Abstract
Microbial lipases owing to their broad substrate specificity are widely used in various industrial applications like food processing, organic synthesis, detergent formulation and oil manufacturing. In the current study the immobilized lipase from Lactobacillus plantarum was found novel in degrading meat which can be applied in medical field and also in synthesizing different short chain fatty acid esters like 2,3,4-hydroxybenzyl acetates and triazole ester which makes a great impingement in natural flavor industry. The 4-hydroxybenzyl acetate obtained can also be used in cosmetics.
Keywords: Lactobacillus plantarum, Lipase, Esterification, Flavor esters
1. Introduction
Lactic acid bacteria gained prominence in recent times due to their potential to produce probiotics. They are gram positive belonging to phylum Firmicutes [1]. They are widely used in fermentation and food industry [2]. Different industrial products like acetic acid, lactic acid, bacteriocins, enzymes, aroma compounds and ethanol that are produced by these bacteria are advantageous and generally regarded as safe [3]. The ubiquitous appearance of them in food, their potential application in production of different industrial products [4] and their contribution to healthy microflora in human and application in bio-medical treatment made them to study and exploit more. Though several lactic acid bacteria have been exploited for production of enzymes [5], [6], [7] yet a lot remains to be explored.
Lipases (triacylglycerol acylhydrolases, EC 3.1.1.3) are one of the largest groups of industrial enzymes which find use in diverse range of industries like detergents, pharmaceuticals, beverages, cosmetics, dairy, degreasing formulations, paper and biofuel [8]. Microbes serve as an excellent source of lipases compared to plant, animal and human because of their rapid growth, limited space for cultivation, withstanding various temperatures and easy genetic manipulation to generate high yields desirable for various applications [9].
2. Materials and methods
2.1. Microorganisms
L. plantarum (MTCC 4461) was used as source of lipase.
2.2. Immobilization using sodium alginate method
Immobilization of lipase was done using 3% sodium alginate suspension. The effect of different parameters like pH and temperature on enzyme activity was studied. The effect of pH on the activity of immobilized lipase was determined by incubating the enzyme in different buffers with pH 5–5.5 (acetate); pH 6–7 (phosphate) and pH 8–8.5 (Tris-HCl) at 4 °C for 24 h. Similarly the effect of temperature was determined by incubating the enzyme at different temperatures ranging from 25 to 50 °C in Tris-HCl buffer with pH 8.0 at an interval of 5 °C and the kinetic parameters Vmax and Km were also determined from Lineweaver-Burk plots [10].
2.3. Application of immobilized lipase in fat degradation
Generally lipases catalyze degradation of fat, oil and grease [11], [12], [13]. This property made them use in treating lipid containing environment. Hence 10 g of adipose tissue (fat) from chicken was weighed and autoclaved. 0.5 ml of enzyme was added to the tissue and incubated at 37 °C. A control was also kept where no enzyme was added and for every 24 h, weight was taken to check the meat degradation process.
2.4. Application of lipase in ester synthesis
Short chain fatty acid esters have high importance in food industry. For this study esterification reaction was carried out in glass vials containing the solvent tetrahydrofuran (THF) varying from (500–1000 µl) in vinyl acetate (varying from 100 to 500 µl) with different substrates (ranging from 50 to 200 mg) like 2-hydroxybenzylalcohol (2-HB), 3-hydroxybenzyl alcohol (3-HB), 4-hydroxybenzylalcohol and glucotriazole (GTRI). Reaction was initiated by addition of immobilized L. plantarum lipase (ranging from 50 to 200 mg). Samples were placed at different time intervals (varying from 24 to 72 h) in an orbital shaker at different temperatures (4 °C, 37 °C, 60 °C) and rpm (100–300) along with the respective controls without immobilized lipase. Solvents were dried using standard methods and distilled before use. Visualization on TLC was achieved by use of UV light (at 254 nm). Column chromatography was performed for purification on silica gel (100–200 mesh, SRL, India) using ethyl acetate and hexane as eluent. The samples were analysed by IR (infrared spectroscopy) and NMR (Nuclear magnetic resonance spectroscopy).
1H NMR (300 MHz and 400 MHz) and 13C (75 MHz and 100 MHz) spectra were recorded in CDCl3 and DMSO-d6 solution with TMS as internal standard. IR spectra were recorded on KBr plates on Jasco FT/IR - 4200 instrument.
3. Results and discussion
3.1. Application of enzyme in meat degradation
The immobilized enzyme was found to be active at pH 6.5 and at temperature 45 °C. The Vmax and Km values of the enzyme were 1.47 µmol/mg/min and 0.37 mM respectively [10]. The immobilized enzyme was applied in meat degradation and esterification reactions.
