Abstract
Agriculture has new challenges against the climate change: the preservation of genetic resources and the rapid creation of new varieties better adapted to abiotic stress, specially salinity. In this context, the agronomic performance of 25 durum wheat (Triticum turgidum subsp. durum Desf.) genotypes (nineteen landraces and six improved varieties), cultivated in two semi-arid regions in the center area of Tunisia, were assessed. These sites (Echbika, 2.2 g l−1; Barrouta, 4.2 g l−1) differ by their degree of salinity of the water irrigation. The results showed that most of the agronomic traits (e.g. spike per meter square, thousand kernels weight and grain yield) were reduced by salinity. Durum wheat landraces, Mahmoudi and Hmira, and improved varieties, Maali and Om Rabia showed the widest adaptability to different quality of irrigation water. Genotypes including Jneh Kotifa and Arbi were estimated as stable genotypes under adverse conditions. Thereafter, salt-tolerant (Hmira and Jneh Khotifa) and the most cultivated high-yielding (Karim, Razzak and Khiar) genotypes were tested for their gynogenetic ability to obtain haploids and doubled haploid lines. Genotypes with good induction capacity had not necessarily a good capacity of regeneration of haploid plantlets. In our conditions, Hmira and Khiar exhibited the best gynogenetic ability (3.1% and 2.9% of haploid plantlets, respectively).
Abbreviations: PLH, plant height; LA, leaf area; NS, number of spike per meter square; NKS, number of kernels per spike; TKW, thousand kernels weight; GY, grain yield; DH plant, doubled haploid plant
Keywords: Durum wheat, Genetic variation, Salinity, In vitro gynogenesis
1. Introduction
Tunisia is considered one of the largest consumers of cereal in the world (≥280 kg/person/year) [1]. In particular, durum wheat (Triticum turgidum subsp. durum Desf.) is a high value crop and the most cultivated cereal (∼60%) [2]. The national production of wheat presents a part of the current demand of this commodity [3]. Crop yield is irregular due to climate fluctuations (e.g. rainfall). Moreover, the poor quality of water irrigation containing dissolved salts and/or rising water tables carrying naturally-deposited salts to the surface is worth noting in semi-arid and arid regions [4]. Salinity reduces wheat production by affecting both growth and yield parameters [5], [6]. Plant height and leaf area are sensitive growth traits to salinity and are considered as valid tools for screening durum wheat germplasm [7], [8]. On the other hand, Saadallah et al. [9] and Asgari et al. [10] reported that salt stress affect grain yield and its components, in particular the number of spike per meter square, the number of kernels per spike and the thousand kernels weight. There is an increasing interest in the selection of stable and high-yielding genotypes to promote the salty water use efficiency in arid and semi-arid regions. Jaradat and Shahid [11] stated that wheat landraces are better adapted than modern cultivars to changing climate conditions. The performance of durum landraces may be attributed to their population genetic structure, buffering capacity, and combination of morpho-physiological traits conferring adaptability to stress environments.
Plant breeders use conventional and biotechnological approaches to obtain homozygous lines of performant genotypes. The traditional method require several cycles of self-pollination, while the haplodiploidization methods (androgenesis, gynogenesis and intergeneric hybridization) are less-time consuming for the development of stable lines in a one-step [12]. This biotechnology is suitable for barley and durum wheat species [13], [14], [15]. Moreover, it offers the possibility to ovoid albino plants (particular form of recalcitrance), the main problem of androgenesis [16], [17], [18].
In this work, we aimed to (i) assess the performance of local germplasm of durum wheat (landraces and improved genotypes) against salt stress in order to identify salt-tolerant genotypes, and (ii) to regenerate durum wheat doubled haploid lines by gynogenesis.
2. Materials and methods
2.1. Plant material
Twenty five (25) durum wheat genotypes (Triticum turgidum ssp. durum) including six (6) improved varieties and nineteen (19) landraces were evaluated for their salinity tolerance (Table 1). To obtain doubled haploid (DH) lines by gynogenesis, five (5) genotypes (Karim, Razzak, Khiar, Hmira and Jneh Khotifa) selected from the 25 durum wheat genotypes were used: Hmira was estimated as salt-tolerant genotype and Jneh Khotifa as a stable genotype. Karim, Razzak and Khiar were chosen as the most cultivated and high yielding improved varieties.
Table 1.
