Abstract
Amylolytic bacterial isolates were obtained by starch-agar plate method from municipal solid wastes. Six amylolytic bacteria were isolated and the best two isolates, named as DY and W1, were selected based on clear zone ratio. The 16S rDNA sequence analysis identified DY and W1 isolates as Chryseobacterium sp. and Bacillus sp., respectively. Amylase production was optimized using basal media. The maximum level of amylase production was achieved from Chryseobacterium and Bacillus isolates after 60 h and 48 h of cultivation, respectively. The optimal temperature, initial pH of the media, agitation and inoculum size were determined for the both isolates. Increased amylase production was observed when basal media were substituted with organic carbon and nitrogen sources. The optimum pH and temperature for amylase activity of the crude amylase of Chryseobacterium sp. were 5.0 and 50 °C, respectively and those of amylase from Bacillus sp. were pH 7.0 and 50 °C, correspondingly. The crude amylase from the Chryseobacterium sp. was stable at pH 5.0–6.0 and up to 40 °C but that from Bacillus sp. was stable at pH 7.0 and up to 30 °C. Amylases from both the isolates lost ∼50% activity when stored at room temperature for two days. Under the optimized fermentation conditions both Chryseobacterium and Bacillus isolates produced almost the similar amount of amylase with organic kitchen wastes compared to the basal media. Results reported herein support the notion that Chryseobacterium sp. and Bacillus sp. can be used to produce industrially important amylases by utilizing organic kitchen wastes.
Keywords: Bacillus sp., Characterization, Chryseobacterium sp., Extracellular amylase, Kitchen wastes, Production optimization
1. Introduction
Among the most important biotechnologically important industrial enzymes, amylases (especially α-Amylases, E.C.3.2.1.1) play a great role as it has multiple applications in various industries such as dairy, soft drinks, chocolates, pharmaceuticals, food processing, leather, textile, paper, wine, meat, fish processing and many others [1]. α-Amylases degrade starch by the hydrolysis of internal α-1,4 and α-1,6-glycosidic linkages to produce smaller carbohydrates such as glucose, maltose and maltotriose units [2]. In starch processing industry, a shift from the acid hydrolysis of starch to enzymatic digestion was notable in the last century [3]. Amylases are found in microbes, plants and animals but industrially important commercial enzymes are produced from microbes [3]. Among all sources of amylase, bacteria are the most significant because of the limited space and short period of time required for their cultivation and their feasibility to genetic manipulation [4], [5]. Several different microorganisms are reported to produce extracellular amylases [6], [7], [8], [9]. Commercially α-amylases are mainly produced from the genus Bacillus. Among many species, Bacillus subtilis, B. stearothermophilus, B. licheniformis and B. amyloliquefaciens are reported extensively as industrial amylase producers [6], [7], [10]. However, there are very few reports on Chryseobacterium sp. as amylase producers. Recently it has been shown that this bacterial species has the capacity to utilize raw organic materials, mung bean, to produce extracellular amylases [11].
Reports on using waste organic matter for the production of different industrially important enzymes had shown increasing trends lately [7], [8], [9], [12]. In general, commercial carbon and nitrogen sources are used for the production of amylase by bacteria. To cut the cost, the use of organic waste matter might be a potential alternative to commercial carbon and nitrogen sources. A decade ago, about 7690 tons of municipal solid wastes (MSW), to which kitchen wastes are the chief source as organic matters, were generated per day in the six major cities of Bangladesh [13]. Based on these data, it is predicted that ∼47,000 tons of wastes will be produced per day by 2025 due to the rapid urbanization and economic growth in Bangladesh. About 75% constituent of the MSW in Bangladesh are organic that comprises mainly carbohydrates, proteins, peptides, fatty acids and their esters. Thus the organic materials of kitchen wastes can be used as raw materials for fermentation to produce many pure and fermented products [12], [14], [15]. The use of waste materials has potentials as the Bangladesh government has taken steps to implement national 3R strategy for waste management including “reducing waste”, “reusing”, and “recycling resources”.
Currently, industrial enzymes are not produced commercially in Bangladesh. As a result, tons of different commercial enzymes are imported every year to fulfill the demand of local industries [12]. As Bangladesh local industrial sectors tend to rise with significant growth rate since last 10 years, it is a demand of time to produce industrial enzymes locally that help to cut the production price greatly and boost export [16]. To develop a high amylase yielding bacterial cell lines, we herein isolated amylolytic bacteria from MSW, screened for higher amylase producers, identified them based on 16S rDNA sequences, optimized amylase production conditions and finally, produced extracellular amylases using kitchen wastes. Data reported herein suggested the notion that amylases can be produced from bacterial isolates, Chryseobacterium sp. and Bacillus sp., by using kitchen wastes as cheap raw materials.
2. Materials and methods
2.1. Isolation and screening of amylolytic bacterial isolates from MSW
To get superior amylolytic bacterial isolates, we have collected MSW from six different municipal dustbins situated in Chittagong and Cox’s Bazar municipalities, Bangladesh. Collected MSW samples were sealed in sterile zipper bags and labeled. All samples were brought in the Molecular Biology Laboratory of the department of Genetic Engineering and Biotechnology, University of Chittagong, Bangladesh, by maintaining cold chain and stored in the refrigerator for further analysis.
