Abstract
In this present study, we have described the diversity of nine Ocimum genotypes naturally grown in the Dakshin Dinajpur district of West Bengal, India. Their diversity was determined on the basis of morphological, chemical and randomly amplified polymorphic DNA (RAPD) to determine the level of variation present in the genus Ocimum. Among nine Ocimum genotypes six (O. americanum, O. × africanum, O. basilicum, O. gratissimum, O. kilimandscharicum and O. tenuiflorum) are found to be different Ocimum species and the rest are as varieties. A total of 18 qualitative and 17 quantitative morphological traits and chemical compositions were evaluated. Significant variations were observed in the morphological traits except O. × africanum and O. basilicum species. Cluster generated from the morphological data showed two different groups viz. basilicum group and sanctum group. Chemical analysis did not show much variation between morphologically similar species viz. O. × africanum and O. basilicum. However, RAPD analyses clearly showed that O. × africanum and O. basilicum are different species. Thus the combined analyses of morphological traits, chemical and molecular markers represent the best possible approach to confirm taxonomic delineation. Moreover, we are reporting O. × africanum for the first time from this region as well as from West Bengal, India.
Keywords: Ocimum, Diversity, Morpho-chemical analysis, GC–MS, RAPD, Genetic variation
1. Introduction
Ocimum (Basil) is the most important genus of the subfamily Nepetoideae under the family Lamiaceae. The word Ocimum is derived from the Greek word “ozo” meaning smell [1] and is called as “king of herbs” due to its immense use in traditional system of medicine, perfumery and pharmaceutical industry [2]. Pushpangadan in 1995 has reported that the genus Ocimum has more than 160 species and is the largest genera in Lamiaceae family worldwide, of which about 65 species are native to Ocimum and the rest should be considered as synonyms [3]. The taxonomy of Basil is considered to be vast and complex. This taxonomic complexity is believed to be due to genetic diversity influenced by crosspollination and several environmental factors. The geographical distribution shows three main centres of Ocimum diversity, viz. (a) Tropical and subtropical regions of Africa, (b) Tropical Asia and (c) Tropical parts of America (Brazil) [4] up to an altitude of about 1800 m from the mean sea level [5]. The maximum number of species is found in the tropical rain forests of Africa however, few species of Basil are native to India [6]. In India, so far about nine species of Ocimum have been reported including three exotic species namely O. americanum L., O. minimum L., and O. × africanum Lour. [7].
Since the distinctness of an Ocimum species from another is always difficult to identify, several characters may need to be considered. Most of the genotypes identified by the earlier authors were based on morphological traits [8], [9], [10]. For morphological characterization taxonomists have formulated a descriptor list, such as leaf shape and colour, flower colour etc. for plants’ taxonomic classifications. Due to extensive cultivation, inter and intra-specific cross hybridization has taken place leading to polyploidy and different numbers of species, subspecies and varieties that are not significantly different in their morphology [11]. Genetic variations are seen in Ocimum species in their inter and intra specific levels. Ocimum species show enormous morphological variations as well as growth characteristics, reproductive behaviour and chemical composition among their species that are affected by environmental factors [12]. Consequently, the morphological descriptors create a lot of confusion on its taxonomy.
Further advancement in the classification of Ocimum was introduced by Lawrence [13] and Grayer et al. [14] based on the prevalent essential oil compositions from the aerial parts. Chemotaxonomy can be exploited to separate the genotypes and to find out the intrinsic variability or variability among genotypes of the same species based on the presence or absence of specific substance at different concentration. For classifying the different basil chemotypes it uses the most abundant aromatic compounds. Essential oils may vary with the cultivar type but the prevalent components are phenylpropanoids and monoterpenes. The chemical composition may be modified furthermore by cross hybridization, morphogenesis, polyploidization, process of oil extraction, drying and storage, stages of harvesting etc. [15]. Thus chemotaxonomy of Ocimum generates severe confusion among scientific community. The traditional evaluation methods and chemotaxonomy are therefore should be combined with molecular markers (which are not influenced by the environmental factors) for a better distinction among Ocimum genotypes [16], [17].
Recently, there are several novel molecular markers used to assess genetic diversity in medicinal plants as well as in the genus Ocimum as because of its independency, highly polymorphism nature and stability [18]. Most widely used molecular markers are RAPD (Random Amplified Polymorphic DNA), RFLP (Restriction Fragment Length Polymorphism), AFLP (Amplified Fragment Length Polymorphism), ISSR (Inter Simple Sequence Repeats), SSRs (Simple Sequence Repeats), VNTRs (Variable Number of Tandem Repeats) etc. [19], [20]. RAPD is the most extensively used technique for the study of genetic diversity in plants especially in medicinal and aromatic plants including the genus Ocimum due to low cost, time saving, easy to handle, sequence information not required, comparative analysis are rapid, cover large genome area and gives high level of polymorphism [21]. Since Ocimum diversity study is very complex, researchers from different parts of the globe have studied the Ocimum diversity based on morphological variability, chemotaxonomy, molecular marker, and cytotaxonomy [6], [22], [23], [24], [25], [26], [27], [28], [29]. Ocimum species are varied across India, however a few reports are available about the Ocimum diversity study. Moreover the contemporary literature is totally lack of similar morpho-chemical and molecular marker based diversity study of different naturally growing Ocimum species from Dakshin Dinajpur, West Bengal, India.
Therefore, the present investigation elaborates the very first report of the genetic diversity and relationship of nine Ocimum genotypes including the natural hybrid O. × africanum based on morpho-chemical and RAPD molecular marker for their characterization.
2. Materials and method
2.1. Plant materials
Total nine genotypes of tulsi (Ocimum sp.) were collected from different places of the district of Dakshin Dinajpur (26° 35′ 15″ N to 25° 10′ 55″ N latitude and 89° 00′ 30″ E – 87° 48′ 37″ E longitude), West Bengal, India and were maintained in the AASM garden of Raiganj University, Raiganj, West Bengal, India. Out of the nine genotypes, two varieties from O. tenuiflorum L. (Purple and Green type, commonly known as Krishna and Radha tulsi respectively), two varieties from O. basilicum L. (Babu and Marua tulsi), two varieties from O. gratissimum L. (Ram and Ajowan tulsi) and single species from O. × africanum Lour. (Lebu/Bon tulsi), O. americanum L. (Bon tulsi) and O. kilimandscharicum Guerke. (Karpur tulsi) were considered for the present investigation. The brief morphological description, accession number, local names, local folk uses, collection centres and altitude are summarized in Table 1. Identification of all the species was made by Botanical Survey of India (BSI), Kolkata and voucher specimens were deposited in the herbarium of Department of Botany, University of North Bengal, Darjeeling, West Bengal, India.
Table 1.
Local name, morphological description and local folk uses of nine Ocimum genotypes collected from different places of the district Dakshin Dinajpur, West Bengal, India.