Degradation of fat is an important property of lipases. From the literature it is evident that the lipase produced from Lactobacillus sp. had the property to degrade meat in 72 h [14]. Similarly the proteolytic activity of lipase towards meat proteins in sausage system was studied and L. plantarum showed degradation of both sarcoplasmic and myofibrillar proteins in 96 h [15]. Lipase was also found to be a target for amelioration of oil pollution. Lee et al. found that the enzymes like lipases and proteases isolated from Bacillus sp. can solve environmental issues [16]. Modification of food and oil is an important aspect in food processing industry. Lipases alter the location of fatty acid chains in the glyceride and replace one with new ones, thus making a less desirable lipid into a higher value fat [17]. In this study, degradation property of lipase was studied and complete degradation of meat was observed at 72 h for L. plantarum as shown in Table 1 with strong smell and froth. This might be due to autooxidation of fattyacids into short chain aldehydes or ketones leading to objectable smell and frothing. From the above results it is evident this enzyme can be applied in removing fats in medical field and also degrading lipid containing waste water preventing water pollution.
Table 1.
Degradation of meat by L. plantarum lipase at different time periods.
| Organism | Initial weight (in g) | Enzyme activity (U/ml) | 24 h (weight in gm) | Enzyme activity (U/ml) | 48 h (weight in g) | Enzyme activity (U/ml) | 72 h (weight in g) | Enzyme activity (U/ml) | 96 h (weight in g) | Enzyme activity (U/ml) |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 10.7 ± 0.02 | – | 10.7 ± 0.03 | – | 10.7 ± 0.02 | – | 9.8 ± 0.02 | 2.25 ± 0.40 U/ml | 9.8 ± 0.02 | 2.25 ± 0.40 U/ml |
| Lipase from L. plantarum | 10.7 ± 0.01 | 55 ± 0.251 U/ml | 8.2 ± 0.02 | 53 ± 0.36 U/ml | 4.8 ± 0.02 | 51 U/ml ± 0.20 | Complete degradation (72 h) | 49 ± 0.25 U/ml | – | – |
Data was mean standard deviation of 9 replicates from 3 experiments (p ≤ 0.05).
3.2. Application of lipase in ester synthesis
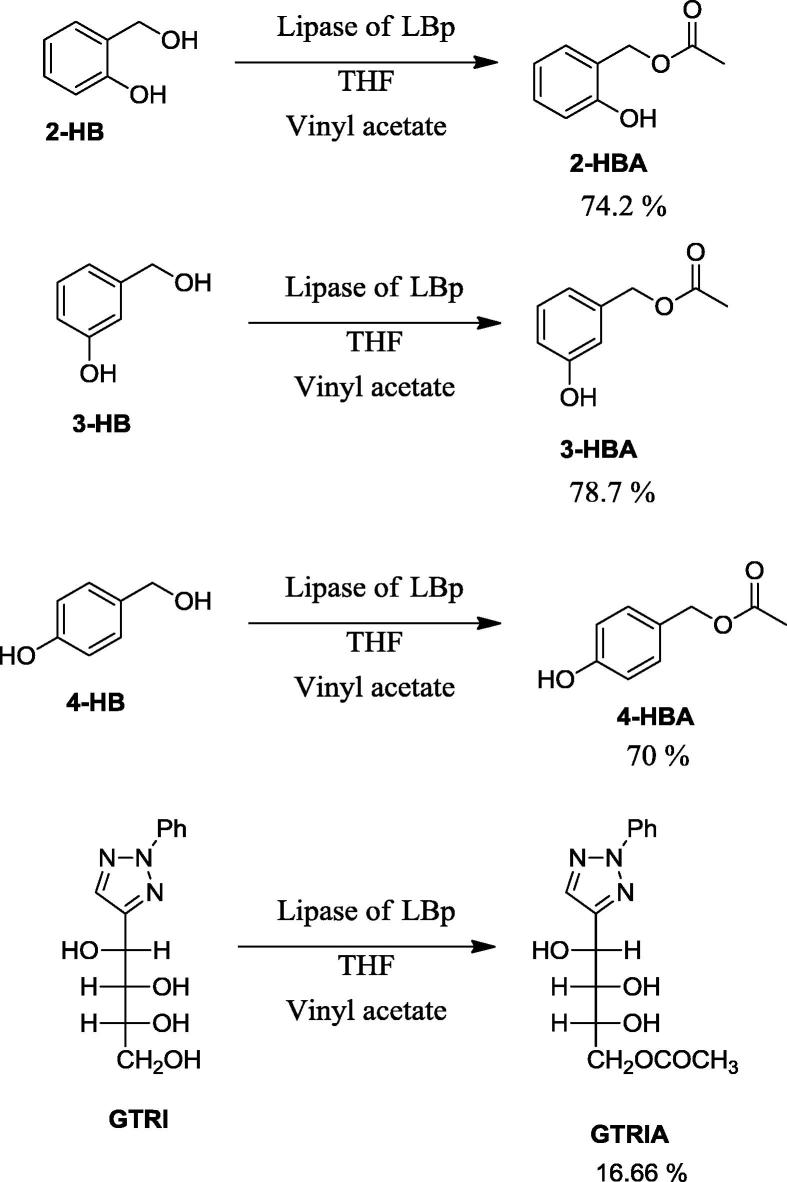
Esterification reactions catalysed by lipases present challenges, which if dealt successfully can result in number of compounds. In the present study, after optimization the short chain fatty acid esters were obtained with 900 µl of the solvent tetrahydrofuran (THF) in 100 µl (1.084 mmol−1) vinyl acetate with 50 mg of different substrates (1.2 mmol−1 of 2,3,4-hydroxybenzylyalcohol, 0.566 mmol−1 of triazole) and 150 mg of enzyme at 37 °C at 110 rpm with 60 h of incubation period as shown in Scheme 1.
