Abstract
The present work illustrates eco-friendly, rapid and cost effective method of AgNPs synthesis using C. pulcherrima stem extract. Initially, various physico chemical factors were optimized. Characterization was done by different spectroscopic and microscopic analysis. AgNPs were spherical in shape with an average size of 8 nm. AgNPs showed good synergistic antimicrobial, antibiofilm and antioxidant activity. The cytotoxicity effect against HeLa cancer cell line was dose dependent while genotoxic study revealed the non toxic nature of AgNPs at lower concentration. The results suggest that AgNPs from C. pulcherrima stem extract have great potential in biomedical applications.
Keywords: Silver nanoparticles, Characterization, Antibiofilm activity, Antioxidant activity, Cytotoxicity, Genotoxicity
1. Introduction
Nanoparticles, especially metal nanoparticles (silver, gold, copper, zinc, titanium, magnesium) are being applied in numerous fields because of their unique properties. They possess properties entirely different from the bulk metal from which they are synthesized. Their application includes diagnosis, wound healing, drug delivery, molecular imaging, water treatment, catalysis, cosmetics, clothing, food industry, sunscreens, etc. They also possess properties like antiviral, antimicrobial, antioxidant, anticancer, antidiabetic, analgesic, antidandruff, anticoagulant, antiinflammatory, antihelmintic, antiproliferative activities and also show properties like antigenotoxic, cytotoxic effect, etc. [13], [11].
Today mankind is faced with two grave problems for which the cure is obscure; multidrug resistant microorganisms responsible for infectious diseases and oxidative stress generated free radicals responsible for innumerable diseases and disorders. The occurrence of cancer is also increasing steadily. Misuse or overuse of antibiotics has led to the development of resistance in the microorganisms and even second line of treatment has become questionable [20]. The cells have antioxidant mechanism to overcome the free radical generation but when this balance is shaken with over production of free radicals and reactive oxygen species, stress condition occurs which leads to many diseases and disorders [16]. Drugs are available to treat any of this pathological condition but their use is being questioned because of many disadvantages they pose like side effects, harmful nature, low efficiency, etc. Hence the need of the hour is new entities with novel mechanism of action. One therapy was the use of natural compounds or medicinal plant extracts, which proved quite successful but synthesis of metal nanoparticles using plant extracts is a novel approach to tackle the infectious disease causing microorganisms or oxidative stress related diseases or cancer [44].
Synthesis of metal nanoparticles using plant extracts is simple, easy and eco friendly. In general, the metal salt of a particular metal is reacted with plant extract and the metals are reduced to metal nanoparticles with the help of secondary metabolites present in the plant extract which act both as reducing and stabilizing agents. Any part of the plant can be used for the synthesis for eg. leaf, stem, flower, fruit, seed, root, bark, etc. [9].
In the present work, silver nanoparticles are synthesized using Caesalpinia pulcherrima stem extract. Caesalpinia pulcherrima is an ornamental plant with several medicinal properties and belongs to Caesalpiniaceae family. C. pulcherrima flower is known for is antiviral, antimicrobial, antioxidant, analgesic, anti inflammatory, anthelmintic activities [31]. The leaves are reported for antimicrobial, antioxidant, antiulcer properties [23], [48], [39]. The stem extract is used for antiplasmodial, abortifacient, emmenagogue, cytotoxic activity [26], [34].
In the present work, we report biosynthesis of AgNPs using the stem extract of C. pulcherrima and its various biological activities (synergistic antimicrobial, antibiofilm, antioxidant, cytotoxic and genotoxic) is reported perhaps for the first time.
2. Materials and methods
The fresh stem of Caesalpinia pulcherrima was collected from Rajkot, Gujarat, India. All the chemicals were obtained from Hi Media Laboratories and Sisco Research Laboratories Pvt. Limited, Mumbai, India. Ultra purified water was used for all the experiments. Extract preparation and optimization of different parameters was followed as described earlier [31].
2.1. Characterization and biological activity of synthesized silver nanoparticles
The AgNPs were characterized by FTIR analysis, XRD analysis, Thermogravimetric analysis, TEM analysis. Antimicrobial activity was measured by measuring the MIC and MBC values of AgNPs [36], [1], synergistic antimicrobial activity [8], [45] and antibiofilm activity [47] against eleven microorganisms. Four Gram positive bacteria (Bacillus cereus, Staphylococcus aureus, Corynebacterium rubrum, Bacillus subtilis), four Gram negative bacteria (Escherichia coli, Klebsiella pneumonia, Pseudomonas aeruginosa, Salmonella typhimurium) and three fungi (Candida albicans, Candida glabrata, Cryptococcus neoformans) were used for antimicrobial activity. The antioxidant activity of synthesized AgNPs was measured by five in vitro antioxidant assays. The antioxidant assays evaluated were 2,2-diphenyl-1-picrylhydrazyl free radical scavenging assay (DPPH), Superoxide anion radical scavenging assay (SO), 2,2′-Azino-bis-(3-ethyl)benzothiazoline-6-sulfonic acid radical cation scavenging assay (ABTS), Reducing capacity assessment (RCA), Ferric reducing antioxidant power assay (FRAP). The details of the method followed are as described earlier [10]. Cytotoxicity by the MTT assay and genotoxicity by comet assay [31]. Human cervical cancer cell line (HeLa) were used for MTT assay.
