Abstract
In this study, copper nanoparticles (Cu-NPs) were synthesized using Corallina officinalis Linnaeus and Corallina mediterranea Areschoug aqueous extracts. Transmission Electron microscope indicated that the biosynthesized Cu-NPs averaged 12.7 nm and 13.6 nm for C. Officinalis and C. mediterranea, respectively. As reported by the FT-IR analyses, the algal extracts contain phyto-chemicals such as proteins, carboxylic acids, complex carbohydrates; these compounds will act as encapsulating agents and be reduced from copper sulphate to Cu-NPs. Energy-dispersive analyses X-ray (EDX) confirmed the copper composition in the synthesized Cu-NPs. The biosynthesized Cu-NPs arrested the growth of Lyngbya majuscula and presented in time and concentration dependent trends. At a concentration of 2 μg/mL, Cu-NPs, synthesized by C. officinalis exerted 85 ± 4% reduction of the algae dry weight. Increasing Cu-NPs concentration led to excellent reduction, which is a very promising result. Cupper-NPs synthesized by C. mediterranea produced moderate effects on L. majuscula. The results also indicated that there were sharp decreases in chlorophyll a content in L. majuscula with the increase in Cu-NPs concentrations. Using 4 μg/mL of Cu-NPs derived from C. officinalis, chlorophyll a decreased by 48 ± 5%. On the other hand, lower reductions in chlorophyll a were recorded upon using Cu-NPs synthesized using C. mediterranea (36 ± 3% and 41 ± 5% reductions at concentrations of 2 μg/mL and 4 μg/mL, respectively). The results of this study suggested that the bioactive and allelopathic compounds derived from the two algal extracts coating the (Cu2+) together with (Cu2+) are responsible for the inhibitive impacts of Cu-NPs on L. majuscula.
Keywords: Phytotoxic, Copper nanoparticles, Corallina officinalis, Corallina mediterranea, Lyngbya majuscula
1. Introduction
Algae represent the principal level of the trophic chain and assume an important role in the equilibrium of aquatic eco-systems. The impact of algae in the ecosystem incorporates both its positive and negative [26]. Cyanobacterial blooms can have detrimental impacts on aquatic ecosystems through altering trophic structure and functionality [20], water column deoxygenation leading to fish mortalities, and decreasing water quality [37]. Different bloom-forming species are known to produce strong toxins that can impact terrestrial and water-based organisms [16], [47].
The absolute, most normal common toxin produces cyanobacteria, the N2-fixing genera including Lyngbya; in tropical and subtropic marine ecosystem worldwide [7]. Lyngbya blooms often bring about oxygen depletion and have caused significant economic effects, diminishing recreational and commercial fisheries, and decreasing the recreational utilization of an affected region [35].
Distinctive species of Lyngbya are able to produce toxins, which may have damaging impacts on the marine ecosystems [30]. Lyngbya majuscula is considered as one of the most common species of Lyngbya worldwide [7]. Severe blooms of L. majuscula will obstruct ambient light reaching the sea grass and other primary producers, with their consequent suppress [46].
In marine ecosystems both nitrogen and phosphorus are usually limiting elements for biological productivity. Both of the two elements can improve the exponential growth of L. majuscula [3]. An increase in the nutrients levels occurs in the system, the alga only needs warm temperatures and high ambient light to stimulate growth [23]. Copper sulphate is usually used to control algae in water bodies at recommended concentrations of 1–2 ppm. According to Ansari and Amjad [6], maximum Chlorella vulgaris growth was observed at 5 ppm Cu (II) and decreased at higher copper concentrations [6]. The toxic component of copper sulphate is the cupric ion (Cu2+) as earlier revealed by Palmer [32]. The toxic effect of Cu+2 on Rhodella reticulata was defined as algae static [43]. Copper toxicity; has been reported for Chlorella sp. with the intent to prevent algal blooms in aquaculture [18]. Furthermore, the toxic effects of Cu (II) on C. vulgaris and its chloroplast were investigated [15]. Moreover, growth inhibition assay of Chlorella kessleri due to copper toxicity was recorded at 72-h growth in the shake flasks [39].
Control of algal blooms through biological techniques such as alteration of normal physiology including the decrease in photosynthetic pigments may be promising ways of ecological recovery [49]. Copper nanoparticles are showing broad spectrum activities. The experiments suggest the possibility to use this material in various fields such as water purification, air antibacterial packaging, and so forth [41]. Copper-based nanoparticles (Cu-NPs) are used in water treatment [40], and as bactericidal in replacement of nano-silver. The chemicals used in the synthesis of copper nanoparticles are commonly available, cheap, and nontoxic, although, toxic impacts of Cu-NPs on different criteria cells have been well documented [2]. Up to current knowledge, not many studies have been accomplished on the impact and potential of Cu-NPs on cyanobacteria [1].
