Abstract
This study investigates the performance of acetate feed membrane less single chamber microbial fuel cell and physical characterization of the bio film present on the anode surface using Scanning Electron Microscope (SEM) and 16S rRNA analyzer. The performance has been investigated using Teflon treated carbon paper with 0.3 mg/cm2 Pt/C loaded as a cathode and carbon paper as an anode. The maximum open circuit potential is noticed as 791 mV, the system successfully revealed a maximum power density of 86.1 mW m−2 at stable current density of 354 mA m−2 with high coulombic efficiency of 65% at maximum degradation rate of 96%. SEM showed the dense adherence of microorganisms on the anode. 16S rRNA sequencing results indicates phylogenetic mixture in the communities of anodic biofilm and there is no single dominant bacterial species. The dominant phyla are Firmicutes, Gamma Proteobacteria, Alpha Proteobacteria, Actinobacteria, with ten dominant microbial strains: Bacillus firmus, Shewanella profunda, Bacillus isronensis, Brevundimonas bullata, Pseudomonas putida, Planococcus citreus, Micrococcus endophyticus, Acinetobacter tandoii, Bacillus safensis and Shewanella xiamenensis.
Keywords: Microbial fuel cell, Acetate, Columbic efficiency, Biofilm, SEM, 16S rRNA
1. Introduction
Clean environment and energy problems are two modern challenges to us, searching for permanent energy resources (renewable energy) is favorable to maintain our life safe with everlasting the environmental energy [1], [2], [3], [4], [5]. In a Bio electrochemical system (BES), microorganisms are interacting with electrode using electrons, which are either removed or supplied through a closed circuit. The most described type of BES is Microbial Fuel Cells (MFCs). MFCs are very promising renewable energy resources, which has the capability to employ microbial communities as the catalyst and capture the electricity from a broad range of organic matters. Microbial fuel cell (MFC) is a galvanic reactor that it can transfer chemical energy that stored in organic substrate into electricity during biocatalytic activity of microorganisms [5], [6], [7], [8], [9], [10], [11], [12], [13]. Produced electrons by microorganisms are transferred to the anode and flooded to the cathode by a resistor. The transfer of the electrons to the cathode of the MFC through a resistor, circuit completing the reaction for sustaining the electric current [14]. The power produced reflects the efficiency of the microbial respiratory chain as a result of the organic substrate degradation [15], [16]. Therefore, from the environmental and economic point of view, MFCs could be exploited as a bifunctional system to facilitate simultaneous wastewater treatment and electricity generation [17], [18]. There are two general techniques of transmitting the electrons to the anode: First way is Mediated Electron Transfer (MET), the use of external redox mediators to wire the electrode biofilm interaction(s) [19], [20]. Second way is a physical adherence between the microorganism and the anode electrode surface surfaces (Direct Electron Transfer, DET) [21], [22]. Therefore, the surface layer of the microorganism has to have a conducive surface (e.g. cytochromes or forming nanowire (pili) [23], [24], [25], [26]. Microbial community that able to release electrons from degradable substrates in addition to consume an easily oxidizable organic substrates are very t crucial feature that have to be considered [27], [28], [29]. In the cathodic electrode, an oxidant is being reduced water molecules. Biodegradable substrate is the source of electrons donor in the MFC [30]. These substrates ranging from a simple molecules as acetate and glucose to complex organic molecules [31]. In the MFC studies, acetate has been the appropriate substrate as many types of wastewater make them more difficult to be utilized as compared to acetate [32]. Acetate is an optimum substrate, it is widely used as carbon source to stimulate electroactive microorganisms [33]. Furthermore, it is usually used as its inertness towards alternative microbial conversions at room temperature [34]. Acetate is the final product of several metabolic pathways for complex carbon source [35]. Activated sludge produce electricity in MFCs from wastewater with electrochemically active bacteria species. Using Pt in cathode materials can enhance the efficiency of a MFC because Pt and Pt black electrodes have a maximum catalytic activity with oxygen rather than graphite materials and carbon-cloth electrodes for cathode constructions [36], [37]. 16S rRNA is the most common technique used to analyse microbial communities involved in electricity production [38]. These molecular methods include clone library analysis and Denaturing Gradient Gel Electrophoresis (DGGE), which commonly used for analyzing microbial community composition, diversity and dynamics [39]. The advantages of DGGE over other methods are that it is rapid and affordable for analyzing multiple unknown organisms [40]. These methods used to understand of how the microbial community dynamics is related to the functioning of a bioreactor such as wastewater treating system, the improvement of system design, treatment process, and the methods to monitor and control bioreactors [41], [42]. The main objective of this paper is to generate electricity and treatment of artificial wastewater simultaneously, improve the performance of Air–Cathode Single-Chamber Mediator-Less Microbial Fuel Cell (ACSCMMFC), and Characterization of the microbial community structure that develops on the anodes, using Scanning Electron Microscope (SEM) and 16S ribosomal RNA (16S rRNA).
2. Materials and methods
2.1. Microbial fuel cell construction
Air–cathode single-chamber mediator-less microbial fuel cell (ACSCMMFCs) testing fixture was used, the cell with 50 ml volume (6 cm length and 4 cm diameter) was manufactured using transparent perspex as a material of construction (Homemade, NRC, Egypt), the cell with an electrode active area of 25 cm2. It consists of an anode and cathode both were made from carbon paper (Laydel), The cathode electrode was treated with Poly tetrafluoroethylene (PTFE) (60 % w/v, dispersion in water) diffusion layers on the air-exposed side [43]. The catalyst layer was prepared by mixing 0.3 mg cm−2 of 30% Pt loading supported on carbon Vulcan xc-72R and Nafion solution (5% Nafion solution from Aldrich) to form catalyst ink, which painted on only the water facing side to reduce water loss and oxygen diffusion into the MFCs causing an increase in both Coulombic Efficiency and power output as mentioned elsewhere [27], [37], [43], [44], [45]. The catalyst ink composition was 13.8 mg of catalyst, 24.21 mg of carbon Vulcan XC-72R (E-TEK), and 5% Nafion solution, the mixture was ultrasonicated at 60 °C for 30 min. The first layer was obtained by spreading the ink on carbon paper electrode area, using a mask and a brush. Several successive layers were then deposited on top of each other to reach the loading of 0.3 mg cm−2; the previous layers were dried before adding another layer. All electrodes were dried at room temperature for 24 h before use [44]. The anode and cathode electrode were placed on opposite sides, it was connected through an external circuit across different external resistance (open circuit, 550 Ω).The performance of MFCs was evaluated with respect to power generation and substrate degradation.
2.2. Preparation of synthetic media solution
MFC reactor was seeded with mixed culture of aerobic activated sludge obtained from the municipal wastewater treatment plant (Benha municipal sanitation unit). The microbial fuel cell was fed with the acetate synthetic media. The growth media prepared as mentioned elsewhere [27].