3. Results and discussion
3.1. Optimization of different parameters
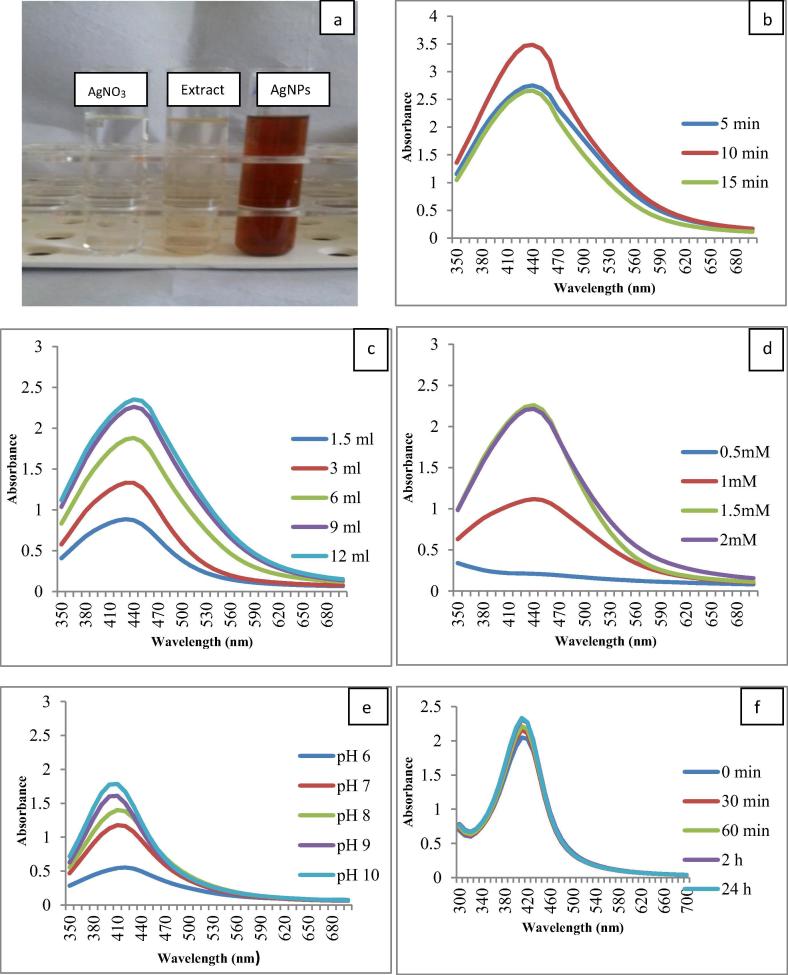
Green synthesis of AgNPs, involves addition of plant extract to silver nitrate solution and incubating the reaction mixture for definite time at room temperature. The phytoconstituents present in the plant extract reduce silver to silver nanoparticles. In order to achieve, good AgNPs, it is essential to optimize different procedure parameters involved like boiling time of extract preparation, extract concentration, AgNO3 concentration, pH and incubation time of reaction mixture, etc. These parameters vary with the plant extract and plant part used; thus it is essential to optimize these conditions as also reported by other researchers [50], [32]. The first indication of AgNPs formation is the colour change that occurs when plant extract is added to silver nitrate solution due to surface plasmon resonance. In the present work also, initially when 6 ml stem extract was added to 40 ml 1 mM AgNO3 and incubated at room temperature, the colourless solution changed to brown colour indicating the formation of AgNPs (Fig. 1a). Moteriya et al. [33] reported such colour change effect for different plants.
Fig. 1.
(a) Colour change image, (b) Effect of boiling time, (c) Effect of extract amount, (d) Effect of silver nitrate concentration, (e) Effect of pH, and (f) UV-vis spectra at different time interval.
3.2. UV–Visible spectroscopic analysis of AgNPs
UV–Vis spectroscopy is an important tool to study the formation of metal nanoparticles in aqueous medium. The synthesized AgNPs show characteristic absorption maxima in the visible region in the range of 350–750 nm. Further, the peak size and peak intensity clearly indicate the number and size of nanoparticles formed; broader peak indicates larger particle formation and narrow peak indicates smaller size of the particles [51] while the intensity of absorption peak indicates the number of particles formed. In other words, the peak intensity is directly proportional to number of particles formed [43]. This selection criterion was used for optimizing various parameters for synthesizing AgNPs, from stem extract of C. pulcherrima.
The first parameter optimized was boiling time of plant extract preparation. The stem extract was boiled for 5, 10 and 15 min. and then 6 ml stem extract was added to 40 ml 1 mM AgNO3. The absorption intensity was higher in 10 min boiled stem extract as compared to 5 and 15 min boiled extract (Fig. 1b). Hence, 10 min boiling time was finalized for the preparation of the stem extract. This is in contrast to AgNPs synthesized using flower extract of the same plant; when flower extract was used for the synthesis of AgNPs, 5 min boiling for extract preparation gave the best results [31].
After confirming the boiling time of extract preparation, the extract concentration was optimized.
The next parameter optimized was by the addition of extract concentration to the reaction mixture. 10 min boiled different extract concentration (1.5, 3, 6, 9 and 12 ml) was added to 40 ml 1 mM AgNO3. In 12 ml extract concentration higher absorbance intensity was observed (Fig. 1c). Absorbance intensity increased with increasing extract concentration because the availability of biomolecules required for the reduction of silver ions to silver nanoparticles is more and results in the formation of more AgNPs. Elavazhagan and Arunachalam [14] used 15 ml extract while [43] used 10 ml extract concentration for AgNP synthesis. Hence, 12 ml extract concentration was finalized for the preparation of AgNPs from the stem extract of C. pulcherrima.
The concentration of silver nitrate also has a tremendous effect on the size of synthesized AgNPs. 10 min boiled 12 ml extract concentration was added to 40 ml different concentrations (0.5 mM, 1 mM, 1.5 mM, 2 mM) of AgNO3. 0.5 mM AgNO3 containing reaction mixture did not show any peak indicating no formation of AgNPs; while 1.0, 1.5 and 2.0 mM AgNO3 containing reaction mixtures showed characteristic peak at 410 nm indicating formation of AgNPs (Fig. 1d). However there are several reports that higher concentration of silver nitrate produces larger particle size [6]. Hence, 1 mM AgNO3 concentration was finalized for the synthesis of AgNPs pH of the reaction mixture affects and also plays an important role in the formation of nanoparticles. In order to evaluate the effect of pH on AgNPs formation, 12 ml 10 min boiled stem extract was added to 40 ml 1 mM silver nitrate and the reaction mixture was adjusted with different pH (6, 7, 8, 9, 10). At pH 6 and pH 7, the absorbance peak was broader and intensity was less (Fig. 1e), indicating less number of particle formation with larger size. As the pH increased, from pH 8 to pH 10, the absorption peak becomes narrowed and the intensity also steadily increased; again clearly indicating smaller size and more number of the particles formed. Best particle formation occurred at pH 10. Using alkaline pH for AgNPs synthesis is also reported by [22].
The effect of reaction time on the biosynthesis of AgNPs was evaluated at various time intervals (30 min, 60 min, 2 h and 24 h). The characteristic maximum absorbance peak of AgNPs was observed at 410 nm at various time intervals. No change in absorption peak or intensity was found after 24 h (Fig. 1f). Kumar et al. [5] and Arunachalam et al. [4] also reported AgNPs formation completed within 24 h.
Therefore optimum conditions for biosynthesis of AgNPs by C. pulcherrima stem extract was 10 min boiling time for stem extract preparation, 12 ml stem extract addition to reaction medium, 1 mM silver nitrate concentration, pH 10 of reaction medium and reaction time for synthesis of AgNPs is 24 h.
