Abstract
Endometriosis is a common chronic gynecological disorder defined as the presence of ectopic functional endometrial tissues, outside uterine cavity, primarily on the pelvic peritoneum and the ovaries. Several studies revealed a correlation between aberrant stem-cell activity in the endometrium and endometriosis. Yet the molecular and cellular behaviors of mesnchymal stem cells in development of endometriosis are hampered by lack of invitro experiments. Our aim was to explore morphological and molecular changes associated with mesenchymal stem cells (MSCs) exposition to serum derived from women with severe endometriosis. Two cell cultures of MSCs isolated from endometrial tissues of two endometriosis-free women. Each cell culture was treated individually with the serum of women with endometriosis (experimental group/n = 7), and serum of women without endometriosis (control group/ n = 4) for 14 days. Quantitative Real-Time PCR was performed later to reveal expression of OCT-4, CDH1 and CDH2, STAT3 and SOX2 genes. Morphologically, cells showed no significant changes. However from molecular point of view, we found increased expression in OCT-4, CDH1 and CDH2. For STAT3 and SOX2 we did not find a significant difference. This study shows that endometriosis serum induced molecular changes in human endometrial MSCs (EnMSCs) that might be related to altered cell behavior which may be a step in differentiation that may be completed invivo by other factors to complete the process of transition. Further researches are needed for optimization to reach differentiation.
Keywords: Endometriosis, Mesnchymal stem cells, OCT-4, SOX2, STAT3, E-cadherin, N-cadherin
1. Introduction
Endometriosis is the growth of endometrial tissue outside the uterine cavity. It is a common gynecological disease that causes chronic pelvic pain and infertility. It affects 8–10% of women. Several theories explain the development of endometriosis. Retrograde menstrual reflux [52], presence of ectopic endometrial stem cells [48] and genetic factors [25] in the etiology of endometriosis have been the main issues in the pathogenesis of endometriosis nowadays [4].
Several factors may play role in development of endometriosis as including abnormal endometrium, altered peritoneal environment, reduced immune surveillance [53], increased angiogenic capacity [14] and endometrium inducing factor(s) [33]. Long-term endometriotic lesions may develop from endometrial stem/progenitor cells, those that may have been established by more mature transient amplifying progenitor cells. Genes encoding proteins involved in cell adhesion, extracellular matrix remodeling, migration, proliferation, immune system regulation, and inflammatory pathways may work by different mechanisms to establish ectopic endometrial implants [12].
Stem cells are undifferentiated cells, featured by their ability to self-renew and differentiate into various specialized cells. EnMSCs derived from ectopic endometriotic lesions exhibit elevated proliferative, migratory, and angiogenic activities. Accordingly, it was reported that EnMSCs represent a major player in endometriosis pathogenesis [11], [45].It was also suggested that the stem cell theory, may account for an alternative endometriosis pathogenesis mechanism and can be involved in all conventional theories as well [49]. Endometrial stem cells are responsible for the rapid cellular proliferation and regeneration of the endometrium [3]. Years ago it was found that an intact endometrial lining could be reached from few endometrial cells regeneration [51].
Transcription Factors (TFs) are proteins that participate in DNA binding, protein–protein interactions, and transcriptional activation or repression. TFs interact with the basal transcriptional machinery and/or chromatin modifying proteins, via altering the rate of gene transcription [55]. Octamer-binding transcription factor 4 (OCT-4), sex determining region Y-box 2 (SOX2) and signal transducer and activator of transcription 3 (STAT3) are three major transcription pluripotency factors. Normally, they contribute to regulation of proliferation and differentiation in stem cells [43], [48]. These factors were reported to be aberrantly expressed in endometriotic tissues [21], [2], [20], [1]. OCT-4 was found to stimulate endometrial cell migration activity leading to ectopic endometrial development [20]. SOX2, together with NANOG, was suggested to enhance cell survival in ovarian endometrial tissues which may further promote endometriotic ectopic growth [29]. STAT3 activation was found to play an important role in the pathogenesis of endometriosis. It contributes to the inflammatory phenotype of eutopic endometriotic tissue [41]. Cadherins are a family of calcium-dependent cell adhesion molecules. They are transmembrane glycoproteins that account for cell-cell contact through adherens junctions. Cadherin family comprises several members such as E-cadherin (CDH1) and N-cadherin (CDH2) and others [50]. They modulate a wide variety of processes, including cell polarization, migration and cancer metastasis [9]. Cadherins were found to take part in the strong adhesion exhibited by the endometriotic cells in ectopic sites [8].
We established our study to understand the pathogenesis of stem cells in development of endometriosis. This will enable us to achieve a definitive treatment or even prophylaxis against this intractable disease in the future.
2. Materials and methods
2.1. Study population
This study accounts an experimental prospective case-control pilot study, including eleven women subjects. It was approved by the Medical Research Ethics committee of the National Research Centre, Cairo, Egypt. Written informed consents were obtained from all participants. The samples were recruited from the Obstetrics and Gynecology Department, Faculty of Medicine, Cairo University. Of the eleven participants, seven had severe endometriosis (the experimental group) and four were endometriosis free (the control group).
The following criteria were met in the study; (1) the endomtriotic women suffered from bilateral endometriomas >5 cm in diameter with peritoneal adhesions and underwent open or laparoscopic surgery for removal, (2) the control women with infertility and underwent diagnostic laparoscopy, (3) endometriosis laparoscopic diagnosis was confirmed by histopathological examination, while, the laparoscopy inspection in control subjects showed that they were clearly free from any endometriotic lesions, (4) all participants did not receive any hormonal therapy 6 months prior to the time of sample collection, as well, (5) they did not have a history of blood malignancies, chronic or immunological diseases.
