Abstract
Twenty streptomycete strains were isolated from marine sediment samples collected from Nabq area, Sharm El-Sheikh, Red Sea Coast, Egypt. Four of them produce exopolysaccharides (EPS) showing marked in vitro antitumor activities. Morphological and cultural characteristics of the most significant strain (No. 3) were shown. Moreover, the sequence of this strain showed similarity with Streptomyces carpaticus. The results reveal that EPS produced by Streptomyces carpaticus No. 3 had high cytotoxicity reaching 51.7% and 59.1% against human tumor cells of breast and colon lines respectively. A chemical analysis of EPS indicated that the composing monosaccharides were galactouronic acid, glucose, xylose, galactose, mannose, and fructose with relative ratio of 3:1:1:2:2:1 respectively, with an average molecular weight (Mw) 1.180 × 105 g/mol and of a number average molecular weight (Mn) 1.052 × 105 g/mol. Also the EPS contained uronic acid (0.5072%) and monosaccharide sulphates (21.753%).
Keywords: Marine streptomycetes, Exopolysaccharide, Antitumor, Radical scavenging activity
1. Introduction
The worldwide attention to cancer as second biggest cause for death in humans has prompted research that thinks about the battle against it. Breast cancer accounts 12% of all new cancer causes and 25% of all cancers in women. Also, colon cancer is the third leading reason for cancer worldwide in human, and fourth in the USA [1]. Most tumor treatments show side effects such as anemia, hair loss, diarrhea, pain, and toxicity. The search for natural anticancer products is needed.
Polysaccharides, which are vital characteristic natural compounds from plants, microorganisms and animal sources are found to show a wide range of biological properties, for instance they are immunostimulant, have anti-inflammatory, antioxidant and antitumor activity [2]. Microbial exopolysaccharides have many uses in numerous fields including food industries, farming and pharmacy because of their different composition, structure, physical and chemical properties [3]. Marine microorganisms regularly deliver secondary metabolites with novel structures and different biological activities [4].
Members of the genus Streptomyces are Gram positive bacteria characterized by a complex morphological cell cycle, belonging to the phylum Actinobacteria, which conceal significant hydrolytic enzymes, antibiotics and medicinally important secondary metabolites. About 70% of all known drugs are produced by actinobacteria, of which 75% were used in medicine [5]. In this way, Streptomyces are considered as common host organisms for industrial and pharmaceutical purposes.
One of the most important discoveries, with respect to bioactivity, is the anticancer properties of exopolysaccharides isolated from numerous prokaryotes and microalgae [6], [7]. Also, some Streptomyces synthesize exopolysaccharides with antioxidant activities [8]. The anticancer activity of exopolysaccharides delivered by Streptomyces is by all accounts promising, however has not been seriously examined yet.
In the current study, 20 streptomycete strains were isolated from Nabq area and checked for exopolysaccharide production. Among these strains, the most promising one, which produced significant amount of exopolysaccharides was subjected to identification. In addition, the radical scavenging activity, the cytotoxic activity against breast and colon cancer cell lines had been investigated.
2. Materials and methods
2.1. Collection of samples and sampling site
The present study is a part of a scientific project concerning the production, characterisation and bioactivity assay of polysaccharides isolated from different marine microbes originating from areas along the Red Sea Coast, Egypt. Sediment samples from the seashore (5 cm depth) of Nabq Nature Reserve area at Sharm El Sheikh were collected in sterile jars and kept in refrigerator till laboratory investigation.
2.2. Isolation of streptomycetes
The serial dilution method of Hayakawa and Nonomura [9] was applied for isolation of streptomycetes. Three agar media were prepared for isolation as follows: starch-nitrate [10], malt yeast extract [11] and brain-heart infusion [12] using 50% sea water. 0.1 ml inoculum of the appropriate dilution was plated on each plate. The plates were incubated at 28 °C for 7–14 days to allow the slow growing forms to develop. Streptomycetes were isolated based on their specific morphological characteristics and then subjected to purification.