Scheme 1.
The analysed samples with NMR were confirmed to be 2-hydroxybenzyl acetate, 3-hydroxybenzyl acetate, 4-hydroxybenzyl acetate and glucose triazole acetate.
3.3. 2-Hydroxybenzyl acetate (2-HBA)
Yield 74.2%, colorless liquid, TLC Rf = 0.5 (EtOAc: n-Hexane, 6:4); 1H NMR (300 MHz, CDCl3) δ 2.13 (CH3, s, 3H), 5.14 (CH2, s, 2H), 6.94 (Ar, m, 2H), 7.30 (Ar, m, 2H), 7.82 (OH, s, 1H); 13C NMR (75 MHz, CDCl3) δ (CH3) 20.95, (CH2) 63.31, 117.81, 120.60, 121.65, 131.20, 132.22, 155.50, 173.80; FT-IR Wavenumber [cm−1] 3401, 2924, 1793, 1706, 1593, 1503, 1455, 1377, 1256, 1095, 1039, 928, 822, 754, 609.396, 528.4, 434.869.
3.4. 3-Hydroxybenzyl acetate (3-HBA)
Yield 78.7%, colorless liquid, TLC Rf = 0.5 (EtOAc: n-Hexane, 5:5); 1H NMR (300 MHz, CDCl3) δ 2.14 (CH3, s, 3H), 5.09 (CH2, s, 2H), 6.04 (OH, s, 1H), 6.84 (m, 2H), 6.92 (d, J = 7.6 Hz, 1H), 7.25 (dd, J = 12.7, 4.9 Hz, 1H); 13C NMR (75 MHz, CDCl3) δ 21.09, 66.30, 115.13, 115.38, 120.34, 129.90, 137.42, 156.01, 171.70; FT-IR Wavenumber [cm−1] 3364, 2878, 1717, 1594, 1458, 1278, 1159, 1034, 921, 865, 785, 750, 695.
3.5. 4-Hydroxybenzyl acetate (4-HBA)
Yield 70%, colorless liquid, TLC Rf = 0.4 (EtOAc: n-Hexane, 5:5); 1H NMR (300 MHz, CDCl3) δ 2.11 (CH3, s, 3H), 5.05 (CH2, s, 2H), 6.86 (m, 2H), 7.37–7.17 (m, 2H); 13C NMR (75 MHz, CDCl3) δ 21.18, 66.53, 115.50, 127.63, 130.41, 156.12, 171.92; IR Wavenumber [cm−1] 3401.82, 2924.52, 1793.47, 1706.69, 1593.88, 1503.24, 1455.03, 1377.89, 1256.4, 1095.37, 1039.44, 928.557, 822.491, 754.031, 609.396, 528.4, 434.869.
In addition to 2,3,4-hydroxyl benzyl alcohol, esterification reaction was carried out with triazole. Triazole is an interesting compound with three 2 hydroxy and one primary hydroxyl groups. But the disadvantage with this substrate is lack of solubility in solvents. Addition of excess vinyl acetate or enzyme did not show complete conversion.