2.2. Serum collection
Peripheral blood samples, from endometriotic (n = 7) and control (n = 4) women were collected. Whole blood of each participant was obtained into vacutainers without anticoagulant then centrifugation of blood sample at 1800 g for 10 min was done followed by separation of resulting supernatant (filtered through 0.2 mm pore size membrane). Collected sera were then stored at −80 °C for later use.
2.3. Tissue sample collection
Endometrial tissue samples were collected under sterile conditions from endometriosis free women (aged 20–49 years), who underwent a diagnostic hysteroscopy as a step of their work up for subfertility. Women who did not receive any type of hormonal therapy six months before sample collection and without history of immunological diseases, malignancies or chronic diseases were eligible for this study.
Full thickness biopsies were taken from healthy endometrium during hysteroscopy. A part of the biopsy is sent for pathological study to confirm healthy endometrium. Endometrial tissue samples were immediately immersed in Dulbecco’s modified Eagle's medium (DMEM) (Lonza, Belgium) low glucose media containing antibiotic/antifungal mix and then transferred to the laboratory to undergo mesenchymal stem cell isolation.
2.4. Isolation and culture of endometrial mesenchymal stem cells (EnMSCs)
Endometrial tissue samples (n = 2) were washed with phosphate buffer saline (PBS), minced into tiny pieces then digested with 1 mg/ml type 1A collagenase (Gibco, life technologies, USA) for 60 min at 37 °C. Afterwards, cells and cell aggregates were filtered through 100 μm pore size cell strainer (Greiner Bio-one, Germany). Obtained cell suspensions were centrifuged, resuspended and cultured in DMEM low glucose medium (Lonza, Belgium) supplemented with 10% FBS, 100 units/ml penicillin (Gibco), 100 μg/ml streptomycin (Pen-Strep, Lonza) and 2 mM/L glutamax (Gibco) [11]. Two cell cultures were obtained, each from a separate endometrial biopsy. Cultured cells were then incubated at 37 °C and humidified atmosphere with 5% CO2 concentration in a CO2 incubator (Sartorius stedim biotech, GmbH, Germany). Media was exchanged every 2–3 days. Subsequent subculture was done at approximately 80% confluence till passage 3.
2.5. Serum application
Previously collected sera (7 endometriotic and 4 control serum samples) were added to the culture media generating an experimental and a control culture group. Two different serum concentrations in the culture medium were investigated, one was 0.2% and the other was 10%. Media containing serum was applied to 30% confluent EnMSCs cultures at passage three for a period of 14 days.
2.6. Microscopic examination and Photo-documentation
The morphology of EnMSCs treated with sera of normal and women with endometriosis was periodically examined, under inverted microscope (Nikon eclipse TS 100, Japan) and cells was serially photographed using digital eyepiece camera (Premiere, MA88-500).
2.7. Gene expression analysis
2.7.1. RNA extraction and cDNA synthesis
Total RNA was extracted from culture cells (both control and experimental culture groups) using RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol. RNA concentration and purity were measured using a NanoDrop ND-2000 spectrophotometer (Thermo Scientific, USA). Extracted RNA was reverse transcribed into complementary DNA (cDNA) using SensiFAST™ cDNA Synthesis Kit (Bioline, Germany) according to the manufacturer’s Instructions. Reactions were done under the following cycling conditions: 25 °C for 10 min (annealing step), 42 °C for 15 min (reverse transcription step), 85 °C for 5 min (inactivation step) then holding at 4 °C using Perkin Elmer thermal cycler (Applied Biosystems).
2.7.2. Quantitative real-time polymerase chain reaction (qRT-PCR)
Gene expression analysis was performed using Power SYBR green PCR Master Mix (Applied Biosystems) using the following primers: STAT3, 5′-ATCGAGCAGCTGACTACACTG-3′and 5′-ATCGAGCAGCTGACTACACTG-3′; OCT-4, 5′- AACGACCATCTGCCGCTTTGA-3′ and 5′- CTCTCACTCGGTTCTCGATAC-3′; SOX2, 5′-ATCGAGCAGCTGACTACACTG-3′ and 5′- TGCGAGTAGGACATGCTGTAG-3′; CDH1, 5′-TGCGAGTAGGACATGCTGTAG-3′and 5′TGAGGATGGTGTAAGCGATGG-3′ and CDH2, 5′- ATGGGGTTCTCCACTTGATTTC-3′ and 5′-CACTGCGGTACAGTGTAACTG -3′. Reactions were performed in an ABI 7500 Detection System (Applied Biosystems) with the following cycling conditions: initial activation at 95 °C for 10 min, 50 cycles of denaturation at 95 °C for 15 s followed by annealing and extension step at 60 °C for 1 min. β-actin gene was expressed in all conditions confirming the integrality of RNA. After normalization to β-actin expression levels, gene expression was calculated by the comparative ct method for relative quantification (2−ΔΔCt) [5].