Seaweeds are an important component of our surroundings and ecosystem. Seaweeds have been one of the richest and most promising sources of bioactive primary and secondary metabolite [12], [17]. Red algae are the most important source of biologically active metabolites in comparison to other algal [29].
Therefore the main objective of current study is to investigate the possibility of inhibiting cyanobacterium; L. majuscula growth using Corallina mediterranea Areschoug and Corallina officinalis Linnaeus cu-NPs. Furthermore, phytotoxic effects of the biosynthesized Cu-NPs, on the growth of the harmful cyanobacterium; L. majuscula will be evaluated.
2. Materials and methods
2.1. Samples collection, biosynthesis and characterization of copper nanoparticles
The red seaweeds C. officinalis Linnaeus and C. mediterranea Areschoug samples were collected from Abu Qir Bay, Mediterranean Sea, Alexandria, Egypt, on December 2015. The algae were brought to laboratory and cleaned thoroughly with fresh water. Seaweeds were spread on blotting paper to remove excess water and shade dried. The algae are classified as Phylum: Rhodophyta; Order: Corallinales; Family: Corallinaceae [4]. Algal aqueous extract was prepared following the method of Aboud et al. [1], and then were used for the synthesis of Cu-NPs.
The precursor (1.0 mM CuSO4 solution) was prepared using (169 mg L−1 in deionized distilled water). For the synthesis of copper nanoparticles, equal volumes of heated CuSO4 solution and heated algal extracts at 1:1 ratio was mixed and stirred for 10 min and then heated at 80 °C for 45 min. Cu-NPs synthesis is indicated by the turning of light brown colour to pale green. The change in the colour of the reactants indicates the formation of Cu-NPs that was confirmed also by UV–vis spectroscopy analyses. The Cu-NPs colloid was centrifuged at 5000 rpm 15 min. The residue was washed in deionized distilled water (DDW), three times. Then, Cu-NPs were collected and stored for further characterization. The biosynthesized Cu-NPs were characterized using TEM and EDX to get details on morphological and structural composition of the particles. FT-IR spectral analyses were carried out to recognize the biomolecules in the algal extracts accountable for synthesis and capping of Cu-NPs. The spectra were reported in the range of 500–4000 cm−1. The UV–visible spectrum was recorded over the 300–700 nm range with a UV 1650 PC-Shimadzu B UV–visible spectrophotometer (Osaka, Japan).
2.2. Algaecide effect of the biosynthesized Cu-NPs against Lyngbya majuscula
The harmful Cyanobacterium species namely, L. majuscula was obtained from Phycology Laboratory, Faculty of Science, Tanta University, Egypt. The alga species was cultured in 1 litre conical flasks containing 400 mL in BG-11 medium for 15 days [5], and incubated in controlled conditions of continuous light (45 μmol/ms) at 25 ± 2 °C.
The stock solution of Cu-NPs (5 mg/mL) was prepared in sterilized deionized water, dispersed for 10 min using a sonicator (60 amplitude) to prevent aggregation and vortexed for 1 min. The stock solution of Cu-NPs was kept at 4 °C and used within one week for the experiments. Prior to each experiment, the stock solution was sonicated for 10 min, vortexed for 1 min and then immediately used in the working concentrations of 2, 4 μg/mL Cu-NPs colloid for estimating the cyanobacterial susceptibility.
2.2.1. Monitoring of algal growth (dry weight-chlorophyll a)
Aliquots of 100 mL of the L. majuscula culture in growth media [5] were exposed to 2 μg/mL and 4 μg/mL of the different biosynthesized Cu-NPs. Furthermore, they were incubated in controlled states of continuous light (45 μmol/ms) and temperature (25 ± 2 °C) for 10 days. Algae dry weight was monitored at 48 h intervals. Similar to the alga dry weight, chlorophyll a was also monitored in the tested alga at 48 h intervals according to Inskeep and Bloom [21]. Aliquots, 2 mL of L. majuscula culture (either treated or untreated) were centrifuged at 15.000 rpm for 10 min. At that point, 1900 μL of the supernatant were discarded and the cells were re-suspended using an ultrasonic bath. After a short time, 900 μL of 100% methanol were added and incubated in the dark at 4 °C for 60 min. Samples were then centrifuged at 15.000 rpm for 10 min and absorption was recorded at 650 nm. Chlorophyll a concentrations in μg/mL were computed by the accompanying equation: Chlorophyll a (μg/mL) = (Abs 650 × 13.9)/2 mL.