2.3. Production of exopolysaccharide (EPS)
Streptomycete strains were grown aerobically for four days in a production medium (containing [g/l]: glucose 30.0, NaNO3 3.0, yeast extract 5.0, NaCl 4.0, MgSO4 0.5, K2HPO4 1.0, and CaCO3 1.0) at pH 7, 28 °C and 150 rpm on a rotary shaker [13]. After incubation the cells were harvested by centrifugation at 5000 rpm for 30 min and the soluble exopolysaccharide was precipitated by adding to the supernatant 4 volumes of absolute ethanol and then agitated vigorously and kept at 4 °C overnight [14]. The precipitate was collected by centrifugation at 5000 rpm for 15 min. The resulting precipitate was re-dissolved in distilled water and dialyzed by dialysis tube (MWCO 3000 Da) using running tap water for 48 h and distilled water for another 48 h [15]. The dialyzed solution was lyophilized to obtain the dry exopolysaccharide.
2.4. Analysis of monosaccharide composition
Twenty milligrams of EPS were hydrolysed with 6N HCl at 100 °C in a sealed tube for 5 h and excess acid was evaporated on water bath at 40 °C and co-distilled with water [16]. The content of monosaccharides was quantified by HPLC on a Shimadzu Shim-Pack SCR-101N column (7.9 mm × 30 cm); deionized water was used as the mobile phase (flow rate 0.5 ml/min), as described by Kwon and Kim [17]. Uronic acid content was determined by the m-hydroxydiphenyl method using glucuronic acid as standard [18]. The sulphate content of the exopolysaccharide, originating from monosaccharide (galactouronic acid, glucose, xylose, galactose, mannose and fructose) sulphates was measured using the turbidimetric method [19] together with sodium sulphate as standard. N-acetyl glucose amine was estimated by the Elson and Morgan reaction [20] and protein was determined using the Bradford method [21].
2.5. Determination of the EPS molecular weight
Polysaccharide molecular weight was determined on an Agilent 1100 HPLC system equipped with a refractive index detector and FPl gel particle (5 μm) columns. Three columns were used of pore type (100, 104, and 105 Å) in series, (1000, 5,000,000 mwt) for N,N-dimethyl formamide (DMF) solvent styrogel high resonance DMF, 3 μm (7.8 × 300 mm) (Waters, Milford, MA, USA). One column (5000–600,000 mwt) was used for water as solvent (polyethylene oxide/glycol standard), OH 7.5 mm and 30 μm pore, 8 um particle size respectively. Sample (0.01 g) was dissolved in 2 ml of solvent and filtrated with 0.45 mm Teflone syringe filter and transferred to a gel-permeation chromatography (GPC) device [22]. The weight average molecular weight (Mw) and number average molecular weight (Mn) were directly calculated according to the definition of Mn and Mw using molecular weight and refractive index signal values at each elution volume [23].
2.6. Infrared spectroscopy
The infrared spectrum of EPS was measured on a Bruker Scientific 500-IR Spectrophotometer. The polysaccharide was mixed with KBr powder, ground and pressed into 1 mm pellets for FTIR measurements in the range of 400–4000 cm−1 [24].
2.7. Radical scavenging activity of EPS toward DPPH radical
The free radical scavenging activity of EPS was measured against 1,1-diphenyl-2-picryl-hydrazyl (DPPH) radicals using the method of Yang et al. [25]. Five ml of DPPH ethanolic solution (freshly prepared at a concentration of 0.1 mM) was added to 1 ml of EPS solution of different concentrations (50–250 µg/ml) in water. After 30 min incubation under ambient temperature in the dark, absorbance was measured at 517 nm using an UV–Vis Spectrophotometer 2401PC (Shimadzu, Japan). Lower absorbance of the reaction mixture indicated higher free radical scavenging activity. The experiment was carried out in triplicate and averaged. The capability to scavenge the DPPH radical was calculated using the following equation:
The EC50 value is the effective concentration (µg) of EPS at which the DPPH radicals were scavenged by 50%.
2.8. Biological activity of EPS
2.8.1. Cell lines
Cell lines of human breast carcinoma (MCF-7) and colon carcinoma (HCT-116) were obtained from Vacsera (Holding Company for Biological Products and Vaccines) at Giza Governorate, Egypt. Skin normal human cell line (BJ-1) “A telomerase immortalized normal foreskin fibroblast cell line” were obtained from Karolinska Center, Department of Oncology and Pathology, Karolinska Institute and Hospital, Stockholm, Sweden.