2.8. Statistical analysis
Data were expressed as mean ± standard deviation. Statistical differences between means of experimental and control groups were analysed using unpaired Student’s t-test. P values less than .05 was considered statistically significant. Statistical analysis was carried out using the SPSS 16.0 software (IBM, New York, USA)
3. Results
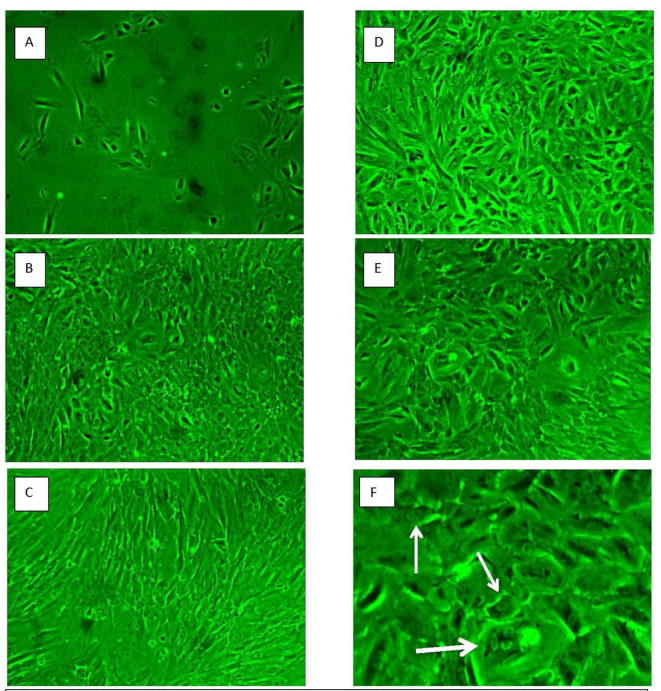
We included 7 serum samples from women with severe endometriosis (bilateral endometriomas >5 cm in diameter with peritoneal adhesions who underwent open or laparoscopic surgery for removal with mean age 25.3 ± 2.1 and 4 serum samples from normal women with mean age 26.2 ± 3.8. In our study we isolated MSCs from normal endometrial stroma collected from two different women into two cell cultures.24 h later some adherent MSCs appeared with heterogeneous appearance, plastic adherent and exhibited short spindle morphology. To compare the effect of different serum concentration preparations on EnMSCs, Cells of the two cell cultures were established in parallel subcultures and supplemented with sera of both control and women with endometriosis. At the third passage (>p3), Images were captured daily on the same cell cultures to gauge the effect of applying control sera or endometriotic women sera using two concentrations a high and low concentration for 14 days (Fig. 1). Cell morphological changes and proliferation were studied using inverted microscopy examination. Ten days after serum application cells in all cultures demonstrated a fibroblast-like, spindle-shaped morphology with round nuclei. Human serum application did not affect the fibroplastic morphology of MSCs. We did not find significant morphological changes in cells treated with control sera at both high and low concentration and there were no colony characteristics changes (Fig.2B and D). Also we did not detect significant changes in the morphology of the cells and/or colony characteristics in culture cells treated with high concentration sera of women with endometriosis (Fig.2C). However, some rounded cells predominately appeared in low-concentration endometriotic women sera-treated EnMSC cultures (Fig.2E and F).
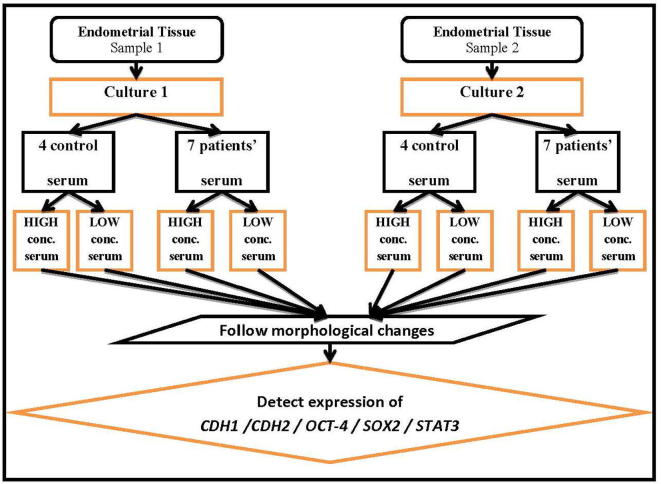
Fig. 1.
Algorithm of the study.
Fig. 2.
Microscopic follow-up for morphological characteristics of EnMSCs cultures during serum challenge phase. (A) Shows passage 3 EnMSCs culture just before serum application. (B vs C) Representative photos for high concentration- control vs endometriotic serum treated MSC cultures, respectively, at 2-week post serum treatment exhibiting fibroplastic morphology (D vs E and F) Representative photos for low concentration- control vs endometriotic serum treated MSC cultures, respectively, at 2-week post serum treatment exhibiting fibroplastic morphology. (F) Some rounded cells prominently appeared in endometriotic fields (white arrows), specifically at the low serum condition.
Gene expression and statistical analyses were performed to assess the differential expression of five markers in endometriotic sera-treated EnMSCs compared with control counterparts. The studied genes were OCT-4, CDH1, CDH2, SOX2 and STAT3. OCT-4 and CDH2 were significantly upregulated in EnMSCs treated with sera of women with endometriosis at both low ((p = .038 and .038, respectively) and high (p = .005 and .011, respectively) serum concentrations. CDH1 was differentially overexpressed only in cells treated with endometriotic serum at low concentration (p = .045). However, expression of both STAT3 and SOX2 was not significantly changed in EnMSCs treated with sera of women with endometriosis compared with those treated with control sera.