4. Characterization of the synthesized AgNPs
4.1. FTIR analysis
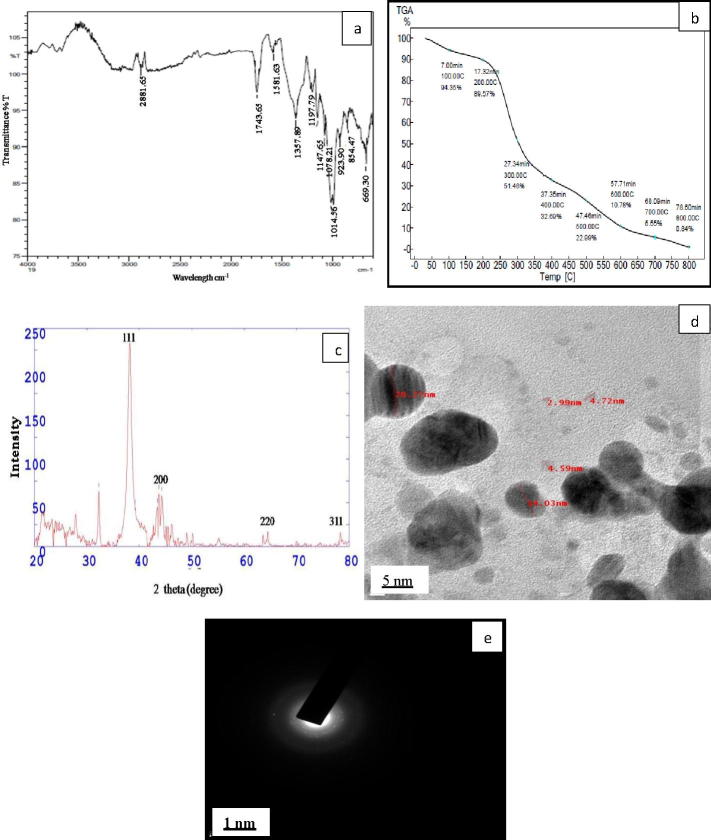
FTIR measurements were carried out to identify the possible biomolecules in stem extract responsible for reduction, capping and stabilization of the silver nanoparticles. FTIR spectrum of AgNPs, recorded in the range of 500–4000 cm−1, showed prominent peaks at 2881.65, 1743.65, 1581.63, 1357.89, 1197.79, 1147.65, 1078.21, 1014.56, 923.90, 854.47 and 669.30 cm−1 (Fig. 2a). The peak at 2881.65 cm−1 corresponds to C—H stretch of alkanes. 1743.65 cm−1 assigned to the C O streach of carbonyls. 1581.63 cm−1 peak is due to N—H bend of primary amines. 1357.89 correspond to N—O symmetric stretch of nitro compounds. 1197.79 cm−1 and 1078.21 cm−1 assigned to the C—N stretching of aliphatic amines. 1147.65 cm−1 and 1014.56 cm−1 correspond to C—O stretch of alcohols. 923.90 cm−1 O—H bend of carboxylic acids. 854.47 cm−1 indicate the C—H bend of alkenes group. 669.30 cm−1 assigned to the C—Cl stretching of alkyl halides. Similar peaks were reported by [35], [2]. It is observed that different functional groups such as alkanes, amino, carbonyl, nitro, alcohols groups etc. are responsible for reduction of silver ions and stabilization of the nanoparticles.
Fig. 2.
(a) FTIR spectrum of AgNPs, (b) TG curve of AgNPs, (c) XRD spectrum of AgNPs, (d) TEM images of AgNPs, and (e) SAED patterns of the AgNPs.
4.2. Thermogravimetric analysis
The thermal stability and capping action of the biomolecules present on the surface of AgNPs was confirmed by TGA (Fig. 2b). The initial weight loss of about 6% at the temperature of 100 °C was due to loss of water molecules from AgNPs. The second weight loss observed in the temperature range of 300–400 °C was found to be around 49%. There was a steady weight loss when the temperature was increased up to 800 °C. This weight loss is due to the degradation of bioorganic molecules present on the surface of AgNPs [27].
4.3. X-ray diffraction analysis
Crystalline nature of AgNPs was determined using Powder XRD. Strong diffraction Braggs peaks at 2θ degrees of 38.21, 43.77, 64.82 and 77.89 which correspond to the (1 1 1), (2 0 0), (2 2 0) and (3 1 1) inter planar reflections of face centered cubic crystal structure respectively (Fig. 2c). The data matched with the standard database of Joint Committee on Powder Diffraction Standards (JCPDS. No. 4.0783) file. Aravinthan et al. [3] also found similar peaks in sunroot synthesized AgNPs.
4.4. TEM analysis
Morphology and particle size of AgNPs was characterized using TEM. The spherical shape of AgNPs was confirmed by TEM images; the size ranged from 3 to 15 nm, with an average size of 8 nm (Fig. 2d). Average particle size of 7 nm is reported in Salmalia malabarica synthesized AgNPs [21]. Crystalline nature of the nanoparticles was further confirmed by selected area electron diffraction (SAED) patterns with diffraction rings (Fig. 2e). The SAED pattern suggests the crystalline nature of AgNPs which is in good agreement with the planes (1 1 1), (2 0 0), (2 2 0), and (3 1 1) of XRD patterns. Similar diffraction rings are also reported by [41].
4.5. Antimicrobial activity
Silver is one of the most and well known universal antimicrobial agent. The individual and combination effect of silver nanoparticles with two antibiotics (Chloramphenicol and Amphotericin B) was observed against 11 microbial strains evaluated using micro well dilution method. The MIC and MBC values of AgNPs alone, AgNPs plus chloramphenicol and their FIC indices is given in Table 1. The MIC values of AgNPs alone ranged from 0.312 to 2.5 mg/ml for Gram positive and Gram negative bacteria while it was 2.5 mg/ml for fungi. E. coli, S. typhimurium and K. pneumoniae were most susceptible bacteria (0.312 mg/ml) followed by S. aureus (0.625 mg/ml). The MIC values of AgNPs plus Chloramphenicol ranged from 0.078 to 0.625 mg/ml for bacteria and AgNPs plus Amphotericin B was 0.156 mg/ml for fungi. S. typhimurium was the most susceptible bacteria. The MIC value of AgNPs plus antibiotic was lower than that of AgNPs alone for both bacteria and fungi. The FIC indices of combination of AgNPs and Chloramphenicol ranged from 0.124 – 0.562 against Gram positive and Gram negative bacteria. AgNPs plus Chloramphenicol combination showed synergistic effect against B. cereus, B. subtilis, S. aureus, C. rubrum and S. typhimurium with FIC indices between 0.124 and 0.373. The combination showed partial synergism against E. coli, P. aeruginosa, K. pneumoniae with FIC indices between 0.5 and 0.562. The combination effect of AgNPs and Amphotericin B and their individual activity is given in Table 2. The FIC indices of combination AgNPs and Amphotericin B ranged from 0.093 to 0.550 against fungi. The combination showed synergistic effect against C. albicans and C. glabrata while partial synergism against C. neoformans.
Table 1.
