Abstract
Vitamins are evaluated for their role in immunity. Recently, vitamin A received a particular attention as a critical micronutrient for regulating immune system. Therefore, the present study aimed to search for new about vitamin A. Forty-eight Egyptian adults aged from 18 to 42 years old from both sexes were subjected to clinical examination and nutrition questionnaire and were screened for vitamin A by using ELISA method. Forty subjects were selected and subdivided into two groups. Group 1 with vitamin A at level >200 µg/dl consists of 10 healthy subjects. Group 2 with vitamin A deficiency at level <50 µg/dl consists of 30 subjects. Tβ4 and CD4 levels were also determined by a commercial ELISA kit. Results showed a significant decrease in serum levels of Tβ4 and CD4 in group 2 than group 1 at P < .003 and P < .019 respectively. Both of Tβ4 and CD4 had positive correlation with vitamin A level at P < .000 and P < .003 respectively as well as with each other at p < .000. We concluded that vitamin A deficiency may be influence the levels of Tβ4 and CD4.
Abbreviations: VAD, vitamin A deficiency; Tβ4, Thymosin-β4; CD4, cluster of differentiation 4; BMI, Body mass index; CBC, complete blood picture; NRC, National Research Center
Keywords: Vitamin A deficiency, Immune function, Thymosin-β4 (Tβ4), cluster of differentiation (CD) 4
1. Introduction
Overall nutritional status is required for the immune system to function efficiently. Deficiency in vitamins can impair phagocytic function in innate immunity [33] and adversely affect several aspects of adaptive immunity [41]. Vitamin A was firstly known as “the anti-infective vitamin” as it is important for immune system to function normally [20]. Vitamin A affected the immune response in both lines of immunity [33]. In innate immunity, it helps to maintain the structural and functional integrity of the skin [51] and mucosal cells of the eye, respiratory, gastrointestinal, and genitourinary tracts [44], [45], [12]. It is also important for many cells including natural killer (NK) cells, macrophages, and neutrophils to function normally [44]. In adaptive immunity, Vitamin A needed for proper function of T and B lymphocytes [37]. Cluster of differentiation 4 (CD4) is a glycoprotein located on the surface of immune cells especially T-helper cells [3]. They are functioning in signal transduction between the T cell receptor (TCR) and an antigen presenting cell; and in T-cell activation [52]. Vitamin A may affect cell-mediated immunity by decreasing the number or distribute ion of CD4+ T-lymphocytes, altering cytokine production, or by decreasing the expression of cell-surface receptors that mediate T-cell signaling [49]. Vitamin A activates T-cell lymphocytes so they can fight off infection [47], while its deficiency prevents proper lymphocyte function [29]. Thymus gland produces beta thymosin hormones [32]. Thymosin-β4 (Tβ4) is the prevailing form, representing 70–80% of the total thymosin content [23]. It is a protein with 43 amino acids, bind to and sequester G-actin to modulate cell migration [2], [8]. It is considered to play a significant role in the cellular metabolism due to its actin-sequestering properties [34]. Tβ4 mRNA has different expression in immune cells suggesting a relationship between Tβ4 and immune response [18]. Several physiological properties of Tβ4 have been reported [14]. It acts as a modulator of wound healing [53] and angiogenesis of heart tissues following injury [25], helping in the development of B cells [19], increasing the efficiency of antigen presentation by macrophages [50], implicated in lymphocyte maturation and differentiation [16], controlling cell morphogenesis and motility [17], regulating immunity [36] and treating liver fibrosis [26]. Vitamin A deficiency (VAD) results in impaired mucosal epithelial regeneration and reductions in the number and killing activity of NK cells, as well as the function of neutrophils and macrophages [5]. In addition, VAD results in altered cytokine signaling which would affect inflammatory responses of innate immunity [45]. The risks of VAD can be reversed by supplementation [4]. Reports regarding the influence of VAD on thymosin β4 and CD4 levels in adults are rare. Consequently, the present study aimed to explore this relationship in a group of Egyptian adults to search a new insight about vitamin A.