Looking closely to culture 1 treated with low concentration sera of women with endometriosis, using RT-PCR, we detected the expression of OCT-4, CDH2 and CDH1 in all cultures treated with serum although the quantities varied. OCT-4 showed increased expression in all samples except only one sample showed no increase in relative quantity. CDH2 showed increase in the relative quantity in all samples except only one sample. CDH1 only 2 samples did not show gene expression. STAT3 showed downregulation in gene expression in most of the samples versus only two sample showed upregulation with no statistical significance. SOX2 showed upregulation in only 4 samples out of 7 but with no statistical significance regarding both cultures. (Table 1).
Table 1.
Showing fold changes in tested transcription factors by RT-PCR.
| Culture | Serum concentration | Sample number | OCT-4 | CDH1 | CDH2 | SOX2 | STAT3 |
|---|---|---|---|---|---|---|---|
| Culture 1 | Low concentration | *S1 | ↑14.77 | ↑37.62 | ↑23.37 | ↑3.06 | ↑1.04 |
| S2 | ↑6.29 | 0.96 | 0.12 | 0.1 | 0.73 | ||
| S3 | ↑8.96 | ↑90.1 | ↑4.55 | 0.2 | 0.8 | ||
| S4 | 0.25 | ↑12.09 | ↑4.07 | ↑13.5 | 0.03 | ||
| S5 | ↑12.49 | ↑14.75 | ↑62.97 | ↑34 | ↑16.67 | ||
| S6 | ↑50.73 | ↑1.47 | ↑5.68 | 0.02 | 0.05 | ||
| S7 | ↑95.33 | 0.19 | ↑89.68 | ↑4.52 | 0.25 | ||
| High concentration | S1 | ↑26.12 | 0.95 | ↑2.72 | 0.002 | 0.93 | |
| S2 | ↑1.91 | 0.03 | ↑8.38 | 0.02 | 0.09 | ||
| S3 | ↑2.23 | ↑1.17 | 0.95 | 0.97 | 0.009 | ||
| S4 | ↑2.37 | ↑3.12 | ↑5.53 | ↑5.6 | 0.06 | ||
| S5 | ↑20.35 | 0.17 | ↑15.75 | 0.006 | 0.92 | ||
| S6 | ↑16.19 | 0.45 | ↑8.04 | ↑13.24 | 0.02 | ||
| S7 | ↑1.38 | ↑1.9 | ↑4.49 | 0.08 | 0.004 | ||
| Culture 2 | Low concentration | S1 | ↑28.68 | ↑10.61 | ↑6.71 | ↑67.90 | 0.65 |
| S2 | ↑4.37 | ↑50.24 | ↑26.48 | ↑39.83 | ↑8.66 | ||
| S3 | ↑4.37 | ↑5.99 | ↑3.98 | ↑13.96 | ↑1.18 | ||
| S4 | ↑23.42 | ↑7.18 | ↑5.97 | ↑4.36 | 0.53 | ||
| S5 | 0.59 | ↑7.69 | ↑6.62 | ↑2.71 | ↑1.06 | ||
| S6 | ↑7.46 | ↑16.31 | ↑25.91 | ↑18.99 | ↑7.58 | ||
| S7 | 0.13 | 1.12 | ↑2.29 | ↑93.54 | ↑1.06 | ||
| High concentration | S1 | ↑2.03 | ↑3.09 | ↑6.69 | ↑3.3 | ↑1.23 | |
| S2 | ↑11.04 | ↑7.01 | ↑3.25 | ↑7.86 | 0.14 | ||
| S3 | ↑3.17 | 0.95 | ↑4.11 | 1.29 | 0.04 | ||
| S4 | ↑6.56 | ↑8.51 | ↑4.6 | ↑3.64 | 0.33 | ||
| S5 | ↑6.12 | ↑2.9 | ↑6.03 | ↑1.98 | 0.76 | ||
| S6 | ↑28.55 | ↑13.93 | ↑19.06 | ↑3.35 | 0.97 | ||
| S7 | ↑5.92 | ↑23.27 | ↑1.3 | 1.26 | 0.71 | ||
*S resembles sample number.
↑means increased expression of transcription factor.
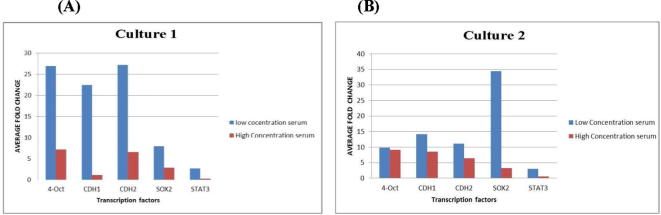
In culture 1, cells treated with high concentration sera of women with endometriosisOCT-4 showed gene expression in all samples. CDH2 gene expression also showed homogenous upregulation in six samples out of seven. CDH1 showed no fold change increase in all samples. STAT3 showed down regulation in all samples. A relative quantity increase was detected in 2 samples regarding SOX2. In Fig.3A we demonstrated the average fold change in both concentrations in culture 1.
Fig. 3.
Culture 1(A) Average fold change of the five genes in cultures treated with low and high concentration serum of women with endometriosis in culture 1. (B) Average fold change of the five genes in cultures treated with low and high concentration serum of women with endometriosis in culture 2.