3. Results and discussion
3.1. Characterization of the biosynthesized Cu-NPs
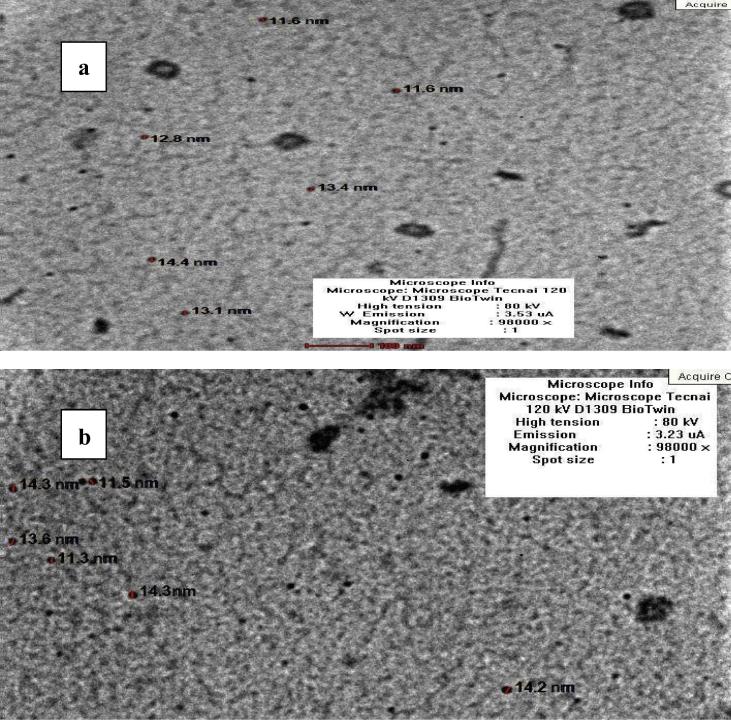
Seaweeds provide a great variety of metabolites and natural bioactive compounds with different biological activities, such as polysaccharides, polyunsaturated fatty acids, phlorotannins, other phenolic compounds, carotenoids and others [34]. In the present work, the extracts of the two Corallina species when exposed to copper sulphate, showed remarkable colour changes indicating the synthesis of Cu-NPs; which is further confirmation that the nanoparticles were subjected to Transmission Electron Microscope. As indicated by Transmission Electron micrographs, the biosynthesized Cu-NPs using C. officinalis extract (Fig.1a) appeared spherical in morphology, well distributed and were in the range of 11.6 and 14.4 nm and average size of 12.7 nm. However, it oscillated between 11.3 nm and 14.3 nm that were synthesized by C. mediterranea extract and the average size was 13.6 nm (Fig.1b). The edges of the particles were lighter than the focuses confirming the existence of the coating materials derived from the algal extracts. The particle sizes of the biosynthesized Cu-NPs via C. officinalis Linnaeus and C. mediterranea Areschoug were in the range reported by Abboud et al. [1], during their biosynthesis of copper oxide nanoparticles using brown alga extract (Bifurcaria bifurcata).
Figure 1.
Transmission electron microscopy images with particles size distribution of the capped Cu-NPs synthesized using Corallina officinalis Linnaeus (a) and Corallina mediterranea Areschoug (b).
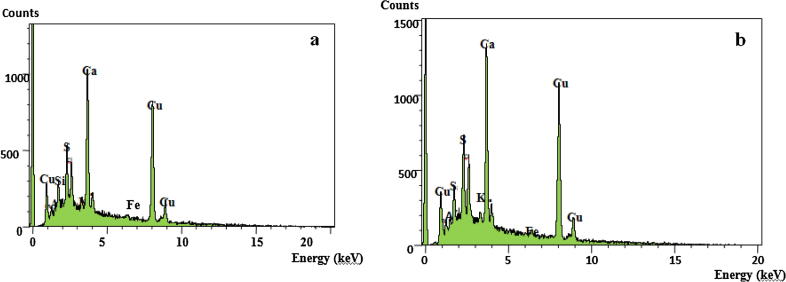
The Cu-NPs were characterized by Energy-dispersive analyses X-ray (EDX) to find out their purity and elemental composition of the Cu-NPs colloids synthesized by C. officinalis and C. mediterranea. Copper (Cu) peaks and other elements were confirmed in EDX spectrum. Strong signals for copper at 1 keV, 8 and 9 keV, respectively (Figs.2a and b) were recorded. Nasrollahzadeh et al. [27], [28], also confirmed the copper composition in green synthesized Cu-NPs through EDX. Thus, the aqueous extract of the two algae species were found to be a potential source bio-reductant to reduce CuSO4 into their nanoparticles [22]. The additional peaks for S, K, Fe and Ca were also observed. These peaks may be attributed to other biomolecules associated with the algal extracts.
Figure 2.
