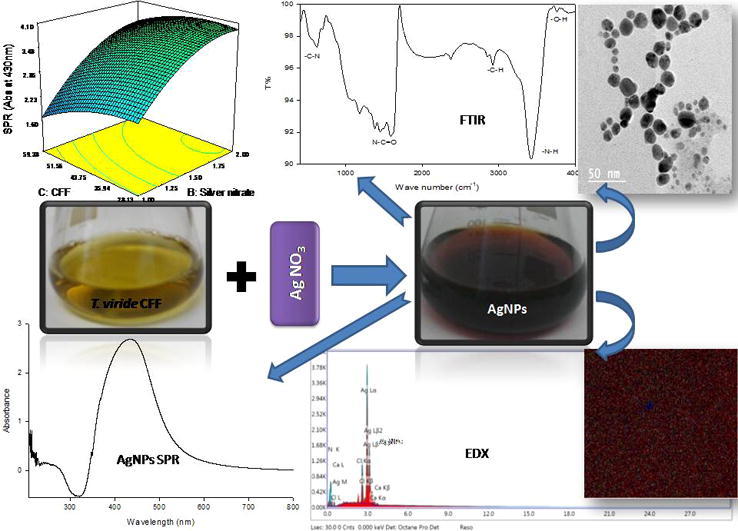
Graphical abstract
Keywords: Nanosilver, Biosynthesis, Characterization, Response surface methodology, Trichoderma viride
Highlights
-
•
The extracellular nanosilver particles (AgNPs) biosynthesis by mediation of Trichoderma viride was achieved.
-
•
Response surface methodology (RSM) was used to optimize and study the interactions between the critical process parameters.
-
•
The characterization of the produced AgNPs revealed the round to oval shape for AgNPs and size of 4–16 nm.
-
•
The results could develop a reliable ecofriendly, simple, and low cost green synthesis process.
Abstract
This study aims to optimize the biosynthesis of nanosilver particles mediated by Trichoderma viride ATCC36838 using response surface methodology (RSM). Silver nanoparticles (AgNPs) were biosynthesized effectively in terms of the factors impacting silver ion (Ag+) reduction to metallic nanosilver (Ag0) using culture filtrate under shaking condition. The results of statistics calculations revealed that 2 mM silver nitrate and 28% (v/v) of culture filtrate at pH 7.0 for 34 h were the optimum values for AgNPs biosynthesis. The characterization of the produced AgNPs was conducted using electron microscopy, energy dispersive X-ray analysis, UV/visible spectrophotometry, and Fourier transform infrared spectroscopy. Round to oval AgNPs were detected with aspects of TEM within diameter range of 4–16 nm. The results of this study could help in developing a reliable ecofriendly, simple, and low cost process for microbial assisted AgNPs green synthesis especially with the continuous increase in its application fields.
1. Introduction
Due to the daily increase in nanotechnology applications fields, the scientific community gives a great attention to develop a low cost, easy and ecofriendly approach for nanoparticles production, especially the biosynthesis of stable, well defined shape and size nanoparticles [1], [2]. Traditional methods for nanoparticles synthesis have many circumstances due to its environmental hazards and expensive costs [3]. The main gate for nanoparticles formation is via reduction of metal salts through the aid of proper reducing agents. Specific attention was directed toward nanosilver particles (AgNPs) due to its wide range of applications in different areas like biomaterial production, optics, catalysis, and antimicrobials [4], [5]. In addition, it expose excellent improved characters as a result of its unique morphology and particles distribution [6], [7].
Green synthesis of nanoparticles using microorganisms is a promising research area for developing simple, inexpensive, and ecofriendly approach. The high capacity of fungi to produce diverse quantities of enzymes able to reduce the metal salts makes it the good choice for biosynthesis of metal nanoparticles [8]. The extracellular pathway through using cell free filtrate (CFF) for AgNPs biosynthesis has many advantages over the intracellular one, as it avoid the need for harvesting, purification, and product recovery techniques which are expensive, time consuming, and cumbersome techniques [9]. As the reductase enzyme which is required for silver ion reduction, secreted into the medium in case of extracellular choice, so there is no need for these separation techniques [3], [10].
Experimental optimization using classical methods through changing one factor per time and fixing the other factors has many disadvantages, where it illustrates the impact of each variable individually via a huge number of experiments, however it doesn’t consider the effect of interaction between different factors under study [11]. We previously described the biosynthesis of AgNPs using a culture supernatant of Penicillium politans NRC510 [3] as well as many previous studies which used these classical methods for AgNPs biosynthesis [12], [13], [14], [15], [16], [17], [18].