In low concentration endometriotic serum treated cells in culture 2, we showed increase in OCT-4 and CDH2 and CDH1 gene expression in a more homogenous manner except of 2 samples showed no fold change increase of OCT-4. Unlike culture 1, SOX2 showed increased expression in all samples. STAT3 showed increase in relative quantity in 5 samples but with no statistical significance. In culture 2 treated with high concentration serum of women with endometriosisOCT-4, CHD2 and CDH1 showed more homogenous fold change increase in samples. STAT3 showed downregulation in most of high concentration serum treated culture 2 plates. SOX2 fold change increase was detected also but with a less evident manner than cell treated with low concentration serum. Fig.3B shows the average fold change of both concentrations in culture 2.
When we searched for a link between the expression of genes and patient characteristics, e.g. age, phase of the menstrual cycle or the different pathologies patients suffered from, we could not establish a correlation between patient’s age or gynecologic disorders and genes expression.
4. Disscussion
The current study focused on quantitative analyses of OCT-4, SOX2, STAT3, CDH1 and CDH2 expression in mesenchymal stem cells treated with serum of patients with endometriosis, in a way to understand the pathogenesis. we detected the increased expression of OCT-4 in all stem cell cultures treated with both high and low concentration of endometriotic women serum. It is known that OCT-4 is important in self-renewal and pluripotency and lineage commitment in embryonic stem cells [42]. Changes in OCT-4 expression are involved in numerous developmental programs. For instance, a transient increase in OCT-4 levels can induce lineage commitment to the primitive endoderm and mesoderm, whereas repression of OCT-4 leads to trophectoderm differentiation. [38], [42] At the top of the pluripotent cell genetic regulatory network, OCT-4 and SOX2 work cooperatively to stimulate the transcription of several target genes, including NANOG, FGF-4, UTF1, Fbx15, microRNA-302 clusters and even SOX2 and OCT-4 themselves [39], [37]. Consistent with their roles in maintaining pluripotency, overexpression of specific transcription factors (OCT-4, SOX2, KLF4 and c-MYC) can induce somatic cells to acquire pluripotency. These induced pluripotent stem cells have characteristics similar to ESCs [31].
Also it was found in a previous study that OCT-4 was expressed in human endometrium [21], ectopic endometrial tissues [2], [19]. Other studies proved highly significant up-regulation of OCT-4 gene expression in ectopic endometrial tissues as adenomyosis and chocolate cysts [26]. OCT-4 was also found to be expressed in cancer cell formation and metastasis [9]. Unknown pathogenic mechanisms lead to neoplastic changes with ovarian endometriomas which could be seen in elevated levels of CA125 in both ovarian cancer and endometriosis [54].
Also we did not find a significant relation between serum concentration or severity of the disease and the amount of OCT-4 expression. Matthai and coworkers also could not correlate between the degree of endometriosis and the amount of OCT-4 expressing cells [35]. Also earlier studies had shown that human meshnchymal stem cells and fibroblasts do not express OCT-4 transcription factor [24], [23] while other studies denied this [6], [36]. This discrepancy may be due to the 4 isoforms of OCT-4 [10] generated by alternative translation initiation.
Palma et al. showed tendency of SOX2 to increase after 48 h of transfection and 120 h after transduction in forced expression of OCT-4 in meshnchymal stem cells [22] while Martin Götte et al. showed expression of the pluripotency factors SOX2, OCT-4, KLF4 and NANOG in human endometrium [2]. Other investigators proved the presence of SOX2 positive cells in perivascular area in endometrium in lower quantities than adult stem cells [10], [28]. These findings may support the notion of bone marrow derived stem cells and the lymphovascular theory as a route of endometriosis [48]. Differentiated Cells already expressingSOX2 &/or OCT-4 could be easily induced by forced expression of SOX2, OCT-4, KLF4 and cMYC to an embryonic stem cell like state [31], [32]. Nevertheless in our study serum treated meshnshymal stem cells did not show significant change in SOX2 expression. This gene disregulation in cells may be due to being in an early transitional cell state to acquire pluripotency in the process of differentiation of EnMSCs to develop endometriosis. Moreover we did not force OCT-4 expression in our study. Signaling events downstream from CDH1 might stimulate OCT-4 to upregulate endogenous OCT-4. It is a tempting to speculate that E-cadherin could control OCT-4 expression [56].
STATs are latent transcription factors that mediate cytokine- and growth factor-directed transcription. In many human cancers and transformed cell lines, STAT3 is persistently activated, and in cell culture, active STAT3 is either required for transformation, enhances transformation, or blocks apoptosis. For example, STAT3 is necessary for differentiation of different cells like M1 leukemia cells, cerebral cortical precursor cells, pro-B cell lines, proliferation of T cells and early development of mouse embryos [47].
A report shows that dominant-negative forms of STAT3 lead to differentiation of embryonic stem cells (ESCs), other findings indicate that STAT3 activation is required for self-renewal of ES cells [44]. One of the possible targets of STAT3 is a POU-domain transcription factor, Oct-3/4. Oct-3/4 is expressed in ES and embryonal carcinoma cells, and is required to maintain the stem cell properties. However, induction of Oct-3/4 expression either by leukemia inhibitory factor or 4HT-stimulation in ES cells expressing STAT3 was never apparent [46]. ByungGak Kim et al. looked for the expression of total STAT3 in endometrium to find no significance difference either in eutopicor normal endometrium. However they found that levels of pSTAT3 were significantly higher in endometrium from women with endometriosis and proposed that consistent activation of STAT3 contributes in the pathogenesis of endometriosis [30]. Regarding our study STAT3 showed downregulation. This observation may point our thinking that activation of STAT3 needs other factors that were not present in the environment of our experiment.