List of used durum wheat genotypes.
| Genotypes | Name |
|---|---|
| Improved varieties | Karim, Razzek, Om Rabia, Maali, Nasr and Khiar |
| Landraces | Aoudhay, Jneh Khotifa, Biskri Pubescent, Agili, Bidi AP4, Azizi, Bayadha, Swebei Algia, Derbessi, Mahmoudi, Souri, INRAT 69, Ward Bled, Arbi, Hmira, Sbei, Chili, Agili Glabre and Richi |
2.2. Experimental condition to test performance of genotypes to salinity
2.2.1. Experimental design
A field experiment was conducted using 25 durum wheat genotypes during the cropping season 2009–2010 in two semi-arid regions in the center area of Tunisia: Echbika (35°37N, 9°56E) and Barrouta (35°34N, 10°02E). These sites differ by their degree of salinity of the water irrigation with 2.2 and 4.2 g l−1, respectively. The experiment was arranged in randomized complete block design with three replications. Each plot was constituted by 10 rows of 1 m long, spaced by 0.20 m. The seeding rate was 300 viable seeds m−2.
2.2.2. Agronomic parameters
Physiological maturity was achieved around mid-May and harvest was performed about one month later. Flag leaf area (LA, cm2) was measured one week after anthesis with Leaf Area Meter-LI-200 as the means of three independent measurements in three different plants in each plot. Plant height (PLH, cm) of five plants randomly chosen was also measured one week after anthesis as the distance from ground to the spike’s tip. Two weeks after anthesis, 1 m was harvested in the two central rows for the determination of total biomass (g m−2). Grains were collected using a shredder (Wentersteiger, LD-180, Germany). Number of spike per meter square (NS), number of kernels per spike (NKS), thousand kernels weight (TKW, g) and grain yield (GY, kg ha−1) were recorded.
2.3. Test of doubled haploid lines
2.3.1. Experimental design
Seeds of the five durum wheat genotypes (Karim, Razzak, Khiar, Hmira and Jneh Khotifa) were sown in the experimental fields at the National Agronomic Institute of Tunis, Tunisia (36°828965N, 10°180883E). This experiment was conducted in a randomized complete block design with three replications. Each plot was constituted by 5 rows of 2 m long, spaced by 0.18 m. The different plots were spaced by 0.30 m.
2.3.2. Donor plants and pretreatment
The choice of the optimal phase of the maturity of ovaries was based on morphological and cytological criteria. The characterization of microspores from the oldest ovaries was established through microscopic examination in aceto-carmine stain. Spikes contained microspores at the late uninucleate or binucleate stage were collected. Consequently, others tillers were collected when they reach a similar stage of morphological development.
Spikes were placed in water and stored in refrigerator at 4 °C for 14 days in the dark [14]. This cold pretreatment aims to change the gametophytic to sporophytic pathway and to improve the gynogenetic ability [19].
2.3.3. Protocol of unpollinated ovary culture
Spikes were surface-sterilized with sodium hypochlorite (12%) for 10 min and washed three times with sterilized water. The ovaries of 1–1.5 mm size were carefully extracted and placed in 5.5 cm diameter Petri dishes (20 ovaries/dish) containing induction medium of Sibi et al. [13] (Table S1). A total of two miles unpollinated ovaries per genotype were approximately used. The Petri dishes were sealed and maintained in incubator in the dark [20] at 27 °C. Calli were regenerated in the differentiation medium at 25 °C, 16 h light/8h dark photoperiod and light intensity of 80–100 µE m−2 s−1. Thereafter, calli with emerging shoots were placed on development medium and maintained in the same conditions of regeneration. After regeneration, the cultures were transferred into beakers containing 125 ml of development medium and grown to plantlets [14].
2.3.4. Chromosome count and chromosome doubling
Systematic checking of ploidy level by chromosome count was released according to Jahier et al. [21] protocol to distinguish the haploid plantlets whose chromosome stock was spontaneously doubled. In order to double the chromosome number, the obtained plants by unpollinated ovaries culture were treated at 2–3 leaves stage by soaking their roots in a colchicine solution (0.1%) for 4 h. After checking ploidy level, the treated plants were transferred in pots (sand: peat, 2:1) and placed in the growth chamber at 25 °C ± 1 °C, 16 h light /8h dark photoperiod and light intensity of 350–450 µE m−2 s−1.