2.3. Microbial fuel cell operation
The MFC was inoculated with the adapted aerobic mixed culture (activated sludge); it was operated under fed batch mode of operation. The aerobic sludge was pre-processed by filtration to remove un-dissolved materials. The cathode was facing to air on one side and the Pt loaded side of cathode was faced to the solution, while the node was set to maintain anaerobic conditions. Cell Potential between anode and cathode was recorded every 5 min with a multimeter and data acquisition system (Lab jack U6 – PRO). After steady state of power and electricity generation, polarization curves were obtained by varying external resistance (Rext) from 100 to 12.5 × 104 Ω. The chemical oxygen demands (COD chromate) of the anodic influent and the effluent were analyzed according to the standard method (closed reflux titrimetric method using chromate as the oxidant) at the end of three reproducible voltage cycles [46]. After electricity generation was stabilized, the MFC was operated for several cycles until the cathode-biofilm was formed and electricity generations of MFC were recorded.
2.4. Scanning electron microscopy
The surface of the anode electrode was characterized (after 46-days from the incubation with the microbial culture in the MFC system) using Scanning Electron Microscope SEM (JEOL, JXA-840A) to determine the microbial-electrode attachment possibility of biofilm formation on the anode electrode surface. Technically, the electrode was fixed with 2.5% glutaraldehyde for 4 h at 40 °C .The samples were then washed three times with water and dehydrated by successive immersion in a series of ethanol solutions of increasing concentration (30%, 50%, 70%, 80%, 90 % and absolute ethanol) for 10 min. Then the specimens were dried, mounted onto specimen stubs using graphite paste, and then the specimens were coated with gold [47].
2.5. Microbial community analysis with DNA purification Kit
The composition of anodic microbial community was one of the most important factors influencing the performance of MFCs. It has been suggested that rare bacteria in anodic biofilm would greatly contribute to the electric power generation [48]. The anodic biofilm (microbial communities) was removed from MSCMFC after inoculation of 2 months to identify the electro active bacteria using ABI 3730xl DNA sequencer. Prior to DNA extraction, bacterial cells were harvested in a 1.5 or 2 ml micro centrifuge tube by centrifugation for 10 min at 5000 g. Then, the pellets were resuspended in (digestion solution; 180 μl, proteinase K solution; 20 μl) and mixed thoroughly by vortexing or pipetting to obtain a uniform suspension. The samples were incubated at 56 °C using a shaking water bath until the cells were completely lysed (∼30 min). The sample, 20 μl of RNase solution was mixed by vortexing then the mixtures were incubated for 10 min at room temperature until a homogeneous mixture was obtained. 400 μl of 50% ethanol was added and mixed by pipetting or vortexing, the prepared lysate was transferred to a Gene JET™ Genomic DNA purification column inserted in a collection tube and centrifugated for 30–60 s at >12,000 g . After thawing, Maxima® Hot Start PCR Master Mix (2X); 25 μl, 16S rRNA Forward primer; 20 μM, 16S rRNA Reverse primer; 20 μM, Template DNA; 5 μl, Water, and nuclease-free; 18 μl were added for each 50 μl reaction at room temperature. Then, the samples were gently vortexed and spinned down. The PCR was performed using the following recommended thermal cycling conditions: initial denaturation at 95 °C for 10 min; 35 cycles of denaturation at 95 °C for 30 s, annealing at 65 °C for 1 min and extension at 72 °C for 1 min; followed by final extension at 72 °C for 10 min . After that, loading was completed to 4ul from the PCR mixture to examine the PCR product on 1% agarose gel against 1 Kb plus ladder. The purification column backed into the collection tube then the empty Gene JET™ purification column was centrifugated for an additional 1 min to remove any residual. The Gene JET™ purification column was transferred to a clean 1.5 ml micro centrifuge tube. Elution buffer (25 μl) was added to the center of the Gene JET™ purification column membrane and centrifugated for 1 min. The Gene JET™ purification column was removed and the purified DNA was stored at −20 °C. Finally, the sequencing of PCR product was done in GATC Company by ABI 3730xl DNA sequencer using forward and reverse primers.
3. Results & discussion
3.1. Microbial fuel cell performance
Acetate MSCMFC is primarily inoculated with aerobic activated sludge through the sampling port at the top. They are operated in batch mode to enrich electrochemically active bacterial community and to reach a reproducible state of the fuel cell. Fig. 1 indicates the MFC performance over three cycles of fed batch process at unlimited resistance and zero current, (open circuit voltage; OCV). Further, it has been found that higher voltage production lead to a reducing the cell life thus it is well known that the voltage generation in microbial fuel cells decreases with time. In the open circuit voltage (OCV), there is a rapid increase to values of approximately 699 mV after 8 days after inoculation with acetate fed MFC, involving that electricity can be generated using mixed aerobic microorganisms. The OCV reached to steady value of approximately (709 mV) for acetate fed MFC in 11 days. The cell voltage decreased sharply to value about 50 mV, once the carbon sources in the MFC are consumed. The maximum voltage of 791 mV for acetate fed batch is observed within 19 days. Then, the voltage dropped quickly to less than 50 mV. For regeneration of MFCs function, as well as to recover the anode activity of the MFC is repeated with the addition of both acetate solution and a new batch of sludge to continue the supplement of the anodic electrode with electrochemically active bacteria. After replicating the procedure of inoculums addition in three successive series, a stable reproducible operation of the MFC is accomplished while the microorganisms are closely attached on the surface of the anode, forming anodic biofilm to produce electrons, protons and CO2 [49], [50], [51]. Anodic bio-film transfers the electrons outside the cell and the proton transferred to final hydrogen acceptor (O2). OCV is used to maximize produced power and obtain the maximum current density.
Fig. 1.
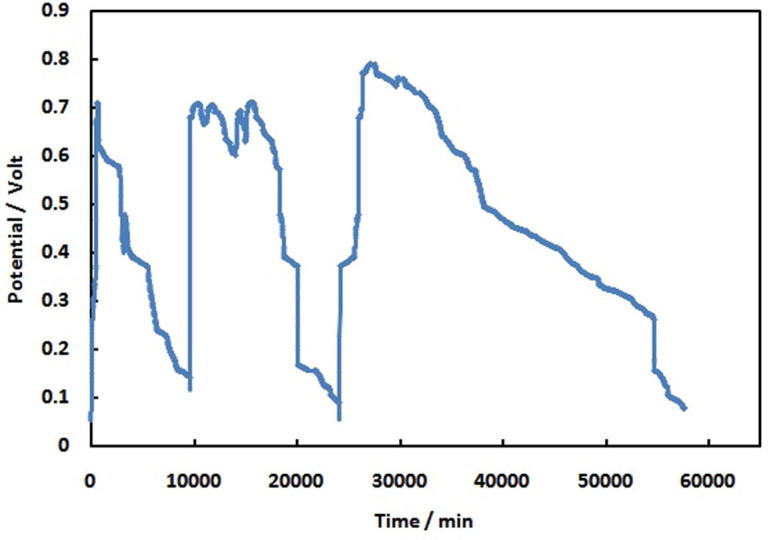
Voltage versus time curve for acetate at no load.