For more understanding of the behavior of EnMSCs treated with endometriotic women serum we looked for E-cadherin and N-cadherin. Poncelet et al. detected lack of E-cadherin expression in peritoneal endometrioticlesions in 22% of the cases [18] which does not correlate with our study as we found a higher percentage 42.8% of the cultures treated with high concentration of serum were positive to E-cadherin and 85.7% in cultures treated with low concentration of serum. Poncelet also showed that lack of E-cadherin expression was characteristic to lesions from advanced stage of the disease though he suggested that lack of E-cadherin expression is related to aggression of the disease. E-cadherin negative cells from endometrial biopsies in an invitro study have an invasive potential while E-cadherin positive ones loses such ability [34]. Regarding our study we found more expression of E-cadherin in culture cells treated with low serum concentration whileit was less expressed in high serum concentration. Low concentration serum may possibly affect the gene level expression rather than high concentration serum in cultured MSCs. These conflicting results may signify our poor understanding of the effect of different concentrations of serum on transcription factors in EnMSCs.
This also can support our assumption that early genetic changes in MSCs may not reach the power to achieve cellular differentiation depending on the concentration of the mediators in serum. Different pathways at different concentration may be another elucidation for such a conflict. Also these could be due to absence of other endogenous factors present invivo that plays an important role at different concentrations.
In the present study both cultures of EnMSCs treated with high and low concentration of endometriotic women serum show significant upregulation of N-cadherin. This may point to an early stage of cadherin switch but without change in cell morphology. At this stage of culture, it was found to be difficult to definitively identify sharp morphological changes in serum treated EnMSCs. This is not particularly surprising, as this test only represents the effect of serum invitro yet there may be many other factors invivo that could play role for sufficient morphological divergence.
There is a growing evidence suggests that black and red lesions may represent different stages of spontaneous evolution of endometriotic implants, with the red lesions being the first stage. Previous studies had shown that N-cadherin were significantly higher in red peritoneal (early) lesions than black peritoneal (late) lesions. Also they did not detect N-cadherin expression epithelial cells in menstrual endometrium [16]. Also Previous studies demonstrated that E-cadherin negative epithelial cells were increased in peritoneal endometriosis compared to eutopic endometrium and that E-cadherin negative, N-cadherin positive endometriotic epithelial cells were invasive invitro [27].
Cadherin switch contributes for Epithilial-mesnchymal transition (EMT) and mesnchymal-epithilial transition (MET). A French research group suggest that endometrial epithelial cells might undergo an EMT-like process after attachment of endometrium to peritoneum resulting in red peritoneal endometriosis. MET-like process may occur later during the evolution of peritoneal endometriotic implants then resulting into black peritoneal endometriosis [13]. MET was suggested to be responsible for migratory and invasive cells with meshnchymal features controlled by environmental triggers [15]. Yet more detailed studies are need for better understanding of the molecular basis of MET processes. we possibly assume that the EnMSC could be the main cell in early migration and invasion on peritoneum via MET-like process and controlled by various invivo factors yet further studies on this issue is required. Early cadherin changes in our study could be an important step in MET and EMT processes that play a role in endometriosis development.
Human MSCs are different from rodent MSCs further more among MSCs prepared from different inbred strains of mice [40].It is why it is difficult to develop a consensus opinion about MSC plasticity [17]. A large number of variables are likely to contribute to the inconsistencies in these observations. The differences in the properties of MSCs prepared in different laboratories make researchers unaware of the controversial molecular characterizes of the cells. In addition, the differentiation of MSCs is largely driven by signals from culture conditions or the microenvironment invivo, particularly the microenvironment of rapidly developing or injured tissues. In most cases the signals that drive differentiation invivo remain indeterminate and therefore cannot be replicated invitro. Under such circumstances it is difficult to design experiments that define the limits of MSC plasticity, and negative outcomes can have multiple explanations.
An earlier research group in the national research centre has proved that the blood of women with endometriosis possesses a factor, endometriosis inducing factor (EIF), that has the ability to differentiate stem cells cultured with that blood into endometrial like cells and glands. Rasheed and coworkers studied previously the effect of serum of endometriotic women on human umbilical cord blood stem cells and on the contrary from our study they showed morphological cell changes and concluded that endometriotic women serum may contain a factor that they called it Endometriosis Inducing Factor [33]. The difference in the source of mesnchymal stem cells between their study and ours may contribute for the discrepancy in the results. A later study by Azmy and coworkers showed that there is an increased expression of MicroRNA 130a in blood of women with endometriosis which is a potent regulator in gene expression in endometriosis causing stem cells to transform to endometriotic cells [7].
In conclusion, we believe that serum treated EnMSCs cells proceed through a process of change in the transcriptional factor(s) which may contribute for early deviation towards an intermediate form of cells without morphological changes. This transient state towards MET which may be affected by other invivo factors that may continue the process of transition which may be hormonal or immunological factors.
Acknowledgements
The authors would like to dedicate this work to the soul of prof. Wael El-Garaf. Fund of this study was provided by National Research Centre, Egypt.
Acknowledgments
Conflict of interest
The authors have no conflict of interest.
Footnotes
Peer review under responsibility of National Research Center, Egypt.