Elemental composition analysis of the capped Cu-NPs synthesized using Corallina officinalis Linnaeus (a) and Corallina mediterranea Areschoug (b).
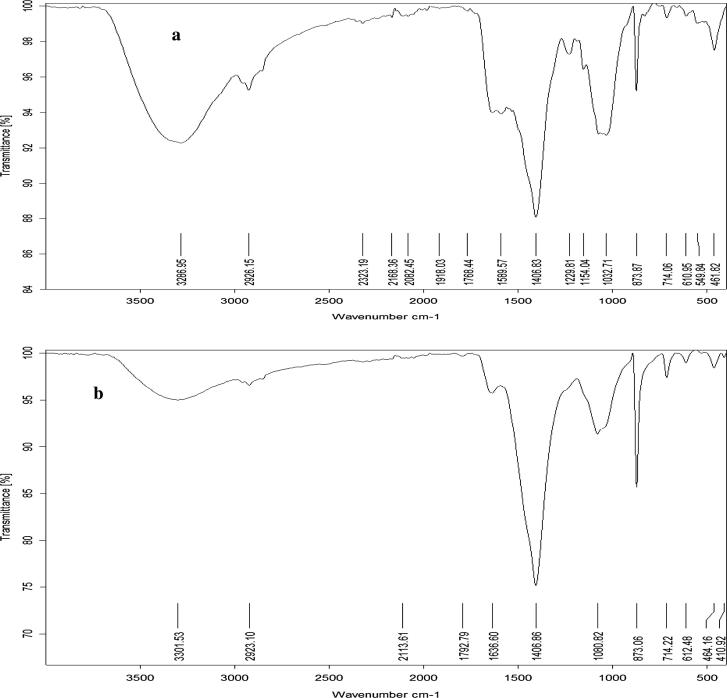
FT-IR spectrum of Cu-NPs that were synthesized using C. officinalis (Table 1 & Fig.3a). The graph shows 6 characteristic peaks over the range of 461 cm−1 and 3288 cm−1. Proteins are also represented by a peak at 1406.0 cm−1 corresponding to Protein δs(CH2). Lipids were represented by 2 main bands at 2926 cm−1 and 1306 cm−1. However, carbohydrates were represented by the bands at 2926 cm−1 and 1033 cm−1 of polysaccharides. In addition to Carboxylic Acid vs(C–O) of COO− groups of carboxylates, Carbonate ion and Aryl disulfides (S–S stretch) at 1406 cm−1, 874 cm−1 and 461 cm−1, respectively. Considering FT-IR spectrum of Cu-NPs C. Mediterranea (Table 1 & Fig.3b) shows 4 characteristic peaks over the range of 612 cm−1 and 3301.5 cm−1. Proteins are represented by 2 main bands at 3301.5 cm−1 which is protein v (N–H) stretching (amide A), at 1406.0 cm−1 corresponding to Protein δs(CH2). The strong band at 1406.6 cm−1 may also represent carboxylic Acid vs (C–O) of COO− groups of carboxylates, and Lipid δs(N(CH3)3) bending of methyl were also represented. However, the band at 873 cm−1 corresponds to carbonate ions. Finally the peak at 612 cm−1 corresponds to Alkynes C–H bend. Based on the phyto-chemical constituents of the two algae extracts that are used as bio-reductants, so far, it has been attributed that phyto-chemical constituents such as carboxylic groups and amino acids [11], diterpenoids [2], enzymes [24] and abundance of hydroxyl and carboxylate groups present in the extracts might have facilitated the formation of Cu (OH)2, which hydrolyzed later into Cu-NPs [42], [43], [44]. None the less, it is presumed that the phyto-chemicals constituents either individually or synergistically could have influenced the bio-reduction in such metal nanoparticles synthesis.
Table 1.
FT-IR analyses of the biosynthesized (Cu-NPs), showing the functional groups responsible for bioreduction of metal precursor and capping of the biosynthesized nanoparticles.
| Bond/stretching | Frequency (cm−1) |
|---|---|
| C. officinalis (Cu-NPs) | |
| Water v(O–H) stretching; Protein v(N–H) stretching (amide A) |
3288 |
| Lipid – carbohydrate Mainly vas(CH2) and vs(CH2) stretching |
2926 |
| Protein δs(CH2) and δs(CH3) bending of methyl Carboxylic Acid vs(C–O) of COO– groups of carboxylates Lipid δs(N(CH3)3) bending of methyl |
1406 |
| Carbohydrate v(C–O–C) of polysaccharides | 1033 |
| Carbonate ion | 874 |
| Aryl disulfides (S–S stretch) | 461 |
| C. mediterranea (Cu-NPs) | |
| Water v(O–H) stretching Protein v(N–H) stretching (amide A) |
3301.5 |
| Protein δs(CH2) and δs(CH3) bending of methyl Carboxylic Acid vs(C–O) of COO− groups of carboxylates Lipid δs(N(CH3)3) bending of methyl |
1406.0 |
| Carbonate ion | 873 |
| Alkynes C–H bend | 612 |
Figure 3.