3.2. Electrode characterization
3.2.1. The effect of external load on cell potential, current, and power density
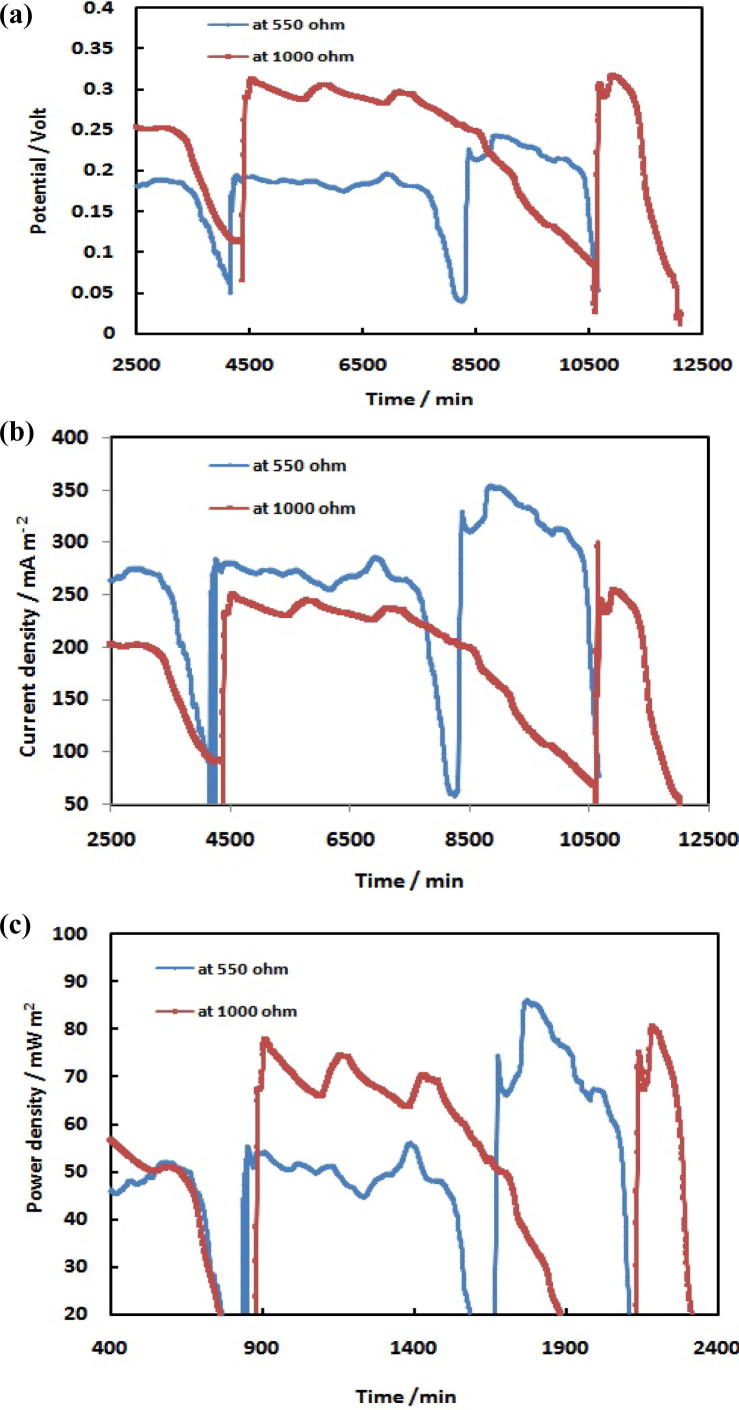
The acetate MSCMFC discharged and operated under a fixed two external resistance 550 and 1000 Ω, in order to explorer the relationship between resistances, potential, current density, and power density. Fig. 2 (a–c) illustrates the performance of acetate MFC inoculated with acetate media with aerobic sludge and it is applied under external resistances (550 & 1000 Ω). Fig. 2(a) represent the effect of both resistance on voltage production versus time, while, Fig. 2 (b & c) represent current and power density production at different resistance (550 (blue line) & 1000 Ω (red line)) versus time respectively. As observed in Fig. 2, the potential, current density, and power density have the same performance. As shown in Fig. 2 (a–c), the output voltage increased slowly during the first day, and then rapidly increased for the next three days. The maximum voltage is 189 mV matching to a current density of 275.14 mA m−2 and power density of 52.05 mW m−2. The voltage output then decreased to about 50 mV, this decrease is corresponding to consumption of acetate. After adding fresh media, it has been observed that there is a spontaneous increase in the voltage output to 196 mV with maximum current density of 285.28 mA m−2 and power density of 55.95 mW m−2. Then, the voltage output is fluctuated to end with 40 mV. After six days, the acetate media is replaced with fresh medium to start the third cycle, through this cycle the voltage output increased from 40 to 243 mV with a maximum current density of 353.82 mA m−2 and power density of 86.07 mW m−2 within three days and then decreased through the next forth day to 50 mV. In case of using 1000 Ω, it is observed that, the voltage output slowly increased during the first day and then increased rapidly during the next three days. Current density values and power density values are the same as that is of the voltage output. In the first cycle, the maximum voltage output value is 2979 mV with a current density of 238.32 mA m−2 and power density of 70.99 mW m−2.
Fig. 2.
Performances of the mediator less single chamber microbial fuel cell operated under acetate fed batch mode at 550 Ω (blue line) and 1000 Ω (a) Potential, (b) Current density, and (c) Power density vs. time.
The voltage is sharply decreased to 50 mV in the end of third day. In the second cycle, the voltage value is increased to reach 312 mV with current density of 249.55 mA m−2 and power density of 77.84 mW m−2. The voltage value decreased to 20 mV after four days. In the third cycle, the maximum voltage output value is 317 mV with maximum current density of 253.79 mA m−2 and maximum power density of 80.51 mW m−2. Then, the voltage decreased gradually to 20 mV in the end of eighth day. From the listed data in Table 1, it can be concluded that, there is a direct relationship between potential and resistance, while in case of current density the relation is inversely. As expected, higher resistance leads to lower the generated current. The maximum voltage peak value at 1000 Ω is higher than that in case of 550 Ω. The maximum current and power densities peak values at 550 Ω are higher than in case of 1000 Ω. Increasing power output, improving coulombic efficiency, and lowering methane production of MFC achieved under operation of the optimal external resistance [52]. The electrons consumption in the anode reducing other electron acceptors such as sulfate and nitrate, or oxygen diffused through the cathode and the sampling ports [11]. Therefore, external resistance 500 Ω give higher performance than 1000 Ω.
Table 1.