2.8.2. Cell culture
Culture was maintained in culture medium RPMI with 1% antibiotic-antimycotic mixture (10,000 U/ml potassium penicillin, 10,000 μg/ml streptomycin sulphate and 25 μg/ml amphotericin B), 1% L-glutamine, and supplemented with 10% heat inactivated fetal bovine serum. Culturing and subculturing were carried out according to Thabrew [26]. Doxorubicin was used as a positive control, while dimethyl sulphoxide (DMSO) used as negative one and viability assays were then carried out.
2.8.3. Cell viability assay
Cell viability assay was done according to the method given by Mosmann [27]. Following culturing for ten days, the cells were seeded at concentration of 10 × 103 cells/well in case of MCF-7, 20 × 103 cells/well in a fresh complete growth medium in case of HCT-116 cell lines using 96-well microtiter plastic plates at 37 °C for 24 h under 5% CO2, in a water jacketed carbon dioxide incubator. Fresh medium (without serum) was added and cells were incubated either alone (negative control) or with EPS to give a final concentration of 100 μg/ml. After 24 h incubation, the medium was aspirated and then 40 μl MTT salt (3-(4, 5-dimethylthiazol-2-yl)-2, 5- diphenyltetrazolium bromide), of 2.5 mg/ml concentration was added to each well and plates were incubated for further four hours at 37 °C under 5% CO2. To stop the reaction and dissolve the formed crystals, 200 μl 10% sodium dodecyl sulphate (SDS) in deionized water was added to each well and incubated overnight at 37 °C. The absorbance was measured using an ELISA reader at 595 nm with 690 nm reference. The cytotoxic activity was calculated according to the following equation:
where X indicates the absorbance of treated sample, av indicates the average absorbance of control and NC indicates the absorbance of negative control.
2.9. Identification of the promising streptomycete strain
2.9.1. Morphological characterisation
The spore chain morphology and the number of spores per chain of the strains of 14 day old cultures grown on inorganic salts-starch agar were examined by light microscope [28]. The spore surface was examined using Em10 Carl-Zeiss transmission electron microscope [29].
2.9.2. Cultural characterisation
The cultural characteristics of the strains were tested on the basis of the methods used in the International Streptomyces Project (ISP), using the media recommended by Shirling and Gottlieb [28]. The colours of mature sporulating aerial mycelium and substrate mycelium were monitored for 7, 14 and 21 day old cultures grown on starch nitrate medium. Diffusible pigments were detected on glycerol asparagine agar medium. Colour determination was carried out using ISCC-NBS colour charts [30].
2.9.3. Physiological and biochemical characterisation
Physiological and biochemical characterisation were determined according to the methods given by several authors as follows: (i) melanin pigment production [31], (ii) nitrate reduction [32], (iii) pectinase [33] and (iv) hydrogen sulphide production [34].
2.9.4. Molecular identification
The most promising streptomycete strain was identified by 16S rRNA gene sequencing. Chromosomal DNA was extracted using the DNeasy Power Soil Kit (Qiagen) according to the manufacturer’s instruction. 16S rRNA gene was amplified using universal primers F (5′-GTGCCAGCAGCCGCGGTA-3′) and R (5′-TTGTAGCACGTGTGTAGCCC-3′) (according to Kröger, M., Institute of Microbiology and Molecular Biology, University of Gießen, Germany). PCR reaction was achieved in a volume of 50 μl containing 1 × green Taq PCR Buffer, 200 mM of each dNTPs, 100 mg BSA, 10 pmole of each primer, 2.5 U of Taq DNA polymerase (Sigma) and 10 ng of DNA. PCR was achieved by the following conditions: 1 min at 98 °C followed by 35 cycles of 1 min at 94 °C, 30 s at 55 °C, and 1 min at 72 °C. The PCR product was purified using the QIAquick PCR Purification Kit (Qiagen) and sequenced in Lab Technology Company (Cairo, Egypt). The 16S rRNA sequence was matched with previously published 16S rRNA sequences of bacteria in the National Center for Biotechnology Information (http: //www.ncbi.nlm.nih. gov) [35] using BLAST, moreover at the EzBioCloud Server [36]. Selected sequences of other microorganisms with the greatest similarity to the 16S rRNA sequences of the bacterial strain were extracted from the nucleotide sequence databases and aligned generating phylogenetic tree. The 16S rRNA gene sequence of the bacterial isolate was deposited in the GenBank nucleotide sequence database with accession number KY355738.