Amylolytic bacteria were isolated from MSW as described earlier [12]. In brief, 1 g of the MSW was suspended in 9 mL of sterile distilled water. After a serial dilution (10−1 to 10−6) of this suspension with sterile distilled water, 50 μL from each diluted suspension was spread on starch agar plates (0.5% peptone, 0.3% beef extract, 2.0% soluble starch, 1.5% agar; pH 7.0). Plates were incubated at 37 °C for 24 h. For the enrichment of amylolytic bacterial isolates, pure bacterial colonies appeared on starch agar were further sub-cultured in the same media. Cultural characteristics of the pure isolates were observed.
The bacterial isolates were subjected to plate assay for screening the best amylolytic strain based on the clear zone ratio. In plate assay, starch agar plates containing the bacterial isolates were flooded with Gram’s iodine (1% Iodine, 2% KI) solution for 1 min and then discarded. The presence of clear zone around a colony indicated its starch hydrolyzing ability whereas blue color around a colony indicated an inability to degrade starch. The higher the clear zone ratio (the ratio of clear zone diameter to bacterial colony diameter), the higher ability to produce extracellular amylases was indicated [12], [17].
2.2. 16S rDNA sequencing of the bacterial isolates
Genomic DNA was extracted from the selected amylolytic bacterial isolates using Favorgen Cultured Cell Genomic DNA Extraction Mini Kit in accordance with the manufacturer instruction (Favorgen® Biotech Corp., Taiwan). The 16S rDNA amplification was carried out in a thermal cycler SimpliAmp™ (Thermo Fisher Scientific Inc; USA) using universal 16S rDNA specific primer pair 5′- AGA GTT TGA TCC TGG CTC AG-3′ (forward) and 5′- ACG GCT ACC TTG TTA CGA CTT-3′ (reverse) [18]. Amplification reactions were performed in a total volume of 25 μl containing 1 μl of each 5 μM primer, 2 μl of template DNA (≤250 ng), 12.5 μl of 2× G2 hot start colorless master mix (Promega, Madison, WI, USA) and 8.5 μl of nuclease free water. The thermocycler was programmed for 1 cycle at 94 °C for 2 min; 35 cycles at 94 °C for 30 s, at 55 °C for 30 s and at 72 °C for 2 min; 1 cycle at 72 °C for 10 min. The PCR products were purified from agarose gel by FavorPrep™ Gel/PCR Purification Mini Kit (Favorgen® Biotech Corp., Taiwan) and sequenced by a DNA sequencer (Model 3130, ABI Automated Genetic Analyzer, Hitachi, Japan).
The 16S rDNA sequences were analyzed using a free computer program Chromas 2.6.2. 16S rDNA sequence of each bacterial isolates was subjected to NCBI BLAST (https://www.ncbi.nlm.nih.gov/blast/) against 16S ribosomal RNA sequences to get most similar sequences. The top matched sequences were retrieved to construct multiple sequence alignment using “Clustal Omega” (www.ebi.ac.uk/Tools/msa/clustalo/). Phylogenetic trees from multiple sequence alignment (statistical method: maximum likelihood method; test of phylogeny: no. of bootstrap replications 500) were constructed using MEGA version 7 [19].
2.3. Crude amylase production
A basal media (1% KH2PO4, 0.25% Na2HPO4, 0.1% NaCl, 0.2% (NH4)2SO4, 0.005% MgSO4.7H2O, 0.005% CaCl2, 0.2% tryptone, 1% soluble starch; pH 7.0) was used for amylase production. For bacterial seed culture, a freshly grown isolated colony was inoculated in 10 mL basal media and incubated at 37 °C and 150 rpm for 20 h. One mL of the culture was then inoculated into 49 mL production medium in a 250 mL conical flask and incubated at 37 °C and 150 rpm for 72 h. The cell-free supernatant obtained by centrifugation at 10,000 rpm for 15 min at 4 °C was used for determining the amylase activity or further study.
2.4. Amylase assay
Amylase activity was determined using soluble starch (1% w/v) as substrate dissolved in 0.05 M sodium phosphate buffer (pH 6.5) as described previously by Gomes et al. [20]. The final reaction mixture, prepared by adding 1.8 mL starch solution and 0.2 mL enzyme solution, was incubated at 50 °C for 10 min. The reaction was stopped by adding 3 mL dinitrosalicylic acid (DNS). The amount of reducing sugar released was determined by taking absorbance at 540 nm with a UV–visible spectrophotometer (Model no. UV-1800; Shimadzu Corporation, Japan) as described by Miller [17], [21]. One unit of amylase activity denotes the amount of enzyme required for releasing 1 μg of reducing sugar (maltose) per minute under assay conditions. The activity of the enzyme was calculated by using following formula [17].
2.5. Optimization of culture conditions for amylase production
To find the optimum incubation period for amylase production, bacterial isolates were inoculated to production media and incubated at 37 °C and 150 rpm. Fermentation was carried out for 72 h and sample was withdrawn 6 h intervals after 24 h of cultivation to monitor the amylase activity and growth.
To study the effects of different initial pH of media on amylase production, isolates were inoculated in the basal media having different initial pH (6.5, 7.0, 7.5, 8.0, 9.0, and 10.0) and incubated at 37 °C and 150 rpm for optimum cultivation period for a specific isolate. The effects of cultivation temperature on amylase production were investigated by culturing the bacteria in the basal media at different temperatures i.e. 30 °C, 35 °C, 37 °C, 40 °C, and 45 °C for optimum cultivation period.