MIC (mg/ml) and MBC (mg/ml) of stem AgNPs, Chloramphenicol and its synergistic effect.
| Microorganisms | Alone |
Stem AgNPs + Chloramphenicol (CH) |
FIC |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC |
MBC |
MIC |
MBC |
MIC |
MBC |
|||||||||
| AgNPs | CH | AgNPs | CH | AgNPs | CH | AgNPs | CH | AgNPs | CH | ∑FICI | AgNPs | CH | ∑FICI | |
| B. cereus | 2.5 | 1.25 | 5 | 5 | 0.156 | 0.156 | 2.5 | 2.5 | 0.062 | 0.124 | 0.186 | 0.5 | 0.5 | 1 |
| B. subtilis | 2.5 | 1.25 | 5 | 5 | 0.156 | 0.156 | 2.5 | 2.5 | 0.062 | 0.124 | 0.186 | 0.5 | 0.5 | 1 |
| S. aureus | 0.625 | 1.25 | >10 | 5 | 0.156 | 0.156 | 2.5 | 2.5 | 0.249 | 0.124 | 0.373 | ND | 0.5 | ND |
| C. rubrum | 2.5 | 2.5 | 5 | 10 | 0.156 | 0.156 | 2.5 | 2.5 | 0.062 | 0.064 | 0.124 | 0.5 | 0.25 | 0.75 |
| E. coli | 0.312 | 2.5 | 2.5 | 10 | 0.156 | 0.156 | 2.5 | 2.5 | 0.5 | 0.062 | 0.562 | 1 | 0.25 | 1.25 |
| P. aeruginosa | 2.5 | 2.5 | 10 | 10 | 0.625 | 0.625 | 10 | 10 | 0.25 | 0.25 | 0.5 | 1 | 1 | 2 |
| S. typhimurium | 0.312 | 1.25 | 2.5 | 5 | 0.078 | 0.078 | 1.25 | 1.25 | 0.25 | 0.062 | 0.312 | 0.5 | 0.25 | 0.75 |
| K. pneumoniae | 0.312 | 2.5 | 5 | 10 | 0.156 | 0.156 | 2.5 | 2.5 | 0.5 | 0.062 | 0.562 | 0.5 | 0.25 | 0.75 |
Table 2.
MIC (mg/ml) and MBC (mg/ml) of Stem AgNPs, Amphotericin B and its synergistic effect.
| Alone |
Leaf AgNPs + Amphotericin B (AMP) |
FIC |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC |
MFC |
MIC |
MFC |
MIC |
MFC |
|||||||||
| AgNPs | AMP | AgNPs | AMP | AgNPs | AMP | AgNPs | AMP | AgNPs | AMP | ∑FICI | AgNPs | AMP | ∑FICI | |
| C. albicans | 2.5 | 5 | 10 | >10 | 0.156 | 0.156 | 2.5 | 2.5 | 0.062 | 0.031 | 0.093 | 0.25 | ND | ND |
| C. glabrata | 2.5 | 5 | 10 | >10 | 0.156 | 0.156 | 2.5 | 2.5 | 0.062 | 0.031 | 0.093 | 0.25 | ND | ND |
| C. neoformans | 2.5 | 5 | 10 | >10 | 0.156 | 0.156 | 2.5 | 2.5 | 0.05 | 0.5 | 0.55 | 0.25 | ND | ND |
Values are expressed in µg ml−1; ∑FIC (Fractional Inhibitory Concentration Index) = FICA + FICB; FICA = (MICA combination/MICA alone); FICB = (MICB combination/MICB alone) ≤ 0.5 = synergistic, ≥0.5–0.75 = partial synergy; 0.76–1.0 = additive >1.0–4.0 = indifferent (non interactive), >4.0 = antagonistic; ND = Note determined because of high MIC value >10 µg ml−1.
The MBC/MFC values of AgNPs alone ranged from 2.5 to 10 mg/ml for Gram positive and Gram negative bacteria while AgNPs plus Amphotericin B was 10 mg/ml for fungi. The MBC/MFC values of AgNPs plus Chloramphenicol ranged from 1.25 to 10 mg/ml for bacteria and AgNPs plus Amphotericin B was 2.5 mg/ml for fungi. The FIC indices of combination of AgNPs and antibiotic ranged from 0.750 to 2 against bacteria while in fungi not determined because of high MIC value.
The AgNPs and antibiotics combination showed excellent synergistic and partial synergy effect against microorganism. Parta et al. [38] observed enhanced synergistic antimicrobial effect of antibiotics kanamycin with AgNPs against five food borne pathogens and antibiotics Amphotericin B with AgNPs against C. albicans and C. glochares. Moteriya and Chanda [29], found synergistic antimicrobial effects of AgNPs with seven different antibiotics against S. aureus. Yallappa et al. [49] reported 1–2-fold increased synergistic antimicrobial activity against Gram positive and Gram negative bacteria with AgNPs and antibiotics Ampicilin and Kanamycin. In general, the combination of AgNPs with antibiotics i.e. synergistic effects were better than AgNPs alone. The reason may be bonding reaction between antibiotic and AgNPs altered the cell membrane or cytoplasmic membrane permeability, morphology, breakdown of DNA and inhibit respiratory activity of microorganisms [15], [24].
4.6. Antibiofilm activity
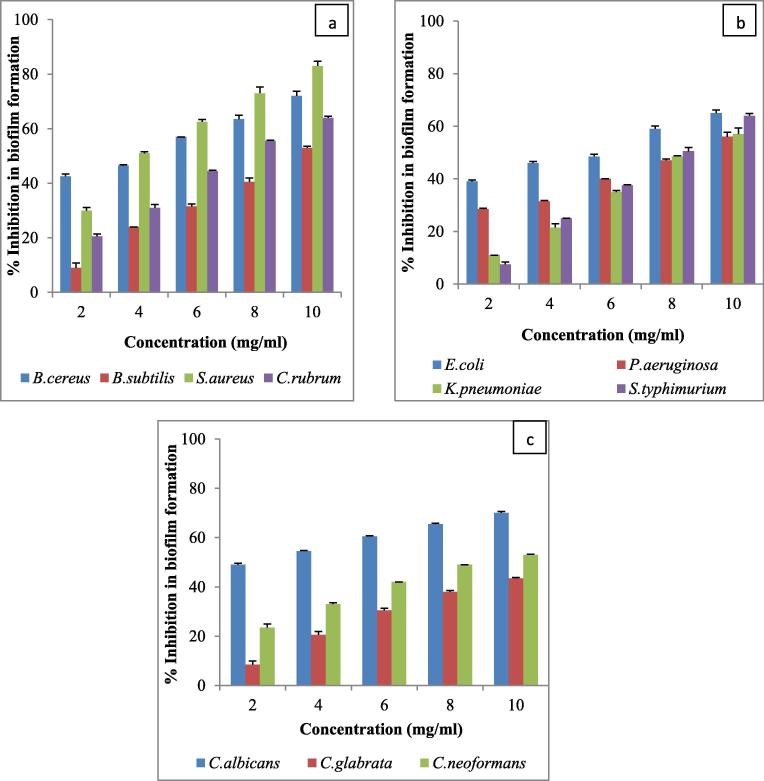
Antibiofilm efficacy was determined using 96 well microtiter plate method. Antibiofilm activity of AgNPs was evaluated against four Gram positive, four Gram negative and three fungi using five different concentrations (2, 4, 6, 8, 10 mg/ml) of AgNPs. In all the 11 microorganisms, biofilm formation decreased with increase in AgNPs concentration. In Gram positive bacteria, maximum antibiofilm inhibition was observed against S. aureus (83%) followed by B. cereus (72%) (Fig. 3a). In Gram negative bacteria, maximum antibiofilm inhibition was found against E. coli (65%) followed by S. typhimurium (64%) (Fig. 3b). In fungi, maximum antibiofilm inhibition was found against C. albicans (70%) followed by C. neoformans (53%) (Fig. 3c). Antibiofilm activity was found against all the 11 microorganism though their levels varied; maximum antibiofilm activity was against Gram positive bacteria S. aureus and minimum against fungi C. glabrata. Barapatre et al. [7] also reported antibiofilm activity against S. aureus followed by E. coli while [12] observed best antibiofilm activity against E. coli followed by S. aureus. Several factors like physical properties, chemical properties and penetration rate of AgNPs are responsible for inhibiting biofilm formation [37].