On the other hand, usage of statistical methods solves the problems of effective variables selection among many case affecting factors. In addition, it helps in understanding the interaction between different important parameters [19], [20]. Response surface methodology (RSM) is a mathematical and statistical analysis method which improves and optimizes the process settings through involving the interactions between different process parameters. RSM has been widely applied to get the optimum conditions for many different biotechnological processes through evaluation of the interaction effects between process variables [21].
In the current study, Trichoderma viride ATCC36838 CFF has been used to mediate the biosynthesis of AgNPs through the reduction of silver ions to metallic nanosilver. Characterization of the biosynthesized AgNPs was conducted using means of spectrophotometry, Fourier Transform Infrared spectroscopy (FTIR), Transmission Electron Microscopy (TEM), Scanning Electron Microscope (SEM), and Energy Dispersive X-ray (EDX) examination. The biosynthesis process was optimized through application of RSM - central composite design (CCD) technique in order to get the proper biosynthesis conditions and understand the interactions effect.
2. Materials and methods
2.1. Microorganism
The fungus Trichoderma viride ATCC36838 has been maintained at 4 °C on modified Czapek-Dox's solid medium at the culture collection of Microbial Chemistry Dept, NRC, Egypt. Then it has been re-cultivated before use in silver nanoparticles biosynthesis.
2.2. Supernatant preparation and AgNPs biosynthesis
For getting the T. viride ATCC36838 supernatant, the fungus was grown on malt glucose yeast peptone (MGYP) broth [18] contained (g/L): 3.0, yeast extract; 3.0, malt extract; 10.0, glucose; and 5.0, peptone, at 28 °C and 100 rpm for 72 h. After that, culture was filtered via Whatman filter paper No.1 and the filtrate was used for the extracellular formation of silver nanoparticles. Silver nitrate (AgNO3) dissolved in distilled water was used as silver source for AgNPs biosynthesis. Reaction mixtures with a total volume of 50 mL contained different T. viride ATCC36838 cell free filtrate (CFF) (%), and silver nitrate concentrations under different pH values for different incubation times according to the central composite design (CCD) described in the next section. Reaction mixtures were incubated at 100 rpm under dark conditions to avoid the photo activation of silver nitrate. The effect of temperature on the AgNPs biosynthesis was determined through incubation of reaction mixtures at 30, 40, 50, 60, 70, 80 or 90 °C for 1 h at pH 5.0. All experiments were done in triplicates and the mean values were presented.
2.3. Experimental design and optimization by RSM
RSM using CCD was applied in order to optimize the levels of the most effective variables in AgNPs biosynthesis and to analyze their relationships. Based on the one-factor experimental results, four critical variables selected were reaction incubation time, silver nitrate concentration, CFF volume, and reaction pH value. Every parameter was studied at different rational five coded levels (−2, −1, 0, 1, 2), and the actual values for these codes was indicated in Table 1.
Table 1.
Coded and actual values of the experimental variables.
| Parameter | Symbol | −2 | −1 | 0 | 1 | 2 |
|---|---|---|---|---|---|---|
| Reaction time (h) | A | 4.00 | 14.00 | 24.00 | 34.00 | 44.00 |
| Silver nitrate conc. (mM) | B | 0.50 | 1.00 | 1.50 | 2.00 | 2.50 |
| Cell free filtrate (%) | C | 12.50 | 28.13 | 43.75 | 59.38 | 75.00 |
| Reaction pH value | D | 4.00 | 5.00 | 6.00 | 7.00 | 8.00 |
Depending on the previous design, the total experimental runs were calculated as: 2k + 2k + x0, where k is variables number and x0 is the repetitions number of the experiments at the center point. Thus, for this design, a total of 30 runs of experiments were performed according to CCD given in Table 2.
Table 2.