| Ocimum Taxa/Local name/Accession No. | Morphological characters | Local folk Uses | Collection site/Altitude |
|---|---|---|---|
| O. tenuiflorum L. (Krishna tulsi) NBU-09795 | Annual to biannual, herb, 70–150 cm tall, leaf ovate-obovate, elliptic-oblong, surface patently hairy to clothed with soft spreading hair, Inflorescence purple, Flowers purplish, Calyx purple, patently hairy to densely pubescent, Seed brown, globose, non-mucilaginous | Fresh leaf used in common cold and fever, inflammation and diabetes, root used as sexual stimulant | Gangarampur 31 m |
| O. tenuiflorum L. (Radha tulsi) NBU-09796 | Annual to biannual, herb, 70–160 cm tall, leaf ovate-obovate, elliptic-oblong, surface patently hairy to clothed with soft spreading hair, Inflorescence green-greenish purple, Flowers purplish, Calyx green, patently hairy to densely pubescent, Seed brown, globose, non-mucilaginous | Leaf used in cold and cough, bronchitis, fiver, fungal skin infection, rheumatic pain and in poisonous insect bites | Daulatpur 37 m |
| O. americanum L. (Bon tulsi) NBU-09797 | Annual, herb, 20–60 cm tall, leaf elliptic-lanceolate, leaf surface glabrous except hairy midrib, veinlets and margin, Inflorescence greenish, Flowers white, Calyx green with sometimes purplish stripe, long hairy, Seed black, narrowly ellipsoid, mucilaginous | Leaf used in flatulence, sexual disabilities, mole and mosquito repellent | Patiram 25 m |
| O. × africanum Lour. (Lebu tulsi) NBU-09798 | Annual, herb, 45–105 cm tall, leaf elliptic – broadly obovate, glabrous except hairy midrib, veinlets and margin, Inflorescence greenish, Flowers white, Calyx green, long hairy, Seed brownish black, ellipsoid, mucilaginous | Fresh leaf and seed used for curing different types of skin diseases including sores and boils and insect bites on the skin | Bansihari 29 m |
| O. basilicum L. (Babu tulsi) NBU-09799 | Annual, herb, 45–100 cm tall, leaf ovate-lanceolate to oblong-lanceolate, glabrous except hairy midrib, veinlets and margin, Inflorescence greenish, Flowers whitish pink, Calyx green, long hairy, Seed brownish black, ellipsoid, mucilaginous | Leaf used in common cold and cough, headache and in sexual problems | Kushmandi 39 m |
| O. basilicum L. (Marua tulsi) NBU-09800 | Annual, herb, 55–100 cm tall, leaf elliptic-lanceolate, glabrous on both sides of the leaf, Inflorescence purple, Flowers pinkish-white, Calyx greenish purple-purple, smooth except sides, Seed black, ellipsoid, mucilaginous | Fresh leaf used in gastric problems | Jordighi 33 m |
| O. gratissimum L. (Ram tulsi) NBU-09801 | Perennial, undershrub or shrub, 140–200 cm tall, leaf lanceolate, ovate or ovate-lanceolate, glabrous except hairy midrib, Inflorescence greenish purple, Flowers yellowish white, Calyx greenish purple, hairy, Seed brown, subglobose, non-mucilaginous | Leaf used in fever, common cold and cough, gastrointestinal problems | Harirampur 32 m |
| O. gratissimum L. (Ajowan tulsi) NBU-09802 | Perennial, undershrub or shrub, 125–260 cm tall, leaf lanceolate, ovate or elliptic-ovate, glabrous except hairy midrib and wavy, Inflorescence greenish, Flowers yellowish white, Calyx green, hairy, Seed brown, subglobose, non-mucilaginous | Leaf used in fever, common cold and cough, gastrointestinal problems and used in poisonous insect stings | Balurghat 27 m |
| O. kilimandscharicum Guerke. (Karpur tulsi) NBU-09803 | Perennial, herb, 60–120 cm tall, leaf ovate-oblong, leaf surface pubescent with white hairs on both sides, much denser and longer on veins beneath, Inflorescence, greenish-greyish, Flowers white, Calyx greenish -greyish, densely hairy, Seed black, narrowly ellipsoid, mucilaginous | Leaf used in headache and sinus problems | Hili 24 m |
2.2. Morphological evaluation
Morphological study was carried out in the year 2013–2015 during the flowering season between September to January. Morphological data were recorded for each species for 35 characters including 18 qualitative and 17 quantitative traits (Table 1, Table 2, see Supporting Information). For morphological characterization, minimal descriptor developed by NBPGR with slight modifications was used [30]. All qualitative and quantitative data were periodically recorded for variation of characters after an interval of fifteen days at peak of the vegetative and flowering period. All the vegetative morphological characters (both qualitative and quantitative) were recorded over the entire growth period but reproductive traits were considered for analysis during full blooming stage (August–November).
Table 2.
Eigen values, variability and cumulative variability among morphological traits (Qualitative and quantitative) of nine genotypes based on principal component analysis.
| Component | Eigen value | Variability (%) | Cumulative% | Major traits contributing the variability |
|---|---|---|---|---|
| PC1 | 13.226 | 40.079 | 40.079 | BL, SM, SC, BW, PL2, PW, IL, IT |
| PC2 | 8.817 | 26.717 | 66.796 | LT, LS1, LA, LW, NW/I, LL, PL |
| PC3 | 5.299 | 16.059 | 82.855 | AC, IC, LS |
PC- Principal Component, BL-bract length, SC-seed colour, SM-seed mucilage, BW-bract width, PL2-petal length, PW-petal width, IL-inflorescence length, IT- inflorescence type, LT-leaf tip, LS1- leaf shape, LA-leaf area, LW-leaf width, NW/I- number of whorls/Inflorescence, LL- leaf length, PL- petiole length, AC-anther colour, IC-inflorescence colour and LS- leaf surface.
2.3. Morphological analysis
The qualitative traits were converted to numerical values according to the above mentioned descriptor and transform into a quantitative data matrix. The mean values of quantitative data and the transform values of qualitative data were used to perform Principal Component Analysis (PCA) using Pearson correlation coefficient at a significant level (α = 0.05) to detect the traits that are most relevant to distinguish among the species. For qualitative characters, detected identical ordinal variables were eliminated (e.g. mode of reproduction and plant growth habit). Agglomerative hierarchical clustering (AHC) of all genotypes was formed on the basis of these morphological characters using dissimilarities with Euclidean Distance by Ward’s method using XLSTAT software (2015).
2.4. Preparation of ethanolic extracts for Gas chromatography/mass spectrometry (GC–MS)
Harvested leaves of each sample were shade dried and ground to powder in a grinder with a 2 mm diameter mesh. The powdered leaf (100 g) was dissolved in 500 mL of ethanol (1:5 w/v) in a volumetric flask in tightly sealed condition and was kept in a shaker for 7 days at room temperature. The extracts were then filtered using Whatman filter paper No. 41. Solvent was recovered using a rotary evaporator (Buchi Rotavapor R-3; Buchi Labortechnik AG, Flawil, Switzerland) at 40 °C with a vacuum controller coupled to a cooling unit. Finally, the yellow-greenish ethanolic extracts were lyophilized and kept in a sealed labelled vial at 4 °C in dark condition until tested and analyzed.
2.5. Gas chromatography/mass spectrometry (GC–MS) analysis
Compositions of the ethanolic extracts of Ocimum species/varieties were determined by GC–MS. 10 μL sample was diluted in 1 mL of ethanol (1:100 dilutions). From this 100 μL of the sample was completely dried using Nitrogen. Sample was derivatized using 30 μL pyridine and 50 μL of BSTFA: TMCS (99:1) and incubated at 60 °C for 60 min. Derivatized samples were subjected to GC–MS.
The GC analysis was performed using an Agilent Technology 7890A equipped with a DB 5 MS capillary column (30 mL × 0.25 mm ID × 0.25 μm film thicknesses dimension). The carrier gas was helium with a flow rate of 1.0 mL/min. Initial column temperature was maintained at 70 °C with 2 min hold time. Then ramp the temperature to 150 °C at the rate of 5 °C/min and again to 280 °C at the rate of 3 °C/min with 2 min hold time and finally to 20 °C temperature at the rate of 10 °C with 3 min hold time. 1.0 μL of sample was subjected to GC–MS using the split mode (split ratio 10:1). The GC–MS analysis was done on the Agilent Technologies 5975CMSD (Mass selective detector). Ionization for MS was Electron Impact Ionization and mass analyzer was single quadrupole. Mass spectra scan range was from 30 m/z to 600 m/z with +ve polarity. AMDIS software was used as a deconvolution tool and National Institute Standard and Technology (NIST 2011) was used to identify the compounds.
2.6. Identification and quantification of components
The compounds were identified based on the comparison of mass spectra (MS) obtained with those of the mass spectra from the library. The relative percentage of each component was calculated by the relative percentage of the total peak area in the chromatogram.