3. Result and discussion
3.1. Isolation of strains, production of polysaccharides, and antitumor activity investigation
Twenty streptomycete strains were isolated from Nabq area; four of them have the ability to produce polysaccharides. The polysaccharides produced after four days are A (6.23 g/l), B (7.54 g/l), C (7.45 g/l) 3 and D (7.96 g/l) from isolates 1, 2, 3 and 4 respectively. The yield of exopolysaccharides was dependent on the Streptomyces species, where Streptomyces nasri produced 2.3 g/l of exopolysaccharide while Streptomyces virginia produced 15.6 g/l, thus our strains were medium producers [13], [37].
The obtained polysaccharides were screened for their cytotoxicity; the results are presented in Table 1.
Table 1.
Cytotoxic activities of the polysaccharides produced by four isolates against breast carcinoma (MCF-7) and colon carcinoma (HCT-116).
| EPS | Cytotoxicity (%) |
Cytotoxicity (%) of normal human cell line (BJ-1) | |
|---|---|---|---|
| MCF-7 | HCT-116 | ||
| A | 38.9 | 36.0 | 46.6 |
| B | 37.3 | 41.6 | 37.8 |
| C | 51.7 | 59.1 | 28.6 |
| D | 28.9 | 30.3 | 46.0 |
The results showed that the exopolysaccharide (C) extracted from strain (No. 3) possesses the highest cytotoxic effect on both breast (MCF-7) and colon (HCT-116) human tumor cell line with 51.7 and 59.1% cytotoxicity respectively. Moreover, the exopolysaccharide (C) showed the lowest cytotoxicity effect on normal human cell lines (28.6%).
The earliest polysaccharide recorded to have antitumor activity was isolated in 1943 from the bacterium Serratia marcescens and got to be distinctly known as Shear's polysaccharide. Wang et al. [38] reported that exopolysaccharides produced from Streptomyces sp. (139) have immune stimulatory, anti-tumoral, anti-rheumatic arthritis and antioxidant activity. Manivasagan et al. [8] extracted extracellular polysaccharides showing antioxidant activity from a marine strain of actinobacterium Streptomyces violaceus MM72. Furthermore, it was found that exopolysaccharides produced by Bacillus sp. and Pseudomonas sp. have cytotoxicity for both colon cancer and human breast cancer cell lines and are promising novel therapeutic agents, since are active at low concentration [39].
3.2. Characterisation, and identification of the selected strain
The promising strain (No. 3) which showed the highest cytotoxic activity was identified according to its morphological, cultural and biochemical characteristics. The spore chains were spiral and the spore surface ornamentation was smooth. Colour of spore mass was dark grey and its substrate mycelium produced yellow brown endopigment while no diffusible pigments were produced. The strain was unable to produce melanin pigment on iron and tyrosine agar. Also, this strain had no ability to decompose pectin but was able to reduce nitrate and produce H2S.
Comparison of 16S rRNA gene sequence of the promising strain (Strain No. 3), and other streptomycetes from GenBank by BLAST analysis and using the EzBioCloud revealed that the closest strains were Streptomyces harbinensis, and Streptomyces carpaticus at less than 94% similarity value. Taking into consideration the species level 16S rRNA gene similarity level of around 98%, this strain most probably represents a new species (Fig. 1). However, Zhu et al. [40] mentioned that Streptomyces carpaticus OUCMDZ-726 isolated from marine carp produced a water soluble crude exopolysaccharide. Haritha et al. [41] indicated that a marine Streptomyces carpaticus strain produced a protease. Moreover, Bhavana et al. [42] emphasized that Streptomyces carpaticus (MTCC-11062 strain) isolated from Visakhapatnam sea coast of Bay of Bengal had antimicrobial activity contrary to Gram +ve, Gram -ve bacteria and yeasts.
Fig. 1.
Phylogenetic tree of the partial sequence of 16S rRNA of the local isolate Streptomyces carpaticus respect to closely related sequences available in GenBank databases.