Response surface central composite design and experiments.
| Run | A | B | C | D | Absorbance (430 nm) |
|---|---|---|---|---|---|
| 1 | 2 | 0 | 0 | 0 | 3.77 |
| 2 | 1 | −1 | −1 | 1 | 4.10 |
| 3 | −1 | −1 | −1 | 1 | 3.81 |
| 4 | 0 | 0 | 0 | 0 | 2.81 |
| 5 | 0 | 0 | 2 | 0 | 2.47 |
| 6 | 0 | 0 | 0 | 0 | 3.19 |
| 7 | −2 | 0 | 0 | 0 | 0.79 |
| 8 | 1 | 1 | −1 | 1 | 6.73 |
| 9 | 1 | −1 | 1 | 1 | 2.43 |
| 10 | −1 | 1 | −1 | −1 | 0.31 |
| 11 | 0 | 0 | 0 | 0 | 3.36 |
| 12 | 0 | 0 | 0 | −2 | 0.16 |
| 13 | 0 | 0 | −2 | 0 | 3.33 |
| 14 | 0 | 0 | 0 | 0 | 3.79 |
| 15 | −1 | 1 | 1 | −1 | 0.88 |
| 16 | −1 | 1 | −1 | 1 | 4.47 |
| 17 | −1 | −1 | 1 | 1 | 1.38 |
| 18 | −1 | −1 | −1 | −1 | 0.26 |
| 19 | 1 | −1 | −1 | −1 | 0.67 |
| 20 | 1 | 1 | 1 | −1 | 0.62 |
| 21 | 0 | −2 | 0 | 0 | 0.60 |
| 22 | 0 | 0 | 0 | 0 | 4.79 |
| 23 | 1 | −1 | 1 | −1 | 0.87 |
| 24 | 0 | 0 | 0 | 0 | 3.43 |
| 25 | 1 | 1 | 1 | 1 | 6.33 |
| 26 | −1 | 1 | 1 | 1 | 5.19 |
| 27 | 0 | 2 | 0 | 0 | 3.51 |
| 28 | −1 | −1 | 1 | −1 | 0.52 |
| 29 | 1 | 1 | -1 | −1 | 0.08 |
| 30 | 0 | 0 | 0 | 2 | 8.66 |
The following second order polynomial equation was used to calculate the relationship between different variables and the response.
where Y is the predicted response, Xi, Xi2, Xj are variables in coded values; β0 is the constant; βi is linear effect; βii is squared effect and βij is interaction effect. The analysis of results was performed with statistical and graphical analysis software (Design Expert®, Version 7.0.0). Design Expert software was used for regression analysis of the data obtained and to estimate regression equation coefficient.
2.4. Characterization of synthesized AgNPs
The spectra (UV–Visible) of AgNPs were measured as a wavelength function using UV/Vis spectrophotometer (Cary 100 UV–Vis; Agilent Technologies, Germany) at 1.0 nm of data intervals. Elemental analysis of the biosynthesized AgNPs was studied using SEM (Quanta FEG250) operated at an accelerating voltage of 20 kV and coupled with energy dispersive X-ray analysis (EDX) for compositional analysis and the conformation of presence of elemental silver. AgNPs solution was centrifuged for 20 min at 10,000 rpm and drop coated on a carbon coated copper grid and dried. AgNPs shape and size were determined by TEM (JEOL JEM-HR-2100) operating at 160 kV, where a drop of aqueous AgNPs was loaded on a carbon coated copper grid, and allowed to dry at room temperature. For FTIR measurements, dry powder of the AgNPs was obtained according to Othman et al. [3] and then used for FTIR spectroscopy measurements on a JASCO FTIR (Japan) instrument in the diffuse reflectance mode at a resolution of 4 cm−1 in KBr pellets.
2.5. Effectiveness of the biosynthesized AgNPs as antimicrobial agent
The assessment of AgNPs as antimicrobial agent was explored using agar well diffusion assay [3]. The microorganisms (Bacillus mycoides, Escherichia coli, and Candida albicans) were seeded in the plates of nutrient agar, and then different concentrations (12.5, 25, 50, and 100%) of the biosynthesized AgNPs solution were added to the agar wells (15 mm) which were made previously using sterile cork borer. The inoculated plates were incubated for 3 h at 37 °C after that; the inhibition zone diameter was measured.
3. Results and discussion
3.1. Explanation of regression analysis
As a result of multiple regression analysis validation for the investigational records, the results obtained from the central composite design were integrated with a full polynomial equation in a second-order. The experiential correlation between the absorbance at 430 nm as indication for surface plasmon resonance (SPR) and the four studied factors in coded units is specified below:
where Y is the obtained absorbance (430 nm) as indication for surface plasmon resonance; A is reaction incubation time; B is silver nitrate concentration; C is CFF volume ratio; D is the reaction pH value. The analysis of variance (ANOVA) outline for the AgNPs SPR model is exposed in Table 3.