FTIR analyses of the capped Cu-NPs synthesized using Corallina officinalis Linnaeus (a) and Corallina mediterranea Areschoug (b).
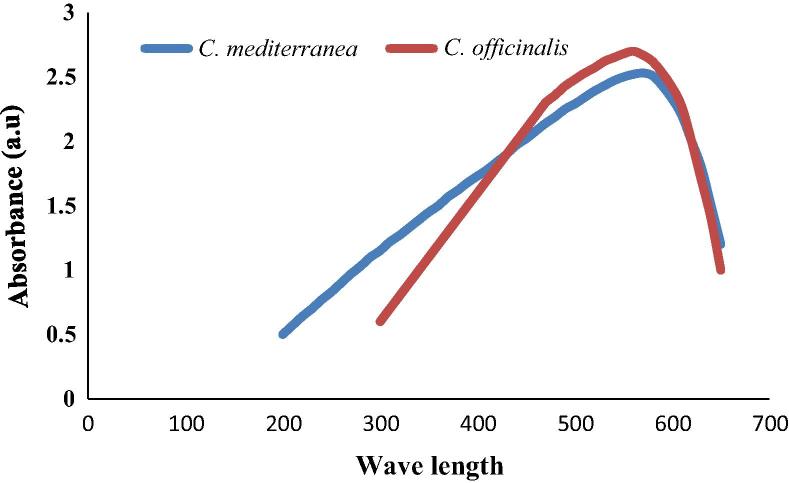
UV–visible spectroscopic analysis and their scanning absorbance vs. wave length (λ) have been established (Fig. 4). The characteristics peaks of Cu-NPs, which are due to charges, transfer spectra the two absorption peaks at wave lengths of 560 nm and 565 nm for C. officinalis Linnaeus and C. mediterranea Areschoug, respectively indicated the formation of Cu-NPs.
Figure 4.
UV-Visible spectrum of Cu-NPs synthesized using Corallina officinalis Linnaeus and Corallina mediterranea Areschoug.
3.2. Algaecide effect of the biosynthesized Cu-NPs against Lyngbya majuscule
The work was extended to study the algaecide effects of the different biosynthesized Cu-NPs on the harmful cyanobacteruim L. majuscula. The aqoues extracts of C. officinalis Linnaeus and C. mediterranea Areschoug, showed no inhibition of L. majuscula growth, when the algae extracts were considered as control.
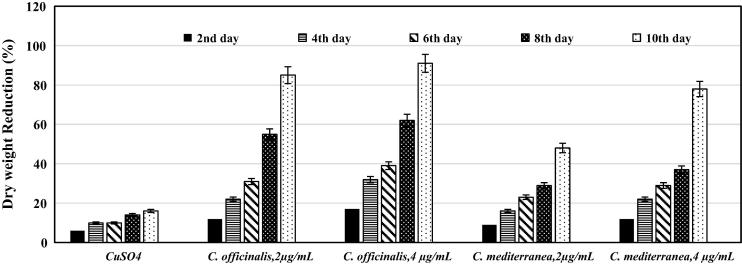
The results (Fig. 5) revealed that the decrease in cell density expressed as dry weight with the increase in Cu-NPs indicated the inhibitory effect with the addition of Cu-NPs. The opacity of different Cu-NPs colloid causing shading effect can also indirectly affect algal growth. Copper-NPs synthesized by C. officinalis came first in their toxic effect on L. majuscula. At a concentration of 2 μg/mL, it exerted 85 ± 4% reduction of the algae dry weight. Increasing the Cu-NPs concentration lead to further reduction to reach 91 ± 6% at Cu-NPs concentration of 4 μg/mL, which is a very promising result. The unexpected antialgal effects of the synthesized Cu-NPs against L. majuscula could be attributed to different bioactive compounds including: terpenoid, polyether, and acetogenin along with some amino acid, acetate and nucleic acid derivatives present in the red algal species [9], [25]. Red algae are also one of the main producers of alkaloids [10]. That may be allelopathic substances in aquatic communities of plants and microorganisms [45].
Figure 5.
Percent reduction of dry weight of Lyngbya majuscula under the stress of different concentrations of Cu-NPs for 10 days.