Effect of different resistance on MSCMFCs.
| External resistance | Voltage, mV | Current density (mA m−2) | Power density (mW m−2) |
|---|---|---|---|
| 550 Ω | 243 | 354 | 86.1 |
| 1000 Ω | 317 | 297 | 80.5 |
3.2.2. Degradation rate of organic substrate
Chemical Oxygen Demand (COD) test is used to determine the availability of converting fuel in the MFC, either into electricity, or growth of biomass or through competitive reactions with other electron acceptors (e.g., oxygen, nitrate, and sulfate) [53]. COD have been measured for different samples that taken from the acetate fed MFCs at different time intervals. Fig. 3 illustrates the degradation of acetate COD values versus time. The acetate fed batch MFC with COD 1210 mg. l−1 at resistance 550 Ω generated the maximum peak power density 86.1 mW m−2 output at stable current density of 354 mA m−2. An increase in COD led to the current production increase, with the decrease in resistance. The COD removal efficiency of acetate at maximum voltage yield of these experiments are 96%. This indicates that, the complete consumption of acetate is achieved after 5 days. The liberating of electrons from the acetate is defined as the coulombic efficiency (CE), the acetate coulombic efficiency is 65%; this result confirmed that there is inverse relation between coulombic efficiencies and concentration of substrate. The coulombic efficiency is reduced due to consumption of competitive electron acceptors by the microorganisms, either those present in the wastewater, or those diffusing through the MFCs such as oxygen [43].
Fig. 3.
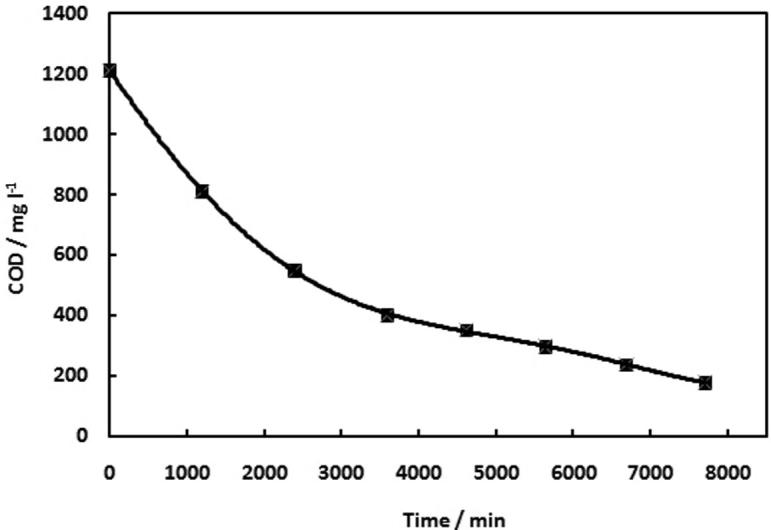
Change of COD with time behavior at external resistance load (550 Ω).
3.2.3. Polarization & power curve
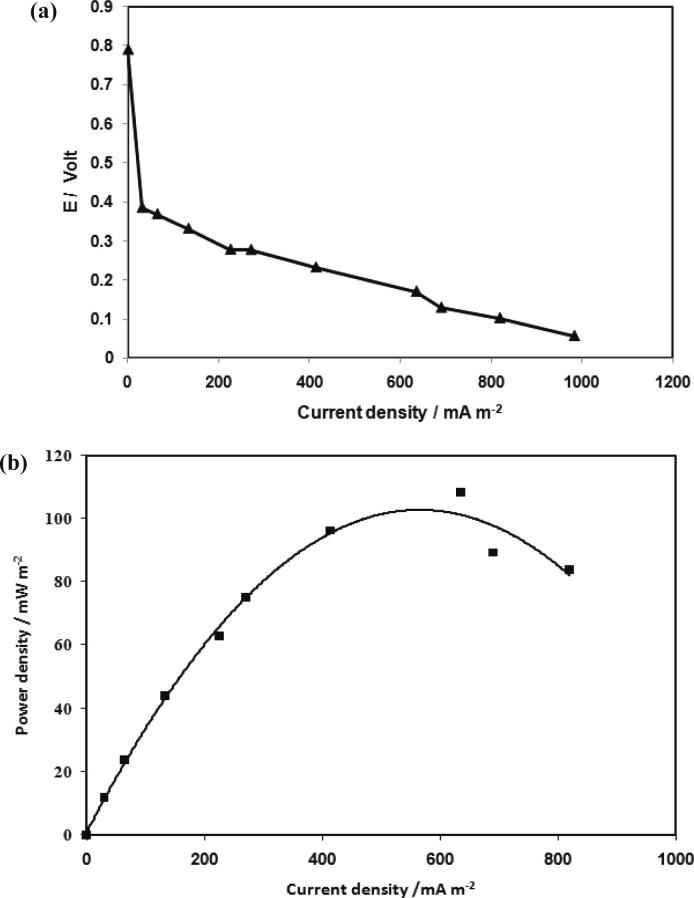
Polarization is the change of MFC electrode potential from its equilibrium state due to a flow of current. It represents, a great tool for the examination and classification of microbial fuel cells [54]. Polarization curves can be plotted as a factor of current density against MFC electrode potential. The yielding data is obtained by varying the supplemented load from 100 to 125,000 Ω after a steady state of MSCMFC operation as shown in Fig. 6. Fig.4a illustrates polarization curve as a factor of current density against potential for acetate at different resistance. Internal resistance is calculated from the polarization curve, whereas the slope of voltage versus current plot is considered [55], the internal resistance is 59 Ω of acetate. The lower the internal resistance the higher the power density, whereas, the high internal resistance consumes amount of power output inside MFCs and low electrochemical activity causing decrease of power generation [56], [57]. The power curve showed the amount of power produced by the system, which considered as the major target of MFCs. When the MFC produce stable electrical current by repeatable power cycles within about 199 h of inoculation (maximum voltage of 243 V, and maximum current density of 235.11 mA m−2), the power curve is obtained by varying the external resistance external resistance (Rext) from 100 to 12.5 × 104 Ω as presented in Fig.4b. The maximum power and current density at 215 Ω, while the minimum value at 125,000 Ω according to the reverse relation between resistance and current, as shown in Table 2. The power and polarization curves demonstrate that the necessary of carbon source to the microbial activity as well as the electrical current generation.
Fig. 6.