Table 3.
ANOVA analysis for the AgNPs surface plasmon resonance model.
| Source | DF | F-value | Prob > F | Significance |
|---|---|---|---|---|
| Model | 14 | 17.53 | <0.0001 | Significant |
| A-Time | 1 | 12.50 | 0.0030 | Significant |
| B-Silver nitrate | 1 | 23.72 | 0.0002 | Significant |
| C-CFF | 1 | 3.22 | 0.0929 | |
| D-pH | 1 | 155.53 | <0.0001 | Significant |
| AB | 1 | 1.57 | 0.2294 | |
| AC | 1 | 1.33 | 0.2675 | |
| AD | 1 | 5.59 | 0.0320 | Significant |
| BC | 1 | 0.26 | 0.6166 | |
| BD | 1 | 19.93 | 0.0005 | Significant |
| CD | 1 | 6.91 | 0.0190 | Significant |
| A2 | 1 | 5.41 | 0.0344 | Significant |
| B2 | 1 | 7.21 | 0.0170 | Significant |
| C2 | 1 | 1.81 | 0.1986 | |
| D2 | 1 | 1.08 | 0.3154 | |
| Residual | 15 | |||
| Lack of fit | 10 | 1.70 | 0.2895 | Not significant |
| Pure error | 5 | |||
| Cor total | 29 | |||
DF: degree of freedom; R2, 0.9424; adjusted R2, 0.8886; Predicted R2, 0.7246; CV, 28.67%; Adequate precision, 15.957.
The F-value of the model (17.53) entails the evidence of model significance. “Prob > F” values fewer than 0.05 point to model terms are siginificant. In the current case A, B, D, AD, BD, CD, A2, B2 model terms are significant. “Prob > F” values superior than 0.10 show the insignificance of the model terms. The “Lack of Fit F-value” of 1.70 means the Lack of Fit is insignificant comparative to the pure error. The insignificance of lack of Fit value is a good indication about the model. The calculated determination coefficient (R2) was 0.9424, implying that the model capability to explain 94.24% of the variability. The “Pred R2”of 0.7246 is in levelheaded concurrence with the “Adj R2” of 0.8886, because the Predicted R2 and adjusted R2 to be in “reasonable agreement” must be inside approximately 0.20 of each other. A comparatively lower rate of variation coefficient (CV = 28.67%) indicated a superior accuracy and reliability of the model experiments. Adequate Precision indicates the signal to noise percentage, where a ratio superior than 4 is advantageous. At this juncture, the proportion obtained in this model (15.957) specified a sufficient signal. In adding up, Tukey Test was used to check the ANOVA, and the outcome showed that diverse levels of each variable had no considerable variation.
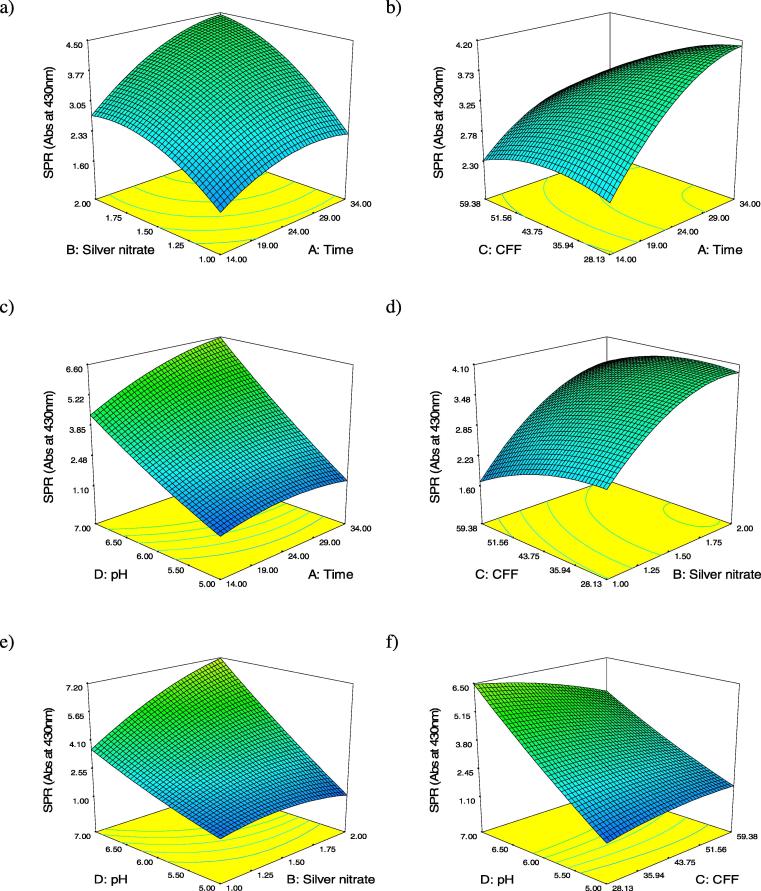
3.2. Interpretation of three dimensions surface plots
The regression model symbolized in the figure of three dimensions surface plots were illustrated to demonstrate the effects of the four studied factors, and collective effects of every independent changeable factor on AgNPs biosynthesis in values of absorbance at 430 nm as indication for AgNPs surface plasmon resonance.