3.3. Partial characterisation of the EPS
It is notable that the biological activities of exopolysaccharides are firmly determined by the chemical composition and polymer structure. Some of the characteristics that must be taken into account in this respect are their molecular weight, as large molecules are difficult to transfer across membranes in order to carry out specific intracellular functions, and their sulphate and uronic acid content (or other constituents that can give the polymers their anionic and acidic properties), as these components seem to have great influence on their biological activity and applications. Furthermore, quantity of monosaccharides, kind of chemical bonds, and also the distribution of different monosaccharides inside the molecule, as well the presence of additional chemical groups such as amino acids, peptides, and/or nucleotides, which could be non-covalently connected to polysaccharide chains. There are other features that are worthy to evaluate, like the rheological properties and resistance to digestion, either acidic or enzymatic.
Exopolysaccharide from strain Streptomyces carpaticus (Strain No. 3) produced its greatest yield (7.45 g/l) after 4 days of incubation. It was subjected for further analysis. It had a positive response to absorption at 280 nm in the UV spectrum and contains peptides (0.012%) and N-acetyl glucosamine (14. 378%) as peptidoglycan. As noticed by m-hydroxydiphenyl colorimetric method, the EPS contained uronic acids (0.5072%) and monosaccharide sulphates (21.753%). These indicate that the EPS is an acidic exopolysaccharide. Numerous authors demonstrated the connection between the acidic polysaccharides and the antitumor activity [43].
3.4. Monosaccharide composition
Molar ratio of monosaccharides after complete hydrolysis with 6N HCl using HPLC was: galactouronic acid, glucose, xylose, galactose, mannose, and fructose in 3:1:1:2:2:1 ratio, which means that it is heteropolymer. It was stated that the greater part of marine microbes produced heteropolysaccharides, comprised of various monosugars arranged in groups of ten or less forming repeating units [44]. Also, glucose and mannose are recognized to have receptors on macrophages which are specific for tumor immunology [45]. Moreover, the suppression of cell proliferation is initiated using EPS because of the induction of apoptosis [46], [47].
3.5. Molecular weight determination of the EPS
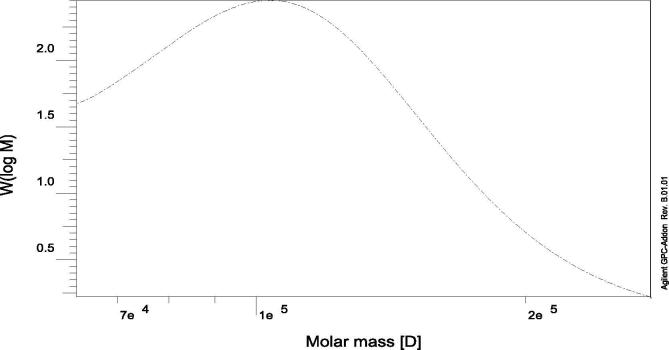
The weight average molecular weight (Mw) of EPS produced by Streptomyces carpaticus (Strain No. 3) was 1.180 × 105 g/mole (Fig. 2); number average molecular weight (Mn) was 1.052 × 105 g/mole and the polydispersity index Pi (Mw/Mn) = 1.121. This result is similar to those found by Sutherland [48] who emphasized that a large portion of polysaccharides delivered from marine sources have an average molecular weight ranging from 1–3 × 105 Da. There is a link between the molecular weight and biological activity of polysaccharides; the activity relies on the extent of molecules as well as the degree of branching and conformation [49].
Fig. 2.