Copper-NPs synthesized by C. mediterranea extract produced moderate effects on L. majuscula. It diminished the growth of the harmful alga by 48 ± 4% at Cu-NPs concentration of 2 μg/mL. The percent reductions of L. majuscula were increased upon increasing the Cu-NPs concentration to reach about 78 ± 6%. In the present study, Cu-NPs exerted dose dependent negative effects on the harmful alga L. majuscula. Similarly, Aruoja et al. [8] reported the toxicity of CuO-NPs on the alga Pseudokirchneriella subcapitata. Furthermore, copper based compounds are usually used for the control of cyanobacteria. The oxidative potential of the copper ion at high concentrations results in the alga cell membrane to rupture, thus lysing and destroying cyanobacteria cell as stated by Burch et al. [13]. Moreover, Pan et al. [33] stated that the immensely high surface areas and uncommon crystal morphologies endow CuO-NPs with dose-dependent antimicrobial efficiency. The results of this study suggested that the bioactive compounds derived from the two algal extracts together with the (Cu2+) are responsible for the negative impacts of Cu-NPs on L. majuscula.
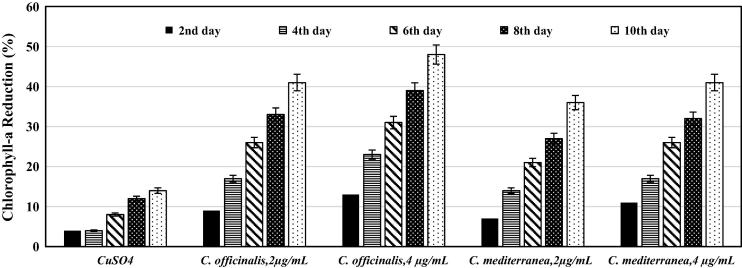
The results presented in Fig. 6 showed that, using varying concentrations of the different synthesized Cu-NPs show sharp decreases in chlorophyll a content in L. majuscula with the increase in Cu-NPs concentrations. Using 2 μg/mL of Cu-NPs derived from C. officinalis, chlorophyll a decreased by 41 ± 4%. The increase of the NPs concentration to 4 μg/mL led to the further increase of chlorophyll a reduction to be 48 ± 5%. On the other hand, lower reductions in chlorophyll a were recorded upon using Cu-NPs synthesized using C. mediterranea. It exerted 36 ± 3% and 41 ± 5% reductions at concentrations of 2 μg/mL and 4 μg/mL, respectively. The biosynthesized Cu-NPs also exhibited the same pattern of effect on the chlorophyll a content of the harmful alga L. majuscula. Additionally FT-IR analyses in this study revealed the presence of different allelopathic compounds that may negatively affect chlorophyll a content of the harmful alga L. majuscula. Our results were consistent with the results achieved by Zhu et al. [50], who found that the allelochemicals such as polyphenols, pyrogallic acid and gallic acid isolated from macrophyte (Myriophyllum spicatum) were key agents in inhibiting the photosynthetic activity of Microcystis aeruginosa. Furthermore, Zhang et al. [48] reported the toxic effects of the aromatic compound; 1, 4-dichlorobenzene on photosynthesis in Chlorella pyrenoidosa. Moreover and in accordance with our results, it was reported that excessive Cu2+ concentration has a detrimental impact on growth and photosynthesis, disturbing the photosynthetic apparatus and plasma membrane permeability of plants [31]. Moreover, Shi et al. [38] indicated that CuO-NPs decline chlorophyll content of the duckweed and that CuO-NPs toxicity is three to four times more than that of ionic Cu because of the larger uptake of NPs-released Cu. Franklin et al. [19], stated that algae require trace amounts of copper for growth, however excess copper can hinder photosynthesis and oxidative phosphorylation in the electron transport chain.
Figure 6.
Percent reduction of chlorophyll a content of Lyngbya majuscula under the stress of different concentrations of Cu-NPs for 10 days.
Qian et al. [36] revealed that photosynthetic pigments capture light energy for photosynthesis; the fall in the abundance of pigments could also stop photosynthesis leading to algal cell death. Cu ions significantly inhibited the content of Chlorophyll a in Microcystis aeruginosa as reported by Chen et al. [14].
Further researches are required to investigate Cu left after end use; this will give a clearer picture of the amount of Cu that will be released during the bioremediation process. We suggest the biosynthesis and application of magnetic iron nanoparticles to attract the excess copper and/or Cu-NPs to complete the bioremediation process. Also, we suggested monitoring the Cu-NP concentration over the course of the exposure experiment (10 days) in a next study.