2.7. DNA extraction
The DNA was isolated from young fresh tender leaves by CTAB (Cetyl trimethyl ammonium bromide) method (Murray and Thompson, 1980) with some modification in the extraction buffer (1.5 M NaCl, 100 mM Tris, 20 mM EDTA, pH- 8.0, 2% CTAB). Before use fresh leaves were surface sterilized with teepol (Extran) for 5 min and rinsed with sterile double distilled water and wiped off with clean tissue paper to remove surface water completely. Concentration of extracted DNA was measured both by running it on 0.8% (m/v) agarose gel as well as by quantification with UV Spectrophotometer (CECIL, CE 7200, Germany). The purity of DNA was calculated by the absorbance ratio of 260:280 nm. Before PCR amplification the DNA samples were diluted to a final concentration of 25 ng/μL with TE buffer (pH- 8.0).
2.8. RAPD-PCR amplification
RAPD Amplification was performed with extracted and purified genomic DNA from nine genomes of Ocimum using ten RAPD primers (Genei, Pvt. Ltd., Bangalore, India). Primers were selected in the present study on the basis of previous works on Ocimum species [21], [31]. Each RAPD PCR were performed in 25 μL volume containing 25 ng genomic DNA as template, 2.5 μL PCR Assay buffer, 200 μM dNTP mix, 1.0 mM MgCl2, 0.5 μL Taq DNA polymerase and 1 μM of each primer (Genei, Pvt. Ltd., Bangalore, India). Amplification reactions were performed with an initial denaturation at 94 °C for 4 min, following the initial steps, PCR was carried out for 35 cycles of 15 s denaturation at 94 °C, followed by primer annealing for 15 s at 40 °C and an extension step of 1.15 min at 72 °C. The last cycle was followed by final extension of 72 °C for 7 min using thermal cycles (Perkin Elmer gene Amp 2400 PCR system).
The PCR amplified products were separated by electrophoresis at a constant voltage of 70 V for 1.5 h in 1× TAE (Tris Acetate EDTA) buffer and resolved on 1.5% agarose gel stained with ethidium bromide. The gel was visualized in a UV-transilluminator and photographed in Gel Doc System (Biorad). A low range DNA ladder (100–3000 bp) (Genei, Pvt. Ltd., Bangalore, India) was used as known molecular weight marker.
2.9. Molecular analysis
The clear and visible amplified bands from the photographic gel were considered for the analysis. The amplified bands were scored as 1 or 0 on the basis of present and absent of bands to generate a binary data matrix. A few bands, which were not reproducible, were excluded for the analysis. Those bands which are present in some species and absent in others are considered to be as “polymorphic band” and if the band is present in all the species is considered as “monomorphic band”. The generated binary data matrix was used to calculate the pair wise genetic similarity coefficient for depicting intra and inter-specific genetic similarities among all the genotypes by Jaccard’s coefficient [32] using the SimQual model of NTSYS-pc (Numerical Taxonomy System version 2.1) software [33]. Based on the genetic similarity matrix a dendrogram was constructed by using the Unweighted Pair Grouped Method with Arithmetic Average (UPGMA) employing the Sequential Agglomerative Hierarchical and Nested (SAHN) algorithm for determining the genetic diversity, inter and intraspecific relationships among all the genotypes.
The polymorphic information content (PIC) is generally used in genetics as a measure of polymorphism for a marker locus using linkage analysis. The PIC value was computed using the following formula, PIC = 1−Σpi2, where pi is the frequency of the ith allele of the locus in the set of nine Ocimum genotypes [34].
Principle coordinate analysis (PCA) was performed by extracting Eigen value and Eigen vectors from a correlation matrix which was generated using a standardized data matrix. 2D and 3D plots were constructed to evaluate the groupings of Ocimum species. In order to highlight the resolving power of the ordination, Principle coordinate analysis (PCA) was performed using the EIGEN and PROJ modules of NTSYS Pc (version 2.1).
3. Results and discussion
3.1. Morphological characterization (Qualitative traits)
Understanding the diversity of a plant species or genus is of great significance, primarily because of its connection to many branches of biological sciences. Morphological studies on Ocimum species showed a high level of variability in recorded traits. For identification of Ocimum species morphological traits including leaf colour, stem, inflorescence, flower and seed; leaf shape, stem and seed play the major role [10], [35].
In the qualitative traits a considerable variability were observed on stem pubescence, stem colour, leaf surface, leaf margin, leaf tip, leaf shape, inflorescence type, flower colour, anther colour, seed shape and seed colour (Table 1, see Supporting Information). However, two traits namely, plant growth habit (erect) and their mode of reproduction (sexual) are found to be monomorphic for all the species and varieties under consideration. Some of the species have pubescent on the stem but with their uneven occurrence. Sparse type of stem pubescent was observed on O. gratissimum (Ajowan tulsi), O. americanum, O. basilicum (Babu tulsi) and O. × africanum but O. kilimandscharicum and O. tenuiflorum (Krishna and Radha tulsi) have dense type of stem pubescent. O. basilicum (Marua tulsi) and O. gratissimum (Ram tulsi) on the other hand showed glabrous stem. Stem colour was also varied from species to species and their varieties. O. gratissimum (Ram and Ajowan tulsi) have brownish stem colour whereas O. kilimandscharicum, O. americanum and O. × africanum have light green stem colour. Purple green stem colour was found on O. basilicum (Babu tulsi) and a distinct deep purple stem colour was observed on O. basilicum (Marua tulsi) and O. tenuiflorum (Krishna tulsi) genotypes.
Leaf surface showed significant level of variations viz. glabrous except hairy midrib, veinlets and margin [O. basilicum (Babu tulsi), O. × africanum and O. americanum], sparse and wavy or undulated O. gratissimum (Ajowan tulsi), patently hairy to clothed with soft spreading hairs [O. tenuiflorum (Purple and Green type) and O. kilimandscharicum], while O. basilicum (Marua tulsi) showed glabrous leaf surface. Notably, most of the species showed same colour of leaf (Light green) except O. gratissimum (Ajowan tulsi) (Deep green) and O. tenuiflorum (Krishna tulsi) (Purple colour). In the present study, O. tenuiflorum showed purple and green type of leaf colour. Earlier Maheshwari et al. 1987 reported [36] the existence of three types of O. tenuiflorum viz. green, purple and purple-green however, recently, Mondello et al. 2002 in their report have claimed the existence of five different types of leaf colour [37].
Leaf margin varied from serrate [O. gratissimum (Ajowan and Ram tulsi), O. kilimandscharicum, O. americanum, O. basilicum (Babu and Marua tulsi) and O. × africanum] to dented [O. tenuiflorum (Krishna and Radha tulsi)]. O. gratissimum (Ajowan and Ram tulsi) showed acute-acuminate leaf tip and broad ovate-lanceolate leaf shape. O. kilimandscharicum, O. americanum and O. basilicum (Babu and Marua tulsi) on the other hand showed acute leaf tip with elliptic leaf shape. But O. tenuiflorum (Krishna and Radha tulsi) have obtuse to acute leaf tip with ovate leaf shape (Fig. 1, see Supporting Information).
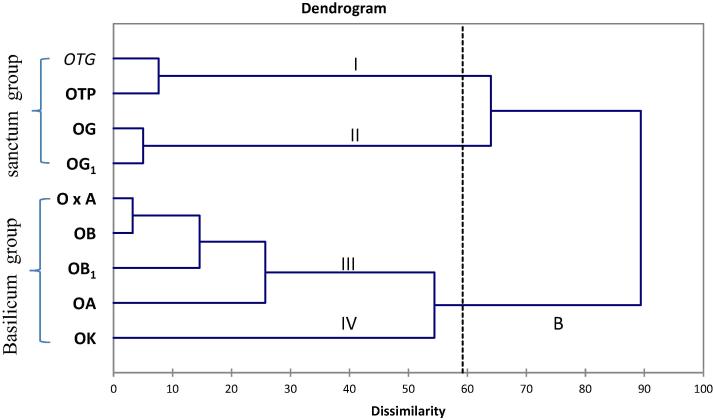
Fig. 1.