2.3.5. Gynogenetic parameters
Five gynogenetic parameters were determined as follow:
The experiment was performed with five replicates for each treatment. In this study, the question was raised concerning the possible correlation between the percentage of haploid plantlets and the percentage of induction, calli and green shoots. The percentage of haploid plantlets was chosen to avoid the toxic effect of colchicine inducing plant mortality.
2.4. Statistical analysis
All experimental data were subjected to analysis of variance (ANOVA) using the SPSS statistical software (16.0) (SPSS for Windows, 2007, Chicago, USA). Means of the different agronomic and gynogenetic parameters were compared by Least Significant Difference (LSD) test (P < .05). A Pearson's correlation was used to determine the relationship between agronomic traits. A principal component analysis (PCA) was carried out to study distribution of the different durum wheat genotypes based on the tested agronomic parameters. This analysis was made using statistical software XLSTAT 2003 version 5.2. A linear regression analysis was also performed between the percentage of haploid plantlets and the percentage of induction, calli and green shoots in order to establish their mutual relationship.
3. Results
3.1. Performance of wheat genotypes under salt stress conditions
Analysis of variance (ANOVA) showed highly significant (P < .01) effect of genotypes (G) on all tested agronomic traits and a significant effect (P < .05) on LA (Table 2). All measured parameters were also highly and significantly (P < .01) affected by the variation of water irrigation among sites (S). Interaction S × G had significant effect for the most traits (e.g. PLH, NS, TKW and GY). Overall, the agronomic parameters of improved varieties cultivated in Barrouta (4.2 g l−1) were reduced as compared to those of Echbika (2.2 g l−1) (Table S2). For wheat landraces, LA, NS, NKS, TKW and GY obtained in Barrouta were also reduced as compared to those of Echbika, while the PLH and Biomass were increased. Improved varieties still the most productive genotypes (GY), although they were affected by salinity.
Table 2.
Analysis of variance of the different agronomic traits according to the sites, genotypes and their interaction.
| Factors | df | PLH | LA | Biomass | NS | NKS | TKW | GY |
|---|---|---|---|---|---|---|---|---|
| Site (S) | 1 | 5010.5** | 296.8** | 208822.67** | 204528.7** | 3099** | 1215.87** | 28706.5** |
| Genotype (G) | 24 | 3718.7** | 1215.87* | 8643.01** | 28745.92** | 82.68** | 123.23** | 1276.84** |
| SxG | 24 | 129.52** | 123.23* | 3421.02* | 14833.99** | 30.23* | 57.94** | 588.32** |
| CV % | 50 | 3.06 | 15.94 | 18.67 | 19.4 | 18.78 | 25.24 | 36.61 |
PLH, plant height; LA, leaf area; NS, number of spike per meter square; NKS, number of kernels per spike; TKW, thousand kernels weight; GY, grain yield.
P < .05.
P < .01.
*** P < .001.
Simple correlation between all the tested parameters showed that GY was highly and positively correlated with NS, NKS and TKW in both sites (Table 4, Table 5). The PLH showed also a high and positive correlation with LA and Biomass. In most cases, GY and its components (NS and NKS) exhibited significant negative associations with PLH, LA and Biomass.
Table 4.
Pearson’s correlation coefficients between the agronomic traits calculated from 25 durum wheat genotypes grown in Echbika site.
| PLH | LA | Biomass | NS | NKS | TKW | GY | |
|---|---|---|---|---|---|---|---|
| PLH | 1 | ||||||
| LA | 0.798*** | 1 | |||||
| Biomass | 0.603** | 0.411* | 1 | ||||
| NS | −0.666*** | −0.665*** | −0.237 | 1 | |||
| NKS | −0.261 | −0.294 | −0.359 | 0.365 | 1 | ||
| TKW | −0.551** | 0.431* | −0.605** | 0.195 | 0.464* | 1 | |
| GY | −0.708*** | −0.683*** | −0.457* | 0.841*** | 0.722*** | 0.585** | 1 |
PLH, plant height; LA, leaf area; NS, number of spike per meter square; NKS, number of kernels per spike; TKW, thousand kernels weight; GY, grain yield.
P < .05.
P < .01.
P < .001.
Table 5.