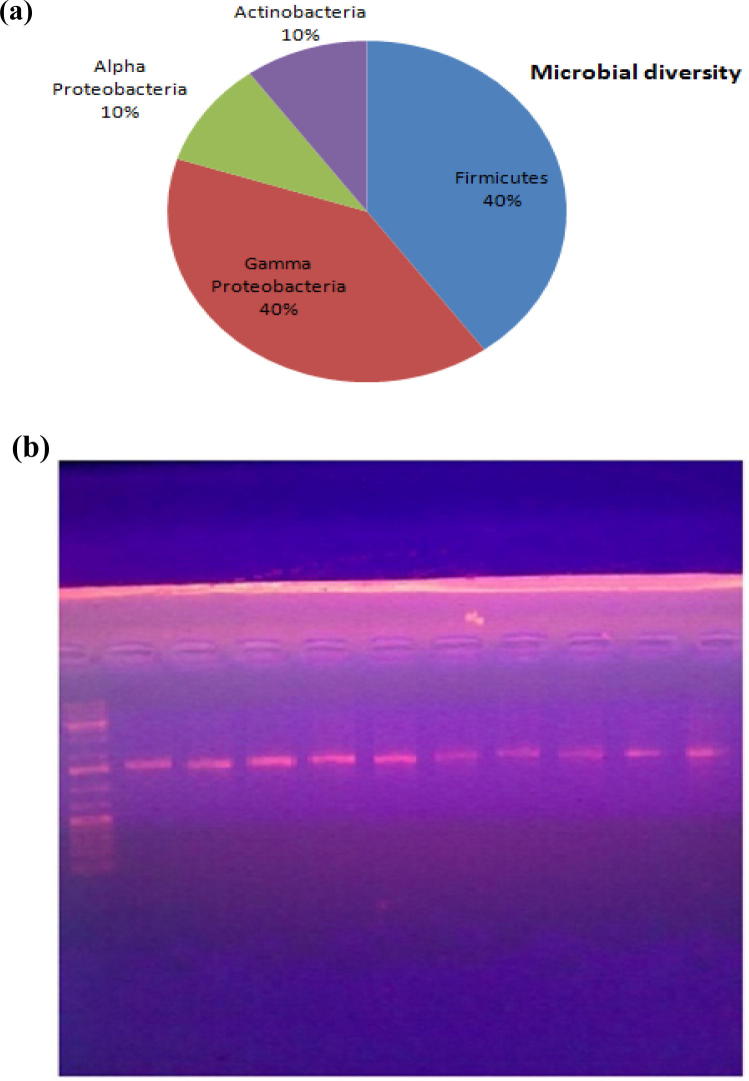
(a) Phylum distributions for taxonomically assigned from the anode biofilm of MFC. (b) Bacterial community profile of single chamber MFC inoculated with aerobic sludge with acetate. Amplified Samples from the anodic bacterial communities are analyzed by denaturing gradient gel electrophoresis (DGGE) of PCR-amplified genes coding for 16S rRNA.
Fig. 4.
Steady-state power and polarization curves of acetate MSCMFC (a) Polarization curve and (b) Power curve.
Table 2.
Effect of applying different load.
| Parameter | Ohm (Ω) | Acetate |
|---|---|---|
| Power density (mW m−2) | 100 | 818.4 |
| Current density (mA m−2) | 100 | 83.7223 |
| Power density (mW m−2) | 125,000 | 0.97394 |
| Current density (mA m−2) | 125,000 | 2.4966 |
| Internal resistance | 59 |
3.2.4. Anode bacterial diversity of acetate biofilm
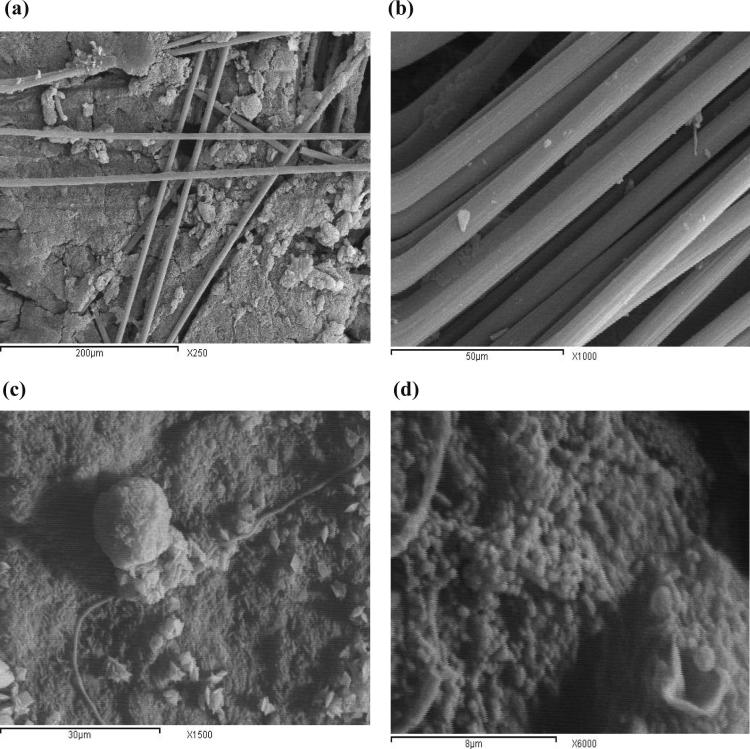
The electrochemically active bacteria referred to as electricigens, anodophilic and exoelectrogens on the anode [14], [58], [59]. Bacterial cell are attached and colonized on the surface of anode forming biofilm which acted as a biocatalyst that able to degrade the substrate and generate electricity [60]. The surface of the anode (carbon paper) is visualized by Scanning Electron Microscope, (SEM (JEOL, JXA-840A) to determine microbial attachment and formation of a biofilm on the anode electrode surface. The tested electrode is removed after 46 days incubation period. It is observed with the naked eye that the anode carbon paper surface covered completely with bacterial cells, then SEM is applied on anodophilic electrode in order to analyse the presence of microbial attachment and biofilm formation. Fig. 5 (a & b) shows the SEM of bare carbon paper electrode surface in the beginning of experiments before inoculation of MFC with aerobic activated sludge, SEM shows the morphological construction of the individual threads, with diameters in the range of 50–200 μm; edges are observed in the axial direction and a cylindrical structure. While, Fig. 5 (c & d) shows the SEM images of inoculated cell with acetate. Both figures revealed the existence of a dense microbial adherence on the carbon paper as compared to the blank figure of plain carbon paper of both, characterize a dense homogenous anodophillic coccoid cells biofilm on the electrode surface (10 μm) as in the previous literature [61]. The anode respiring bacteria connect themselves and aggregate to the surface of anode, forming a living matrix of protein and sugar. The sticky aggregation (biofilm) is mainly rod shaped, (10 μm) and coccoid shape (20 μm). Differentiation in the anodophillic structure is thought to be caused by both difference in electroactive bacteria and substrate feeding. Which have the ability to acclimate and colonize to the anode electrode by secreting matrix materials.
Fig. 5.
SEM images (a & b) for carbon paper- free anode before adding microbial community at different magnification. SEM images of (c & d) showing morphological characters of acetate oxidizing bacteria under different magnifications.
3.2.5. Molecular analysis of biofilm formed on the electrodes
Microbial fuel cells enhanced their performance gradually as communities developed on the anode, and the density of the biofilm as measured using the DNA content is also positively associated with the MFC power density. Analysis shows enriched anode with the phylogenetic diversity with no single dominant bacterial species. The 16S rDNA gene clone libraries for anodic biofilm at the end of the fed batch operation (day 130) of MFC are identified phylogenetically and are grouped by phylum or class, using ABI 3730xl DNA sequencer. Fig.6a Shows pie bar for dominant phyla. The dominant phyla are Firmicutes, Gamma Proteobacteria, Alpha Proteobacteria, Actinobacteria, with ten dominant microbial strains: Bacillus firmus, Shewanella profunda, Bacillus isronensis, Brevundimonas bullata, Pseudomonas putida, Planococcuscitreus, Micrococcus endophyticus, Shewanella xiamenensis, Acineto bactertandoii, and Bacillus safensis. All these species were facultative anaerobes, this may reflect the fact that only species in the anodic biofilm can participate in electron transfer.