Dissimilarity dendrogram generated by Ward’s method showing major clusters among nine Ocimum genotypes based on their morphological traits. OTP-O. tenuiflorum (Krishna tulsi); OTG- O. tenuiflorum (Radha tulsi); OG-O. gratissimum (Ram tulsi); OG1-Ocimum gratissimum (Ajowan tulsi); O×A-O. × africanum (Lebu tulsi); OB-O. basilicum (Babu tulsi); OB1-O. basilicum (Marua); OA-O. americanum (Bon tulsi); OK-O. kilimandscharicum (Karpur tulsi).
Variation was also observed in inflorescence type. Out of nine genotypes O. gratissimum (Ajowan and Rum tulsi), O. tenuiflorum (Krishna and Radha tulsi) and O. kilimandscharicum showed branched inflorescence and rest of the species showed unbranched or simple type of inflorescence. There are four types of flower colour observed in the studied species. These are yellowish white [O. gratissimum (Ajowan and Ram tulsi)], white (O. kilimandscharicum, O. americanum and O. × africanum), whitish pink [O. basilicum (Babu and Marua tulsi)] and purple [O. tenuiflorum (Krishna and Radha tulsi)]. Pollen colour is an important character to distinguish Ocimum species. O. gratissimum (Ajowan and Ram tulsi) and O. tenuiflorum (Krishna and Radha tulsi) showed yellow coloured pollen while O. americanum, O. basilicum (Babui and Marua tulsi) and O. × africanum showed white coloured pollen. O. kilimandscharicum has brick red or gray coloured pollen which was entirely different from other genotypes. Colour of pollen is therefore may serve as an additional morphological traits to understand the diversity among Ocimum species and varieties.
So far we have discussed different morphological traits to evaluate the morphological diversity of Ocimum species. Very recently, few reports are available where people used seed morphology to differentiate morphologically close Ocimum species [38]. Seeds of all the species vary from brown to black in colour. Seed shape also showed significant difference among the species studied. The observed seed shapes were subglobose-globose [O. gratissimum (Ajowan and Ram tulsi)], globose [O. tenuiflorum (Krishna and Radha tulsi)], small elliptic (O. kilimandscharicum and O. americanum) and broadly elliptic [O. basilicum (Marua and Babu tulsi) and O. × africanum]. It was observed that few seeds were mucilaginous [O. basilicum (Babu and Marua tulsi), O. × africanum, O. americanum and O. kilimandscharicum] and few were non-mucilaginous [O. gratissimum (Ajowan and Ram tulsi) and O. tenuiflorum (Krishna and Radha tulsi)] when wetted in water. These observations are in agreement to those reported by Patel et al. in 2015 [38].
3.2. Quantitative traits
The descriptive analysis of nine genotypes in the present investigation showed significant difference in their quantitative traits (Table 2, see Supporting Information). The mean plant height varied from 35 to 204 cm. The shortest was O. americanum and the tallest was O. gratissimum (Ajowan tulsi). O. basilicum (Babu and Marua tulsi) and O. × africanum did not show any significant difference in height (73.25–74.25 cm). The present literature has an ambiguity about the plant height of O. tenuiflorum. This variation may be due in part on the agro climatic condition and their habitat. Some authors reported the height ranging from 95 to 120 cm [39], whereas some authors have reported different values about the palnts’ height. Both the varieties of purple and green type of O. tenuiflorum, in our case showed heights ranging from 103.25 to 105.75 cm. These data agree well with the earlier reports [39]. Interestingly, the two varieties of O. gratissimum (Ram and Ajowan tulsi) differ in their height. The average height of Ajowan tulsi (125–260 cm) was found to be larger than Ram tulsi which grows up to 140–200 cm in the studied area. This difference in plant’s height helps to classify the two varieties of O. gratissimum. Our results differed from the reports of pervious workers [25] who have reported the variation of plants’ height from 80.53 to 84.26 cm. But the present results unequivocally agree with some of the previous reports [39], [40]. On the other hand plant height of O. basilicum (Babu tulsi) and O. americanum was ranging from 45–100 cm and 20–60 cm respectively. Similar results had also been reported [23], [40], [41] previously.
We also carefully studied the leaf length and size. A great variation of leaf length and leaf area was observed that ranged from 3.76 cm2 (O. americanum) to 31.26 cm2 [O. gratissimum (Ajowan tulsi)]. Petiole length varied from 1.5 cm [O. basilicum (Babu tulsi)] to 4.46 cm [O. gratissimum (Ram tulsi)]. The leaf area variations (3.76–57.3cm2) were in accordance with the earlier report [42].
Inflorescence length varied from 10.04–23.48 cm. Highest inflorescence length showed in O. basilicum (Babu tulsi) and lowest in O. tenuiflorum (Purple). Maximum number of whorls per inflorescence was observed in O. gratissimum (Ram tulsi) and minimum in O. tenuiflorum. Bract length varied from 0.28 cm [O. tenuiflorum (Krishna and Radha tulsi)] to 0.91 cm [O. basilicum (Marua tulsi)] with green to purple colour respectively. Petal length varied from 0.39 cm [O. gratissimum (Ajowan and Ram tulsi)] to 1.0 cm [O. basilicum (Marua tulsi)]. Stigma and style length ranged from 0.2–0.4 cm (O. americanum) to 0.7–0.8 cm (O. kilimandscharicum) and 0.4–0.5 cm [O. tenuiflorum (Krishna and Radha)] to 1.1–1.2 cm (O. kilimandscharicum) respectively. We found that O. basilicum (Babui tulsi) and O. × africanum (Lebu tulsi) showed maximum similarities among the morphological traits except their aroma. O. × africanum has lemon or citronella flavour aroma where O. basilicum (Babu tulsi) has the sweet odour.
Since both inter or intra-specific hybridization is enormous in case of Ocimum species, natural inter and/or intra-specific cross hybridization and the consequent genetic diversity may, therefore, be the leading cause of huge morphological variations. Nevertheless, depending on qualitative and quantitative morphological parameters these morphological variations allow distinguishing some of the genotypes amidst studied germplasm.
3.3. Principal component analysis
Principal component analysis is one of the most useful statistical tools for screening multivariate data with significantly high correlation [43]. Total 35 morphological traits (18 qualitative and 17 quantitative traits) were analyzed to find out principal components are presented in Table 1, Table 2, see Supporting Information. The first three components contributed 82.85% of the cumulative variability, while first two 66.79% of the variability and first component accounted total 40.07% variability. In each principal component the maximum variability was contributed by the first principal component (40.07) followed by second PC (26.71) and third PC (16.05). The first principal component contributed the traits i.e. bract length, seed colour, seed mucilage, bract width, petal length, petal width, inflorescence length and inflorescence type. In second principal component the traits contributing to the total variability were leaf tip, stamen length, leaf area, leaf width, number of whorls/Inflorescence, leaf length and petiole length. The third principal component was mostly influenced by the traits that were anther colour, inflorescence colour and leaf surface (Table 2). Based on the PCA results of morphological traits species were differentiated on the biplot (Fig. 2, see Supporting Information).
Fig. 2.
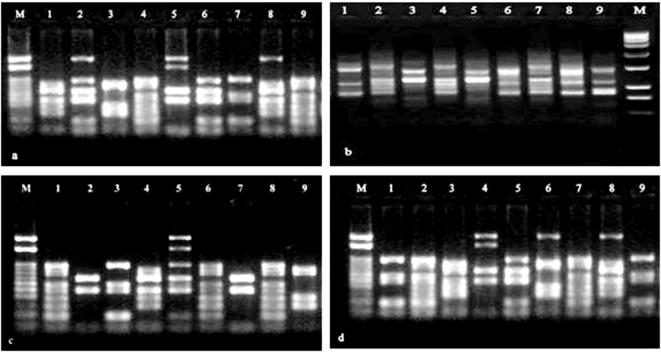
RAPD band profiling was visualized on 1.5% agarose gel electrophoresis after stained with ethidium bromide in nine Ocimum genotypes and photograph for analysis. a-BGM-1, b-BGM-3, c-BGM-4 & d-BGM-7. M-Marker (100-3000 bp ladder); lane 1-Ocimum gratissimum L.; 2-O. × africanum Lour.; 3 -Ocimum basilicum L.; 4 -Ocimum tenuiflorum L. (Purple); 5 -Ocimum basilicum L. (Marua); 6 -Ocimum americanum L. ; 7 -Ocimum tenuiflorum L. (Green); 8 -Ocimum gratissimum L. (Ajowan); 9 -Ocimum kilimandscharicum Guerke.