Fig. 1a–c shows reaction incubation time (A) had significant effect on the AgNPs biosynthesis. When incubation time (A) increased, the AgNPs biosynthesis increased to extent limit depending on the second tested variable. Microbial biosynthesis of metal nanoparticles through metal ions reduction requires between 24 and 124 h, and the rate of metal reduction process to its corresponding nanoparticles depending basically on the reduction potential of biomass [3]. Fig. 1a, d, and e shows silver nitrate concentration (B) had a strong significance on AgNPs biosynthesis in the interaction effect with reaction incubation time (A), CFF volume (C), and reaction pH value (D). When it increased, the AgNPs biosynthesis increased clearly and affected by the interaction of second parameter (A), (C) or (D). Silver nitrate concentration and the biological mass affect silver nanoparticles synthesis significantly, where there is a correlation between Ag+concentration and the quantity of enzymes and proteins present in reaction medium to get the proper balance for AgNPs biosynthesis [22]. Fig. 1b, d and f demonstrates that concentration of CFF (C) had a lesser momentous outcome on the AgNPs biosynthesis than the effects of other three variables. This is due to the sufficiency of the biomolecules present in the used range of CFF for nanoparticles formation via participation in silver ion reduction process [14]. Fig. 1c, e and f explains that reaction pH value (D) had the most noteworthy effect on the AgNPs biosynthesis. The biosynthesis yield increased with the increase in pH value from 5.0 toward neutral pH values, which agree with our previous findings with Penicillium. politans NRC510 [3].
Fig. 1.
(a–f) Three dimensions surface plots of AgNPs biosynthesis: The result of two active parameters whereas the other two are detained at 0 levels.
The three dimensions surface plots also illustrate that the effects of the four studied factors on the AgNPs biosynthesis were dissimilar, and their influence was ordered as D > B > A > C, respectively. The experimental models were tested in the optimized biosynthesis conditions using reaction incubating time of 34 h, silver nitrate concentration of 2mM, CFF volume of 28.13 (%), and reaction pH value of 7.00. The predicted absorbance at 430 nm was 8.90 which is close to the value of the real response, that confirmed the strength of the models.
3.3. Effect of temperature
It has been recorded that incubation temperature is greatly affect the silver ion reduction process, where at low temperature degrees the AgNPs biosynthesis process require longer time than ion metal reduction at higher degrees. At 70 °C, the maximum intensity for SPR peak was detected (Fig. 2), indicating the formation of Ag nanoparticles at high and fast rate, leading to formation of smaller size nanoparticles [9]. Sintubin et al. [10] stated that at higher temperatures silver ion reduction was favorable and proceed at higher rate.
Fig. 2.
Biosynthesized silver nanoparticles UV-Visible absorption spectra, indicating the peak of silver nanoparticles surface plasmon resonance at different reaction temperatures. The experiment was carried out in triplicates and the average data was presented.