References
- 1.Kim B.G., Yoo J.-Y., Kim T.H., Shin J.-H., Langenheim J.F., Ferguson S.D., Fazleabas A.T., Young S.L., Lessey B.A., Jeong J.-W. Hum Reprod. 2015;30(5):1069–1078. doi: 10.1093/humrep/dev050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Götte M., Wolf M., Staebler A., Buchweitz O., Kiesel L., Schüring A.N. Fertil Steril. 2011;95(1):338–341. doi: 10.1016/j.fertnstert.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Gargett C.E., Masuda H. Mol Hum Reprod. 2010;16(11):818–834. doi: 10.1093/molehr/gaq061. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy S., Bennett S., Weeks D.E. Hum Reprod Update. 2001;7(4):411–418. doi: 10.1093/humupd/7.4.411. [DOI] [PubMed] [Google Scholar]
- 5.Livak K.J., Schmittgen T.D. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 6.Berg J.S., Goodell M.A. Cell Stem Cell. 2007;1(4):359–360. doi: 10.1016/j.stem.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Garf W, Anwar M., (2010).
- 8.Witz CA. Seminars in reproductive medicine, Copyright copyright 2003 by Thieme Medical Publishers Inc, 333 Seventh Avenue, New York, NY 10001, USA. Tel.:+ 1 (212) 584–4662, 2003, vol. 21, p. 173–182.
- 9.Chiou SH,Wang ML, Chou YT, Chen CJ, Hong CF, Hsieh WJ, et al. Can Res 2010; 70(24):10433–44. [DOI] [PubMed]
- 10.Schwab K.E., Gargett C.E. Hum Reprod. 2007;22(11):2903–2911. doi: 10.1093/humrep/dem265. [DOI] [PubMed] [Google Scholar]
- 11.Kao A.-P., Wang K.-H., Chang C.-C., Lee J.-N., Long C.-Y., Chen H.-S., Tsai C.-F., Hsieh T.-H., Tsai E.-M. Fertil Steril. 2011;95(4):1308–1315. doi: 10.1016/j.fertnstert.2010.09.064. [DOI] [PubMed] [Google Scholar]
- 12.Meola J, e Silva JCR, Dentillo DB, da Silva WA, Veiga-Castelli LC, de Souza Bernardes LA, et al. Fertil Steril 2010; 93(6):1750–73. [DOI] [PubMed]
- 13.Wells A., Yates C., Shepard C.R. Clin Exp Metas. 2008;25(6):621–628. doi: 10.1007/s10585-008-9167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giudice LC, Kao LC, CrossRef, PubMed, Web of Science® Times Cited 423 (n.d.).
- 15.Hugo H., Ackland M.L., Blick T., Lawrence M.G., Clements J.A., Williams E.D., Thompson E.W. J Cell Physiol. 2007;213(2):374–383. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 16.Matsuzaki S, Darcha C, Hum Reprod; 2012 der442. [DOI] [PubMed]
- 17.Sekiya I., Larson B.L., Smith J.R., Pochampally R., Cui J.-G., Prockop D.J. Stem Cells. 2002;20(6):530–541. doi: 10.1634/stemcells.20-6-530. [DOI] [PubMed] [Google Scholar]
- 18.Poncelet C., Leblanc M., Walker-Combrouze F., Soriano D., Feldmann G., Madelenat P., Scoazec J.-Y., Daraï E. Acta Obstet Gynecol Scand. 2002;81(3):195–203. doi: 10.1034/j.1600-0412.2002.810302.x. [DOI] [PubMed] [Google Scholar]
- 19.Pacchiarotti A., Caserta D., Sbracia M., Moscarini M. Fertil Steril. 2011;95(3):1171–1173. doi: 10.1016/j.fertnstert.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 20.Chang J.-H., Au H.-K., Lee W.-C., Chi C.-C., Ling T.-Y., Wang L.-M., Kao S.-H., Huang Y.-H., Tzeng C.-R. Fertil Steril. 2013;99(5):1332–1339. doi: 10.1016/j.fertnstert.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 21.Forte A., Schettino M.T., Finicelli M., Cipollaro M., Colacurci N., Cobellis L., Galderisi U. Mol Med. 2009;15(11–12):392–401. doi: 10.2119/molmed.2009.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palma C.S., Tannous M.A., Malta T.M., Russo E.M., Covas D.T., Picanço-Castro V. Genet Mol Res. 2013;12(2):1054–1060. doi: 10.4238/2013.April.2.22. [DOI] [PubMed] [Google Scholar]
- 23.Go M.J., Takenaka C., Ohgushi H. Exp Cell Res. 2008;314(5):1147–1154. doi: 10.1016/j.yexcr.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 24.Greco S.J., Liu K., Rameshwar P. Stem Cells. 2007;25(12):3143–3154. doi: 10.1634/stemcells.2007-0351. [DOI] [PubMed] [Google Scholar]
- 25.Albertsen H.M., Chettier R., Farrington P., Ward K. PLoS ONE. 2013;8(3):e58257. doi: 10.1371/journal.pone.0058257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathieu J, Zhang Z, Zhou W, Wang AJ, Heddleston JM, Pinna CM, et al. Can Res 2011;71(13):4640–52. [DOI] [PMC free article] [PubMed]
- 27.Zeitvogel A., Baumann R., Starzinski-Powitz A. Am J Pathol. 2001;159(5):1839–1852. doi: 10.1016/S0002-9440(10)63030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwab K.E., Hutchinson P., Gargett C.E. Hum Reprod. 2008;23(4):934–943. doi: 10.1093/humrep/den051. [DOI] [PubMed] [Google Scholar]