The cluster analysis was performed to classify all the genotypes according to their most important components. From the Agglomerative hierarchical clustering (AHC) four clearly distinct groups were obtained on the basis of the morphological traits using Euclidean distance by Ward’s method (Fig. 1). In the first group O. tenuiflorum (Green type), O. tenuiflorum (Purple type) and in II cluster O. gratissimum (Ram tulsi) and O. gratissimum (Ajowan tulsi) are grouped together and they were close to each other. Cluster III constituted largest species including O. × africanum, O. basilicum (Babu tulsi), O. basilicum (Marua tulsi) and O. × americanum. However, cluster IV contained only O. kilimandscharicum. This study highlighted the separation between different species isolated groups such as sanctum and the basilicum group [5]. From the morphological traits (both qualitative and quantitative) four distinct groups of species were plotted in the two dimensional plot of PCA that was confirmation of the dendrogram constructed based on the morphological traits by Ward’s method (Fig. 2, see Supporting Information).
3.4. Chemical analysis
Chemical compositions were determined by GC–MS analyses from the ethanolic extracts of dried leaves of nine Ocimum genotypes and are shown in Table 3. In these analyses, total 73 compounds were identified of which twelve are aliphatic acids, three aliphatic alcohols, seven amino acids, two aromatic compounds, one fused ring aromatic hydrocarbon, twenty-three carbohydrates, five phenolic compounds, one quinone, three steroids, twelve terpenoids, vitamin E and three are unidentified compounds.
Table 3.
Chemical composition of nine different Ocimum genotypes through GC–MS analysis.
| Compounds | Relative area percentage (peak area relative to the total peak area, expressed as percentage) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| OA | OB | OB1 | OG | OG1 | OK | OTP | OTG | O × A | |
| Aliphatic acid | |||||||||
| Acetic acid | – | – | 0.74 | – | – | – | – | – | – |
| Butanedioic acid | 0.36 | – | – | 0.72 | 0.3 | – | 0.27 | 0.09 | – |
| Butanoic acid | – | – | – | – | – | – | 0.03 | – | – |
| Dodecanoic acid | – | – | 0.37 | – | – | – | – | – | – |
| Eicosanoic acid | 0.04 | – | – | – | – | – | – | 0.93 | – |
| Hexadecanoic acid | 0.39 | 29.64 | 13.46 | 10.37 | 6.87 | 0.69 | 0.34 | 15.01 | 6.8 |
| n-Pentadecanoic acid | – | – | 0.12 | – | – | – | – | – | – |
| Octadecanoic acid | 1.55 | 6.26 | 2.83 | 1.92 | 0.48 | 2.08 | 0.07 | 8.62 | 2.49 |
| Propanedioic acid | – | – | – | – | – | – | 0.11 | – | – |
| Propanoic acid | 1.05 | 1.93 | 0.02 | 0.04 | 1.77 | 1.42 | 0.5 | 0.05 | 1.77 |
| α-Linolenic acid | 2.94 | 15.04 | 3.8 | 1.08 | 7.05 | 8.23 | – | 15.2 | 16.61 |
| Aliphatic alcohol | |||||||||
| Glycerol | 7.36 | 15.42 | 7.17 | 0.48 | 0.07 | 53.29 | 5.45 | 21.84 | 10.87 |
| Hexadecenol | 3.87 | 12.76 | 4.62 | 7.1 | – | 6.3 | – | 9.94 | 10.06 |
| Meso-Erythritol | – | 5.27 | 0.48 | – | – | 5.13 | – | 0.14 | 0.74 |
| Amino acid | |||||||||
| l-Alanine | – | – | – | – | – | – | 0.25 | – | – |
| l-Norleucine | – | – | – | – | – | – | 0.01 | – | – |
| l-Proline | 0.74 | – | – | – | – | – | 1.23 | – | – |
| l-Threonic acid | – | – | – | – | – | – | 0.14 | – | – |
| l-threonine | – | – | – | – | – | – | 0.08 | – | – |
| l-Valine | 0.08 | – | – | – | – | – | – | – | – |
| Pipecolic acid | – | – | – | – | – | – | – | 0.05 | – |
| Aromatic acid | |||||||||
| Benzoic acid | 0.14 | – | 0.19 | – | – | – | 0.01 | – | – |
| Cinnamic acid | – | – | – | 0.21 | – | – | – | – | – |
| Fused ring aromatic hydrocarbon | |||||||||
| Naphthalene | 0.07 | – | – | – | – | – | – | – | – |
| Carbohydrates | |||||||||
| Arabinitol | 0.04 | 2.22 | – | – | – | – | – | 0.01 | 0.01 |
| Fructose | 3.65 | – | 4.2 | 5.08 | 3.47 | – | 27.24 | – | 3.56 |
| d-(+)-Talofuranose | – | – | – | – | – | – | 0.37 | – | – |
| d-Allofuranose | – | – | – | – | – | – | 0.41 | – | – |
| d-Arabinopyranose | – | – | – | – | – | – | 0.06 | – | – |
| d-galactose | – | – | 0.56 | – | – | 2.7 | 0.04 | – | – |
| d-gluconic acid | 0.2 | – | – | – | – | – | 0.36 | – | – |
| d-Mannitol | 0.49 | – | – | – | – | 1.53 | – | – | – |
| d-Psicose | 4.04 | – | – | – | – | – | – | – | – |
| d-Sorbitol | – | – | – | – | – | – | 0.55 | – | – |
| Furanone | – | – | – | – | – | – | 0.03 | – | – |
| l-(-)-Arabitol | – | – | – | – | – | – | 0.68 | – | – |
| l-(-)-Sorbose | 6.91 | – | – | 5.9 | – | – | 9.1 | – | – |
| Myo-Inositol | 0.3 | – | 0.18 | 0.01 | – | – | 1.16 | – | – |
| Sucrose | – | – | 1 | – | – | – | 5.65 | – | – |
| α-d-(-)-Tagatose | – | – | 4.95 | – | 3.46 | – | 19.02 | – | – |
| α-d-(+)-Mannose | 0.5 | – | 14 | – | – | – | 0.24 | 0.22 | 13.75 |
| α-d-(+)-Talose | – | – | 0.14 | 13.35 | – | – | 1.31 | – | – |
| α-d-Glucose | 55.32 | – | – | – | – | – | 22.15 | 0.02 | 17.23 |
| β-d-allopyranose | 0.24 | – | – | – | – | – | – | – | – |
| β-d-glucose | 4.29 | – | 17.76 | 26.98 | 16.14 | 2.78 | 2.65 | 0.73 | 4.23 |
| l-Threitol | 1.73 | – | – | – | 0.34 | 0.21 | – | – | – |
| Xylitol | – | – | 0.26 | – | – | 1.75 | – | – | – |
| Phenolic compounds | |||||||||
| Caffeic acid | – | – | – | – | 0.21 | – | – | – | – |
| Catechol | – | – | – | – | – | – | 0.08 | – | – |
| Vanillin | – | – | – | 0.71 | – | – | – | – | – |
| Eugenol | – | – | – | 12.46 | – | – | – | 8.61 | – |
| Methyl eugenol | – | – | 15.53 | – | – | – | – | – | – |
| Quinone | |||||||||
| Tert-Butylhydroquinone | – | – | – | – | 0.09 | – | – | – | – |
| Steroid | |||||||||
| Ergosterol | – | – | 0.65 | – | – | – | – | 1.26 | 0.88 |
| Stigmasterol | – | – | 0.79 | 0.88 | 2.29 | – | – | 3.59 | 1.51 |
| β-Sitosterol | 0.99 | 3.62 | 2.55 | 2.7 | 3.36 | 6.31 | – | 4.53 | 2.5 |
| Terpenoids | |||||||||
| Carvacrol | – | – | – | – | 0.54 | – | – | – | – |
| Caryophyllene oxide | – | – | – | 1.36 | – | – | – | 0.48 | – |
| Germacrene D | – | – | – | – | – | – | – | 0.01 | – |
| Norpinene | – | – | 0.01 | – | – | – | – | – | – |
| Phytol | – | – | 0.54 | – | 14.68 | – | – | – | 2.94 |
| Squalene (Precursor) | – | – | – | 1.69 | 4.78 | – | – | – | – |
| Tau-Cadinol | – | – | 0.36 | – | – | – | – | – | – |
| Thymol | – | – | – | – | 29.8 | – | – | 0.69 | 0.02 |
| α-Amyrin | – | – | 0.79 | 1.42 | – | – | – | – | – |
| α-Selinene | – | – | – | – | – | – | – | 0.43 | – |
| β-Elemene | – | – | – | – | – | – | – | 1.15 | – |
| β-Selinene | – | – | – | – | – | – | – | 0.64 | – |
| Vitamin E | |||||||||
| α-Tocopherol | 0.16 | – | 1.01 | 2.39 | 1.4 | 0.44 | – | 0.5 | 0.4 |
| Unknown | 2.55 | 7.83 | 0.91 | 3.16 | 2.91 | 7.14 | 0.43 | 5.28 | 3.79 |
OA- O. americanum (Bon tulsi), OB- O. basilicum (Babu tulsi), OB1- O. basilicum (Marua tulsi), OG- O. gratissimum (Ram tulsi), OG1- O. gratissimum (Ajowan tulsi), OK- O. kilimandschericum (Karpur tulsi), OTP- O. tenuiflorum (Krishna tulsi), OTG- O. tenuiflorum (Radha tulsi), O×A- O. × africanum (Lebu tulsi).