2.6. Effect of carbon and nitrogen sources on amylase production
Effects of various carbon and nitrogen sources were determined by supplementing the basal media with 1% different carbon sources (glucose, sucrose, lactose, and wheat flour) for amylase production under optimized culture conditions. To investigate the effects of different nitrogen sources, 0.2% w/v yeast extract, peptone, tryptone, beef extract, and casein were added to the basal media in separate experiments under optimized culture conditions.
2.7. Effects of the temperature and pH on amylase activity and stability
To investigate the effects of temperature on amylase activity, amylase assay was done at temperature 30–70 °C in the water bath. The thermostability of amylase was studied by treating the crude amylase at different temperatures as indicated for 2 h. The residual amylase activities were determined as mentioned above.
Effects of pH on amylase activity were observed by conducting amylase assay with buffers of different pH (4.0–8.0) in which 1% soluble starch solutions were prepared. The pH stability of amylase was studied as previously described [12], [22]. Each 1 mL of crude amylase was treated for 2 h with 1 mL of different buffers covering the range of pH 4.0–8.0.
2.8. Determination of reaction time and storage stability of crude amylase
To optimize the enzyme-substrate reaction time, the enzyme assay was carried out at different reaction times ranging from 5 to 30 min in a water bath under optimum reaction temperature and pH.
To determine the storage stability of crude amylases, the enzyme solution was stored at room temperature for 6 days and amylase activity was measured after 2 day intervals.
2.9. Formulation of kitchen waste media for amylase production
To investigate the capacity of isolated bacteria to utilize the organic materials of kitchen wastes, a media were formulated that consisted of the peels of vegetables from kitchen wastes. The vegetables’ peels from kitchen wastes were then blended and used as carbon source to develop a kitchen waste media. The composition of the kitchen waste media was 2% kitchen waste, 0.1% NaCl, 0.2% (NH4)2SO4, 0.005% MgSO4·7H2O, and 0.005% CaCl2. The pH of the kitchen waste media was adjusted to 8. After sterilization, this medium was inoculated with isolated bacteria and fermentation was carried out under optimized conditions. Finally, the amylase activities were determined as mentioned above.
2.10. Statistical analysis
Student’s t-test was performed for statistical analysis by using IBM SPSS Statistics 20 computer program. A P value of <0.05 was considered to be statistically significant. Data were presented as the means ± standard errors of the means (SEM) of at least three independent experiments, or as noted in the figure legends.
3. Results and discussion
3.1. Isolation, screening, and identification of amylolytic bacteria
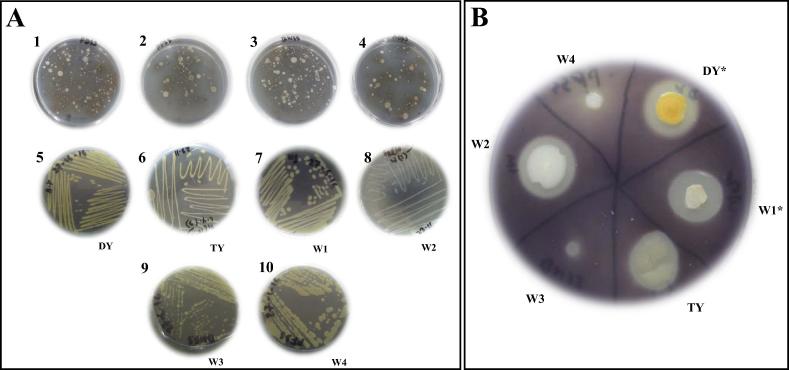
We used enrichment technique to isolate amylolytic bacteria. For isolation of bacterial isolates having specific characteristics, enrichment culture was highly recommended [23], [24]. After inoculating the MSW suspension on starch agar, isolated colonies were obtained. To enrich amylolytic bacteria, all the isolated colonies were further sub-cultured on starch agar to obtain pure cultures (Fig. 1). These pure cultures were further subjected to plate assay by flooding them with Gram’s iodine and the ratio of clear zone was determined. Only six bacterial isolates were selected as potential producer of amylase and named as DY, TY, W1, W2, W3, and W4. Isolates DY and W1 were the best amylase producers with clear zone ratio of 2.2 and 2.1, respectively. All other isolates had the clear zone ratio below 2. Any bacterial isolates having clear zone ratio 2 or more reported as potential producers of extracellular enzymes or proteins [12]. Based on the clear zone ratio, DY and W1 were selected for further experiments (Fig. 1).
Fig. 1.
Isolation of amylolytic bacteria from MSW. (A) Amylolytic bacteria enriched in starch-agar plates (1–4) and pure cultures of different isolates are shown (5–10). DY (5), TY (6), and W1–W4 (7–10) isolates were selected as potential strains for amylase production. (B) Amylase activity of the selected six isolates was observed on starch agar-plate which was flooded with Gram’s iodine solution. * indicated the isolates having clear zone ratio above 2.
