PiNK Plus Prestained Protein Ladder. Result of Western blot test using Anti-chicken IgY (IgG) (whole molecule) alkaline phosphatase antibody produced in rabbit to detect the heavy chains and the light chains of chicken IgY. The LC: light chain ≈ 65 kDa, the HC: heavy chain ≈ 27 kDa.
4. Discussion
Our results show that it is possible to generate IgY antibodies from chicken eggs with chicken immune system boosted by vaccination with Salmonella typhimurium. We confirmed the validity of the vaccine by culturing the S. typhimurium (data not shown). Compared to antibody production in rabbits, the IgY technology offers several advantages; no blood sampling, only eggs are needed following immunization and low quantities of antigen are required to obtain high and long-lasting IgY titers in the yolk of immunized hen eggs. Therefore, the production of polyclonal antibodies through the chicken immunization makes IgY an excellent alternative, producing the antibodies in large amount and quality from simple methods of production without the need for invasive techniques.
We have successfully purified chicken IgY antibody in egg yolks of hyperimmunized hens, which can be used for development of oral passive immunotherapy as well as a diagnostic reagent for Salmonella typhimurium detection and has the potential of expanding to other diseases. PEG600 precipitation is a cheap and rapid method to precipitate IgY from mixture of proteins, but we found it is better to be followed by a dialysis step due to the problem in the removal of lipids which are present in high quantities in chicken eggs. This indicates that an efficient method should consist of three successive PEG precipitations, starting with 3.5%, followed by 8.5% to remove lipid substances, and then 12% PEG to precipitate the IgY. Pauly et al., [13] study also explained the extraction of total IgY from egg yolk by using PEG precipitation, with purity of the extract around 80%. Consequently, the appropriate IgY purification method is influenced by the scale of purification, cost effectiveness, laboratory apparatus, and the effect of the environment. Furthermore, De Meulenaer and Huyghebaert, [14] demonstrated that filtration technology seems to offer the greatest opportunities for industrial applications, even though precipitation with polyethylene glycol display a cheap and easy methodology for laboratory use.
Our results also showed that the selection of chicken breed for IgY production is quite important for obtaining high antibody yield. Rhode Island Red chicken eggs yielded more than double quantities (8.37 mg/ml) of yolk IgY compared to Single Comb White Leghorns egg (3.66 mg/ml).
In conclusion, IgY should be used as an alternative to mammalian antibodies, and it is better to immunize chickens before they begin to produce eggs, since the stress induced by handling them could have an adverse effect on egg production, as might the nature of the antigen or adjuvant used.
The benefits of IgY technology and its universal application in both research and medicine is expected to expand at a large-scale. It is expected that IgY will play an increasing role in research, diagnosis, and immunotherapy in the future.
Acknowledgments
The authors would like to thank Dr. Robin Abu Ghazaleh for advice, Mr. Hasan Al-Taradeh and Mrs. Asma Tamimi for technical support. The project was partially supported by NARC-Ministry of Agriculture and the Quality Improvement Fund-Ministry of Higher Education, Palestine.
Acknowledgments
Authors’ contribution
WAA carried out the experimental work and provided the first draft of the manuscript. WQ provided supervision and coordinates the financial assistance received from NARC.F.AL-R designed and supervised the work. All authors have read and approved the final manuscript.
Footnotes
Peer review under responsibility of National Research Center, Egypt.
References
- 1.Schade R., Calzado E.G., Sarmiento R., Chacana P.A., Porankiewicz-Asplund J., Terzolo H.R. Chicken egg yolk antibodies (IgY-technology): a review of progress in production and use in research and human and veterinary medicine. Altern Lab Anim. 2005;33(2):129–154. doi: 10.1177/026119290503300208. [DOI] [PubMed] [Google Scholar]
- 2.Chalghoumi R, Beckers Y, Portetelle D, Théwis A. Focus on: hen egg yolk antibodies (IgY), production and use for passive immunization against bacterial enteric infections in chicken: a review. Biotechnol Agron Soc Environ 2009;13(2):295.
- 3.Raj G.D., Latha B., Chandrasekhar M.S., Thiagarajan V. Production, characterization and application of monoclonal antibodies against chicken IgY. VeterinarskiArhiv. 2004;74(3):189–199. [Google Scholar]
- 4.Schade R, Terzolo HR. IgY-technology: application and trends. In: EPC 2006-12th European poultry conference, Verona, Italy, 10–14 September, 2006. World's Poultry Science Association (WPSA); 2006.
- 5.Larsson A., Karlsson-Parra A., Sjöquist J. Use of chicken antibodies in enzyme immunoassays to avoid interference by rheumatoid factors. Clin Chem. 1991;37(3):411–414. [PubMed] [Google Scholar]
- 6.Hodek H.P., Trefil P., Simunek J., Hudecek J., Stiborova M. Optimized protocol of chicken antibody (IgY) purification providing electrophoretically homogenous preparations. Int J Electrochem Sci. 2013;8:113–124. [Google Scholar]
- 7.Bird C.R., Thorpe R. Purification of immunoglobulin Y (IgY) from chicken eggs. The protein protocols handbook. 2009:1779–1781. [Google Scholar]
- 8.Rose M.E., Orlans E., Buttress N. Immunoglobulin classes in the hen's egg: their segregation in yolk and white. Eur J Immunol. 1974;4(7):521–523. doi: 10.1002/eji.1830040715. [DOI] [PubMed] [Google Scholar]
- 9.Polson A., vonWechmar M.B., Van Regenmortel M.H.V. Isolation of viral IgY antibodies from yolks of immunized hens. Immunol Commun. 1980;9(5):475–493. doi: 10.3109/08820138009066010. [DOI] [PubMed] [Google Scholar]
- 10.Fischer M., Hlinak A. An ELISA for the quantification of chicken immunoglobulin (IgY) in various liquid media. Altex. 1996;13(4):179–183. [PubMed] [Google Scholar]
- 11.Son A.P. Isolation of IgY from the yolks of eggs by a chloroform polyethylene glycol procedure. Immunol Invest. 1990;19(3):253–258. doi: 10.3109/08820139009041840. [DOI] [PubMed] [Google Scholar]
- 12.Tan S.H., Mohamedali A., Kapur A., Lukjanenko L., Baker M.S. A novel, cost-effective and efficient chicken egg IgY purification procedure. J Immunol Methods. 2012;380(1):73–76. doi: 10.1016/j.jim.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Pauly D., Chacana P.A., Calzado E.G., Brembs B., Schade R. IgY technology: extraction of chicken antibodies from egg yolk by polyethylene glycol (PEG) precipitation. J Vis Exp: JoVE. 2011;51 doi: 10.3791/3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Meulenaer B., Huyghebaert A. Isolation and purification of chicken egg yolk immunoglobulins: a review. Food Agric Immunol. 2001;13(4):275–288. [Google Scholar]