The variation of the chemical constituents in the studied Ocimum species and varieties is quite remarkable. Nature and extent of chemical constituents varied from species to species. In O. basilicum (Babu tulsi) and O. tenuiflorum (Radha tulsi) the main constituents in the ethanolic extracts were found to be aliphatic acids with a total of 52.87% and 39.9% respectively while aliphatic alcohol became the main constituent in O. kilimandschericum having 64.72% natural abundance. Hexadecanoic acid and α-Linolenic acid were the main aliphatic acids with 29.64% and 15.2% respectively in O. basilicum (Babu tulsi) and O. tenuiflorum (Radha tulsi). Similar results were reported earlier by Domokos et al. (1993). Notably, α-linolenic acid and α-sitosterol were found to present in the ethanolic extract of O. sanctum. This finding is in accordance with that reported by Nadkarni and Patwardhan in1992 and Singh et al. in 1996 [44], [45].
Eugenol (12.46%) was the main phenolic compound in clove like flavour O. gratissimum (Ram tulsi) whereas in carom seed like spicy flavoured O. gratissimum (Ajowan tulsi) was reach with terpenoids thymol (29.8%) and phytol (14.68%). Chemical analysis result clearly describes the availability of eugenol and thymol rich two different chemotypes of O. gratissimum [23], [46], [47], [48]. Along with eugenol (8.61%) and thymol (0.69%) O. tenuiflorum (Radha tulsi) also contained β-elemene (1.15%). Previously, Verma et al. in 2013 reported the occurrence of β-elemene in O. tenuiflorum [23]. But in the other variety of O. tenuiflorum (Krishna tulsi) eugenol, thymol and β-elemene were not detected.
Interestingly, methyl eugenol (15.53%) was present as chief compound of O. basilicum (Marua tulsi) whereas in O. basilicum (Babu tulsi) methyl eugenol was absent. Except methyl eugenol, however, there is no other volatile component (phenylpropanoids and monoterpenes) based on which we can make a clear distinction between the two varieties of O. basilicum. Thus, chemical method of distinction is insufficient to identify the varieties at the intra-specific level.
Though morphologically similar O. × africanum (Lebu tulsi) and O. basilicum (Babu tulsi) differ abruptly in their chemical constituent content, ergosterol, stigmasterol, phytol and thymol were present in O. × africanum (Lebu tulsi), but all were found to be absent in O. basilicum (Babu tulsi). O. × africanum is therefore a different species other than O. basilicum (Babu tulsi). Hence, chemical method is a better way out over morphological methods to identify the intra specific level of diversity of various Ocimum species. This difference was further verified by RAPD analysis.
3.5. Molecular analysis
In the present investigation a total 17 (BG 1-17) RAPD primers were used to detect inter and intra-specific diversity of Ocimum species found in Dakshin Dinajpur district. Out of 17 primers only 10 primers produced clear scorable bands (Fig. 2). A total of 88 distinct and scorable amplified bands were produced by the ten primers (Table 4). As presented in Table 4 the number of bands for each different primers ranging from 5 (BGM-5 and BGM-13) to 13 (BGM-7) with an average 8.8 loci per primer. The number of polymorphic amplicons ranged from 5 (BGM-5 and BGM-13) to 12 (BGM-7) with an average of 8.5 loci per primer and three primers (BGM-1, BGM-7 & BGM-17) produced monomorphic band in each. The highest number of bands (13) obtained from the primer (BGM-7) with 92.31% polymorphism while the lowest number of bands (5) obtained from the primers (BGM-5 and BGM-13) with 100% polymorphism respectively. Therefore, different primers showed different levels of polymorphism varying from 83.33% (BGM-17) to 100% (BGM-3, BGM-4, BGM-5, BGM-9, BGM-12, BGM-13 & BGM-15) with an average of 96.56%. The variation of size ranges of the amplicons with different primers were 200 bp to 3000 bp (BGM-4).
Table 4.
Analysis of polymorphism percentage among nine genotypes of the genus Ocimum using RAPD primers.
| Primer code | Sequence (5′-3′) | Total No. of bands | No. of polymorphic bands | Polymorphism (%) | PIC | Fragment size range (bp) |
|---|---|---|---|---|---|---|
| BGM-1 | TGCCGAGCTG | 10 | 9 | 90 | 0.350 | 400–3000 |
| BGM-3 | GTGACGTAGG | 8 | 8 | 100 | 0.437 | 500–800 |
| BGM-4 | AGGTCTTGGG | 11 | 11 | 100 | 0.412 | 200–3000 |
| BGM-5 | GGTGCTGCGC | 5 | 5 | 100 | 0.424 | 390–890 |
| BGM-7 | CTGGGCAACT | 13 | 12 | 92.31 | 0.378 | 500–3000 |
| BGM-9 | GAAACGGGTG | 10 | 10 | 100 | 0.353 | 400–700 |
| BGM-12 | GGAACGGGTG | 9 | 9 | 100 | 0.416 | 600–1200 |
| BGM-13 | CATCCCGACA | 5 | 5 | 100 | 0.375 | 260–700 |
| BGM-15 | GCACGCCGGA | 11 | 11 | 100 | 0.470 | 390–880 |
| BGM-17 | CTATCGCCGC | 6 | 5 | 83.33 | 0.386 | 530–1200 |
| Total | 88 | 85 | 965.64 | 4.001 | ||
| Average | 8.8 | 8.5 | 96.564 | 4 | ||
PIC values were calculated for each primer. It was observed that primer BGM-15 showed the highest PIC value (0.470), whereas primer BGM-1 showed lowest PIC value (0.350) with an average of (4.00). The RAPD primers generated 5 highly informative polymorphic loci (PIC > 0.4) among 50 percent polymorphic fragments. However, the highest PIC value (0.470) was observed in the primer BGM-15, which is recommended for germplasm analysis. This may be due to the polyallelic nature of RAPD markers.
A similarity matrix was obtained among all the nine genotypes of the genus Ocimum based on Jaccard’s coefficient. According to the similarity matrix, the genetic similarity ranged from 0.215 to 0.620. The highest similarity (0.620) was measured between O. gratissimum (Ajowan tulsi) and O. gratissimum (Ram tulsi). On the other hand least similarity (0.215) was observed between O. basilicum (Babui tulsi) and O. gratissimum (Ram tulsi) (Table 5). Genetic similarity was measured through the RAPD binary data matrix analysis of nine genotypes belonging to six Ocimum species and three varieties. This genetic similarity study revealed varying degrees of genetic relatedness among different Ocimum genotypes belonging to different species.