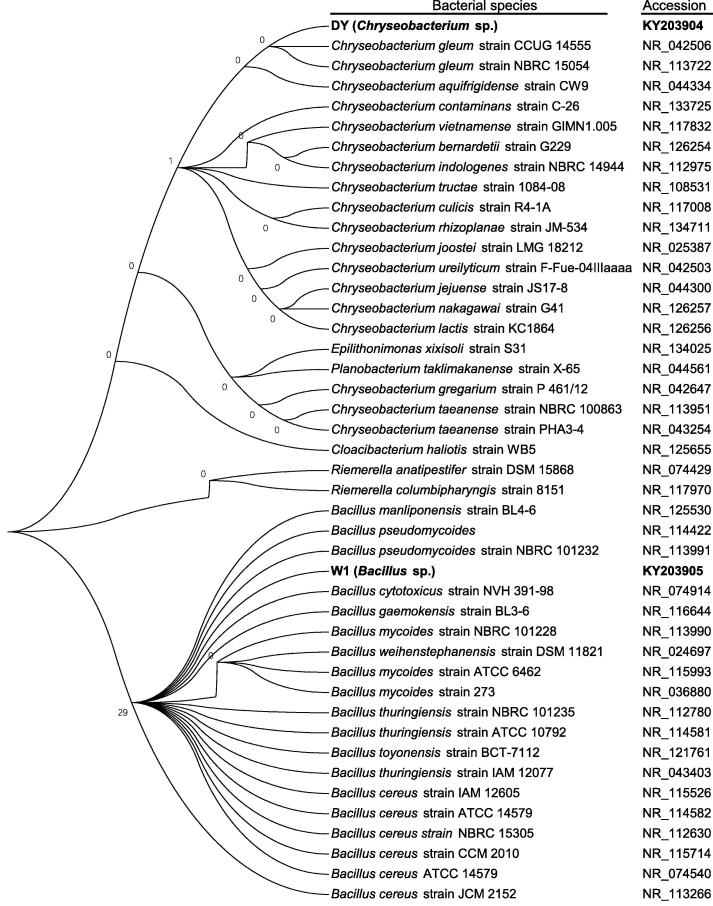
The 16S rDNA was amplified by PCR from the genomic DNA extracted from DY and W1 isolates and sequenced. The 16S rDNA sequences of DY (758 bp) and W1 (1082 bp) isolates were deposited to NCBI GenBank with the accession no KY203904 and KY203905, respectively. BLAST similarity search and the phylogenetic analysis revealed that the DY and W1 isolates were closely related to Chryseobacterium sp. and Bacillus sp., respectively (Fig. 2). The use of 16S rDNA sequence in the characterization of microorganisms is more dependable and sensitive than culture-dependent techniques alone and the results obtained in this investigation are consistent with other related studies [11], [17], [22], [25]. The amylase producing ability of Bacillus sp. was highly reported in the literature and used tremendously in industrial production of amylases [5], [10]. There were very few reports on Chryseobacterium sp. as amylase producers. However Chryseobacterium sp. in our study showed higher clear zone ratio in plate assay compared to that of Bacillus isolate (Fig. 1). In the present study, we therefore, performed the comparative study between a Bacillus and a Chryseobacterium isolate.
Fig. 2.
Phylogenetic relationship of Chryseobacterium sp. (isolate DY) and Bacillus sp. (isolate W1) with other Chryseobacterium and Bacillus species.
3.2. Optimization of amylase production conditions
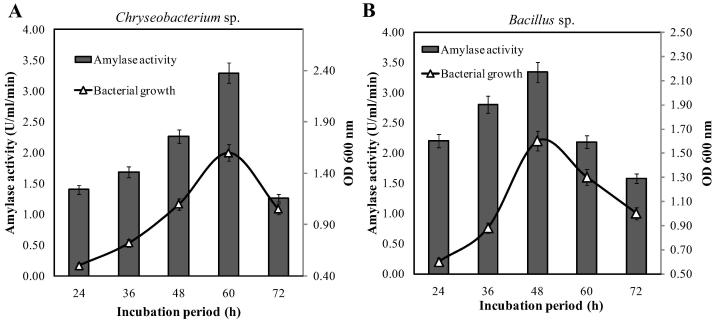
To get the maximum production of amylase, fermentation conditions were optimized as these were important for growth and extracellular enzyme production of microbes [26]. Cultivation period plays a vital role in the maximum amylase production [27]. The time course study in the basal media showed that the incubation period for optimum production of amylase was 60 h and 48 h for Chryseobacterium sp. and Bacillus sp., respectively. Growth analysis with the optical density from the same experiment revealed that Chryseobacterium sp. and Bacillus sp. showed maximum OD 600 nm after 60 h and 48 h of cultivation, respectively (Fig. 3). A positive correlation between growth and amylase activity for both strains was observed indicating that the amylase production was growth associated for the both isolates. According to the previous reports, different species of Bacillus have shown similar cultivation period of 48 h [17], [22], [27] whereas Chryseobacterium sp. took 72 h for maximum amylase production [11].
Fig. 3.
Time course study of amylase production and growth of Chryseobacterium sp. (A) and Bacillus sp. (B) in the basal media. The fermentation at shake flask was carried out at 37 °C for 72 h at 120 rpm. The initial pH of the media was adjusted to 7.0.
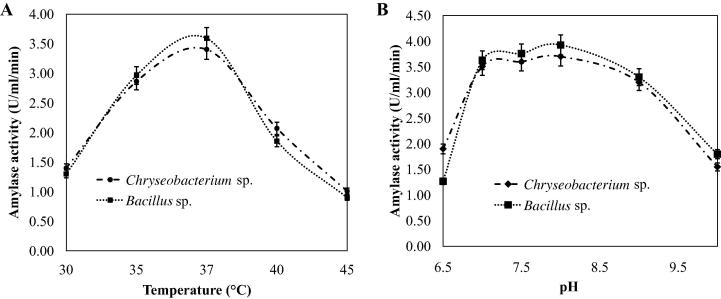
The optimum temperature for growth and amylase production was determined by incubating Bacillus sp. and Chryseobacterium sp. isolates in the basal medium at different temperatures (30–45 °C) for optimum cultivation period. It was found that the both isolates were able to produce amylase between 30 and 45 °C but maximum amylase production was observed at 37 °C for both Chryseobacterium and Bacillus isolates (Fig.4A). The experiments with the initial pH of the production media showed that amylase production was supported by a wide range of pH with the peak at pH 8.0 for the both isolates. (Fig.4B). These observations were similar with previous reports for both the Chryseobacterium sp. and Bacillus sp. [11], [17], [22].