Molecular weight distribution of EPS produced by Streptomyces carpaticus (strain No. 3), where W (logM) is depicted as a function of Molar mass [D]
3.6. Infrared spectroscopy analysis of the EPS
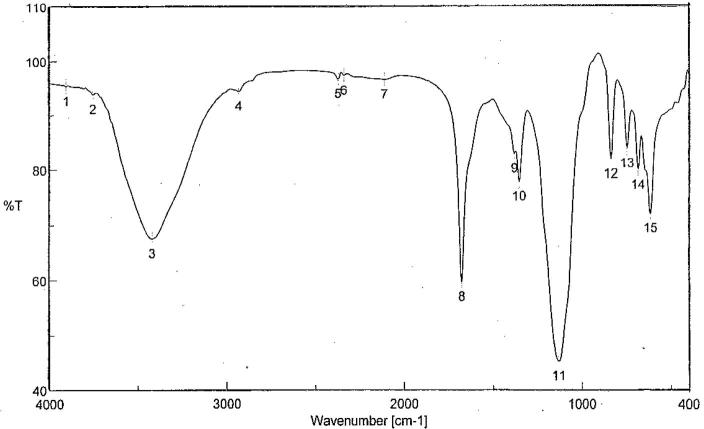
Infrared spectra show the presence of many groups, stretching bands at 3700–3900 cm−1 are probably associated with the —OH group of carbohydrate residues which may be linked with the uronic acid and the internal hydrogen bonds (Fig. 3). The band at 2372 cm−1 was correlated with the stretching vibration of C—H bonds in the sugar ring. The presence of peak at 1132 indicates C—N group. The band at 1675 cm−1 was due to the stretching vibration of the COO— and C O 1380 cm−1 indicated the presence of sulphate ester [50] and 1353 cm−1 indicate monosaccharide sulphates. Also the absorbance at 836 cm−1 indicates the α-glycosidic bonds. Therefore, the infrared spectroscopy analysis suggested that it was highly likely that the EPS belonged to α-type acidic heteropolysaccharide [51].
Fig. 3.
Fourier-transform infrared spectrum of EPS produced from Streptomyces carpaticus (strain No. 3).
3.7. Radical scavenging activity
The model of scavenging the DPPH-radical is extensively used technique for evaluating the free radical scavenging capability [52], [53]. The DPPH radical-scavenging activities of EPS are illustrated in Fig. 4, from which it is clear that the investigated EPS exhibits scavenging efficiency with EC50 value of 111 μg/ml. These outcomes demonstrated that EPS had a detectable effect on the scavenging of free radicals, particularly at high concentration. Such results are in accordance with those found by many workers [3], [54], [55], [56].
Fig. 4.
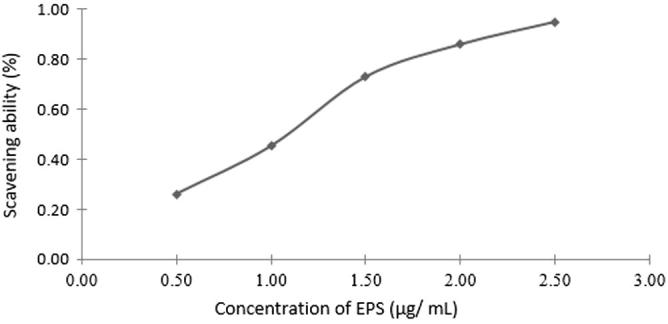
Free radical scavenging effects of EPS from Streptomyces carpaticus (strain No. 3) using DPPH.
4. Conclusion
It was concluded that the marine strain No. 3 isolated from Nabq area, Red Sea Coast, Egypt, was identified as a relative of Streptomyces carpaticus, but which is most probably a new species, produced exopolysaccharide. The EPS exhibits free radical scavenging activity and antitumor activity against both breast and colon cell lines. Marine actinobacteria have become increasingly popular sources of EPS. Therefore, search of new EPS producing microorganisms is still promising.
Footnotes
Peer review under responsibility of National Research Center, Egypt.
Contributor Information
Manal S. Selim, Email: manalsleem@yahoo.com.
Shaimaa K. Amer, Email: Shaymaa_amer@sci.asu.edu.eg.
Sahar S. Mohamed, Email: sahar_2009sm@yahoo.com.
Marwa M. Mounier, Email: marwa_m3@yahoo.com.
Hala M. Rifaat, Email: halamohamed6@yahoo.com.