2. Subjects and methods
Our study was held through a project at National Research Center (NRC) and was approved by the Ethical Committee. The Center of Medical Excellence of NRC guaranteed and gave the permission to perform our study at the outpatient clinic. After taking a written informed consent, forty-eight Egyptian adults from both sexes, aged from 18 to 42 years old were subjected to clinical examination and nutrition questionnaire to evaluate their nutrition status and to detect any symptom or sign of vitamin A deficiency that are dry eyes, dry and rough skin, eye inflammation, night blindness, respiratory and urinary infections. They were also screened for vitamin A by using ELISA method. Accordingly, forty subjects were selected and enrolled in this study. They were divided according to the international reference range of vitamin A for adult [9] & [30] into two groups: Group 1 includes healthy subjects (n = 10) with sufficient vitamin A at level >200 µg/dl. Group 2 includes subjects (n = 30) with vitamin A deficiency at level <50 µg/dl. Adults who had genetic disorders, chronic or autoimmune diseases, systemic failure or any malignant tumors were excluded. Subjects on daily vitamin A supplement were also excluded from the study. A careful medical history and clinical examination were taken including: demographic data in the form of age and sex. Vital signs including blood pressure, Radial pulse, respiratory rate and body temperature were recorded. Adults were asked about repeated attacks of upper respiratory tract infection and/or gastrointestinal infection. Anthropometric measures regarding height and weight were recorded for each subject. The height was measured to the nearest 0.5 cm on a Holtain portable anthropometer, and the weight was determined to the nearest 0.1 kg on a Seca scale Balance with the subject dressed minimum clothes and no shoes. Body mass index (BMI) was calculated as Weight (kg)/Height (m2).
3. Samples collection
Blood samples (5 ml) were drawn from all subjects, a part of blood (2 ml) was taken immediately in EDTA-containing vacutainers to estimate complete blood picture (CBC) and the remaining (3 ml) was centrifuged 3000 rpm for 10 min then sera were isolated and stored at −20 until the determination of vitamin A and other laboratory investigations.
4. Research methods and procedures
4.1. Biochemical assays
Serum levels of vitamin A, Tβ4 and CD4 were measured by using a commercial enzyme linked immunosorbent assay ELISA kit, produced by Glory Science Co., Ltd. 2400 Veterans Blvd. Suite 16 – 101, Del Rio, TX 78840, USA. Tel: 001-830-734-0090 www.glorybioscience.com., performed at National Research Centre, medical physiology department.
4.2. Statistical analysis
All values are expressed as mean ± SE and the differences between the two groups were calculated by student’s t test. The correlation was done between different parameters using Pearson’s correlation. A Chi-square (χ2) test was used to test the significance associations among non-parametric data. All analyses were carried out using Statistical Package for Social Science (SPSS) version 16 (IBM, Chicago IL, USA. Statistical software). The statistical significance was set at p < .05.
5. Results
Forty participants were enrolled in our study. Thirty-four were females (85%) and six were males (15%). We screened all the cases for symptoms of vitamin A deficiency (VAD). The results revealed a high rate of vitamin A deficiency 62.5% (group 2 (n = 30)) versus 20.8% (group 1 (n = 10)) with sufficient level of vitamin A. There were 9 cases suffering from night blindness out of 30 adults (group 2) representing 30% of vitamin A deficient subjects.
The results revealed that age was nearly comparable in the two groups at P = .414. In the same time, no significant difference was observed between the two groups for CBC parameters. The results of anthropometric measures were recorded in Table 1 and showed that there was no statically difference between the two groups in height and BMI at p = .3 and p = .89 respectively. While a significant difference between the two groups in weight at p < .017.
Table 1.
Anthropometric measures in the two groups.
| Mean ± SD | Group I (n = 10) | Group II (n = 30) | Significance |
|---|---|---|---|
| Weight /kg | 71.7 ± 5.49 | 62.88 ± 16.7 | P < .017 |
| Height/m2 | 1.60 ± 8.5 | 1.57 ± 8.9 | P = .3 NS |
| BMI kg/m2 | 26.4 ± 3.7 | 26.2 ± 6.37 | P = .89 NS |
BMI: body mass index P < .01 = significant difference NS = no significant difference.*
There was a high significant decrease in serum levels of vitamin A, Tβ4 and CD4 at P < .001, P < .003, P < .001 in the two groups as shown in Fig. 1, Fig. 2, Fig. 3 respectively.
Fig. 1.
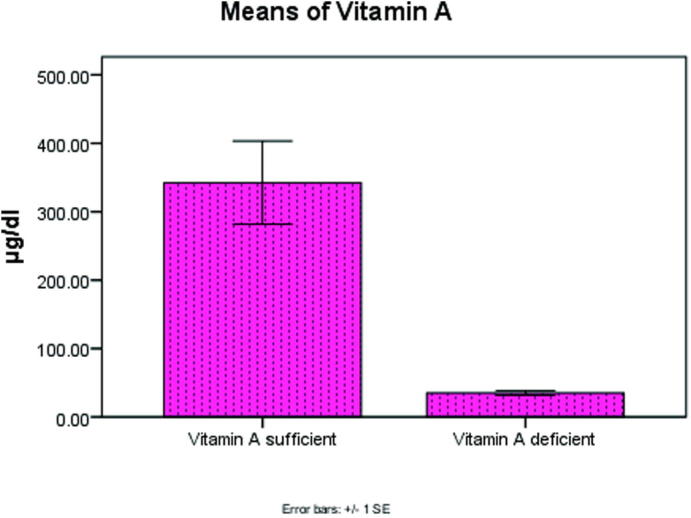
Significant decrease in vitamin A level at P < .001 in the two groups.