3.5.1. 2-Phenyl-4-(D-arabino-4′-acetoxy-1′,2′,3′-trihydroxybutyl)-2H-1,2,3-triazole (GTRIA)
Yield 16.66%, White solid, TLC Rf = 0.3 (EtOAc); 1H NMR (600 MHz, DMSO-d6) δ 2.03 (s, 3H), 3.56–3.53 (m, 1H), 3.85–3.81 (m, 1H), 7.97 (s, 1H), 5.37 (d, J = 7.0 Hz, 1H), 4.85 (d, J = 7.9 Hz, 1H), 7.40 (t, J = 7.4 Hz, 1H), 7.57–7.55 (m, 2H), 7.99 (d, J = 7.7 Hz, 2H), 4.27 (dd, J = 11.3, 2.5 Hz, 1H), 4.00 (dd, J = 11.3, 6.5 Hz, 1H), 5.11 (dd, J = 7.1, 2.0 Hz, 1H), 5.09 (d, J = 6.2 Hz, 1H); 13C NMR (151 MHz, DMSO-d6) δ 21.33, 65.65, 66.97, 68.50, 74.30, 118.56, 127.80, 130.14, 135.80, 139.78, 153.48, 171.03; IR Wavenumber [cm−1] 3392.17, 2955.38, 2916.81, 2848.35, 1734.66, 1519.63, 1472.38, 1462.74, 1365.35, 1231.33, 1024.98, 728.961, 719.318, 420.406.
4. Conclusion
The present study is focused on the catalytic property or esterification efficacy of the immobilized lipase from L. plantarum in synthesizing different industrial products. The main objective of the study is to explore the efficacy of bacterial lipases derived from Lactobacillus class of probiotics, which are healthy microflora of human mucosal surfaces, towards meat degradation and ester synthesis. Due to their avirulent nature, they do not cause any secondary pollution and health hazard. Lactobacillus plantarum has ability to adapt to different environments and substrates and it is highly versatile lactic acid bacterial strain [18]. Lipase produced from such versatile strain can be useful in bio-medical field and pollution control. The esterification reactions performed with the immobilized lipase from L. plantarum was novel in synthesizing different short chain fatty acid esters which find use as flavoring agents in food industry. The 4-hydroxybenzyl acetate obtained can also be used in cosmetics as anti-tanning agent [19]. This signifies that the non-pathogenic class of lipases can be engineered towards efficient ester synthesis and meat degradation.
Acknowledgement
We acknowledge BITS-Pilani Hyderabad Campus for providing all the facilities for the present work.
Acknowledgments
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Peer review under responsibility of National Research Center, Egypt.
References
- 1.Yusuf M.A. IOSR J Pharm. 2013;3:44–50. [Google Scholar]
- 2.Rattanachaikunsopon P., Phumkhachorn P. Ann Biol Res. 2010;4:218–228. [Google Scholar]
- 3.Yang E., Fan L., Jiang Y., Doucette C., Fillmore S. AMB Express. 2012;2:48. doi: 10.1186/2191-0855-2-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kannahi M., Viji N. Int J Pharm Sci Rev Res. 2014;29:183–186. [Google Scholar]
- 5.Patel A., Shah N., Prajapati J.B. Croat J Food Sci Technol. 2013;5:85–91. [Google Scholar]
- 6.Zareian M., Ebrahimpour A., Abu Bakar F., Mohamed A.K.S., Forghani B., Ab-Kadir M.S.B. Int J Mol Sci. 2012;13:5482–5497. doi: 10.3390/ijms13055482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gurung N., Ray S., Bose S., Rai V. Biomed Res Int. 2013;2013:6. doi: 10.1155/2013/329121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ray A. Asian J Pharm Technol. 2012;2:33–37. [Google Scholar]
- 9.Anbu P., Subash C., Gopinath B., Cihan A.C., Chaulagain B.P. Biomed Res Int. 2013;2013:2. [Google Scholar]
- 10.Ramyasree S., Dutta J.R. Int J Chem Technol Res. 2015;8:680–685. [Google Scholar]
- 11.Rocha D., Gomes B.M., Gomes S.D., Dilcemara L.S., Zenatti C. Eng Agríc. 2013;33:332–340. [Google Scholar]
- 12.Subash C., Gopinath B., Anbu P., Lakshmipriya T., Hilda A. Biomed Res Int. 2013;2013:10. doi: 10.1155/2013/154549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Odeyemi A.T., Aderiye B.I., Bamidele O.S. J Microbiol Res. 2013;3:43–52. [Google Scholar]
- 14.Padmapriya B., Rajeswari T., Noushida E., Sethupalan D.G., Venil C.K. World Appl Sci J. 2011;12:1798–1802. [Google Scholar]
- 15.Fadda S., Oliver G., Vignolo G. J Food Sci. 2002;67:1179–1183. [Google Scholar]
- 16.Lee L.P., Karbul H.M., Citartan M., Gopinath S.C.B., Lakshmipriya T., Tang T.H. Biomed Res Int. 2015;2015:1–9. doi: 10.1155/2015/820575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clausen K. Eur J Lipid Sci Technol. 2001;103:333–340. [Google Scholar]
- 18.Siezen R.J., Wilson G. Microbiol Biotechnol. 2010;3:1–9. doi: 10.1111/j.1751-7915.2009.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michael A., Ash I. Synapse Info Resources; New York: 2004. Hand book of preservatives. [Google Scholar]