Pearson’s correlation coefficients between the agronomic traits calculated from 25 durum wheat genotypes grown in Barrouta site.
| PLH | LA | Biomass | NS | NKS | TKW | GY | |
|---|---|---|---|---|---|---|---|
| PLH | 1 | ||||||
| LA | 0.633** | 1 | |||||
| Biomass | 0.832*** | 0.602** | 1 | ||||
| NS | −0.491* | −0.427* | −0.481* | 1 | |||
| NKS | −0.556** | −0.167 | −0.473* | 0.502** | 1 | ||
| TKW | −0.099 | 0.314 | −0.100 | 0.006 | 0.644** | 1 | |
| GY | −0.601** | −0.308 | −0.540** | 0.850*** | 0.839*** | 0.445* | 1 |
PLH, plant height; LA, leaf area; NS, number of spike per meter square; NKS, number of kernels per spike; TKW, thousand kernels weight; GY, grain yield.
P < .05.
P < .01.
P < .001.
The distribution of genotypes and agronomic traits was performed on the main plan of the PCA formed by the first two axes. For the Echbika site, these axes presented 65% of the total variability (Fig. 1A). The PCA 1 was positively correlated with NKS, NS, TKW and GY, and negatively correlated with PLH and Biomass. However, the PCA 2 was weakly correlated with these parameters (Table 3). Three groups of genotypes with differential sensitivities to salt stress were identified. The first group contained improved varieties including Khiar, Om Rabia, Razzek, Nasr, Karim and Maali. These genotypes were separated from the landraces based on high grain yield and its components (i.e. NKS and TKW) and a low straw yield (PLH) (Fig. 1A, Table S2). The second group included Mahmoudi, Bayadha and INRAT 69 genotypes that were moderately productive. The last group presented the remains of wheat landraces with low grain production and a large straw yield. For the Barrouta site, the PCA 1 and PCA 2 presented 61% of the total variability (Fig. 1B). The GY and NKS still significantly correlated with the PCA 1 (Table 3). However, NS and TKW became weakly correlated with this axis. In fact, salinity reduced tillers fertility and grain filling. The PCA 2 became strongly correlated with NS, Biomass and LA parameters. In these conditions, four groups were identified. The first group included improved varieties, Maali and Om Rabia. These genotypes were distinguished by their tolerance to salinity and still among the most productive genotypes (Fig. 1B, Table S2). The same group included wheat landraces, Mahmoudi and Hmira that became strongly correlated with yield parameters. Nevertheless, Khiar, Razzek, Nasr and INRAT 69, negatively correlated with the Biomass, PLH and LA traits, formed the second group. The third and the fourth groups were mainly constituted by wheat landraces (e.g. Jneh Khotifa, Arbi and Swebei Algia) which proved as the most stable genotypes in term of salinity tolerance and kept their production level.
Fig. 1.
Distribution of the tested agronomic traits and durum wheat genotypes, cultivated in Echbika (A) and Barrouta (B) sites, on the main plane of PCA. PLH, plant height; LA, leaf area; NS, number of spike per meter square; NKS, number of kernels per spike; TKW, thousand kernels weight; GY, grain yield.
Table 3.
Correlation of the studied agronomic traits with the first two discriminant axes of PCA for the Echbika and Barrouta sites.
| Echbika |
Barrouta |
|||
|---|---|---|---|---|
| PCA 1 | PCA 2 | PCA 1 | PCA 2 | |
| PLH | −0.753 | −0.197 | −0.752 | 0.02 |
| LA | 0.052 | 0.926 | −0.711 | 0.493 |
| Biomass | −0.601 | −0.04 | −0.101 | 0.755 |
| NS | 0.922 | 0.06 | 0.17 | 0.84 |
| NKS | 0.754 | 0.20 | 0.632 | 0.725 |
| TKW | 0.745 | −0.501 | 0.037 | 0.101 |
| GY | 0.665 | −0.168 | 0.839 | 0.17 |
PLH, plant height; LA, leaf area; NS, number of spike per meter square; NKS, number of kernels per spike; TKW, thousand kernels weight; GY, grain yield.
3.2. Doubled haploid lines regeneration
The evaluation of the gynogenetic ability was based on five successive phases: induction, differentiation, development of green shoots, regeneration of haploid plantlets and production of DH lines phases.