Fig. 3.
% antibiofilm inhibition of AgNPs against (a) Gram positive bacteria, (b) Gram negative bacteria, and (c) fungi.
4.7. Antioxidant activity
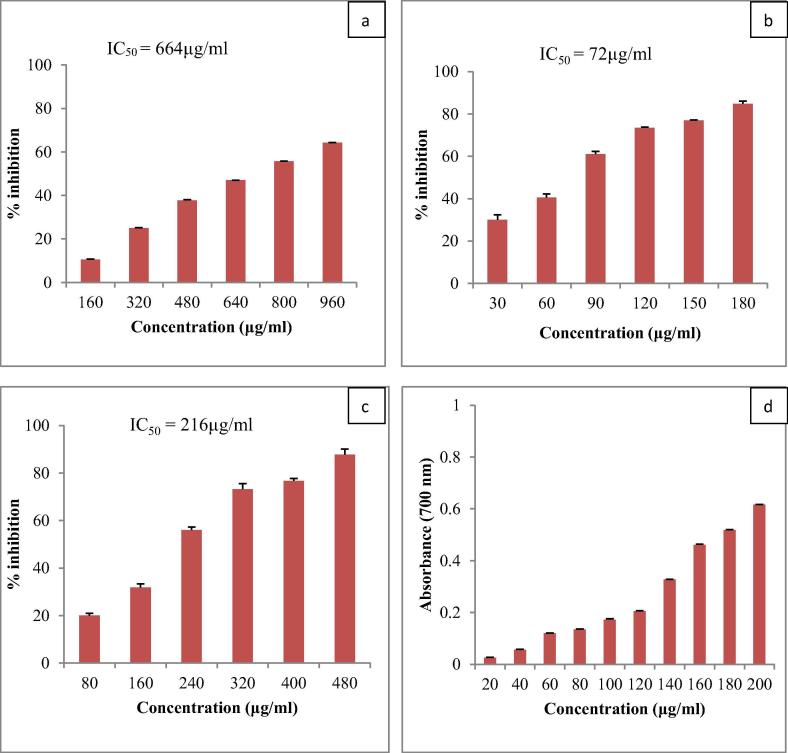
The antioxidant activity of AgNPs was determined using five different colorimetric assays. DPPH assay is stable, simple and more feasible assay. The DPPH radical scavenging activity of AgNPs is given in Fig. 4a. The absorbance at 517 nm decreased when the concentration of AgNPs increased from 160 µg/ml to 960 µg/ml clearly indicating increase in free radical scavenging activity. IC50 value of AgNPs was 664 µg/ml. Similar dose dependent DPPH activity was found in Salicornia brachiata synthesized AgNPs [46].
Fig. 4.
Antioxidant activity of AgNPs (A) DPPH free radical scavenging activity, (B) Superoxide anion radical scavenging activity, (C) ABTS radical scavenging activity, and (D) Reducing capacity assessment.
Superoxide anion is a weak oxidant but it gives rise to the generation of powerful and dangerous hydroxyl radicals as well as singlet oxygen, both of which contribute to oxidative stress. If not quenched, they cause damage to DNA and proteins in the living cells. In the PMS/NADH-NBT system, superoxide anions are generated by the oxidation of NADH coupling reaction reduces NBT. Antioxidants consume superoxide anions and decrease the absorbance of reduced NBT at 560 nm. At concentrations 30–180 µg/ml, AgNPs showed scavenging rate ranging from 30% to 85% (Fig. 4b). The IC50 value of standard gallic acid was 185 μg/ml while that of AgNPs was 72 µg/ml indicating a much better superoxide anions scavenging ability of synthesized AgNPs. Superoxide anions are very dangerous radicals and if not quenched will lead to the formation of other dangerous radicals like singlet oxygen (O2), superoxide (O2•), hydroxyl (OH•), peroxyl (ROO•), Hydrogen peroxide (H2O2), peroxinitrite (•ONOO), nitric oxide (NO•) and cyanide (CN) thus it is noteworthy and appreciable to find a source with this low IC50 value. Inbathamizh et al. [18] reported superoxide radical scavenging activity was 34% at 100 μg/ml of M. pubescens synthesized AgNPs while [25] found superoxide radical scavenging activity 52% at 100 μg/ml of Abutilon indicum synthesized AgNPs.
ABTS cation radical scavenging activity is given in Fig. 4C. In the concentration range of 80–480 µg/ml, an inhibition of 20–88% was envisaged. There was a steady increase in the ABTS cation radical scavenging activity by increasing the concentration of AgNPs. The IC50 value of AgNPs was 216 µg/ml. Moteriya and Chanda [30] and Patra et al. [38] also observed concentration dependent ABTS cation radical scavenging activity in phytomediated AgNPs.
The reducing capacity of AgNPs is given in Fig. 4d. The reducing power increased by increasing the concentration of AgNPs. The increased absorbance at 700 nm indicated an increase in reductive ability of AgNPs. Kanipandian et al. [19] also reported similar result in Cleistanthus collinus extract synthesized AgNPs.
FRAP assay is simple and inexpensive procedure to evaluate the antioxidant capacity of the sample. In the ferric reducing power assay, the reduction of Fe3+ to Fe2+ occurred in the presence of antioxidants and the amount of Fe2+ complex monitored by measuring the formation of blue colour at 593 nm. Ferric reducing antioxidant power of AgNPs was 9.32 (M/g). FRAP activity of synthesized AgNPs was also reported by 32.63 ± 0.019 µmol FeSO4/g sample [40].