Fig. 2.
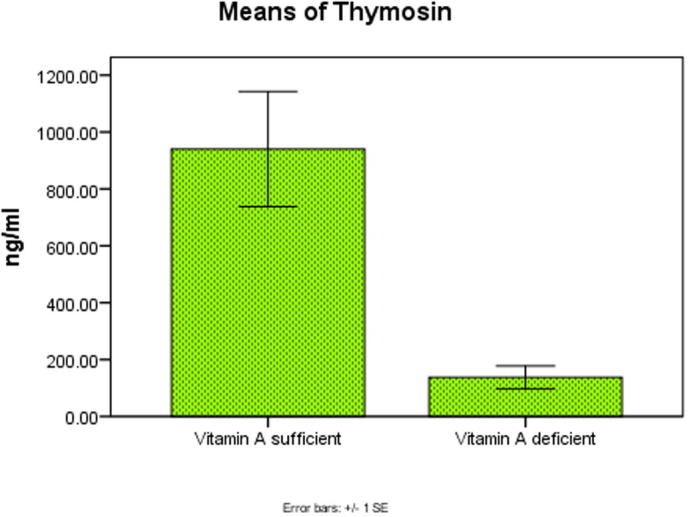
Significant decrease in Thymosin beta 4 level at P < .003 in the two groups.
Fig. 3.
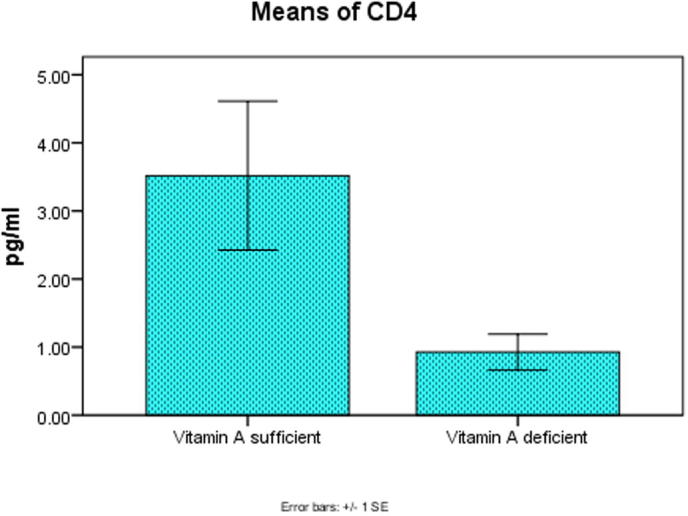
Significant decrease in CD4 level at P < .001 in the two groups.
Figs.4 and 5 represented a high significant positive correlation between vitamin A and both of Tβ4 and CD4 at (r = .579∗∗, P < .000) and (r = 0.451∗∗, P < .003) respectively, at the same time Fig. 6 demonstrate a high significant positive correlation between Tβ4 and CD4 at (r = 0.567∗∗, P < .000).
Fig. 4.
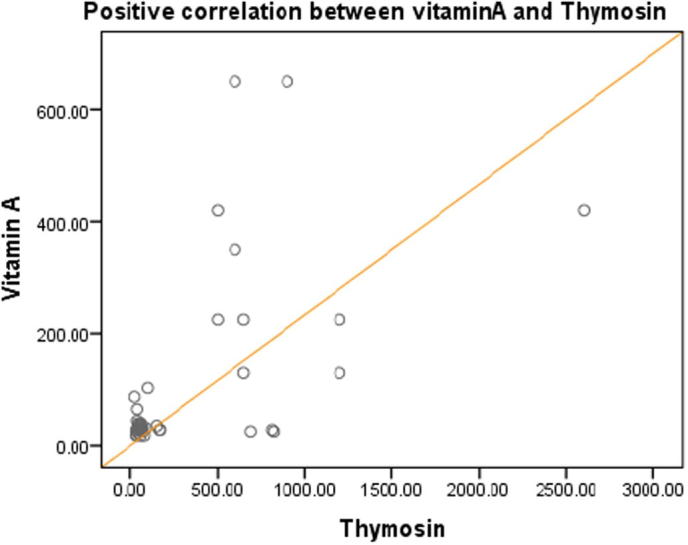
Positive correlation between vitamin A and Thymosin at r = 0.579**, P < .000.
Fig. 5.