3.2.1. Stages of unpollinated ovary culture
After fifteen (15) days of culture on induction medium in darkness, enlarged ovaries were obtained (Fig. 2A). Calli structures were developed after six weeks on differentiation medium (Fig. 2B). Thereafter, green shoots were observed from callus transferred on development medium after light exposure (Fig. 2C). Haploid plantlets were regenerated in the same conditions (Fig. 2D). DH lines (Fig. 2G) were recovered after chromosome doubling by 0.1% colchicine (Fig. 2F). A check of the ploidy level was made on haploid (Fig. 2E) and DH plants after the colchicine treatment (Fig. 2H).
Fig. 2.
Different stages of durum wheat unpollinated ovaries development for the doubled haploid regeneration.
3.2.2. Genotypic effect
The ANOVA showed significant variations between genotypes for the gynogenetic parameters (P < .05), except the green shoots (P = .146) and DH plants (P = .931) (Table S3). Both Hmira and Razzek showed the highest induction rates with 71% and 55.7%, respectively (Fig. 3A). However, Razzek were the most efficient genotype for the development of calli (23.8%) followed by Hmira (18%) (Fig. 3B). Hmira (7%) presented the best rate of green shoots followed by Karim (6.2%) (Fig. 3C). On the other hand, Hmira and Khiar showed the highest percentage of regenerated haploid plantlets (3.1% and 2.9% respectively) (Fig. 3D). Finally, Khiar and Hmira exhibited the highest percentage of DH plants (0.8% and 0.7% respectively) (Fig. 3E).
Fig. 3.
Classification of the six-durum wheat genotypes according to the percentage of induction (A), calli (B), green shoots (C), haploid plantlets (D) and doubled haploid plants (E). Graph bars (mean ± SE) with the same letter are not significantly different (P < .05; LSD test).
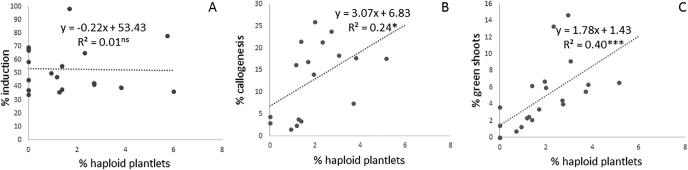
The results indicated a non-significant and negative correlation between the percentage of haploid plantlets and the percentage of induction (r = 0.02; P = .924), (Fig. 4). However, a significant and positive correlation between the percentage of haploid plantlets and the percentage of calli (r = 0.49; P = .044) and green shoots (r = 0.64; P = .001) were obtained.
Fig. 4.
Correlation between the percentage of durum wheat haploid plantlets and the percentage of induction (A), calli (B) and green shoots (C). nsP > .05; *P < .05; **P < .01; ***P < .001.
4. Discussion
In normal conditions (i.e. salinity of 2.4 g l−1), the improved durum wheat genotypes (e.g. Khiar, Om Rabia, Razzek, Nasr, Karim and Maali) showed a better responsiveness to good irrigation water quality as compared with landraces (Table S2). Implementation of stress condition (i.e. salinity of 4.2 g l−1) caused haying off; that is, it decreased grain yield and its components. Similar findings were reported by Díaz De León et al. [8] and Yousfi et al. [22]. In our conditions, the NKS was the most sensitive trait. This result was also in agreement with previous reports on durum wheat [23] and bread wheat [24]. The decrease in grain yield across saline site might be explained by the decrease of NS and the number of fertile spike per plant, which could also affect the number of kernels per area and the thousand kernels weight. In fact, El-Hendawy et al. [24] and Abbassenne et al. [25] showed that salinity might reduce the fertility of the spike and the translocation of assimilates to the grain in bread wheat and barley.
The present research showed that the tested genotypes exhibited a differential response to salinity. The improved varieties still clearly outyielded the landraces regardless the salinity conditions. Interestingly, the wheat landraces, Mahmoudi, Hmira, and improved varieties, Om Rabia and Maali were distinguished by a large adaptation to different quality of irrigation water (2.4 and 4.2 g l−1), (Table S2, Fig. 1). Genotypes including Jneh Kotifa and Arbi seem to be a stable genotypes under adverse conditions.
In both sites, the relationships between GY and yield components (NS, NKS and TKW) was positive. The NS and NKS were better associated with GY than TKW, as previously reported by del Pozo et al. [26] in full irrigation and water stress conditions. In the same conditions, PLH, LA and Biomass were positively correlated. Increasing LA is known as one of the ways to increase the light interception capacity of a crop and biomass production [27].