- 29.Song Y., Xiao L., Fu J., Huang W., Wang Q., Zhang X., Yang S. Reprod Biol Endocrinol. 2014;12(1):42. doi: 10.1186/1477-7827-12-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim C.M., Oh Y.J., Cho S.H., Chung D.J., Hwang J.Y., Park K.H., Cho D.J., Choi Y.M., Lee B.S. Hum Reprod. 2007;22(3):843–849. doi: 10.1093/humrep/del425. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 32.Huangfu D., Osafune K., Maehr R., Guo W., Eijkelenboom A., Chen S., Muhlestein W., Melton D.A. Nat Biotechnol. 2008;26(11):1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 33.Azmy O.M., Elgarf W.T. Med Res J. 2012;11(2):40–47. [Google Scholar]
- 34.Gaetje R., Kotzian S., Herrmann G., Baumann R., Starzinski-Powitz A. Am J Pathol. 1997;150(2):461. [PMC free article] [PubMed] [Google Scholar]
- 35.Matthai C., Horvat R., Noe M., Nagele F., Radjabi A., Van Trotsenburg M., Huber J., Kolbus A. Mol Hum Reprod. 2006;12(1):7–10. doi: 10.1093/molehr/gah254. [DOI] [PubMed] [Google Scholar]
- 36.Lengner C.J., Camargo F.D., Hochedlinger K., Welstead G.G., Zaidi S., Gokhale S., Scholer H.R., Tomilin A., Jaenisch R. Cell Stem Cell. 2007;1(4):403–415. doi: 10.1016/j.stem.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Card D.A.G., Hebbar P.B., Li L., Trotter K.W., Komatsu Y., Mishina Y., Archer T.K. Mol Cell Biol. 2008;28(20):6426–6438. doi: 10.1128/MCB.00359-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palmieri S.L., Peter W., Hess H., Schöler H.R. Develop Biol. 1994;166(1):259–267. doi: 10.1006/dbio.1994.1312. [DOI] [PubMed] [Google Scholar]
- 39.Kuroda T., Tada M., Kubota H., Kimura H., Hatano S., Suemori H., Nakatsuji N., Tada T. Mol Cell Biol. 2005;25(6):2475–2485. doi: 10.1128/MCB.25.6.2475-2485.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phinney D.G., Kopen G., Isaacson R.L., Prockop D.J. J Cell Biochem. 1999;72(4):570–585. [PubMed] [Google Scholar]
- 41.Yoo JY, Jeong JW, Fazleabas AT, Tayade C, Young SL, Lessey BA. Biol Reprod 2016;95(1):Article–11. [DOI] [PMC free article] [PubMed]
- 42.Niwa H., Miyazaki J., Smith A.G. Nat Genet. 2000;24(4):372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- 43.Fong H., Hohenstein K.A., Donovan P.J. Stem Cells. 2008;26(8):1931–1938. doi: 10.1634/stemcells.2007-1002. [DOI] [PubMed] [Google Scholar]
- 44.Niwa H., Burdon T., Chambers I., Smith A. Genes Dev. 1998;12(13):2048–2060. doi: 10.1101/gad.12.13.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moggio A., Pittatore G., Cassoni P., Marchino G.L., Revelli A., Bussolati B. Fertil Steril. 2012;98(6):1521–1530. doi: 10.1016/j.fertnstert.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Matsuda T., Nakamura T., Nakao K., Arai T., Katsuki M., Heike T., Yokota T. EMBO J. 1999;18(15):4261–4269. doi: 10.1093/emboj/18.15.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gkouveris I., Nikitakis N., Sauk J. J Can Therapy. 2015;6(08):709. [Google Scholar]
- 48.Sasson I.E., Taylor H.S. Ann NY Acad Sci. 2008;1127(1):106–115. doi: 10.1196/annals.1434.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ulukus M. Women’s Health. 2015;11(5):587–595. doi: 10.2217/whe.15.43. [DOI] [PubMed] [Google Scholar]
- 50.Perez-Moreno M., Jamora C., Fuchs E. Cell. 2003;112(4):535–548. doi: 10.1016/s0092-8674(03)00108-9. [DOI] [PubMed] [Google Scholar]
- 51.Smalley M.J., Clarke R.B. J Mammary Gland Biol Neoplasia. 2005;10(1):37–47. doi: 10.1007/s10911-005-2539-0. [DOI] [PubMed] [Google Scholar]
- 52.Vinatier D., Orazi G., Cosson M., Dufour P. Eur J Obstet Gynecol Reprod Biol. 2001;96(1):21–34. doi: 10.1016/s0301-2115(00)00405-x. [DOI] [PubMed] [Google Scholar]
- 53.Sourial S., Tempest N., Hapangama D.K. Int J Reprod Med. 2014;2014 doi: 10.1155/2014/179515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Socolov R., Butureanu S., Angioni S., Sindilar A., Boiculese L., Cozma L., Socolov D. Eur J Obstet Gynecol Reprod Biol. 2011;154(2):215–217. doi: 10.1016/j.ejogrb.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 55.Lee T.I., Young R.A. Annu Rev Genet. 2000;34(1):77–137. doi: 10.1146/annurev.genet.34.1.77. [DOI] [PubMed] [Google Scholar]
- 56.Kelly K.F., Ng D.Y., Jayakumaran G., Wood G.A., Koide H., Doble B.W. Cell Stem Cell. 2011;8(2):214–227. doi: 10.1016/j.stem.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]