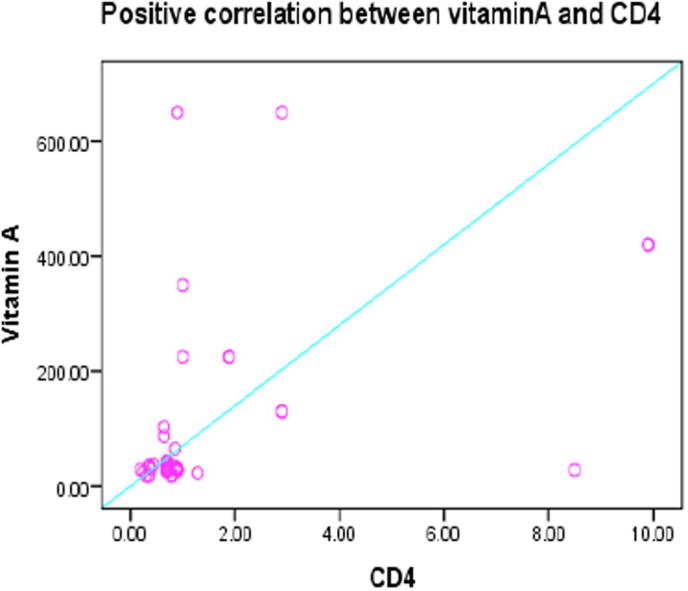
Positive correlation between vitamin A and CD4 r = 0.451**, P < .003.
Fig. 6.
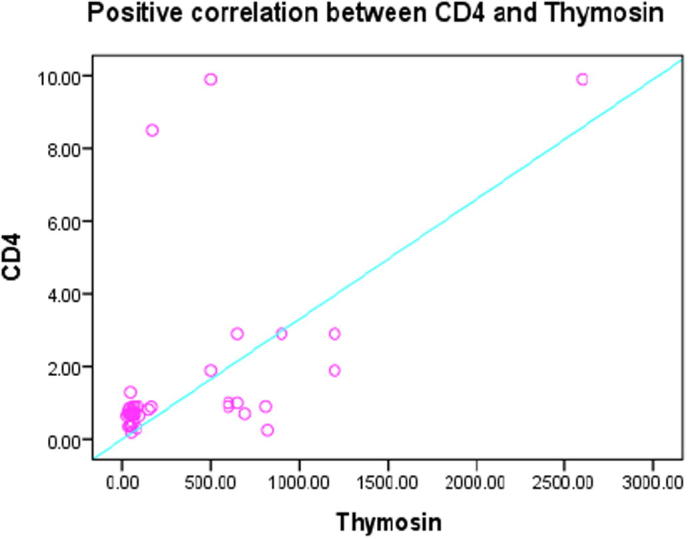
Positive correlation between CD4 and Thymosin r = 0.567**, P < .000.
The results of Chi-square χ2 test to examine the relation between non-parametric variables in the studied groups were represented in Table 2. There was a high significant (p < .001) association for VAD symptoms, food type and eating vegetables and fruits at (χ2 = 5.400b, df = 3, P < .002), (χ2 = 9.800b, df = 3, P < .029) and (χ2 = 43.000a, df = 2, P < .000) respectively.
Table 2.
Association of the non-parametric variables in the two groups.
| Variables | Chi – square value | df | Association |
|---|---|---|---|
| VAD symptoms | 5.400b | 3 | P < .002 |
| Food type | 9.800b | 3 | P < .029 |
| Eating vegetables and fruits | 43.000a | 2 | P < .000 |
p < .001 = significant difference.
0 cells (0.0%)have expected frequencies less than 5.
0 cells (0.0%)have expected frequencies less than 5 the minimum expected cell frequency is 10.0.
6. Discussion
Our previous studies demonstrated the effect of vitamins B12 and D deficiencies on thymosin β4 (Tβ4) and the cluster of differentiation CD4 levels as they are important elements to maintain proper immune system [13] and [46]. Recently, vitamin A has received a particular attention regarding its crucial effect for regulating immune system [33]. The present study showed a high rate of vitamin A deficiency (VAD) affecting 62.5% of the study group versus 20.8% sufficient controls at p < .001 which could be attributed to several reasons. According to the previous studies, it could be due to fat malabsorption, impaired bile production and release or chronic exposure to oxidants [10], zinc deficiency which act as a cofactor in conversion of retinol to retinal [54] or iron deficiency that affect vitamin A uptake [6]. Furthermore, our study revealed a high significant (p < .001) association between the two groups for VAD symptoms, food type and eating vegetables and fruits which revealed that the 62.5% of our subjects deficient in vitamin A, did not care with the type of food that they eat and their meals did not include adequate amount of fresh vegetables and fruits in accordance with [1] who stated that VAD could be due to dietary problems as a result of inadequate intake of vitamin A and parallel with Sommer [48] who stated that primary VAD occurs among subjects who do not consume sufficient amount of carotenoids from fruits and vegetables or preformed vitamin A from animal and dairy products. A far more focused role of vitamin A has emerged for several aspects of T cell differentiation and function as well as development of lymphoid organ [7]. VAD found to induce inflammation [39]. Retinoic acid (RA), the main metabolite of vitamin A found to promote CD4+ T cell effector response via retinoic acid receptor alpha (RARα) [21]. RA involved in the activation of effector CD4+ T cells during inflammation. Thus, in a pro-inflammatory context, the RA-signaling pathway in CD4+ T cells is enhanced at the site of inflammation [35]. CD4+ T cell effector function and migration to the site of inflammation were inhibited by removal of RA signaling in T cells [38]. Recent evidence has shown that vitamin A through RA plays a key role in the migration of T cells into tissues, the proper development of T cell–dependent antibody responses [40] and promotes T cell activation and differentiation into different T helper subsets such as Th1, Th2 and Th17 cells [7]. On the other hand, in vitro studies have shown that RA is able to induce regulatory T cells [22]. Furthermore, RA found to have a role in gut immunity, it is able to induce gut homing receptors on T and B cells, allowing their trafficking to the intestine to perform their functions [24]. The data in the current study showed a high significant decrease in serum levels of Tβ4 and CD4 at P < .003 and P < .001 respectively in vitamin A-deficient group when compared to vitamin A-sufficient group. Adding our results to the above mentioned reports reveals that vitamin A may play an important role in cell-mediated immunity, particularly with respect to CD4 and Tβ4, and this was supported by