Fig. 4.
Effects of temperature (A) and pH (B) on amylase production by Chryseobacterium and Bacillus isolates. The shake flask fermentation was carried out at different temperatures (A) and pH (B) as indicated at 120 rpm for the optimum cultivation period for amylase production. The initial pH of the basal media in (A) was adjusted to 7.0.
3.3. Effects of inoculum size, agitation, carbon, and nitrogen sources on amylase production
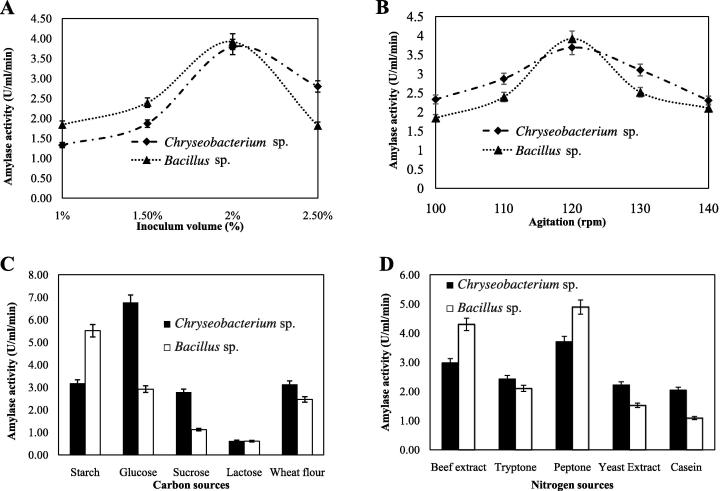
The effects of inoculum size (v/v) and agitation on amylase production were investigated. Maximum amylase production was obtained with 2% inoculum of the both isolates. Further increase in inoculum size causes the reduction in amylase production (Fig.5A). It was previously reported that Bacillus amyloliquefaciens showed maximum amylase production with 1% inoculum [22]. The decrease in amylase production with the increase in the percentage of inoculum might be due to the initial rapid growth of bacterium and lack of sufficient nutrient available in the medium for higher volume of bacteria. Our study just replicated previous results about the negative effects of high inoculum size to extracellular amylase production [22], [28]. Optimum amylase production was obtained at 120 rpm for the both isolates. Further increase in agitation decreased in amylase production might be due to cell lysis at higher agitation (Fig.5B). Previous reports showed that Bacillus produced maximum amylase at 120–150 rpm [17], [22], [27].
Fig. 5.
Effects of inoculum volume (A), agitation (B), carbon (C), and nitrogen sources (D) on amylase production. Shake flask fermentation was carried out at optimum growth conditions for Chryseobacterium and Bacillus isolates.
The carbon and nitrogen sources in bacterial media play important roles for the production of extracellular enzymes [27], [29], [30]. To determine the effects of various carbon sources on amylase production, the fermentation at shake flask culture was carried out by setting optimum conditions and substituting carbon source of the basal media. Glucose was found to be the best carbon source for the extracellular production of amylase by Chryseobacterium sp. whereas Bacillus sp. utilized soluble starch (Fig.5C) for maximum production of amylase. Both of the isolates produced a reasonable amount of amylase by utilizing wheat flour. The utilization of soluble starch by Bacillus sp. and the moderate utilizing ability of complex substrate like flour were reported previously [22], [30]. Lactose was the least suitable for the amylase production by the both isolates (Fig.5C). Based on data reported herein, glucose was considered as the best carbon source for Chryseobacterium sp. for amylase production.
The effects of nitrogen sources on amylase production by the both bacterial isolates were determined by using different organic nitrogen sources with basal media. Among different organic nitrogen sources, peptone was the most stimulatory for maximum amylase production by both Chryseobacterium sp. and Bacillus sp. whereas casein was found as the least stimulatory among the nitrogen sources tested. Chryseobacterium and Bacillus isolates produced 2.5-fold and 4-fold more amylase with peptone when compared with casein, respectively (Fig.5D). The beef extract was also found as a potential nitrogen source for amylase production by the both isolates. Tryptone, peptone, and casein were reported as good sources for the production of extracellular amylase [22], [31].