Distinctive differences in anodic bacterial communities formed at the anode of MFC are revealed by Denaturing Gradient Gel Electrophoresis (DGGE) of polymerase chain reaction (PCR) amplified 16S rDNA gene of the excised bands. Fig.6b illustrates the sequencing results of the bands of the PCR- DGGE analysis profiles of the 16SrDNA gene fragments amplified from the removed DNA of the biofilm on the electrodes and the bacteria in the solution at the end of the batch. Each band on the DGGE profile represents a specific species in the microbial aggregation and the discoloration concentration of a band represents the comparative population of the related microbial population. Changes in the banding pattern and band intensity of the bacterial biofilm community are observed. Among the detectable bands in the DGGE profiles, five bands are demonstrated with a very high intensity. Moreover, band 9 and 10 are obtained at high intensity in the solution. Low intensity is found at bands 6, 7, and 8. This analysis is showed increasing relative abundances of sequences related to Firmicutes and Gamma Proteobacteria, with decreasing the abundance of Alpha Proteobacteria, and Actinobacteria. In addition, sequences closely matched to B. firmus, B. isronensis, M. endophyticus, and Acinetobacter tandoii (98%), P. putida (99%), B. bullata (97%), S. profunda (96%), S. xiamenensis (95%), and Planococcus citreus (87%). The banding patterns similarities of different staining intensity indicate that there are dominant bacterial species on the electrodes as represented in Table 3.
Table 3.
Identity of the 16S rRNA gene sequences obtained from DGGE bands of anode carbon paper of mediator less MFC.
| Band | Accession number | Phylum | Bacterial description | Similarity(%) |
|---|---|---|---|---|
| 1 | NR 118955 | Firmicutes | Bacillus firmus strain NCIMB 9366 | 98 |
| 2 | NR 104770 | Gamma Proteobacteria | Shewanella profunda strain LT13a | 96 |
| 3 | NR 115952 | Firmicutes | Bacillus isronensis strain B3W22 | 98 |
| 4 | NR 113611 | Alpha Proteobacteria | Brevundimonas bullata strain NBRC 13290 | 97 |
| 5 | NR 074596 | Gamma Proteobacteria | Pseudomonas putida KT2440 strain KT2440 | 99 |
| 6 | NR 113814 | Firmicutes | Planococcus citreus strain NBRC 15849 | 87 |
| 7 | NR 044365 | Actinobacteria | Micrococcus endophyticus strain YIM 56238 | 98 |
| 8 | NR 116732 | Gamma Proteobacteria | Shewanella xiamenensis strain S4 | 95 |
| 9 | NR 117630 | Gamma Proteobacteria | Acinetobacter tandoii strain DSM 14970 | 98 |
| 10 | NR 113945 | Firmicutes | Bacillus safensis strain NBRC 100820 | 98 |
The phylogenetic tree analysis is constructed based on partial archaeal 16S rRNA gene sequences of anodic biofilm of membrane-less MFCs revealed a diverse bacterial community in the anodic biofilm, consisting of Gamma Proteobacteria, Alpha Proteobacteria subdivisions of the phylum Proteobacteria and the phyla of Firmicutes, and Actinobacteria.
4. Conclusion
Analysis of anodic biofilm using the Scanning Electron Microscope (SEM) and 16S rRNA support the hypothesis that the diversity of the surface anodophilic bacteria are the main responsible factor for the acetate fed mediator-less single chamber microbial fuel cell (MLSCMFC) behavior. The acetate is completely oxidized with a capacity to direct transfer of electrons to the anode. The COD removal efficiency of acetate at maximum voltage yield of 96% with coulombic efficiency of 65% is achieved after 5 days. This experimental data confirm that, the increase in COD leads to an increase in current production and decrease in the internal resistance, but high substrate concentrations inhibit power generation in MFCs. There is inversely relationship between coulombic efficiency and concentration of substrate. A high concentration of substrates inhibited the bacteria activities and decreased the CE.
Acknowledgments
This work is supported by the Egyptian Academy of Scientific Research and Technology (ASRT) Funds for Scientist of Next Generation (SNG) and National Research Centre (NRC).
Footnotes
Peer review under responsibility of National Research Center, Egypt.
References
- 1.Reddy L.V., Kumar S.P., Wee Y. Microbial Fuel Cells (MFCs) – a novel source of energy for new millennium. Appl. Microbiol. Microb. Biotechnol. 2010:956–964. [Google Scholar]
- 2.Liu H., Logan B.E. Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of aproton exchange membrane. Environ. Sci. Technol. 2004;38(2):4040–4046. doi: 10.1021/es0499344. [DOI] [PubMed] [Google Scholar]
- 3.Min B., Logan B.E. Continuous electricity generation from domestic wastewater and organic substrates in a flat plate microbial fuel cell. Environ. Sci. Technol. 2004;38(21):5809–5814. doi: 10.1021/es0491026. [DOI] [PubMed] [Google Scholar]
- 4.Cheng K.Y., Cord-ruwisch R., Ho G. Short communication using a potentiostat. Bioelectrochemistry. 2008 doi: 10.1016/j.bioelechem.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Allen R. Microbial fuel-cells: electricity production from carbohydrates. Appl. Biochem. Biotechnol. 1993;39(40):27–40. [Google Scholar]
- 6.Zhang D., Yang F., Shimotori T., Wang K., Huang Y. Performance evaluation of power management systems in microbial fuel cell-based energy harvesting applications for driving small electronic devices. J. Power Sources. 2012;217:65–71. [Google Scholar]
- 7.Chang I., Jang J., Gil G., Kim M., Kim H., Cho B., Seop I., Kyung J., Cheol G., Kim M., Joo H., Won B., Hong B. Continuous determination of biochemical oxygen demand using microbial fuel cell type biosensor. Biosens. Bioelectron. 2004;19(6):607–613. doi: 10.1016/s0956-5663(03)00272-0. [DOI] [PubMed] [Google Scholar]
- 8.Joo H., Soo H., Sik M., Seop I., Kim M., Hong B. A mediator-less microbial fuel cell using a metal reducing bacterium, Shewanella putrefaciens. Enzyme Microb. Technol. 2002;30:145–152. [Google Scholar]
- 9.Chang I.S., Moon H., Bretschger O., Jang J.K., Park H.I., Nealson K.H., Kim B.H. Electrochemically active bacteria (EAB) and mediator-less microbial fuel cells. J. Microbiol. Biotechnol. 2006;16(2):163–177. [Google Scholar]