4. Conclusions
The different goals of this work were successfully achieved. Copper nanoparticles (Cu-NPs) from the aqueous extracts of the two red seaweeds; C. officinalis Linnaeus and C. mediterranea Areschoug have been biosynthesized. The particles’ diameters averaged 12.7 nm and 13.6 nm for C. officinalis and C. mediterranea, respectively. The results indicated that proteins, carboxylic acids, complex carbohydrates acted as encapsulating agents and were reduced from copper sulphate to Cu-NPs. The copper composition in the synthesized Cu-NPs was also confirmed. Metal toxicity effects have received significant attention. Copper-NPs synthesized by C. officinalis have excellent toxic effect on L. majuscula in a dose dependent manner. Copper-NPs synthesized by C. mediterranea extract produced moderate effects on L. majuscula dry weight. The results also indicated that there were sharp decreases in chlorophyll a content in L. majuscula with the increase in Cu-NPs concentrations derived from either C. officinalis or C. mediterranea.
Footnotes
Peer review under responsibility of National Research Center, Egypt.
References
- 1.Abboud Y., Saffaj T., Chagraoui A., El Bouari A. Appl. Nanosci. 2014;4:571–576. [Google Scholar]
- 2.Ahmad J., Alhadlaq H.A., Alshamsan A., Siddiqui M.A. J. Appl. Toxicol. 2016;36(10):1284–1293. doi: 10.1002/jat.3299. [DOI] [PubMed] [Google Scholar]
- 3.Ahern K.S., Ahern C.R., Udy J.W. Harmful Algae. 2008;7:389–404. [Google Scholar]
- 4.A.A. Aleem, Mar. algae Alexandria, Egypt. Alexandria: Privately published, 1 (1993) 135.
- 5.Allen’s M.M., Stanier S.T.J. Gen. Microbiol. 1968;51:302. [Google Scholar]
- 6.Ansari Z., Amjad M. Pak. J. Sci. 2008;60:64–66. [Google Scholar]
- 7.Arquitt S., Johnstone R. Syst. Dyn. Rev. 2004;20:179–198. [Google Scholar]
- 8.Aruoja V., Dubourguier H.C., Kasemets K., Kahru A. Sci. Total Environ. 2009;407:1461–1468. doi: 10.1016/j.scitotenv.2008.10.053. [DOI] [PubMed] [Google Scholar]
- 9.Ayyad S.N., Al-Footy K.O., Alarif W.M., Sobahi T.R. Chem. Pharm. Bull. 2011;59(10):1294–1298. doi: 10.1248/cpb.59.1294. [DOI] [PubMed] [Google Scholar]
- 10.Barbosa M., Valentão P., Andrade P.B. Mar. Drugs. 2014;12:4934–4972. doi: 10.3390/md12094934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beveridge T.J., Murray R.G. J. Bacteriol. 1980;141:876–887. doi: 10.1128/jb.141.2.876-887.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhakuni D.S., Rawat D.S. Anamaya Publisher; New Delhi: 2005. Bioactive Marine Natural Products; pp. 1–10. [Google Scholar]
- 13.M. Burch, C.W.K. Chow, P. Hobson, Algicides for control of toxic cyanobacteria. In: Proceedings of the American Water Works Association Water Quality Technology Conference, Nashville, Tennessee, CD-ROM, November 12–14, 2001.
- 14.Chen J., Qian Y., Li H., Cheng Y., Zhao M. Environ. Sci. Pollut. Res. 2015;22:12407–12414. doi: 10.1007/s11356-015-4492-9. [DOI] [PubMed] [Google Scholar]
- 15.Chen Z., Song S., Wen Y., Zou Y., Liu H. Environ. Sci. Pollut. Res. 2016;23:17910–17918. doi: 10.1007/s11356-016-6997-2. [DOI] [PubMed] [Google Scholar]
- 16.Codd G.A. Water Sci. Technol. 1995;32:149–156. [Google Scholar]
- 17.El Gamal A.A. Saudi Pharm. J. 2010;18(1):1–25. doi: 10.1016/j.jsps.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franklin N.M., Stauber J.L., Markich S.J., Lim R.P. Aquat. Toxicol. 2000;48:275–289. doi: 10.1016/s0166-445x(99)00042-9. [DOI] [PubMed] [Google Scholar]
- 19.Franklin N.M., Stauber J.L., Markich S.J., Lim R. Supervising Sci. Rep. 1998;133 http://www.environment.gov.au/science/supervising Available online: [Google Scholar]
- 20.Havens K.E. Adv. Exp. Med. Biol. 2008;619:733–747. doi: 10.1007/978-0-387-75865-7_33. [DOI] [PubMed] [Google Scholar]
- 21.Inskeep W.P., Bloom P.R. Plant Physiol. 1985;77:483–485. doi: 10.1104/pp.77.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jayalakshmi, Yogamoorthi A. Int. J. Nanomater. Biostructures. 2014;4(4):66–71. [Google Scholar]
- 23.Johnstone S., Fielding F., Hamilton G., Mengersen K. Mar. Environ. Res. 2010;69:27–37. doi: 10.1016/j.marenvres.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Mandal D., Bolander M.E., Mukhopadhyay D., Sarkar G., Mukherjee P. Appl. Microbiol. Biotechnol. 2006;69:485–492. doi: 10.1007/s00253-005-0179-3. [DOI] [PubMed] [Google Scholar]
- 25.J. A. Maschek, B. J. Baker, “The Chemistry of Algal Secondary Metabolism” in Algal Chemical Ecology, Amsler, C.D., Springer-Verlag, Berlin Heidelberg, Germany, 1–24.