3.4. Partial characterization of crude amylase produced by Bacillus and Chryseobacterium isolates
3.4.1. Effects of pH, temperature, and time on amylase activity
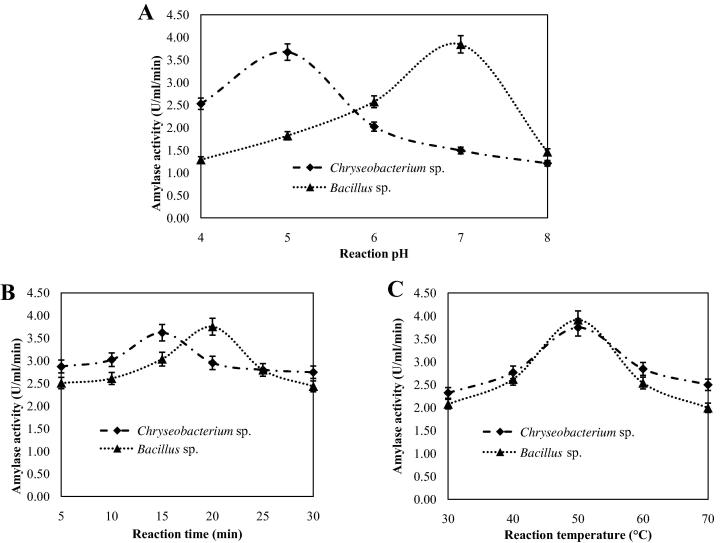
The optimum pH for amylase activity was estimated and the enzymes from Chryseobacterium sp. showed maximum activity at pH 5.0 whereas that from Bacillus sp. showed maximum activity at pH 7.0 (Fig.6A). According to Divakaran et al. amylases from Bacillus showed maximum activity at pH 7 [32]. It is in good agreement with our results. Wang et al. found that α-amylase from Chryseobacterium taeanense TKU001 was active at pH 9.0 [11]. However, our study revealed that the Chryseobacterium isolate in the current study might be novel and acidic α-amylase producer. The optimum reaction time of the enzyme solution was studied. Amylases from Chryseobacterium sp. and Bacillus sp. showed maximum activity after 15 min and 20 min of reaction, respectively (Fig.6B). Amylase activity was determined at different temperatures (30–70 °C). Amylases from Chryseobacterium and Bacillus isolates showed maximum activity at 50 °C (Fig.6C). However, amylase from the both sources was active within 30–70 °C. The similar reports have been done [11], [33].
Fig. 6.
Effects of reaction pH (A), time (B), and temperature (C) on amylase activity.
3.4.2. Determination of the thermal, pH, and storage stability of amylases
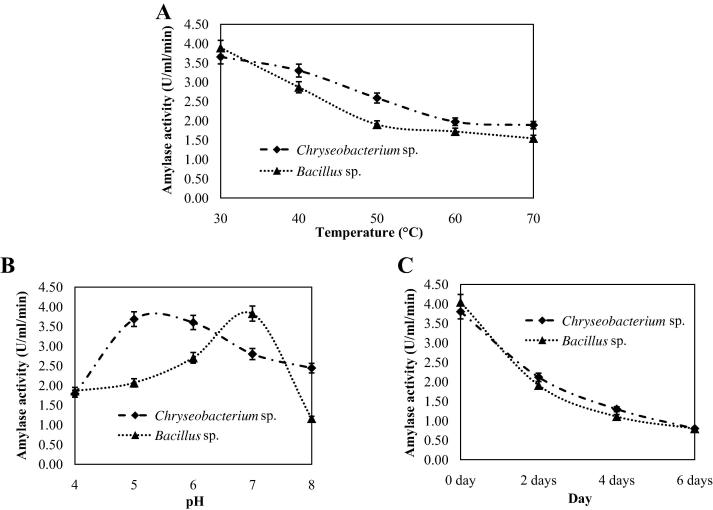
Amylases produced by Chryseobacterium isolate were stable up to 40 °C during the treatment period but lost its activity gradually after 50 °C and lost approximately 50% activity at 70 °C (Fig.7A). On the other hand, amylases from Bacillus isolate were stable up to 30 °C and lost 50% activity at 50 °C (Fig.7A). The results indicated that amylases from Chryseobacterium isolate were more thermostable than those from Bacillus isolate. The thermo-stability data of Bacillus isolate in our study are in good agreement with the report of Deb et al. [22]. Amylase from Chryseobacterium isolate was highly stable at pH 5.0 (Fig.7B). However it was significantly stable between pH 5.0 and 8.0. The amylases from Bacillus isolate were highly stable at pH 7.0 but labile at acidic or alkaline conditions (Fig.7B). It was found that room temperature was not suitable for storage of amylase as it from both isolates lost ∼60% of their activity after 4 days of storage (Fig.7C). The data indicated that the crude amylases from both the isolates were heat sensitive and inefficient to retain their activity when stored at room temperature.
Fig. 7.
Determination of the thermal (A), pH (B), and storage stability (C) of amylases from Chryseobacterium and Bacillus isolates.