3.4. Characterization of silver nanoparticles
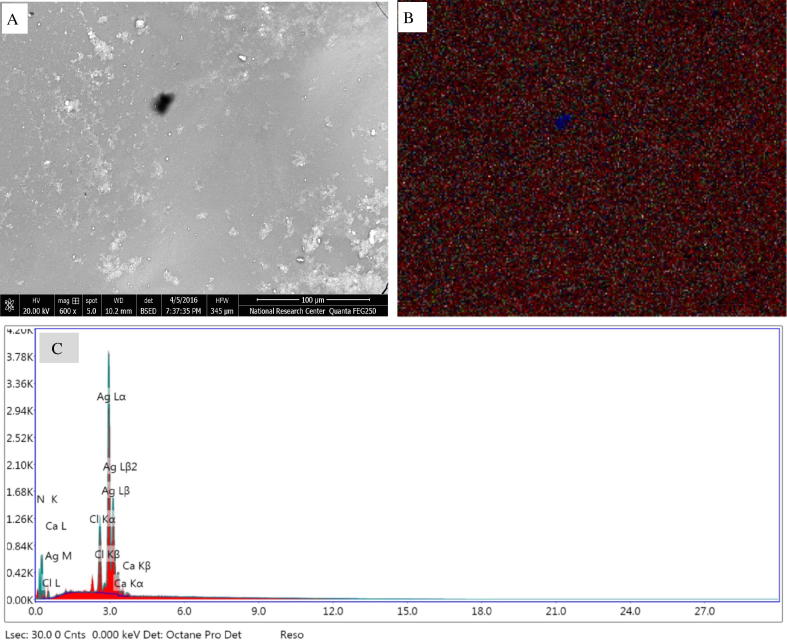
3.4.1. SEM and EDX analysis
SEM micrograph for the biosynthesized AgNPs obtained by mediation of T. viride CFF is represented in Fig. 3a. At the same time, the existence of silver element in the biosynthesized AgNPs was confirmed by EDX and mapping spectrum. The obtained micrograph from EDX mapping indicated that, the AgNPs were well dispersed in a homogenous distribution over the biosynthesized sample (Fig. 3a and b). Quantitative and qualitative elements estimation that involved in synthesis of nanoparticles was done through EDX analysis. Usually, as cause of its SPR, higher counts at 3 keV was shown by silver nano-crystals [14]. EDX analysis results (Fig. 3c) confirmed the presence of silver element through strong signals of silver nanoparticles energy peaks in range around 3.0 keV, which confirmed that AgNPs were biosynthesized successfully by T. viride CFF. On the other hand, high proportions of Ag element (80.6%) were detected by EDX elemental analysis as expected, followed by Cl (9.9%).
Fig. 3.
Biosynthesized silver nanoparticles profiles: (A) Magnified SEM micrograph 600×, (B) EDX mapping micrograph showing nanosilver as ( ) red dots, and (C) EDX profile representing the percentage of nanosilver and other metals.
) red dots, and (C) EDX profile representing the percentage of nanosilver and other metals.
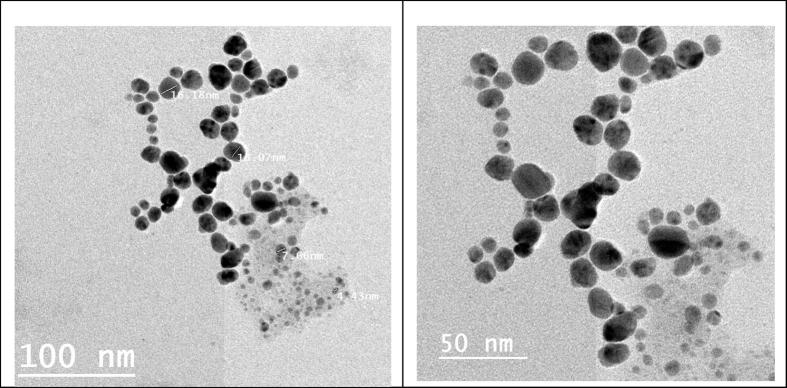
3.4.2. TEM analysis
The AgNPs biosynthesized by means of T. viride CFF mediation was examined using TEM through drop-coated films. The obtained micrographs revealed the range of the biosynthesized AgNPs to be more or less between spherical and oval shapes within diameter range of 4–16 nm (Fig. 4). The obtained TEM micrographs show silver nanoparticles in different nano-sizes as individual particles or in some aggregates. The lack of direct contact of nanoparticles even in forms of aggregates, confirming the role of a capping agent (protein) in nanoparticles stabilization [13].
Fig. 4.