The GY and its components, however, were negatively associated with growth traits (PLH, LA and Biomass). Indeed, improved varieties of durum wheat showed lower biomass and PLH than landraces. Meanwhile, NS, NKS, TKW and GY were higher in the improved varieties compared to the landraces. This is might be explained by the introduction of semi-dwarfing genes Rht during the breeding programs. These genes reduce cell elongation with subsequent plant height reduction to avoid lodging risk and to increase the grain yield and its components, especially number of spikes per plant and number of kernels m−2 [28], [29].
Our results suggest that growth traits (Biomass, LA or PLH) and grain yield may respond differentially against stress in wheat genotypes as mentioned in other studies [30], [31]. Therefore, the phenotyping of these parameters (i.e. green biomass, LA and PLH) cannot be used as a general approach to predict grain yield.
The performance of salt-tolerant genotypes (e.g. Jneh Khotifa and Hmira) with high-yielding genotypes (e.g. Karim, Razzak and Khiar) for the regeneration capacity of DH lines by gynogenesis were assessed. All the tested genotypes showed no recalcitrance and regenerated a DH lines. A genotypic variation was observed for the tested parameters except for the green shoots and DH plants regeneration (Table S3, Fig. 3). Generally, the wheat landrace Hmira and the improved variety Khiar showed the best gynogenetic capacity. Similar pattern was also obtained in barley [20] and durum wheat [13], [32] by gynogenesis or intergeneric wheat x maize crosses [33]. Altogether, the results confirming that DH lines regeneration is controlled by genetic factors.
This work showed that genotypes with good induction capacity have not necessarily a good capacity of haploid plantlets and DH plants regeneration and vice-versa (Fig. 4). In fact, Karim showed good induction while the rates of haploid and DH regeneration were low. On the other hand, Khiar presented, despite its low rate of induction, a good capacity of haploid and DH regeneration. The percentage of differentiated callus seems to be a determinant parameter of haploid and DH plants regeneration. Nevertheless, many other factors might affect this regeneration rate, in particular the culture conditions of donor plants in the field. In fact, Chlyah and Saidi [34] and Jacquard et al. [35] reported the impact of environmental factors: the date of collection of plant material, the effects of annual cycle and spike position on the androgenetic response of genotypes grown in the field. Moreover, the stage of ovary culture, the pretreatment [36], [37], the chromosome doubling technique [28], and the culture medium of induction and regeneration [38], [39], [40] showed an evident relevance in the regeneration process. The low frequency of DH plants as compared to the haploid plantlets and the absence of significant differences between genotypes might be also due to the toxic effect of the colchicine [41]. In conclusion, the efficiency of gynogenesis protocol seems to be valuable for a wide range of genotypes. Further work is needed to optimize the gynogenetic ability of durum wheat by acting on other factors (e.g. pretreatment). Furthermore, it will be interesting to combine the performance of salt-tolerant genotypes with high-yielding genotypes and to be fixed by gynogenesis.
Acknowledgements
This study was supported by the International Atomic Energy Agency (IAEA, Austria), the Tunisian Ministry of Higher Education and Scientific Research and the Institution of Agricultural Research and Higher Education of Tunisia.
Footnotes
Peer review under responsibility of National Research Center, Egypt.
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jgeb.2017.11.004.
Appendix A. Supplementary data
References
- 1.Chahed Y. Tunisia GRAIN, Grain and Feed Annual, USDA Foreign Agriculture service, Grain Report; 2009. <https://www.fas.usda.gov/data/tunisia-grain-and-feed-annual-0>.
- 2.Boukef S., Karmous C., Trifa Y., Rezgui S. Durum wheat grain quality traits as affected by nitrogen fertilization sources under Mediterranean rainfed conditions. J Agr Sustain. 2013;4:99–114. [Google Scholar]
- 3.USDA. Tunisia Grain and Feed Annual; 2016. <http://gain.fas.usda.gov/Recent%20GAIN%20Publications/Grain%20and%20Feed%20Annual_Tunis_Tunisia_3-30-2016.pdf>.