a high significant positive correlation between Tβ4 and CD4 that we had in our study which could be attributed to the fact that Tβ4 is the predominant form of thymic hormones in mammalian cells [15] & [27] and that its initial function is to stimulate the production of T lymphocytes which are targets of thymosin activity [28]. Tβ4 mRNA has also different expression in lymphocytes, macrophages, granulocytes and platelets suggesting a relationship between Tβ4 and the immune response [18]. Supporting our results, an experimental study by Kramer [29] found that splenic T-cells mitogenic responsiveness was depressed in vitamin A-deficient rats as compared to controls. Another study conducted by Miller [31] revealed that vitamin A affects T-lymphocyte function, VAD shifts the immune response towards the Th1 cell-mediated activity and impairs Th2 response, while supplementation with this vitamin increase total T-cell numbers, particularly CD4+cells and tends to boost Th2 type responses. A study by de Azevedo et al. [11] found also that children with VAD and anemia showed a significant increase in absolute CD4 and CD8 T-cell counts after vitamin A supplementation. Our current study found a strongly significant positive correlation between vitamin A and both of Tβ4 and CD4 at p < .000 and p < .003 respectively, in accordance with Savino and Dardenne [43] who stated that severe thymus atrophy due to the depletion of CD4+CD8+ thymocytes and decrease in T cell proliferation was observed in vitamins deficiencies following malnutrition status. Another explanation for the decreased thymus output of thymosin hormone and CD4+ T lymphocytes could be the extreme susceptibility of thymus to free radical and oxidative damage caused by stress, infection and poor diet [42]. These studies in addition to ours reveal that vitamin A may play an important role in cellular immunity, especially with respect to CD4 level and Tβ4 hormone, and this is confirmed by the significant positive correlation between vitamin A and both of Tβ4 and CD4 that we had. In conclusion, the thymus might be a target of VAD. This was clearly observed from a great influence of VAD on Tβ4 and CD4 levels that we had. It is obviously that our study is the first to demonstrate these relations.
7. Recommendations
We urgently need to increase health awareness, the importance of vitamin A supplementation and healthy eating and we need further studies in future, either clinical or experimental to explore the crucial effect of vitamin A on thymus output in adults.
Acknowledgements
This work was supported by the National Research Center through a project entitled: “Interaction between Nutrition and Infectious Diseases in Egyptian family in Relation to Immunological and Physiological Function“, (project ID: 10010402).
Declaration.
This work described has not been published previously, and the publication was approved by all authors and if accepted it will not be published elsewhere in English or any other language.
Conflicts of Interest.
There are no conflicts of interest.
Footnotes
Peer review under responsibility of National Research Center, Egypt.
References
- 1.Akhtar S. Prevalence of vitamin A deficiency in South Asia: causes, outcomes, and possible remedies. J Health, Popul, Nutr. 2013;31(4):41323. doi: 10.3329/jhpn.v31i4.19975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballweber E, Hannappel E, Huff T, Stephan H, Haener M, Taschner N et al. Polymerisation of chemically cross-linked actin: thymosin beta(4) complex to filamentous actin: alteration in helical parameters and visualisation of thymosin beta (4) binding on F actin. J Mol Biol 2002; 315: 613–25. https://doi.org/10.1006/jmbi.2001.5281. [PMid: 11812134]. [DOI] [PubMed]
- 3.Bernard A, Boumsell L, Hill C. 1984. Joint Report of the First International Workshop on Human Leucocyte Differentiation Antigens by the Investigators of the Participating Laboratories. In Bernard A, Boumsell L, Dausset J, Milstein C, Schlossman SF. Leucocyte typing: human leucocyte differentiation antigens detected by monoclonal antibodies: specification, classification, nomenclature. Berlin: Springer. pp. 45–48. Report on the first international references workshop sponsored by INSERM, WHO and IUIS.