T. viride mediated AgNPs biosynthesis TEM micrograph.
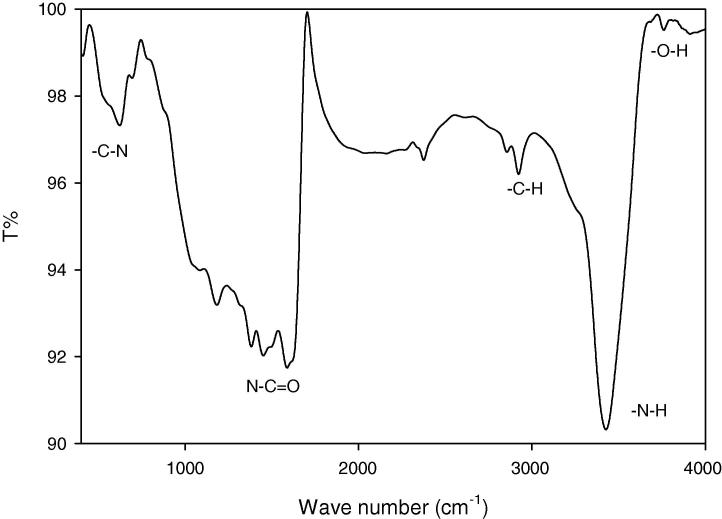
3.4.3. FTIR spectroscopy
The major functional groups present in the biosynthesized AgNPs by T. viride CFF was detected through FTIR spectroscopic analysis. These functional groups may be responsible for AgNPs biosynthesis, stabilization, and capping. The FTIR spectrum of AgNPs produced by T. viride CFF mediation was represented in Fig. 5 and showed different transmission peaks at different positions. From the spectrum obtained, two peaks were observed at 1183, and 1084 cm−1 which can be assigned to C—N aliphatic and aromatic amines stretching vibration. Other peak observed at 1588 cm−1 could refer to the protein amide bond N—C O due to proteins’ carbonyl stretching. On the other hand, methoxy, methylene, and methyl groups stretching vibrations via C—H could be detected at 2924, and 2856 cm−1, whereas stretching of O—H in flavonoids, alcohols, and phenols was observed at 3761, 3683, and 3426 cm−1 [16]. Typical proteins’ phenomenon of overlapping —NH stretching vibration could be detected at the sharp peak at 3426 cm−1 [15]. The results obtained, indicate the participation of the proteinaceous matter in reduction of Ag+, and hence the interaction of these biological components with Ag+ via the detected functional groups in order to mediate the nanoparticles formation process.
Fig. 5.
Fourier transform infrared (FTIR) spectroscopy of the AgNPs biosynthesized by means of T. viride CFF mediation.
3.5. Antimicrobial activity of silver nanoparticles
The antimicrobial effect of AgNPs against different microorganisms could be due to the binding to cell surface, and hence affecting cell wall and cell membrane functions, or through interacting with cell inside components. In the current study, AgNPs biosynthesized by means of T. viride exhibited the ability to act as antimicrobial agent, depending on the tested microorganism (Fig. 6). From the results obtained, it is clear that the biosynthesized nanoparticles has a good anti microbial activity against Bacillus mycoides as a Gram positive bacteria, Escherichia coli as a Gram negative bacteria, and Candida albicans as a non filamentous fungus, even in the lowest tested concentration (12.5%).
Fig. 6.
Antimicrobial activity of the AgNPs biosynthesized by means of T. viride CFF mediation.
4. Conclusions
The current study was designed to look for optimizing and studying the interaction relations between variables impacting AgNPs biosynthesis, in order to accomplish elevated effectiveness of AgNPs biosynthesis process. Through application of RSM, the models for AgNPs biosynthesis were established in relation to the reaction incubation time, silver nitrate concentration, CFF volume ratio, and pH value of reaction, as four effective parameters. The outcome of this study could assist to get high efficiency AgNPs biosynthesis through simple, non-toxic, inexpensive, and eco-friendly fungal mediated approach.
Acknowledgements
The authors would like to acknowledge the National Research Centre, Dokki, Giza, Egypt, for lab facilities and financial support.
Acknowledgments
Conflict of interest
The authors declare that the research was conducted in absence of any conflict of interest.
Footnotes
Peer review under responsibility of National Research Center, Egypt.
Contributor Information
Abdelmageed M. Othman, Email: othmanam_nrc@yahoo.com, am.othman@nrc.sci.eg.
Maysa A. Elsayed, Email: maysaelsayed@yahoo.com.
Ali M. Elshafei, Email: alielshafei@yahoo.com.
Mohamed M. Hassan, Email: mmm.hassan_21@yahoo.com.