- 4.Rengasamy P. Soil processes affecting crop production in salt affected soils. Funct Plant Biol. 2010;37:613–620. [Google Scholar]
- 5.Husain S., Munns R., Condon A.G. Effect of sodium exclusion trait on chlorophyll retention and growth of durum wheat in saline soil. Aust J Agric Res. 2003;54:589–597. [Google Scholar]
- 6.Roy S.J., Negrão S., Tester M. Salt resistant crop plants. Curr Opin Biotechnol. 2014;26:115–124. doi: 10.1016/j.copbio.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Munns R., James R.A. Screening methods for salinity tolerance: a case study with tetraploid wheat. Plant Soil. 2003;253:201–218. [Google Scholar]
- 8.Díaz De León J.L., Escoppinichi R., Zavala-Fonseca R., Castellanos T., Röder M.S., Mujeeb-Kazi A. Phenotypic and genotypic characterization of salt-tolerant wheat genotypes. Cereal Res Commun. 2010;38:15–22. [Google Scholar]
- 9.Saadallah K., Drevon J.J., Hajji M., Abdelly C. Genotypic variability for tolerance to salinity of N2-fixing common bean (Phaseolus vulgaris) Agronomie. 2001;21:675–682. [Google Scholar]
- 10.Asgari H.R., Cornelisb W., Van Dammeb P. Effect of salinity on wheat (Triticum aestivum L.) grain yield components and ion uptake. Desert. 2011;16:169–175. [Google Scholar]
- 11.Jaradat A., Shahid M. How diverse a farmer-managed wheat landrace can be? Emir J Food Agric. 2014;26:93–118. [Google Scholar]
- 12.Germana M.A. Gametic embryogenesis and haploid technology as valuable support to plant breeding. Plant Cell Rep. 2011;30:839–857. doi: 10.1007/s00299-011-1061-7. [DOI] [PubMed] [Google Scholar]
- 13.Sibi M.L., Kobaissi A., Shekafandeh A. Green haploid plants from unpollinated ovary culture in tetraploid wheat (Triticum durum Defs.) Euphytica. 2001;122:351–359. [Google Scholar]
- 14.Slama Ayed O., Trifa Y., SlimAmara H., DeBuyser J., Picard E. Production of doubled haploids in Tunisian durum wheat (Triticum durum Desf.) cultivars through unpollinated ovary culture. Plant Mutat. Rep. 2010;2:33–39. [Google Scholar]
- 15.Getahun T., Feyissa T., Gugsa L. Regeneration of plantlets from unpollinated ovary cultures of Ethiopian wheat (Triticum turgidum and Triticum aestivum) Afr J Biotechnol. 2013;12:5754–5760. [Google Scholar]
- 16.Kumari M., Clarke H.J., Small I., Siddique K.H.M. Albinism in plants: a major bottleneck in wide hybridization, androgenesis and double haploid culture. Crit Rev Plant Sci. 2009;28:393–409. [Google Scholar]
- 17.Islam S.M., Tuteja N. Enhancement of androgenesis by abiotic stress and other pretreatments in major crop species. Plant Sci. 2012;182:134–144. doi: 10.1016/j.plantsci.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Makowska K., Oleszczuk S. Albinism in barley androgenesis. Plant Cell Rep. 2014;33:385–392. doi: 10.1007/s00299-013-1543-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soriano M., Li H., Boutilier K. Microspore embryogenesis: establishment of embryo identity and pattern in culture. Plant Reprod. 2013;26:181–196. doi: 10.1007/s00497-013-0226-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sibi M, Fakiri M. In: AUPELF-UREF editors. Quel avenir pour l’amélioration des plantes? John Libbey Eurotext, Paris; 1994, p. 337–44.
- 21.Jahier J, Chèvre AM, Eber F, Delourme R, Tanguy AM. Techniques de cytogénétique végétale, INRA –France; 1992.