4.8. Cytotoxicity study
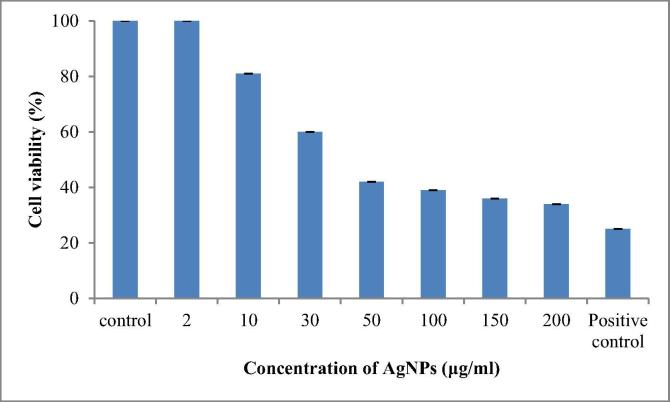
In vitro cytotoxicity on HeLa cell line was tested using different (2 μg, 10 μg, 30 μg, 50 μg, 100 μg, 150 μg, 200 μg) concentrations of AgNPs. The cytotoxic effect on HeLa cell line was dose dependent and cancer cell death increased with increasing concentration of AgNPs. The cell viability was 100% at lower concentration of AgNPs and at 50 µg/ml concentration 42% cells were viable. Maximum inhibition was found at 200 µg/ml concentrations (67%) (Fig. 5). Rajkuberan et al. [42] also found dose dependent cytotoxicity against HeLa cell line. Husseiny et al. [17] found IC50 was 121.23 μg cm−3 of biosynthesized AgNPs against MCF-7 cell line. AgNPs induce cytotoxic effect due to the physicochemical interaction between AgNPs and cancerous cells, which results in generation of reactive oxygen species which damage DNA leading to cell death.
Fig. 5.
In vitro cytotoxicity of biosynthesized AgNPs against HeLa cell line.
4.9. Genotoxicity study
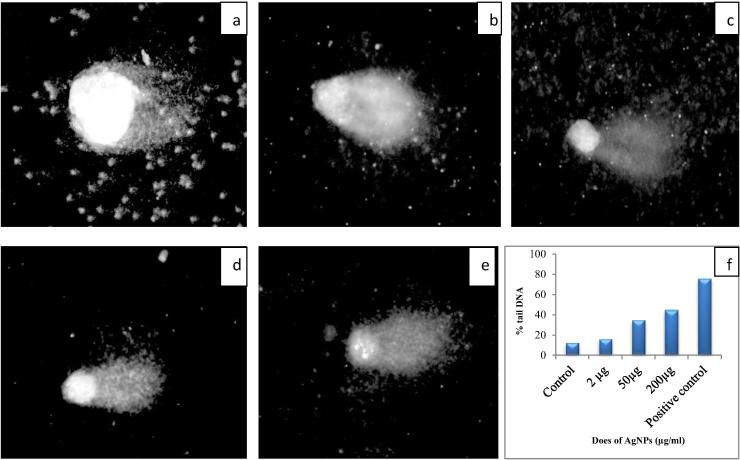
The genotoxicity of biosynthesized AgNPs to damage DNA in the normal human peripheral blood lymphocyte culture was evaluated by alkaline comet assay. Lymphocyte culture was treated with three different concentrations of AgNPs (2 µg, 50 µg and 200 µg) and DNA damage was measured according to comet length or tail length (Fig. 6). Halo surrounding nuclei was clearly found in negative control (Fig. 6a). In positive control, cells were treated with mitomycin C drug (Fig. 6b). 2 µg and 50 µg AgNPs treated cells showed round and intact nuclei without any fragmented DNA while 200 µg treated cells, fragmented DNA was found (Fig. 6c–e). The comet length increased with increasing concentration of AgNPs in dose dependent manner, maximum comet length was found at 200 µg concentration but it was less than positive control (Fig. 6f). The dose dependent genotoxicity of AgNPs is also reported in bone marrow cells and sperms cells of mice [28].
Fig. 6.
DNA damage by comet assay a – Control, b – Positive control, c – 2 µg of AgNPs, d – 50 µg of AgNPs, e – 200 µg of AgNPs, and f – DNA damage in cell after exposure of AgNPs.
5. Conclusion
The biosynthesis and characterization of silver nanoparticles using stem extract of C. pulcherrima was performed and confirmed by spectroscopic and microscopic techniques. The biosynthesized AgNPs were spherical in shape and average size was 8 nm. The synthesized AgNPs showed good synergistic antimicrobial, antibiofilm and antioxidant activity. AgNPs exhibited a strong inhibitory effect on HeLa cancer cells, with a dose-dependent effect. In vitro genotoxic study confirmed that C. pulcherrima stem extract synthesized AgNPs were not toxic at lower concentration. It is suggested that further research should be focused on molecular mechanism and in vivo effects of AgNPs.
Acknowledgements
The authors thank Department of Biosciences (UGC-CAS) for providing excellent research facilities. One of the authors Ms. Pooja Moteiya is thankful to UGC, New Delhi for providing meritorious Junior Research Fellowship.
Footnotes
Peer review under responsibility of National Research Center, Egypt.
References
- 1.Akinyemi K.O., Oladapo O., Okwara C.E., Ibe C.C., Fasure K.A. Screening of crude extract of six medicinal plants used in South West Nigerian unorthodox medicine for anti-methicillin resistant Staphylococcus aureus activity. BMC Complement Altern Med. 2005;5:6–12. doi: 10.1186/1472-6882-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.AlSalhi M.S., Devanesan S., Alfuraydi A.A., Vishnubalaji R., Munusamy M.A., Murugan K., Nicoletti M., Benelli G. Green synthesis of silver nanoparticles using Pimpinella anisum seeds: antimicrobial activity and cytotoxicity on human neonatal skin stromal cells and colon cancer cells. Int J Nanomed. 2016;11:4439–4449. doi: 10.2147/IJN.S113193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aravinthan A., Govarthanan M., Selvam K., Praburaman L., Selvankumar T., Balamurugan R., Kamala-Kannan S., Kim J.H. Sunroot mediated synthesis and characterization of silver nanoparticles and evaluation of its antibacterial and rat splenocyte cytotoxic effects. Int J Nanomed. 2015;10:1977–1983. doi: 10.2147/IJN.S79106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arunachalam K.D., Arun L.B., Annamalai S.K., Arunachalam A.M. Potential anticancer properties of bioactive compounds of Gymnema sylvestre and its biofunctionalized silver nanoparticles. Int J Nanomed. 2015;10:31–41. doi: 10.2147/IJN.S71182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashok Kumar D., Palanichamy V., Roopan S.M. Green synthesis of silver nanoparticles using Alternanthera dentata leaf extract at room temperature and their antimicrobial activity. Spectrochim Acta Part A Mol Biomol Spectrosc. 2014;127:168–171. doi: 10.1016/j.saa.2014.02.058. [DOI] [PubMed] [Google Scholar]
- 6.Balashanmugam P., Balakumaran M.D., Murugan R., Dhanapal K., Kalaichelvan P.T. Phytogenic synthesis of silver nanoparticles, optimization and evaluation of in vitro antifungal activity against human and plant pathogens. Microbiol Res. 2016;192:52–64. doi: 10.1016/j.micres.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Barapatre A., Aadil K.R., Jha H. Synergistic antibacterial and antibiofilm activity of silver nanoparticles biosynthesized by lignin degrading fungus. Bioresour Bioprocess. 2016 doi: 10.1186/s40643-016-0083-y. [DOI] [Google Scholar]