References
- 1.Daba A.S., Ezeronye O.U. Afr J Biotechnol. 2003;2:672–678. [Google Scholar]
- 2.Ooi V.E., Liu F. Curr Med Chem. 2000;7:715–729. doi: 10.2174/0929867003374705. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Q., Li N., Liu X., Zhao Z., Li Z., Xu Z. Carbohydr Res. 2004;339:105–111. doi: 10.1016/j.carres.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Ruocco N., Costantini S., Guariniello S., Costantini M. Molecules. 2016;21:1–16. doi: 10.3390/molecules21050551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanaka Y.T., Mura S.O. Annu Rev Microbiol. 1953;47:57–87. doi: 10.1146/annurev.mi.47.100193.000421. [DOI] [PubMed] [Google Scholar]
- 6.Roposo M.F.I., de Morais R.M.S.C., de Morais A.M.M.B. Marine Drugs. 2013;11:233–252. doi: 10.3390/md11010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel AK, Laroche C, Marcati A, UrsuJubeau AVS, Marchal L, Petit E, Djelveh G, Michaud P. Biores. Technol. 2013;145: 345–50. [DOI] [PubMed]
- 8.Manivasagan P., Sivasankar P., Venkatesan J., Senthilkumar K., Sivakumar K., Kim S. Int J Biol Macromol. 2013;59:29–38. doi: 10.1016/j.ijbiomac.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Hayakawa M., Nonomura H. J Ferment Technol. 1987;65:501–509. [Google Scholar]
- 10.Naguib M.I., Zeinat K.M., Mansour F.A. Egypt J Bot. 1978;21:9–17. [Google Scholar]
- 11.Pridham TG, Anderson P, Foley C, Lindenfelser HA, Hesseltine CW, Benedict RG. Antib Annu 1956–1957: 947–53. [PubMed]
- 12.Lantz M.S., Ciborowski P. Meth Enz. 1994;235:563–594. doi: 10.1016/0076-6879(94)35171-6. [DOI] [PubMed] [Google Scholar]
- 13.Gohar Y., Beshay U., Daba A., Hafez E. Polish. J Microbiol. 2006;55:179–187. [PubMed] [Google Scholar]
- 14.Whistler R.L., Lauterbach G.E. Arch Biochem Biophys. 1958;77:62–67. doi: 10.1016/0003-9861(58)90041-9. [DOI] [PubMed] [Google Scholar]
- 15.Liu S., Ye S.H., Wang J.H. Food Sci Technol. 2010;35:111–114. [Google Scholar]
- 16.Sudhamani S.R., Tharanathan R.N., Prasad M.S. Carbohydr Polym. 2004;56:423–427. [Google Scholar]
- 17.Kwon H., Kim J. Anal Biochem. 1993;215:243–252. doi: 10.1006/abio.1993.1582. [DOI] [PubMed] [Google Scholar]
- 18.Filisetti-Cozzi T.M.C., Carpita C. Anal Biochem. 1991;197:157–162. doi: 10.1016/0003-2697(91)90372-z. [DOI] [PubMed] [Google Scholar]
- 19.Dodgson K.S., Price R.G. Biochem J. 1962;84:106–110. doi: 10.1042/bj0840106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elson L.A., Morgan W.T. Biochem J. 1934;28:988–995. doi: 10.1042/bj0280988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bradford M.M. Anal Biochem. 1976;7:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 22.Jun H.I., Lee H., Song G.S., Kim Y.S. Food Sci Technol. 2006;39:554–561. [Google Scholar]
- 23.You L., Gao Q., Feng M., Yang B., Ren J., Gu L., Cui C., Zhao M. Food Chem. 2013;138:2242–2249. doi: 10.1016/j.foodchem.2012.11.140. [DOI] [PubMed] [Google Scholar]
- 24.Ray B. Carbohydr Polym. 2006;66:408–416. [Google Scholar]
- 25.Yang Z.F., Zheng Z.H., Cao S.F. J Agr Food Chem. 2009;57:176–181. doi: 10.1021/jf803007j. [DOI] [PubMed] [Google Scholar]
- 26.Thabrew M., Hughes R.D., Mcfarlane I.G. J Pharm Pharmacol. 1997;49:1132–1135. doi: 10.1111/j.2042-7158.1997.tb06055.x. [DOI] [PubMed] [Google Scholar]
- 27.Mosmann T. J Immune Meth. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 28.Shirling E.B., Gottlieb D. Int J Syst Bacteriol. 1966;16:313–340. [Google Scholar]
- 29.Tresner H.D., Davies M.C., Backus E.J. J Bacteriol. 1961;1:70–80. doi: 10.1128/jb.81.1.70-80.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kenneth L.K. J Res NBS. 1958;16:427. [Google Scholar]
- 31.Pridham T.G., Anderson P., Foley C., Lindenfelser L.A., Hesselting C.W., Benedict R.G. Antibiot Ann. 1957:947–953. [PubMed] [Google Scholar]
- 32.Gordon R.E. J General Microbiol. 1966;45:355–364. doi: 10.1099/00221287-45-2-355. [DOI] [PubMed] [Google Scholar]
- 33.Hankin L., Zucker M., Sands D.C. Appl Microbiol. 1971;22:205–509. doi: 10.1128/am.22.2.205-209.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cowan ST, Steel S, Manual for the identification of medical bacteria 2nd ed. Cambridge, Univ. Press; 1974
- 35.Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoon S.H., Ha S.M., Kwon S., Lim J., Kim Y., Seo H., Chun J. Int J Syst Evol Microbiol. 2017;87:1613–1617. doi: 10.1099/ijsem.0.001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He F, Yang Y, Yang G, Yu L, Naturforsch Z. 2008; 63c: 181–88.