- 22.Yousfi S., Serret M.D., Araus J.L. Effect of salinity and water stress during the productive stage on growth, ion concentrations, D13C and d15N of durum wheat and related amphiploids. J Exp Bot. 2010;61:3529–3542. doi: 10.1093/jxb/erq184. [DOI] [PubMed] [Google Scholar]
- 23.Turki N., Shehzad T., Harrabi M., Tarchi M., Okuno K. Variation in response to salt stress at seedling and maturity stages among durum wheat varieties. J Arid Land Stud. 2014;24:261–264. [Google Scholar]
- 24.El-Hendawy S.E., Hu Y., Yakout G.M., Awad A.M., Haifz S.E., Schmidhalter U. Evaluating salt tolerance of wheat genotypes using multiple parameters. Euro Agron. 2005;22:243–253. [Google Scholar]
- 25.Abbassenne F., Bouzerzour H., Hachemi L. Phénologie et production du blé dur (Triticum durum Desf.) en zone semi-aride. Ann Agron INA. 1998;18:24–36. [Google Scholar]
- 26.del Pozo A., Yáñez A., Matus I.A., Tapia G., Castillo D., Sanchez-Jardón L., Araus J.L. Physiological traits associated with wheat yield potential and performance under water-stress in Mediterranean environment. Front Plant Sci. 2016;7:987. doi: 10.3389/fpls.2016.00987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weraduwage S.M., Chen J., Anozie F.C., Morales A., Weise S.E., Sharkey T.D. The relationship between leaf area growth and biomass accumulation in Arabidopsis thaliana. Front Plant Sci. 2015;6:167. doi: 10.3389/fpls.2015.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kowalski A.M., Gooding M., Ferrante A., Slafer G.A., Orford S., Gasperini D., Griffiths S. Agronomic assessment of the wheat semi-dwarfing gene Rht8 in contrasting nitrogen treatments and water regimes. Field Crops Res. 2016;191:150–160. doi: 10.1016/j.fcr.2016.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casebow R., Hadley C., Uppal R., Addisu M., Loddo S., Kowalski A., Griffiths S., Gooding M. Reduced height (Rht) alleles affect wheat grain quality. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0156056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petcu E., Petcu G., Lazãr C., Vintilã R. Relationship between leaf area index, biomass and winter wheat yield obtained at Fundulea, under conditions of 2001 year. Rom Agric Res. 2003;19–20:21–29. [Google Scholar]
- 31.Paul K., Pauk J., Deák Z., Sass L., Vass I. Contrasting response of biomass and grain yield to severe drought in Cappelle Desprez and Plainsman V wheat cultivars. Peer J. 2016;4 doi: 10.7717/peerj.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mdarhri-Alaoui M., Saidi N., Chlayah A., Chlayah H. Obtention par gynogenèse in vitro de plantes haploïdes chlorophyliennes chez le blé dur. Sciences de la vie / Life Sciences. 1998;321:25–30. [Google Scholar]
- 33.Gert D., Baumann A., Schmucker S. Production of wheat doubled haploids (Triticum aeativum L.) by wheat x maize crosses using colchicine enriched medium for embryo regeneration. Cereal Res Commun. 2005;33:461–468. [Google Scholar]
- 34.Chlyah H, Saidi N. In: AUPELF-UREF editors. Amélioration des plantes pour l'adaptation aux milieux arides. Paris: John Libbey Eurotext; 1991, p. 135–48.
- 35.Jacquard C., Asakaviciute R., Hamalian A.M. Barley anther culture: effects of annual cycle and spike position on microspore embryogenesis and albinism. Plant Cell Rep. 2006;25:375–381. doi: 10.1007/s00299-005-0070-9. [DOI] [PubMed] [Google Scholar]
- 36.Rituraj K., Meenakshi S., Baenziger P.S., Dipak K.S. Effect of cold-mediated pretreatment on microspore culture in winter and spring wheat. Am J Plant Sci. 2013;4:2259–2264. [Google Scholar]
- 37.Castillo A.M., Sanchez-Diaz R.A., Vallés M.P. Effect of ovary induction on bread wheat anther culture: ovary genotype and developmental stage, and candidate gene association. Front Plant Sci. 2015;6:1–12. doi: 10.3389/fpls.2015.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Díaz De León J.L., Garibaldi-Meza C. Potential practical applications of in vitro culture of mature wheat embryos. Cereal Res Commun. 1995;23:19–25. [Google Scholar]
- 39.González J.M., Jouve N. Improvement of anther culture media for haploid production in triticale. Cereal Res Commun. 2000;28:65–72. [Google Scholar]
- 40.Martinez L. In: Doubled haploid production in crop Plants. A manual. Maluszynski M., Kasha K.J., Forster B.P., Szarejko I., editors. Kluwer Academic Publishers; Netherlands: 2003. pp. 275–280. [Google Scholar]
- 41.Tadesse W., Inagaki M., Tawkaz S., Baum M., Van Ginkel M. Recent advances and application of doubled haploids in wheat breeding. Afr J Biotechnol. 2012;11:15484–15492. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.