Table 5.
Genetic similarity matrix of nine Ocimum genotypes based on RAPD.
| OG | O × A | OB | OTP | OB1 | OA | OTG | OG1 | OK | |
|---|---|---|---|---|---|---|---|---|---|
| OG | 1 | ||||||||
| O × A | 0.303 | 1 | |||||||
| OB | 0.215 | 0.413 | 1 | ||||||
| OTP | 0.356 | 0.406 | 0.239 | 1 | |||||
| OB1 | 0.254 | 0.483 | 0.577 | 0.258 | 1 | ||||
| OA | 0.403 | 0.516 | 0.475 | 0.338 | 0.406 | 1 | |||
| OTG | 0.304 | 0.456 | 0.226 | 0.604 | 0.288 | 0.313 | 1 | ||
| OG1 | 0.620 | 0.264 | 0.313 | 0.393 | 0.273 | 0.438 | 0.322 | 1 | |
| OK | 0.373 | 0.358 | 0.273 | 0.441 | 0.254 | 0.415 | 0.368 | 0.344 | 1 |
OG- O. gratissimum (Ram tulsi); O×A- O. × africanum (Lebu tulsi); OB- O. basilicum (Babui/Babu tulsi); OTP- O. tenuiflorum (Krishna tulsi); OB1- O. basilicum (Marua); OA- O. americanum (Bon tulsi); OTG- O. tenuiflorum (Radha tulsi); OG1- Ocimum gratissimum (Ajowan tulsi); OK- O. kilimandscharicum (Karpur tulsi).
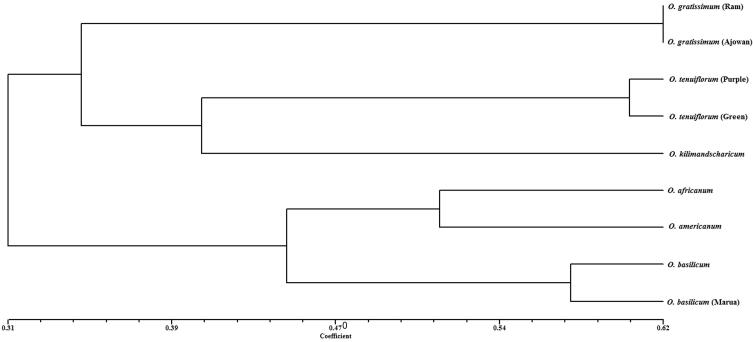
A dendrogram was generated using UPGMA and SHAN clustering method from the genetic similarity data matrix (Fig. 3). The dendrogram grouped nine genotypes of Ocimum species into two main clusters. Further, within the clusters those genotypes closely similar to each other were sub clustered together. Cluster-I constituted three distinct subclusters included the genotypes belonging to O. gratissimum (Ram tulsi), O. gratissimum (Ajowan tulsi) in subcluster- i, O. tenuiflorum (Purple) and O. tenuiflorum (Green) in subcluster-ii and O. kilimandscharicum in subcluster-iii. Cluster-II again divided into two subclusters. O. × africanum and O. americanum was in subcluster-i while subcluster-ii contained O. basilicum (Babu tulsi) and O. basilicum (Marua tulsi).
Fig. 3.
Dendrogram represented inter and intra specific relationship among nine genotypes of the Ocimum based on RAPD similarity matrix.
The PCA (Principal Coordinate Analysis) was performed to determine the consistency of the differentiation among the genotypes defined by the cluster analysis. The PCA indicated that the effect of individual amplification products on the overall variation observed was lesser, hence a total of ten RAPD products were required to explain 72.90% of the variation among the nine Ocimum genotypes. The analysis indicated that, the first two principal coordinates accounted for 60.10% of the total variation. The RAPD-based PCA revealed that the genotypes belonging to a particular cluster were grouped together in the PCA plot. Similar results were also observed in two and three dimensional representations (Fig. 3, see Supporting Information).
Evaluation of the genetic diversity in intra and inter-specific level of plant is a precondition for plant breeding programs and has a vital role in the conservation of plant genetic resources. The use of the RAPD marker is well established in the characterization of individual species, morphotypes and cultivars for assessment of genetic variability in germplasm collections and as a general guide in the choice of parents for breeding programs. In this present investigation, nine genotypes of the genus Ocimum under six species were characterized with RAPD genetic marker for genetic diversity analysis. Results indicated the presence of a wide genetic variability among different genotypes. Polymorphism in a given population is often due to the existence of genetic variants represented by the number of alleles at a locus and their frequency of distribution in a population.
According to genetic characterization, it was observed that O. × africanum (Lebu tulsi), O. americanum (Bon tulsi) and two types of O. basilicum (Babu and Marua tulsi) were closely related to each others. This can be explained by the hybrid origin of O. × africanum [O. americanum and O. basilicum (Babu tulsi)] [16], [14], [25], [26], [49]. Though, taxonomic origin of lemon scented basil is very much confusing, still now it is considered as O. x africanum, a natural hybrid between O. basilicum and O. americanum. Also, it is treated as a variety of O. basilicum var. citriodorum or even as a variety of a separate species, O. americanum var. pilosum [29], [50].
4. Conclusion
In this paper, we have described the diversity of nine Ocimum genotypes grown naturally in the Dakshin Dinajpur district of West Bengal, India. All the species are wide spread across the district. Their diversity was described in terms of morphological, chemical and RAPD analyses. In a combination of these three analyses clear distinction has been made between O. basilicum (Babu tulsi) – O. basilicum (Marua tulsi), O. gratissimum (Ram tulsi) – O. gratissimum (Ajoyan tulsi) and O. tenuiflorum (Krishna tulsi) – O. tenuiflorum (Radha tulsi). Among nine Ocimum genotypes six (O. americanum, O. × africanum, O. basilicum, O. gratissimum, O. kilimandscharicum and O. tenuiflorum) are different species of Ocimum and the rest are varieties. This study strongly recommended that both morpho-chemical and molecular assay could be used as complementary methods in describing the diversity of Ocimum genotypes, their correct identification and taxonomic classification. Notably, we have been able to identify a local genotype namely, Lebu tulsi as O. × africanum for the first time from this region as well as from West Bengal, India and believe that the present work will shed a clear light in the diversity of Ocimum genotype in this region.
Acknowledgement
Authors are thankful to Raiganj University for providing lab facilities and Botanical Survey of India (BSI), Kolkata for identification of nine Ocimum genotypes. Indian Institute of Science, Bangalore for GC–MS analyses. Thanks are due to Dr. P. D. Ghosh, Emeritus Professor, Cytogenetics & Plant Breeding Section, Department of Botany, University of Kalyani, West Bengal, India and Dr. Soumen Saha, Assistant Professor, Department of Sericulture, Raiganj University, Raiganj, West Bengal for their help. Dr. Abhik Chatterjee, Department of Chemistry, Raiganj University, Raiganj is cordially acknowledged by TC for his constant help and inspiration and Dr. P.D. Deepalakshmi, Division of Biological Sciences, Indian Institute of Science, Bengaluru-560 012, India.
Footnotes
Peer review under responsibility of National Research Center, Egypt.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jgeb.2016.12.004.