- 38.Wang L., Li S., Li Y. FEMS Microbiol Lett. 2003;220:21–27. doi: 10.1016/S0378-1097(03)00044-2. [DOI] [PubMed] [Google Scholar]
- 39.Vidhyalakshmi R., Vallinachiyar C. J Cancer Sci. 2013;5:31–34. [Google Scholar]
- 40.Zhu W, Mao W, Sun H, New Streptomyces carpaticus OUCMD-726 derived from carp useful in preparation of marine polysaccharide. Patent No. CN103396957 A, Univ. China Ocean (UYOC); 2013.
- 41.Haritha R., SivaKumar K., Swathi A., Jagan Mohan Y.S.Y.V., Ramana T. Microbiol J. 2012;2:23–35. [Google Scholar]
- 42.Bhavana M., Prasad Talluri V.S.S.L., Sivakumar S.V., Rajagopal S.V. Inter. J Pharm Sci. 2014;6:281–285. [Google Scholar]
- 43.Zhang Y., Zhou T., Wang H., Cui Z., Cheng F., Wang K.P. Carbohydr Polym. 2016;20:401–408. doi: 10.1016/j.carbpol.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 44.Decho A.W. Oceanogr Mar Biol Ann Rev. 1990;28:73–153. [Google Scholar]
- 45.Kim J.Y., Yoon Y.D., Ahn J.M., Kang J.S., Park S.K. Immunopharmacol. 2007;7:78–87. doi: 10.1016/j.intimp.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 46.Fang N., Li Q., Yu S., Zhang J., He L. J Altern Complement Med. 2006;12:125–132. doi: 10.1089/acm.2006.12.125. [DOI] [PubMed] [Google Scholar]
- 47.Oh J.Y., Baek Y.M., Kim S.W., Hwang H.J., Hwang H.S. J Microbiol Biotechnol. 2008;18:512–519. [PubMed] [Google Scholar]
- 48.Sutherland I.W. Pure Appl Chem. 1997;69:1911–1917. [Google Scholar]
- 49.Ohno N., Nameda S., Harada T. Int J Med Mushr. 2003;5:359–368. [Google Scholar]
- 50.Parikh A., Madamwar D. Bioresour Technol. 2006;97:1822–1827. doi: 10.1016/j.biortech.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 51.Shina E.-J., Jeong J.H., Chung Y.H., Kim W.-K., Ko K.-H., Bach J.-H., Hong J.-S., Yoneda Y., Kim H.-C. Neurochem Int. 2011;59:122–137. doi: 10.1016/j.neuint.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leong L.P., Shui G. Food Chem. 2002;76:69–75. [Google Scholar]
- 53.Nagai T., Inoue R., Inoue H., Suzuki N. Food Chem. 2003;80:29–33. [Google Scholar]
- 54.Wang Q., Li H., Chen T.T., Han J.R. Sci Hortic. 2011;134:222–226. [Google Scholar]
- 55.Agili F.A., Mohamed S.F. Aust J Basic Appl Sci. 2012;6:277–283. [Google Scholar]
- 56.Shengjie Li, Renhui H, Nagendra PS, Xueying T, Yonghua X, Yonghua W, J Dairy Sci 2014; 97:7334–43.