3.5. Production of crude amylases using kitchen wastes
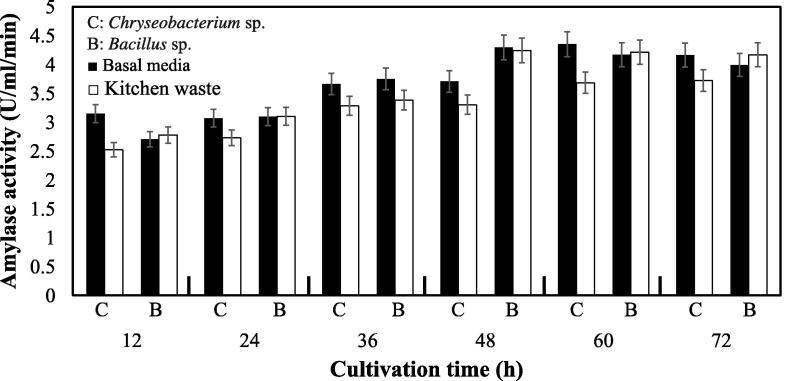
Use of readily available raw materials is considered as economical for commercial production of valuable enzymes using fermentation process. Utilization of wastes materials, i.e. agricultural, MSW, vegetable wastes, is considered as common practice in this regard because these waste materials are a good source of carbon necessary for microbial growth [12], [22], [34], [35], [36], [37]. In order to produce amylase using organic kitchen wastes, we replaced starch of the basal media by 2% of starchy kitchen wastes as described by Azad et al. for utilizing organic MSW [12]. The fermentation with kitchen waste media at shake flask level was performed at the optimum conditions. Results showed that Chryseobacterium isolate produced the highest amount of the enzyme after 60 h of fermentation in the optimized basal media and kitchen waste media (Fig. 8). The Bacillus isolate produced maximum level of amylase after 48 h incubation as it did with the basal media (Fig. 8). The results found in this experiments indicated that both of the bacterial isolates were very much potential to utilize organic kitchen waste material as a carbon source. Statistical analysis showed that there is no significance difference in the amylase production between basal and organic kitchen waste media. Although both of the isolates produce an impressive amount of amylase in the presence of starchy wastes, Chryseobacterium sp. showed slightly higher ability to utilize the starchy kitchen wastes and higher amylase production than the Bacillus sp. These results indicated that Chryseobacterium and Bacillus isolates might be potential strain to produce extracellular amylase by using organic kitchen wastes as sole carbon source. The ability of Bacillus to produce amylases by using different agro-industrial wastes is highly reported [7], [34], [38].
Fig. 8.
Production of amylase from Chryseobacterium and Bacillus isolates by using starchy kitchen wastes. Shake flask fermentation was carried out at optimum growth conditions for both Chryseobacterium and Bacillus isolates optimized with the basal media.
This study was conducted as a foundation work, screening and identification of high amylase producing strains, for further improvement of amylase production by utilizing readily available waste materials in Bangladesh. We have identified rarely reported Chryseobacterium sp. as extracellular amylase producing bacterial isolate. Our results demonstrated that kitchen wastes can be successfully used as cheap carbon sources in fermentation for crude amylase production, allowing further studies for molecular characterization and production of recombinant amylase by using organic kitchen waste.
4. Conclusions
We have isolated extracellular amylase producing bacterial isolates from different dustbins located Chittagong and Cox’s Bazar, Bangladesh. Among them, best two isolates were selected after screening and identified as Chryseobacterium sp. and Bacillus sp. using 16S rDNA sequencing. Chryseobacterium sp. was rarely reported as amylase producing bacteria. Moreover, amylase production conditions for the both identified strains were optimized. Amylases produced by Chryseobacterium sp. and Bacillus sp. were partially characterized. Finally, it has been shown that the both isolates could successfully utilize starchy kitchen wastes as carbon source in the basal media for production of amylase. Results reported herein support the notion that Chryseobacterium sp. and Bacillus sp. can be used to produce industrially important amylases by utilizing kitchen wastes.
Competing interests
The authors declare that they have no competing interests.
Authors' contribution
LWM and MMH conceived and designed the project. MMH, AH1, and AH2 carried out the laboratory experiments. LWM and MMH supervised the laboratory works and interpreted the results. MMH prepared the manuscript. AKA finally interpreted data by repeating some experiments and edited the manuscript. All authors read and approved the final manuscript.
Acknowledgments
This research was done in the ‘Molecular Biology Laboratory’ of the Department of Genetic Engineering and Biotechnology, University of Chittagong and ‘TWAS Lab’ of the Department of Genetic Engineering and Biotechnology, Shahjalal University of Science and Technology, Sylhet 3114. This work was funded by the Planning and Development Office of the University of Chittagong (Grantee: Md. Mahbub Hasan, Lecturer, Department of Genetic Engineering and Biotechnology, University of Chittagong; Official memo no.: 132/P&D/7-34(17)/206; Date: 29/03/2016).
The nucleotide sequences data reported in this paper have been deposited in NCBI GenBank and will be found in the DDBJ/EMBL/GenBank nucleotide sequence databases with the accession number KY203904 and KY203905 for Chryseobacterium sp. and Bacillus sp., respectively.
Footnotes
Peer review under responsibility of National Research Center, Egypt.
Contributor Information
Md. Mahbub Hasan, Email: mahbub.hasan@cu.ac.bd.
Lolo Wal Marzan, Email: marzan.geb@cu.ac.bd.