- 4.Benn CS. We need studies of the mortality effect of vitamin A supplementation, not surveys of vitamin A deficiency. Nutrients 2017; 15; 9(3): E280. https://doi.org/10.3390/nu9030280. [DOI] [PMC free article] [PubMed]
- 5.Bhaskaram P. Immunobiology of mild micronutrient deficiencies. Br J Nutr. 2011;85(2):S75–S80. doi: 10.1079/bjn2000297. [DOI] [PubMed] [Google Scholar]
- 6.Black R.E., Allen L.H., Bhutta Z.A., Caulfield L.E., de Onis M., Ezzati M., Mathers C., Rivera J. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371(9608):243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 7.Bono M.R., Tejon G., Flores-Santibañez F., Fernandez D., Rosemblatt M., Sauma D. Retinoic acid as a modulator of T cell immunity. Nutrients. 2016;8:349. doi: 10.3390/nu8060349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bubb MR. Thymosin beta 4 interactions. Vitamins Hormones. 2003; 66: 297–316. https://doi.org/10.1016/S0083-6729 (03)01008-2. [DOI] [PubMed]
- 9.Chernecky C.C., Berger B.J. Vitamin A (retinol) - serum. In: Chernecky C.C., Berger B.J., editors. Laboratory Tests and Diagnostic Procedures. 6th ed. Elsevier Saunders; Philadelphia, PA: 2013. pp. 1175–1177. [Google Scholar]
- 10.Combs GF. The Vitamins: Fundamental Aspects in Nutrition and Health (3rd ed.). Burlington: Elsevier Academic Press;2008. ISBN 978-0-12-183493-7.
- 11.de Azevedo P.A., Rondó P.H., Rehder Vaz-de-Lima L., de Freitas Oliveira C., Ueda M., Gonçalves-Carvalho C., Reinaldo L.G. The impact of vitamin A supplementation on the immune system of vitamin A-deficient children. Int J Vitam Nutr Res. 2010;80(3):188–196. doi: 10.1024/0300-9831/a000017. [DOI] [PubMed] [Google Scholar]
- 12.Dong P., Tao Y., Yang Y., Wang W. Expression of retinoic acid receptors in intestinal mucosa and the effect of vitamin A on mucosal immunity. Nutrition. 2010;26(6–8):740–745. doi: 10.1016/j.nut.2009.08.011. PMID: 19932006. [DOI] [PubMed] [Google Scholar]
- 13.El-Zayat S.R., Sibaii H., Abd El-Shaheed A., Mahfouz N.N., Sallam S.F., El Azma M.H. Did vitamin B12 difficiency affect Thymosin β4 Level? Eur J Sci Res. 2016;141(2):116–125. http://www.europeanjournalofscientificresearch.com [Google Scholar]
- 14.Freeman KW, Bowman BR, Zetter BR. Regenerative protein thymosin-4 is a novel regulator of purinergic signaling. FASEB J 2011; 25: 907–15. https://doi.org/10.1096/fj.10-169417. [PMid: 21106936]. [DOI] [PubMed]
- 15.Galy AH, Hadden EM, Touraine JL, Hadden JW. Effects of cytokines on human thymic epithelial cells in culture: IL1 induces thymic epithelial cell proliferation and change in morphology. Cell Immunol 1989; 124(1):13–27. https://doi.org/10.1016/0008-8749 (89)90108-1. [DOI] [PubMed]
- 16.Goldstein AL. History of the discovery of the thymosins. Ann N Y Acad Sci. 2007; 1112:1–13. https://doi.org/10.1196/annals. [1415.045 PMid: 17600284]. [DOI] [PubMed]
- 17.Goldstein AL, Hannappel E, Sosne G and Kleinman HK. Thymosin β4: a multi-functional regenerative peptide. Basic properties and clinical applications. Expert Opin Biol Ther. 2012; 12(1):37–51. https://doi.org/10.1517/14712598.2012. [634793 PMid: 22074294]. [DOI] [PubMed]
- 18.Gondo H, Kudo J, White JW, Barr C, Selvanayagam P, Saunders GF. Differential expression of the human thymosin-beta 4 gene in lymphocytes, macrophages, and granulocytes. J Immunol. 1987; 1; 139(11): 3840–8. PMID: 3500230 [PubMed - indexed for MEDLINE]. [PubMed]
- 19.Górski A, Korczak-Kowalska G, Nowaczyk M, Gaciong Z, Skopińska-Rózewska E. Thymosin: an immunomodulator of antibody production in man. Immunology. 1982; 47(3):497–501. PMid: 6215339 PMCid: PMC1555552]. [PMC free article] [PubMed]