- 10.Ramanaviciene A. Hemoproteins in design of biofuel cells. Fuel Cell. 2009;1:25–36. [Google Scholar]
- 11.Gil G., Chang I., Kim B.H., Kim M., Jang J., Park H.S., Kim H.J. Operational parameters affecting the performance of a mediator-less microbial fuel cell. Biosens. Bioelectron. 2003;18(4):327–334. doi: 10.1016/s0956-5663(02)00110-0. [DOI] [PubMed] [Google Scholar]
- 12.Moon H., Chang I.S., Kang K.H., Jang J.K., Kim B.H. Improving the dynamic response of a mediator-less microbial fuel cell as a biochemical oxygen demand (BOD) sensor. Biotechnol. Lettters. 2004;26:1717–1721. doi: 10.1007/s10529-004-3743-5. [DOI] [PubMed] [Google Scholar]
- 13.Hassan S.H.A., Seong Y., Oh S. Enzyme and Microbial Technology Power generation from cellulose using mixed and pure cultures of cellulose-degrading bacteria in a microbial fuel cell. Enzyme Microb. Technol. 2012;51(5):269–273. doi: 10.1016/j.enzmictec.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Logan B.E., Regan J.M. Electricity-producing bacterial communities in microbial fuel cells. Trends Microbiol. 2006;14(12):512–518. doi: 10.1016/j.tim.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Yan E., Aaron L., Gostomski P.A. Gaseous pollutant treatment and electricity generation in microbial fuel cells (MFCs) utilising redox mediators. Environ. Sci. Biotechnol. 2014;13:35–51. [Google Scholar]
- 16.Heiskanen A. Bioelectrochemical probing of intracellular redox processes in living yeast cells–application of redox polymer wiring in a microfluidic environment. Anal. Bioanal. Chem. 2013;405(11):3847–3858. doi: 10.1007/s00216-013-6709-4. [DOI] [PubMed] [Google Scholar]
- 17.Beitler J.J. Cosmetic control of parotid gland hypertrophy using radiation therapy. AIDS Patient Care. 1995;9(6):271–275. doi: 10.1089/apc.1995.9.271. [DOI] [PubMed] [Google Scholar]
- 18.Wang H., Ren Z.J. A comprehensive review of microbial electrochemical systems as a platform technology. Biotechnol. Adv. 2013;31(8):1796–1807. doi: 10.1016/j.biotechadv.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Sun J., Li W., Li Y., Hu Y., Zhang Y. Redox mediator enhanced simultaneous decolorization of azo dye and bioelectricity generation in air-cathode microbial fuel cell. Bioresour Technol. 2013 doi: 10.1016/j.biortech.2013.05.039. [DOI] [PubMed] [Google Scholar]
- 20.Hassan R.Y.A., Bilitewski U. A viability assay for Candida albicans based on the electron transfer mediator 2,6-dichlorophenolindophenol. Anal. Biochem. 2011;419(1):26–32. doi: 10.1016/j.ab.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 21.Khater Dena Z., El-Khatib K.M., Hazaa M.M., Hassan R.Y.A. Development of bioelectrochemical system for monitoring the biodegradation performance of activated sludge. Appl. Biochem. Biotechnol. 2015 doi: 10.1007/s12010-015-1522-5. [DOI] [PubMed] [Google Scholar]
- 22.Hassan R.Y.A., Hassan M.H.N.A., Abdel-Aziz S., Khaled E. Nanomaterials-based microbial sensor for direct electrochemical detection of Streptomyces Spp. Sens. Actuators, B. 2014;203:848–853. [Google Scholar]
- 23.Reguera G., Nevin K.P., Nicoll J.S., Covalla S.F., Woodard T.L., Lovley D.R. Biofilm and nanowire production leads to increased current in Geobacter sulfurreducens fuel cells. Appl. Environ. Microbiol. 2006;72(11):7345–7348. doi: 10.1128/AEM.01444-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schroder U. Anodic electron transfer mechanisms in microbial fuel cells and their energy efficiency. Phys. Chem. Chem. Phys. 2007;9(21):2619–2629. doi: 10.1039/b703627m. [DOI] [PubMed] [Google Scholar]
- 25.Stams A.J.M., de Bok F.A.M., Plugge C.M., Eekert V.M.H.A., Dolfing J., Schraa G. Exocellular electron transfer in anaerobic microbial communities. Environ. Microbiol. 2006;8(3):371–382. doi: 10.1111/j.1462-2920.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- 26.Torres C., Marcus A., Lee H., Parameswaran P., Krajmalnik-Brown R., Rittmann B. A kinetic perspective on extracellular electron transfer by anoderespiring bacteria. FEMS Microbiol. Rev. 2010;34:3–17. doi: 10.1111/j.1574-6976.2009.00191.x. [DOI] [PubMed] [Google Scholar]
- 27.Khater D., Hazaa M., Hassan R.Y.A. Activated sludge-based microbial fuel cell for bio-electricity generation. J. Basic Environ. Sci. 2015;2:63–73. [Google Scholar]
- 28.Kelly P.T., He Z. Nutrients removal and recovery in bioelectrochemical systems: a review. Bioresour. Technol. 2013 doi: 10.1016/j.biortech.2013.12.046. [DOI] [PubMed] [Google Scholar]
- 29.Trinidad-Hernandez M. Reversible gastroparesis: functional documentation of celiac axis compression syndrome and postoperative improvement. Am. Surg. 2006;72(4):339–344. [PubMed] [Google Scholar]
- 30.Liu Z., Liu J., Zhang S., Su Z. Study of operational performance and electrical response on mediator-less microbial fuel cells fed with carbon- and protein-rich substrates. Biochem. Eng. J. 2009;45:185–191. [Google Scholar]
- 31.Fornero J., Rosenbaum M., Angenent L. Electric power generation from municipal, food, and animal wastewaters using microbial fuel cells. Electroanalysis. 2010;22(7–8):832–843. [Google Scholar]
- 32.Sun M., Sheng G., Mu Z., Liu X., Chen Y., Wang H., Yu H. Manipulating the hydrogen production from acetate in a microbial electrolysis cell-microbial fuel cell-coupled system. J. Power Sources. 2009;191:338–343. [Google Scholar]
- 33.Bond D., Holmes D., Tender L., Lovley D. Electrode-reducing microorganisms that harvest energy from marine sediments. Science. 2002;295(5554):483–485. doi: 10.1126/science.1066771. [DOI] [PubMed] [Google Scholar]
- 34.Aelterman P. Gent Univ. Belgium.; 2009. Microbial Fuel Cells for the Treatment of Waste Streams with Energy Recovery. (Ph.D. Thesis) [Google Scholar]