References
- 1.Saxena S. Microbial enzymes and their industrial applications. Springer; India, New Delhi: 2015. (Appl. Microbiol.). [Google Scholar]
- 2.Rana N., Walia A., Gaur A. Natl. Acad. Sci. Lett. 2013;36(1):9–17. [Google Scholar]
- 3.Van der Maarel M.J., Van Der Veen B., Uitdehaag J.C., Leemhuis H., Dijkhuizen L. J. Biotechnol. 2002;94(2):137–155. doi: 10.1016/s0168-1656(01)00407-2. [DOI] [PubMed] [Google Scholar]
- 4.Prakasham R., Rao C.S., Rao R.S., Rajesham S., Sarma P. Appl. Biochem. Biotechnol. 2005;120(2):133–144. doi: 10.1385/abab:120:2:133. [DOI] [PubMed] [Google Scholar]
- 5.Selvamohan T., Sherin S. Plant Arch. 2010;10(2):651–656. [Google Scholar]
- 6.Khire J., Pant A. World J. Microbiol. Biotechnol. 1992;8(2):167–170. doi: 10.1007/BF01195840. [DOI] [PubMed] [Google Scholar]
- 7.Hayashida S., Teramoto Y., Inoue T. Appl. Environ. Microbiol. 1988;54(6):1516–1522. doi: 10.1128/aem.54.6.1516-1522.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sani A., Awe F.A., Akinyanju J.A. J. Ind. Microbiol. 1992;10(1):55–59. [Google Scholar]
- 9.Uguru G., Akinyanju J., Sani A. Enzyme Microb. Technol. 1997;21(1):48–51. [Google Scholar]
- 10.Souza P.M.d. Braz. J. Microbiol. 2010;41(4):850–861. doi: 10.1590/S1517-83822010000400004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang S.-L., Liang Y.-C., Liang T.-W. Process Biochem. 2011;46(3):745–750. [Google Scholar]
- 12.Azad A.K., Nahar A., Hasan M.M., Islam K., Azim M.F., Hossain M.S., Rahman M.R., Ojha R.K., Mahmud G.M.S., Kayes R. Asian J. Microbiol. Biotechnol. Environ. Sci. 2013;15:365–374. [Google Scholar]
- 13.Alamgir M., Ahsan A. J. Environ. Health Sci. Eng. 2007;4(2):67–76. [Google Scholar]
- 14.Barnabé S., Sasseville J., Tyagi R., Valéro J. Vecteur Environ. 2003;36(2):50–62. [Google Scholar]
- 15.Champagne P. Resour. Conserv. Recycl. 2007;50(3):211–230. [Google Scholar]
- 16.Onneshan Unnayan. Recent trends in agriculture, industry, and service sectors. Bangladesh Economic Update. 2016:5–13. [Google Scholar]
- 17.Dash B.K., Rahman M.M., Sarker P.K. BioMed Res. Int. 2015;2015:9. doi: 10.1155/2015/859805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fouad A.F., Barry J., Caimano M., Clawson M., Zhu Q., Carver R., Hazlett K., Radolf J.D. J. Clin. Microbiol. 2002;40(9):3223–3231. doi: 10.1128/JCM.40.9.3223-3231.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar S., Stecher G., Tamura K. Mol. Biol. Evol. 2016;33(7):1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomes I., Sultana M., Uddin K., Gomes J., Steiner W., Gomes D.J. Bangladesh. J. Microbiol. 2001;18(2):141–150. [Google Scholar]
- 21.Miller G.L. Anal. Chem. 1959;31(3):426–428. [Google Scholar]
- 22.Deb P., Talukdar S.A., Mohsina K., Sarker P.K., Sayem S.A. SpringerPlus. 2013;2(1):154. doi: 10.1186/2193-1801-2-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogol M., Shpak B., Rothman D., Sechter I. Appl. Environ. Microbiol. 1985;50(1):125–126. doi: 10.1128/aem.50.1.125-126.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown D.F.J., Walpole E. J. Antimicrob. Chemother. 2003;51(2):289–296. doi: 10.1093/jac/dkg076. [DOI] [PubMed] [Google Scholar]
- 25.Dey U., Chatterjee S., Mondal N.K. Biotechnol. Rep. 2016;10:1–7. doi: 10.1016/j.btre.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kathiresan K., Manivannan S. Afr. J. Biotechnol. 2006;5:10. [Google Scholar]
- 27.Gupta R., Gigras P., Mohapatra H., Goswami V.K., Chauhan B. Process Biochem. 2003;38(11):1599–1616. [Google Scholar]
- 28.Riaz N., Haq I., Qadeer M. Int. J. Agric. Biol. 2003;5(3):249–252. [Google Scholar]
- 29.Sumrin A., Ahmad W., Ijaz B., Sarwar M.T., Gull S., Kausar H., Shahid I., Jahan S., Asad S., Hussain M. Afr. J. Biotechnol. 2011;10(11):2119–2129. [Google Scholar]
- 30.Saxena R.K., Dutt K., Agarwal L., Nayyar P. Bioresour. Technol. 2007;98(2):260–265. doi: 10.1016/j.biortech.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 31.Sharma N., Vamil R., Ahmad S., Agnihotri R. Int. J. Pharm. Sci. Res. 2012;3(4):1161. [Google Scholar]
- 32.Divakaran D., Chandran A., Pratap R. Chandran. Braz. J. Microbiol. 2011;42(4):1397–1404. doi: 10.1590/S1517-838220110004000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aygan A., Arikan B., Korkmaz H., Dinçer S., Çolak Ö. Braz. J. Microbiol. 2008;39(3):547–553. doi: 10.1590/S1517-838220080003000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ikram ul H., Ashraf H., M.A Q., Iqbal J. Bioresour. Technol. 2005;96(10):1201–1204. doi: 10.1016/j.biortech.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 35.Djekrif-Dakhmouche S., Gheribi-Aoulmi Z., Meraihi Z., Bennamoun L. J. Food Eng. 2006;73(2):190–197. [Google Scholar]
- 36.Swamy M.V., Seenayya G. Process Biochem. 1996;31(2):157–162. [Google Scholar]
- 37.Afrisham S., Badoei-Dalfard A., Namaki-Shoushtari A., Karami Z. J. Mol. Catal. B Enzym. 2016;132:98–106. [Google Scholar]
- 38.Bhange K., Chaturvedi V., Bhatt R. Biotechnol. Rep. 2016;10:94–104. doi: 10.1016/j.btre.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]