- 20.Green H.N., Mellanby E. Vitamin A as an anti-infective agent. Br Med J. 1928;2(3537):691–696. doi: 10.1136/bmj.2.3537.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall J.A., Cannons J.L., Grainger J.R., Dos Santos L.M., Hand T.W. Essential Role for retinoic acid in the promotion of CD4+T cell effector responses via retinoic acid receptor alpha. Immunity. 2011;34:435–447. doi: 10.1016/j.immuni.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall JA, Grainger JR, Spencer SP and Belkaid Y. The role of retinoic acid in tolerance and immunity. Immunity. 2012; 35, 13–22. [CrossRef] [PubMed]. [DOI] [PMC free article] [PubMed]
- 23.Huff T, Müller CS, Otto AM, Netzker R, Hannappel E. beta Thymosins, small acidic peptides with multiple functions. Int J Biochem Cell Biol. 2001; 33: 205–20. https://doi.org/10.1016/S1357-2725 (00)00087-X. [DOI] [PubMed]
- 24.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2008; 21, 527–38. [CrossRef] [PubMed]. [DOI] [PubMed]
- 25.Kannan L., Rath N.C., Liyanage R., Lay J.O., Jr. Effect of toll-like receptor activation on thymosin beta-4 production by chicken macrophages. Mol Cell Biochem. 2010;344(1–2):55–63. doi: 10.1007/s11010-010-0528-0. [DOI] [PubMed] [Google Scholar]
- 26.Kim J, Jung Y. Potential role of thymosin Beta-4 in liver fibrosis. Int J Mol Sci 2015; 16(5):10624–35, https://doi.org/10.3390/ijms160510624. [DOI] [PMC free article] [PubMed]
- 27.Knutsen AP, Freeman JJ, Mueller KR, Roodman ST, Bouhasin JD. Thymosin-α1 stimulates maturation of CD34+ stem cells into CD3+4+ cells in an in vitro thymic epithelia organ coculture model. Int J Immunopharmacol 1999; 21: 15–26. https://doi.org/10.1016/S0192-0561 (98)00060-5. [DOI] [PubMed]
- 28.Kouttab NM, Goldstein A, Lu M, Lu L, Campbell B, Maizel AL. Production of human B and T cell growth factors is enhanced by thymic hormones. Immuno-pharmacology 1988; 16: 97–105. https://doi.org/10.1016/0162-3109 (88)90018-5. [DOI] [PubMed]
- 29.Kramer T.R. Relationship between vitamin A status and T-lymphocyte responsiveness. J Nutr Immunol. 2008;4(1–2):77–85. [Google Scholar]
- 30.Mason JB. Vitamins, trace minerals, and other micronutrients. In: Goldman L, Schafer AI, editors. Goldman's Cecil Medicine. 25th ed. Philadelphia, PA: Elsevier Saunders; 2016 [chap 218].
- 31.Miller J.F. The discovery of thymus function and of thymus-derived lymphocytes. Immunol Rev. 2002;185(1):7–14. doi: 10.1034/j.1600-065x.2002.18502.x. [DOI] [PubMed] [Google Scholar]
- 32.Mizuki N., Simon K.A., Olivier N.K., Robert L.T., Phillip M.B., Hiroto H. The thymus: a comprehensive review radio graphics. 2006;26(3):335–348. [Google Scholar]
- 33.Mora J.R., Iwata M., Von Andrian U.H. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2010;8(5):685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piludu M, Piras M, Pichiri G, Coni P, Orrù G, Cabras T et al. Thymosin Beta 4 may translocate from the cytoplasm in to the nucleus in HepG2 cells following serum starvation. An ultra structural study. PLoS One 2015; 10(4): e 0119642. https://doi.org/10.1371/journal.pone.0119642 [PMid: 25835495 PMCid: PMC4383617]. [DOI] [PMC free article] [PubMed]
- 35.Pino-Lagos K., Guo Y., Brown C., Alexander M.P., Elgueta R., Bennett K.A., De Vries V., Nowak E., Blomhoff R., Sockanathan S. A retinoic acid dependent checkpoint in the development of CD4+ T cell-mediated immunity. J Exp Med. 2011;208:1767–1775. doi: 10.1084/jem.20102358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rath N.C., Kannan L., Liyanage R., Lay JrJO. Thymosin beta in macrophage. J Endocrin Reprod. 2007;2:55–61. [Google Scholar]
- 37.Raverdeau M., Mills K.H. Modulation of T cell and innate immune responses by retinoic Acid. J Immunol. 2014;192(7):2953–2958. doi: 10.4049/jimmunol.1303245. [DOI] [PubMed] [Google Scholar]
- 38.Raverdeau M., Kingston H., Mills G. Modulation of T cell and innate immune responses by retinoic acid. J Immunol. 2014;192(7):2953–2958. doi: 10.4049/jimmunol.1303245. doi: 10.4049/jimmunol.1303245. [DOI] [PubMed] [Google Scholar]