- 26.Merchant R.E., Andre C.A. Altern. Ther. Health Med. 2001;7:79. [PubMed] [Google Scholar]
- 27.Nasrollahzadeh M., Sajadi S., Mehdi M. RSC Adv. 2015;5:40628–40635. [Google Scholar]
- 28.Nasrollahzadeh M., Mehdi M., Mohammad S.S. J. Colloids Int. Sci. 2015;455:245–253. [Google Scholar]
- 29.NoerKasanah N., Triyanto, Seto D.S., Amelia W. Indones. J. Chem. 2015;15:201–209. [Google Scholar]
- 30.Osborne N.J.T., Webb P.M., Shaw G.R. Environ. Int. 2001;27:381–392. doi: 10.1016/s0160-4120(01)00098-8. [DOI] [PubMed] [Google Scholar]
- 31.Ouzounidou G., Symeonidis L., Babalonas D., Karataglis S. J. Plant Physiol. 1994;144:109–115. [Google Scholar]
- 32.C.M. Palmer, Control of algae. In: Algae in Water Supplies. An illustrated manual on the identification, significance and control of algae in water supplies, U.S. Department of Health, Education and Welfare Public Health Service, Washington DC, 1962. pp 63–66.
- 33.Pan X., Redding J.E., Wiley P.A., Wen L. Chemosphere. 2010;79:113–116. doi: 10.1016/j.chemosphere.2009.12.056. [DOI] [PubMed] [Google Scholar]
- 34.Pérez M.J., Falqué E., Domínguez H. Mar. Drugs. 2016;14:52. doi: 10.3390/md14030052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pittman S.J., Pittman K.M. Estuar. Coast. Shelf Sci. 2005;63:619–632. [Google Scholar]
- 36.Qian H.F., Yu S.Q., Sun Z.Q., Xie X.C. Aquat. Toxicol. 2010;99:405–412. doi: 10.1016/j.aquatox.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 37.Robarts R.D., Waiser M.J., Arts M.T., Evans M.S. Lakes Reservoirs Res. Manage. 2005;10:167–177. [Google Scholar]
- 38.Shi J., Abid A.D., Kennedy I.M., Hristova K.R. Environ. Pollut. 2011;159:1277–1282. doi: 10.1016/j.envpol.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Snell T.W., Persoone G. Aquat. Toxicol. 1989;14:65–80. [Google Scholar]
- 40.Suleiman M., Mousa M., Hussein, Amjad, I.A. J. Mater. Environ. Sci. 2015;6(7):1924–1937. [Google Scholar]
- 41.Theivasanthi T., Alagar M. Arch. Phys. Res. 2010;1(2):112–117. [Google Scholar]
- 42.ThekkaePadil V.V., Černík M. Int. J. Nanomed. 2013;8:889–898. doi: 10.2147/IJN.S40599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toncheva–Panovaa T., Merakchiyskaa M., Djingovab R., Ivanovaa J. Gen. Appl. Plant Physiol. 2006:53–60. [Google Scholar]
- 44.Premkumar T., Geckeler K.E. Small. 2006;2(5):616–620. doi: 10.1002/smll.200500454. [DOI] [PubMed] [Google Scholar]
- 45.Volk R.B., Furkert F.H. Microb. Res. 2006;161:180–186. doi: 10.1016/j.micres.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 46.Watkinson A.J., O’Neil J.M., Dennison W.C. Harmful Algae. 2005;4:697–715. [Google Scholar]
- 47.Wu X., Jiana J., Wan Y., Giesy J.P., Hua J. Proc. Natl. Acad. Sci. U.S.A. 2012;109:9477–9482. doi: 10.1073/pnas.1200062109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang J., Wang J., Feng J., Lv J. Environ. Monit. Assess. 2016;188:526. doi: 10.1007/s10661-016-5537-3. [DOI] [PubMed] [Google Scholar]
- 49.Zhang J., Xie Z., Jiang X., Wang Z. Clean Soil Air Water. 2014;42:1–6. [Google Scholar]
- 50.Zhu J.Y., Liu B.Y., Wang J., Gao Y.N. Aquat. Toxicol. 2010;2:196–203. doi: 10.1016/j.aquatox.2010.02.011. [DOI] [PubMed] [Google Scholar]