- 8.Bassole I.N.H., Lamien- Meda A., Bayala B., Obame C.L., Ilboudo A.J., Franz C., Novak J., Nebie R.C., Dicko M.H. Chemical composition and antimicrobial activity of Cymbopogon citrates and Cymbopogon giganteus essential oils alone and in combination. Phytomedicine. 2011;18(12):1070–1074. doi: 10.1016/j.phymed.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Chanda S. In: Microbial pathogens and strategies for combating them: science, technology and education. Mendez-Vilas A., editor. FORMATEX Research Center; Badajoz (Spain): 2013. Silver nanoparticles (medicinal plants mediated): a new generation of antimicrobials to combat microbial pathogens – a review; pp. 1314–1323. [Google Scholar]
- 10.Chanda S., Rakholiya K., Dholakia K., Baravalia Y. Antimicrobial, antioxidant and synergistic properties of two nutraceutical plants: Terminalia catappa L. and Colocasia esculenta L. Turk J Biol. 2013;37:81–91. [Google Scholar]
- 11.Chaudhuri R.G., Paria S. Core/shell nanoparticles: classes, properties, synthesis mechanisms, characterization and applications. Chem Rev. 2012;112:2373–2433. doi: 10.1021/cr100449n. [DOI] [PubMed] [Google Scholar]
- 12.Das B., Dash S.K., Mandal D., Ghosh T., Chattopadhyay S., Tripathy S., Das S., Dey S.K., Das D., Roy S. Green synthesized silver nanoparticles destroy multidrug resistant bacteria via reactive oxygen species mediated membrane damage. Arab J Chem. 2015 doi: 10.1016/j.arabjc.2015.08.008. [DOI] [Google Scholar]
- 13.De M., Ghosh P.S., Rotello V.M. Applications of nanoparticles in biology. Adv Mater. 2008;20:4225–4241. [Google Scholar]
- 14.Elavazhagan T., Arunachalam K.D. Memecylon edule leaf extract mediated green synthesis of silver and gold nanoparticles. Int J Nanomed. 2011;6:1265–1278. doi: 10.2147/IJN.S18347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fayaz A.M., Balaji K., Girilal M., Yadav R., Kalaichelvan P.T., Venketesan R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against Gram-positive and Gram-negative bacteria. J Nanomed Nanotechnol. 2010;6:103–109. doi: 10.1016/j.nano.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Finkel T., Holbrook N.J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 17.Husseiny S.M., Salah T.A., Anter H.A. Biosynthesis of size controlled silver nanoparticles by Fusarium oxysporum, their antibacterial and antitumor activities. Beni-Suef Univ J Basic Appl Sci. 2015;4(3):225–231. [Google Scholar]
- 18.Inbathamizh L., Mekalai Ponnu T., Jancy Mary E. In vitro evaluation of antioxidant and anticancer potential of Morinda pubescens synthesized silver nanoparticles. J Pharm Res. 2013;6:32–38. [Google Scholar]
- 19.Kanipandian N., Kannan S., Ramesh R., Subramanian P., Thirumurugan R. Characterization, antioxidant and cytotoxicity evaluation of green synthesized silver nanoparticles using Cleistanthus collinus extract as surface modifier. Mater Res Bull. 2014;49:494–502. [Google Scholar]
- 20.Kar D., Bandyopadhyay S., Dimri U., Mondal D.B., Nanda P.K., Das A.K., Batabyal S., Dandapat P., Bandyopadhyay S. Antibacterial effect of silver nanoparticles and capsaicin against MDR-ESBL producing Escherichia coli: an in vitro study. Asian Pac J Trop Dis. 2016;6(10):807–810. [Google Scholar]
- 21.Krishna I.M., Reddy G.B., Veerabhadram G., Madhusudhan A. Eco-friendly green synthesis of silver nanoparticles using Salmalia malabarica: synthesis, characterization, antimicrobial and catalytic activity studies. Appl Nanosci. 2015;6(5):681–689. [Google Scholar]
- 22.Krishnaraj C., Harper S.L., Choe H.S., Kim K., Yun S. Mechanistic aspects of biologically synthesized silver nanoparticles against food and water borne microbes. Bioprocess Biosyst Eng. 2015;38(10):1943–1958. doi: 10.1007/s00449-015-1436-1. [DOI] [PubMed] [Google Scholar]
- 23.Kumar A., Nirmala V. Gastric antiulcer activity of the leaves of Caesalpinia pulcherrima. Indian J Pharmaceut Sci. 2004;66(5):676–678. [Google Scholar]
- 24.Li W.R., Xie X.B., Shi Q.S., Duan S.S., Ouyang Y.S., Chen Y.B. Antibacterial effect of silver nanoparticles on Staphylococcus aureus. Biometals. 2011;24:135–141. doi: 10.1007/s10534-010-9381-6. [DOI] [PubMed] [Google Scholar]
- 25.Mata R., Nakkala J.R., Sadras S.R. Biogenic silver nanoparticles from Abutilon indicum: their antioxidant, antibacterial and cytotoxic effects in vitro. Colloids Surf B. 2015;128:276–286. doi: 10.1016/j.colsurfb.2015.01.052. [DOI] [PubMed] [Google Scholar]
- 26.McPherson D.D., Cordell G.A., Soejarto D.D., Pezzuto J.M., Fong H.H.S. Peltogynoids and homoisoflavonoids from Caesalpinia pulcherrima. Phytochemistry. 1983;22:2835–2838. [Google Scholar]
- 27.Mittal A.K., Bhaumik J., Kumar S., Banerjee U.C. Biosynthesis of silver nanoparticles: elucidation of prospective mechanism and therapeutic potential. J Colloid Interface Sci. 2014;415:39–47. doi: 10.1016/j.jcis.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 28.Mohamed H.R.H. Studies on the genotoxicity behavior of silver nanoparticles in the presence of heavy metal cadmium chloride in mice. J Nanomater. 2016 doi: 10.1155/2016/5283162. [DOI] [Google Scholar]
- 29.Moteriya P., Chanda S. Biosynthesis of silver nanoparticles using flower extract of Cassia roxburghii DC and its synergistic antibacterial efficacy. Sci Iran. 2014;21(6):2499–2507. [Google Scholar]
- 30.Moteriya P., Chanda S. Low cost and ecofriendly phytosynthesis of silver nanoparticles using Cassia roxburghii stem extract and its antimicrobial and antioxidant efficacy. Am J Adv Drug Delivery. 2014;2(4):557–575. [Google Scholar]
- 31.Moteriya P., Chanda S. Synthesis and characterization of silver nanoparticles using Caesalpinia pulcherrima flower extract and assessment of their in vitro antimicrobial, antioxidant, cytotoxic, and genotoxic activities. Artif Cells Nanomed Biotechnol. 2016;45(8):1556–1567. doi: 10.1080/21691401.2016.1261871. [DOI] [PubMed] [Google Scholar]