References
- 1.Gopinath V., MubarakAli D., Priyadarshini S., Priyadharsshini N.M., Thajuddin N., Velusamy P. Colloids Surf B Biointerfaces. 2012;96:69–74. doi: 10.1016/j.colsurfb.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 2.Kathiresan K., Manivannan S., Nabeel M.A., Dhivya B. Colloids Surf B Biointerfaces. 2009;71(1):133–137. doi: 10.1016/j.colsurfb.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 3.Othman A.M., Elsayed M.A., Elshafei A.M., Hassan M.M. Int J ChemTech Res. 2016;9(12):433–444. [Google Scholar]
- 4.Bagherzade G., Tavakoli M.M., Namaei M.H. Asian Pac J Tropical Biomed. 2017;7(3):227–233. doi: 10.1016/j.apjtb.2016.12.014. [DOI] [Google Scholar]
- 5.Helaly F.M., El-Sawy S.M., Hashem A.I., Khattab A.A., Mourad R.M. Contact Lens Anterior Eye. 2017;40(1):59–66. doi: 10.1016/j.clae.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 6.AbdelRahim K., Mahmoud S.Y., Ali A.M., Almaary K.S., Mustafa A.E.-Z.M.A., Husseiny S.M. Saudi J Biol Sci. 2017;24(1):208–216. doi: 10.1016/j.sjbs.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riaz Ahmed K.B., Nagy A.M., Brown R.P., Zhang Q., Malghan S.G., Goering P.L. Toxicol in Vitro. 2017;38:179–192. doi: 10.1016/j.tiv.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Deb S. Int J ChemTech Res. 2014;6(7):3909–3917. [Google Scholar]
- 9.Fayaz A.M., Balaji K., Girilal M., Yadav R., Kalaichelvan P.T., Venketesan R. Nanomed Nanotechnol Biol Med. 2010;6(1):103–109. doi: 10.1016/j.nano.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Sintubin L., Verstraete W., Boon N. Biotechnol Bioeng. 2012;109(10):2422–2436. doi: 10.1002/bit.24570. [DOI] [PubMed] [Google Scholar]
- 11.Bhattacharya S.S., Garlapati V.K., Banerjee R. New Biotechnol. 2011;28(1):31–39. doi: 10.1016/j.nbt.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Anand B.G., Thomas C.K.N., Prakash S., Kumar C.S. Biocatal Agric Biotechnol. 2015;4(2):150–157. doi: 10.1016/j.bcab.2015.01.002. [DOI] [Google Scholar]
- 13.Devi L.S., Joshi S.R. J Microsc Ultrastruct. 2015;3(1):29–37. doi: 10.1016/j.jmau.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibrahim H.M.M. J Radiat Res Appl Sci. 2015;8(3):265–275. doi: 10.1016/j.jrras.2015.01.007. [DOI] [Google Scholar]
- 15.Mishra P. Manjari, Sahoo S. Kumar, Naik G. Kumar, Parida K. Mater Lett. 2015;160:566–571. doi: 10.1016/j.matlet.2015.08.048. [DOI] [Google Scholar]
- 16.Mittal A.K., Bhaumik J., Kumar S., Banerjee U.C. J Colloid Interface Sci. 2014;415:39–47. doi: 10.1016/j.jcis.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 17.Muthukrishnan S., Bhakya S., Senthil Kumar T., Rao M.V. Ind Crops Prod. 2015;63:119–124. doi: 10.1016/j.indcrop.2014.10.022. [DOI] [Google Scholar]
- 18.Singh D., Rathod V., Ninganagouda S., Hiremath J., Singh A.K., Mathew J. Bioinorg Chem Appl. 2014;2014:1–8. doi: 10.1155/2014/408021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daâssi D., Frikha F., Zouari-Mechichi H., Belbahri L., Woodward S., Mechichi T. J Environ Manage. 2012;108:84–91. doi: 10.1016/j.jenvman.2012.04.039. [DOI] [PubMed] [Google Scholar]
- 20.Poonkuzhali K., Palvannan T. Carbohyd Polym. 2011;86(2):860–864. doi: 10.1016/j.carbpol.2011.05.028. [DOI] [Google Scholar]
- 21.Lu S.-Y., Qian J.-Q., Wu Z.-G., Ye W.-D., Wu G.-F., Pan Y.-B., et al. J Biochem Technol. 2009;1(3):79–84. [Google Scholar]
- 22.El-Rafie M.H., Shaheen T.I., Mohamed A.A., Hebeish A. Carbohyd Polym. 2012;90(2):915–920. doi: 10.1016/j.carbpol.2012.06.020. [DOI] [PubMed] [Google Scholar]