Appendix A. Supplementary data
References
- 1.Hereman S. Bradbury Evans and Co.; London: 1868. Paxton’s Botanical Dictionary. [Google Scholar]
- 2.Simpson B.B., Corner O.M. McGraw-Hill Book Company; Hamburg: 1986. Economic Botany-Plants in our World. [Google Scholar]
- 3.Pushpangadan P., Bradu B.L. In: Advances in Horticulture. Chadha K.L., Gupta R., editors. Malhotra Publishing House; New Delhi: 1995. [Google Scholar]
- 4.Paton A., Harley R.M., Harley M.M. In: Ocimum: An Overview of Classification and Relationship. Hiltunen R., Holm Y., editors. Harwood Academic Publishers; Amsterdam: 1999. [Google Scholar]
- 5.Khosla M.K. Ind. J. Genet. 1995;55:71–83. [Google Scholar]
- 6.Mishra D., Awasthi A., Mishra P. Sci. Secure J. Biotechnol. 2014;3:188–197. [Google Scholar]
- 7.Balyan S.S., Pushpangadan P. PAFAI J. 1988;10:13–19. [Google Scholar]
- 8.Malav P., Pandey A., Bhatt K.C., Krishnan S.G., Bisht I.S. Genet. Res. Crop Evol. 2015;62:1245–1256. [Google Scholar]
- 9.Conn B.J. Telopea. 2014;17:169–181. [Google Scholar]
- 10.Agarwal C., Sharma N.L., Gaurav S.S. Ind. J. Fund. Appl. Life Sci. 2013;3:521–525. [Google Scholar]
- 11.De Masi L., Siviero P., Esposito C., Castaldo D., Siano F., Laratta B. Eur. Food Res. Technol. 2006;223:273–281. [Google Scholar]
- 12.Carovi'c-Stanko K., Liber Z., Besendorfer V., Javornik B., Bohanec B., Kolak I., Satovic Z. Plant Syst. Evol. 2010;285:13–22. [Google Scholar]
- 13.Lawrence B.M. In: Advances in Labiate Science. Harley R.M., Reynolds T., editors. Royal Botanic Garden; Kew, UK: 1992. pp. 399–436. [Google Scholar]
- 14.Grayer R.J., Kite G.C., Goldstone F.J., Bryan S.E., Paton A., Putievsky E. Phytochemistry. 1996;43:1033–1039. doi: 10.1016/s0031-9422(96)00429-3. [DOI] [PubMed] [Google Scholar]
- 15.Simon J.E., Quinn J., Murray R.G. In: Basil: A Source of Essential Oils. Janik J., Simon J.E., editors. Timber Press; Portland, Ore, USA: 1990. [Google Scholar]
- 16.Carovi'c-Stanko K., Liber Z., Polite O., Strikic F., Kolak I., Milos M., Satovic Z. Plant Syst. Evol. 2011;294:253–262. [Google Scholar]
- 17.Labra M., Miele M., Ledda B., Grassi F., Mazzei M., Sala F. Plant Sci. 2004;167:725–731. [Google Scholar]
- 18.Pourmohammad A. Acta Universitatis Sapientiae Agric. Environ. 2013;5:80–90. [Google Scholar]
- 19.Patel K.H., Fougat S.R., Kumar S., Mistry G.J., Kumar M. 3, Biotechnology. 2015;5:697–707. [Google Scholar]
- 20.Harisaranraj R., Prasitha R., Babu S.S., Suresh K. Ethnobot. Leafl. 2008;12:609–613. [Google Scholar]
- 21.Singh A.P., Dwivedi S., Bharti S., Srivastava A., Singh V., Khanuja S.P.S. Euphytica. 2004;136:11–20. [Google Scholar]
- 22.Erum S., Naeemuulah M., Massod S., Khan M.I. Pak. J. Agric. Res. 2011;24:1–4. [Google Scholar]
- 23.Verma R.S., Padalia R.C., Chauhan A., Thul S.T. Ind. Crops Prod. 2013;45:7–19. [Google Scholar]
- 24.Sundaram S., Purwar S., Singh K.S., Dwivedi P. J. Med. Plants Res. 2014;8:640–645. [Google Scholar]
- 25.Patel R.P., Singh R., Saikia K.S., Rao B.R.R., Sastry K.P., Zaim M., Lal R.K. Ind. Crops Prod. 2015;77:21–29. [Google Scholar]
- 26.Vieira R.F., Goldsbrough P., Simon J.E. J. Am. Soc. Hortic. Sci. 2003;128:94–99. [Google Scholar]
- 27.Lal S., Mistry K., Thaker R., Shah S., Vaidya P. Int. J. Adv. Biol. Res. 2012;2:279–288. [Google Scholar]
- 28.Bernhardt B., Fazekas G., Ladanyi M., Inotai K., Zambori-Nemeth E., Bernath J., Szabo K. J. Appl. Res. Med. Aromat. Plants. 2014;1:23–29. [Google Scholar]
- 29.Pushpangadan P., Sobti S.N. Cytologia. 1982;47:575–583. [Google Scholar]
- 30.B.M. Singh, R.K. Mahajan, S. Umesh, S.K. Pareek, Minimal descriptors of agri-horticultural crops. Part-iv: medicinal and aromatic plants, National Bureau of Plant Genetic Resources, New Delhi, India, 2003.
- 31.Saha S., Kader A., Sengupta C., Ghosh P.D. Am. J. Plant Sci. 2012;3:64–74. [Google Scholar]
- 32.Jaccard P. Bull. Soc. Vaud. Sci. Nat. 1908;44:223–270. [Google Scholar]
- 33.Rohlf F.J. Exeter Publications; New York: 2000. Numerical Taxonomy and Multivariate Analysis System, version 2.1. [Google Scholar]
- 34.Anderson J.A., Churchill G.A., Autrique J.E., Tanksley S.D., Sorrells M.E. Genome. 1993;36:181–186. doi: 10.1139/g93-024. [DOI] [PubMed] [Google Scholar]
- 35.Svecova E., Neugebauerov J. Acta Univ. Sapientiae Alimentaria. 2010;3:118–135. [Google Scholar]
- 36.Maheshwari M.L., Singh B.M., Gupta R., Chien M. Indian Perfumer. 1987;31:137–145. [Google Scholar]
- 37.Mondello L., Zappia G., Cotroneo A., Bonaccorsi I., Chowdhury J.U., Yusuf M., Dugo G. Flavour Fragrance J. 2002;17:335–340. [Google Scholar]
- 38.Patel D.S., Khare P.K., Chaurasia B. Indian J. Plant Sci. 2015;4:16–18. [Google Scholar]
- 39.Kritikar K.R., Basu B.D. Lalit Mohn Pub; Allahabad, India: 1984. Indian Medicinal Plants. [Google Scholar]
- 40.Sastry K.P., Kumar R.R., Kumar A.N., Sneha G., Elizabeth M. J. Plant Dev. 2012;19:53–64. [Google Scholar]
- 41.Omer E.A., Said-al ahl H.A.H., Hendawy S.F. Res. J. Agric. Biol. Sci. 2008;4:293–300. [Google Scholar]
- 42.Ahmad S.D., Khaliq I. Pak. J. Biol. Sci. 2002;5:1084–1087. [Google Scholar]
- 43.Johnson D.E. Duxbury Press; New York: 1998. Applied Multivariate Methods for Data Analysis. [Google Scholar]
- 44.Nadkarni G.B., Patwardan V.A. Curr. Set. 1952;21:68–69. [Google Scholar]
- 45.Singh S., Majumdar D.K., Yadav M.R. Indian J. Exp. Biol. 1996;34:1212–1215. [PubMed] [Google Scholar]
- 46.Jirovetz L., Buchbauer G., Shafi M.P., Kaniampady M.M. Eur. Food Res. Technol. 2003;217:120–124. [Google Scholar]
- 47.Tchoumbougnang F., Zollo P.H.A., Avlessi F., Alitonou G.A., Sohounhloue D.K., Ouamba J.M., Tsomambet A., Okemy-Andissa N., Dagne E., Agnaniet H., Bessiere J.M., Menut C. J. Essent. Oil Res. 2006;18:194–199. [Google Scholar]
- 48.Dambolena J.S., Zunino M.P., Lopez A.G., Rubinstein H.R., Zygadlo J.A., Mwangi J.W., Thoithi G.N., Kibwage I.O., Mwalukumbi J.M., Kariuki S.T. Inn. Food Sci. Emerg. Technol. 2010;11:410–414. [Google Scholar]
- 49.Satović Z., Liber Z., Karlović K., Kolak L. Acta biologica Cracoviensia Ser. Botanica. 2002;44:155–160. [Google Scholar]
- 50.Paton A., Putievsky E. Kew. Bull. 1996;51:509–524. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.