- 35.Biffinger J.C., Pietron J., Bretschger O., Nadeau L.J., Johnson G.R., Williams C.C., Nealson K.H., Ringeisen B.R. The influence of acidity on microbial fuel cells containing Shewanella oneidensis. Biosens. Bioelectron. 2008;24:900–905. doi: 10.1016/j.bios.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 36.Schroder U., Niessen J., Scholz F. A generation of microbial fuel cells with current outputs boosted by more than one order of magnitude. Angew. Chem. Int. Ed. 2003;42(25):2880–2883. doi: 10.1002/anie.200350918. [DOI] [PubMed] [Google Scholar]
- 37.Cheng S., Liu H., Logan B.E. Power densities using different cathode catalysts (Pt and CoTMPP) and polymer binders (Nafion and PTFE) in single chamber microbial fuel cells. Environ. Sci. Technol. 2006;40(1):364–369. [PubMed] [Google Scholar]
- 38.Rittmann B.E., Krajmalnik-Brown R., Halden R.U. Pre-genomic, genomic and post-genomic study of microbial communities involved in bioenergy. Nat. Rev. Microbiol. 2008;6:604–612. doi: 10.1038/nrmicro1939. [DOI] [PubMed] [Google Scholar]
- 39.Green S.J., Leigh M.B., Neufeld J.D. Denaturing gradient gel electrophoresis (DGGE) for microbial community analysis. In: Timmis K.N., editor. Springer; Heidelberg, Germany: 2009. pp. 4137–41580. (Microbiology of hydrocarbons, oils, lipids, and derived compounds). [Google Scholar]
- 40.Muyzer G., De Waal A.G., Uitterlinden E. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993;59:695–700. doi: 10.1128/aem.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Briones A., Raskin L. Diversity and dynamics of microbial communities in engineered environments and their implications for process stability. Curr. Opin. Biotechnol. 2003;14:270–276. doi: 10.1016/s0958-1669(03)00065-x. [DOI] [PubMed] [Google Scholar]
- 42.Wittebolle L., Vooren N.V., Verstraete W., Boon N. High reproducibility of ammonia-oxidizing bacterial communities in parallel sequential batch reactors. J. Appl. Microbiol. 2009;107:385–394. doi: 10.1111/j.1365-2672.2009.04222.x. [DOI] [PubMed] [Google Scholar]
- 43.Cheng S., Liu H., Logan B. Increased performance of single-chamber microbial fuel cells using an improved cathode structure. Electrochem. Commun. 2006;8(3):489–494. [Google Scholar]
- 44.Mahmoud M., Gad-allah T.A., El-khatib K.M., El-gohary F. Power generation using spinel manganese – cobalt oxide as a cathode catalyst for microbial fuel cell applications. Bioresour. Technol. 2011;102(22):10459–10464. doi: 10.1016/j.biortech.2011.08.123. [DOI] [PubMed] [Google Scholar]
- 45.Liu H., Cheng S., Logan B.E. Power generation in fed-batch microbial fuel cells as a function of ionic strength, temperature, and reactor configuration. Environ. Sci Technol. 2005;39(14):5488–5493. doi: 10.1021/es050316c. [DOI] [PubMed] [Google Scholar]
- 46.Andrew D.E., Clescenri L.S., Breenberg A.E. nineteenth ed. Washington, DC, USA; APHA, AWW, WEF: 1995. Standard Method for the Examination of Water and Wastewater. [Google Scholar]
- 47.Sun M., Mu Z., Chen Y., Sheng G., Liu X., Chen Y. Microbe-assisted sulfide oxidation in the anode of a microbial fuel cell. Environ. Sci Technol. 2009;43:3372–3377. doi: 10.1021/es802809m. [DOI] [PubMed] [Google Scholar]
- 48.Kiely P.D., Call D.F., Yates M.D., Regan J.M., Logan B.E. Anodic biofilms in microbial fuel cells harbor low numbers of higher-power-producing bacteria than abundant genera. Appl. Microbiol. Biotechnol. 2010;88(1):371–380. doi: 10.1007/s00253-010-2757-2. [DOI] [PubMed] [Google Scholar]
- 49.Song T.S., Jiang H.L. Effects of sediment pretreatment on the performance of sediment microbial fuel cells. Bioresour. Technol. 2011;102:10465–10470. doi: 10.1016/j.biortech.2011.08.129. [DOI] [PubMed] [Google Scholar]
- 50.Song T.S., Yan Z.S., Zhao Z.W., Jiang H.L. Removal of organic matter in freshwater sediment by microbial fuel cells at various external resistances. Chem. Technol. Biotechnol. 2010;85:1489–1493. [Google Scholar]
- 51.Lu J.-R., Zhou N., Zhuang S.-G., Zhnag L., Ni J.-T. No title. Electrochem. Commun. 2008;10:1641–1643. [Google Scholar]
- 52.Pinto R.P., Srinivasan B., Guiot S.R., Tartakovsky B. The effect of real-time external resistance optimization on microbial fuel cell performance. Water Res. 2010;45(4):1571–1578. doi: 10.1016/j.watres.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 53.Logan B.E., Hamelers B., Rozendal R., Schroder U., Keller J., Freguia S., Verstraete A.P.W., Rabaey K. Microbial fuel cells: methodology and technology. Environ. Sci. Technol. 2006;40(17):5181–5192. doi: 10.1021/es0605016. [DOI] [PubMed] [Google Scholar]
- 54.Hoogers G. CRC Press; Boca Raton, FL: 2003. Fuel Cell Technology Handbook. [Google Scholar]
- 55.Picoreanu C., Head I.M., Katuri K.P., van Loosdrecht M.C.M., Scoot K. Computational model for biofim-based microbial fuel cells. Water Res. 2007;41:2921–2940. doi: 10.1016/j.watres.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 56.Kim J.R., Jung S.H., Regan J.M., Logan B.E. Electricity generation and microbial community analysis of alcohol powered microbial fuel cells. Bioresour. Technol. 2007;98(13):2568–2577. doi: 10.1016/j.biortech.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 57.Nam J.-Y., Kim H.-W., Lim K.-H., Shin H.-S. Effect of organic loading on the continuous electicity generation from fermented waste water using a single chamber- microbial fuel cell. Bioresour. Technol. 2010;101:533–537. doi: 10.1016/j.biortech.2009.03.062. [DOI] [PubMed] [Google Scholar]
- 58.Lovley D.R. Bug juice: harvesting electricity with microorganisms. Nat. Rev. Microbiol. 2006;4(7):497–508. doi: 10.1038/nrmicro1442. [DOI] [PubMed] [Google Scholar]
- 59.Logan B.E., Hamelers B., Rozendal R., Schrorder U., Keller J., Freguia S., Aelterman P., Verstraete W., Rabaey K. Microbial fuel cells: methodology and technology. Environ. Sci Technol. 2006;17(40):5181–5192. doi: 10.1021/es0605016. [DOI] [PubMed] [Google Scholar]
- 60.Cheng B.E.L.S. Ammonia treatment of carbon cloth anodes to enhance power generation of microbial fuel cells. Electrochem. Commun. 2007;9:492–496. [Google Scholar]
- 61.Enren Z., Wei X., Guowang D., Chendong S. Electricity generation from acetate and glucose by sedimentary bacterium attached to electrode in microbial – anode fuel cells. Power Sources. 2006;161:820–825. [Google Scholar]