- 39.Reifen R. Vitamin A as an anti-inflammatory agent. Proc Nutr Soc 2012; 61(3):397–400. [PMID: 12230799] https://doi.org/10.1079/PNS2002172. [DOI] [PubMed]
- 40.Ross A.C. Vitamin A and retinoic acid in T cell–related immunity. Am J Clin Nutr. 2012;96(5) doi: 10.3945/ajcn.112.034637. 1166S-1172S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salva S.M.C., Merino G.A., Gruppi A., Alvarez S. Dietary supplementation with probiotics improve hematopoiesis in malnourished mice. PLoS ONE. 2012;7(2):e31171. doi: 10.1371/journal.pone.0031171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savino W. The thymus gland is a target in malnutrition. Eur J Clin Nutr. 2002; 56(3): S46–S49. PMID:12142962. [PubMed - indexed for MEDLINE]. [DOI] [PubMed]
- 43.Savino W., Dardenne M. Nutritional imbalances and infections affect the thymus: consequences on T-cell-mediated immune responses. Proc Nutr Soc. 2010;69(4):636–643. doi: 10.1017/S0029665110002545. [DOI] [PubMed] [Google Scholar]
- 44.Semba RD. Vitamin A and immunity to viral, bacterial and protozoan infections. Proc Nutr Soc. 2204a; 58, 719–27. [| PubMed | ISI | ChemPort |]. [DOI] [PubMed]
- 45.Semba R.D. Vitamin A. In: Hughes D.A., Darlington L.G., Bendich A., editors. Diet and human immune function. Humana Press Inc.; Totowa, New Jersey: 2004. pp. 105–131. [Google Scholar]
- 46.Sibaii H., El-Zayat S.R., Abd El-Shaheed A., Mahfouz N.N., Sallam S.F., El Marwa H., Azma M.H. The hidden function of vitamin D Macedonian. J Med Sci. 2016;4(4):591–595. doi: 10.3889/oamjms.2016.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Solomons NW. 2012. Vitamin A. In: Erdman JJ, Macdonald I, Zeisel S, editors. Present Knowledge in Nutrition. 10th ed: John Wiley & Sons Ltd: 149–84.
- 48.Sommer A. Vitamin A deficiency and clinical disease: an historical overview. J Nutr. 2008;138:1835–1839. doi: 10.1093/jn/138.10.1835. [DOI] [PubMed] [Google Scholar]
- 49.Spencer S.P., Wilhelm C., Yang Q., Hall J.A., Bouladoux N., Boyd A., Nutman T.B., Urban J.F., Jr, Wang J., Ramalingam T.R., Bhandoola A., Wynn T.A., Belkaid Y. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science. 2014;343(6169):432–437. doi: 10.1126/science.1247606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tzehoval E, Sztein MB, Goldstein AL. Thymosins alpha 1 and beta 4 potentiate the antigen-presenting capacity of macrophages. Immunopharmacology 1989; 18(2):107–13. [PMID: 2807872, PubMed - indexed for MEDLINE]. [DOI] [PubMed]
- 51.Villamor E, Fawzi WW. Effects of vitamin a supplementation on immune responses and correlation with clinical outcomes. Clin Microbiol Rev 2005; 18(3):446–64. [PMID: 16020684 PMCID: PMC1195969] https://doi.org/10.1128/CMR.18.3.446-464.2005. [DOI] [PMC free article] [PubMed]
- 52.Wang M, He HJ, Turko IV, Phinney KW, Wang L. Quantifying the cluster of differentiation 4 receptor density on human T-lymphocytes using multiple reaction monitoring mass spectrometry. Anal Chem 2013; 85(3):1773–7. https://doi.org/10.1021/ac3031306. [PMid: 23286534]. [DOI] [PubMed]
- 53.Xu TJ, Wang Q, Ma XW, Zhang W, Xue XC, CunZang C, et al. A novel dimericthymosin beta 4 with enhanced activities accelerates the rate of wound healing. Drug Des Devel Ther. 2013; 7: 1075–1088. [PMid: 24109178. PMCid: PMC3792846]. [DOI] [PMC free article] [PubMed]
- 54.Zeba A.N., Sorgho H., Rouamba N., Zongo I., Rouamba J., Guiguemdé R.T., Hamer D.H., Mokhtar N., Ouedraogo J.B. Major reduction of malaria morbidity with combined vitamin A and zinc supplementation in young children in Burkina Faso: a randomized double blind trial. Nutr J. 2008;7:7. doi: 10.1186/1475-2891-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