- 32.Moteriya P., Chanda S. Characterization, synergistic antibacterial and free radical scavenging efficacy of silver nanoparticles synthesized using Cassia roxburghii leaf extract. J Genetic Eng Biotechnol. 2017;15:505–513. doi: 10.1016/j.jgeb.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moteriya P., Padalia H., Jadeja R., Chanda S. In: Gupta V.K., editor. vol. 3. Daya Publishing House; New Delhi: 2016. Review: screening of silver nanoparticle synthetic efficacy of some medicinal plants of Saurashtra region; pp. 63–83. (Natural products: research review). [Google Scholar]
- 34.Ogu G.I., Aisuodionoe M.E., Nwachukwu P.U. Anti-plasmodial activity of Caesalpinia pulcherrima (Swarts) stem bark extract against Plasmodium berghei in albino mice. Int J Biol Pharm Allied Sci. 2012;1(2):168–178. [Google Scholar]
- 35.Padalia H., Moteriya P., Chanda S. Green synthesis of silver nanoparticles from marigold flower and its synergistic antimicrobial potential. Arab J Chem. 2015;8:732–741. [Google Scholar]
- 36.Palomino J.C., Martin A., Camacho M., Guerra H., Swings J., Portaels F. Resazurin microtiter assay plate: simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2002;46:2720–2722. doi: 10.1128/AAC.46.8.2720-2722.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park H., Park S., Roh J., Kim S., Choi K., Yi J., Kim Y., Yoon J. Removal characteristics of engineered nanoparticles by activated sludge. J Ind Eng Chem. 2013;19:614–619. doi: 10.1016/j.chemosphere.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 38.Patra J.K., Das G., Baek K.H. Phyto-mediated biosynthesis of silver nanoparticles using the rind extract of watermelon (Citrullus lanatus) under photo-catalyzed condition and investigation of its antibacterial, anticandidal and antioxidant efficacy. J Photochem Photobiol, B. 2016;161:200–210. doi: 10.1016/j.jphotobiol.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 39.Pawar C.R., Mutha R.E., Landge A.D., Jadhav R.B., Surana S.J. Antioxidant and cytotoxic activities of Caesalpinia pulcherrima wood. Indian J Biochem Biophys. 2009;46(2):198–200. [PubMed] [Google Scholar]
- 40.Phongtongpasuk S., Poadang S., Yongvanich N. Environmental-friendly method for synthesis of silver nanoparticles from dragon fruit peel extract and their antibacterial activities. Energy Procedia. 2016;89:239–247. [Google Scholar]
- 41.Rahimi-Nasrabadi M., Pourmortazavi S.M., Shandiz S.A.S., Ahmadi F., Batooli H. Green synthesis of silver nanoparticles using Eucalyptus leucoxylon leaves extract and evaluating the antioxidant activities of extract. Nat Prod Res. 2014;28(22):1964–1969. doi: 10.1080/14786419.2014.918124. [DOI] [PubMed] [Google Scholar]
- 42.Rajkuberan C., Sudha K., Sathishkumar G., Sivaramakrishnan S. Antibacterial and cytotoxic potential of silver nanoparticles synthesized using latex of Calotropis gigantea L. Spectrochim Acta Part A Mol Biomol Spectrosc. 2015;136:924–930. doi: 10.1016/j.saa.2014.09.115. [DOI] [PubMed] [Google Scholar]
- 43.Ramesh P.S., Kokila T., Geetha D. Plant mediated green synthesis and antibacterial activity of silver nanoparticles using Emblica officinalis fruit extract. Spectrochim Acta Part A Mol Biomol Spectrosc. 2015;142:339–343. doi: 10.1016/j.saa.2015.01.062. [DOI] [PubMed] [Google Scholar]
- 44.Roy N., Gaur A., Jain A., Bhattacharya S., Rani V. Green synthesis of silver nanoparticles: an approach to overcome toxicity. Environ Toxicol Pharmacol. 2013;36(3):807–812. doi: 10.1016/j.etap.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 45.Sabate D.C., Gonzalez M.J., Porrini M.P., Eguaras M.J., Audisio M.C., Marioli J.M. Synergistic effect of surfactin from Bacillus subtilis C4 and Achyrocline satureioides extracts on the viability of Paenibacillus larvae. World J Microbiol Biotechnol. 2012;28:1415–1422. doi: 10.1007/s11274-011-0941-x. [DOI] [PubMed] [Google Scholar]
- 46.Seralathan J., Stevenson P., Subramaniam S., Raghavan R., Pemaiah B., Sivasubramanian A., Veerappan A. Spectroscopy investigation on chemo-catalytic, free radical scavenging and bactericidal properties of biogenic silver nanoparticles synthesized using Salicornia brachiata aqueous extract. Spectrochim Acta Part A Mol Biomol Spectrosc. 2014;118:349–355. doi: 10.1016/j.saa.2013.08.114. [DOI] [PubMed] [Google Scholar]
- 47.Stepanovic S., Vukovic D., Hola V., Bonaventura G., Djukic S., Cirkovic I., Ruzicka F. Quan-tification of biofilmin microtiter plates: over view of testing conditions and practical recommendations for assessment of biofilm production by Staphylococci. APMIS. 2007;115:891–899. doi: 10.1111/j.1600-0463.2007.apm_630.x. [DOI] [PubMed] [Google Scholar]
- 48.Sudhakar M., Rao C.V., Rao P.M., Raju D.B., Venkateswarlu Y. Antimicrobial activity of Caesalpinia pulcherrima, Euphorbia hirta and Asystasia gangeticum. Fitoterapia. 2006;77:378–380. doi: 10.1016/j.fitote.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 49.Yallappa S., Manjanna J., Dhananjaya B.L. Phytosynthesis of stable Au, Ag and Au–Ag alloy nanoparticles using J. Sambac leaves extract and their enhanced antimicrobial activity in presence of organic antimicrobials. Spectrochim Acta Part A Mol Biomol Spectrosc. 2015;137:236–243. doi: 10.1016/j.saa.2014.08.030. [DOI] [PubMed] [Google Scholar]
- 50.Zayed M.F., Eisa W.H., Abdel-Moneam Y.K., El-kousy S.M., Atia A. Ziziphus spina-christi based bio-synthesis of Ag nanoparticles. J Ind Eng Chem. 2015;23:50–56. [Google Scholar]
- 51.Zayed M.F., Eisa W.H., Shabaka A.A. Malva parviflora extract assisted green synthesis of silver nanoparticles. Spectrochim Acta Part A Mol Biomol Spectrosc. 2012;98:423–428. doi: 10.1016/j.saa.2012.08.072. [DOI] [PubMed] [Google Scholar]