Abstract
Maternal prenatal symptoms of depression and anxiety have been suggested to impose differential effects on later offspring development, depending on their characteristics, such as timing, intensity and persistence. Paternal symptoms have been less investigated. While knowledge on these trajectory characteristics is essential for improved comprehension of prenatal stress, prospective studies including both expecting parents have been scarce. We aim at identifying and comparing the trajectories of prenatal depressive and anxiety symptoms in both parents in a pregnancy cohort design. The sample included 3202 mothers and 2076 fathers who were recruited to the FinnBrain Birth Cohort study (www.finnbrain.fi). Depressive symptoms were assessed using the Edinburgh Postnatal Depression Scale (EPDS) and general anxiety by the anxiety scale of the Symptom Checklist -90 (SCL-90) repeatedly at 14, 24, and 34 gestational weeks. Five differential depressive and four anxiety symptom trajectories were identified across pregnancy both in mothers and in fathers. The trajectories of consistently low depressive or anxiety symptoms were associated with higher educational level in both parents, and with nulliparity and non-smoking during pregnancy in mothers. Parents with consistently high or increasing levels of symptoms had more often prenatal SSRI medication. The congruences between elevated depressive and anxiety symptoms at any point in pregnancy, as well as parental trajectories within families were low. However, in this population-based sample, the self-reported symptom levels of both parents were generally very low. Variance in timing and persistence of parent-reported prenatal depressive and anxiety symptoms is potentially important, while symptom trajectories are very similar in mothers and fathers. These differential symptom trajectories and the significance of their correlates should be acknowledged when studying prenatal stress exposures and the related outcomes in children.
Introduction
Maternal psychiatric symptoms during pregnancy reportedly have diverse and often adverse influences on child development, and ultimately on offspring adult health [1–5]. Paternal symptoms during the pre- and postnatal period and their associations with offspring development have been investigated to a lesser extent [6–8]. Depression and anxiety are the most common psychiatric symptoms during the prenatal and early postnatal periods both in women as well as men. Women commonly present with more pronounced depressive and anxiety symptomatology than men, also during pregnancy [9,10]. Clinically significant prenatal depressive symptoms affect approximately 10–20% of mothers and 2–13% of fathers [8,10–16]. The prevalence of prenatal anxiety symptoms in different studies varies from 9.5% to 29% in mothers and from 8% to 14% in fathers [9,10,15,17,18]. The variation in prevalence estimates is partly explained by the timing of the measurement and the method of assessment. Many background factors, such as history of previous psychiatric disorders, low socioeconomic status and pregnancy-related somatic risk factors increase the risk for prenatal depression or anxiety [19,20]. Comorbidity between depression and anxiety has shown to be high also during pregnancy [15,19,21]. However, depression and anxiety are also partially distinct conditions, supported by the notions of differential symptom course trajectories [22], clinical prognostic factors [22,23] and pathophysiology [24]. Furthermore, previous evidence suggests that prenatal anxiety and depressive symptoms may also have differences in their biological correlates [25] as well as distinct effects on fetal development [2,26]. Therefore, parental anxiety and depression should be studied independently, especially in the context of prenatal fetal exposures.
Previous reports on the longitudinal course of parental prenatal depressive and anxiety symptoms comprising both parents have been scarce and inconsistent. In several studies, depressive symptoms have shown to decline throughout the prenatal period and onwards, both in mothers and in fathers [9,10] However, one recent study reported highest levels of depressive symptoms during the first and third trimesters, in mothers [27]. Similarly, prenatal anxiety symptoms have shown to follow a U-shaped pattern with highest levels of symptoms during the first and third trimesters in both parents [9,10,27]. On the other hand, some studies have reported the peak in paternal anxiety symptoms to occur at mid-pregnancy, where after anxiety would decrease steadily and across the postpartum period [28,29]. As a result of these discrepancies, it has been suggested that the timing and duration of depressive and anxiety symptoms in the prenatal period do not follow a uniform course, implying considerable heterogeneity in symptoms between individuals [30,31]. This has important implications for the concept of prenatal stress exposure and for studies investigating offspring outcomes related to parental symptoms. By studying symptom trajectories, more detailed information on the individual differences in timing, intensity and consistency of parental symptoms could be identified.
Parental mental health trajectories have previously been studied mainly during the transition from the third trimester of pregnancy to postpartum or from the perinatal period into childhood years [32–36]. Studies based on general population samples concur in that most parents report only minimal or mild symptoms across the prenatal period and postpartum [33–37]. In addition, most studies have identified a group of continuously high depressive or anxiety symptom levels [32–37]. These studies have mainly included only one prenatal symptom assessment, usually during the third trimester [8,32,34–36]. However, the importance of investigating the courses of depressive and anxiety symptoms across trimesters has been highlighted in several reports on the negative effects of prenatal psychiatric symptoms on child development and well-being [5,38]. Identifying differential courses of symptoms during pregnancy may provide a basis for a more detailed investigation of diverse offspring outcomes, related to variation in timing and chronicity of exposure to prenatal psychological distress.
Despite the strong evidence that fathers have a crucial role in child well-being [39], the research on parental mental health trajectories has mainly focused on mothers. A few studies have suggested that elevated paternal depressive or anxiety symptoms during transition from the third pregnancy trimester to the postpartum period increase the risk for later problems in parenting [26] and child socio-emotional development [7,8]. The mechanisms behind the possible effects of maternal and paternal psychiatric symptoms on child’s outcome are at least partially different [8,40,41]. Maternal depression or anxiety during pregnancy has suggested to affect the child mainly through different intrauterine processes, including HPA-axis activity, functioning of the placenta, changes in the immunological milieu, or nutritional supply [38,42,43]. Instead, the effects of paternal psychiatric symptoms on the child may either be mediated by the mother experiencing higher psychosocial distress due to the father’s psychological distress [40,41] or by potential epigenetic effects of in paternal sperm, resulting in DNA methylation in the offspring [44,45]. Moreover, direct genetic effects are among the potential mechanisms to explain the effects of prenatal distress on child outcomes, for both parents, as child characteristics and prenatal distress experienced by the parents may indicate a shared underlying genotype [46,47]. Overall, very few studies focus on paternal and maternal prenatal psychiatric symptoms simultaneously within the same study. In addition, as far as we know, there are no studies on the trajectories of paternal or spousal psychiatric symptoms covering most of the prenatal period by repeated prospective measurements.
The aim of this study was to analyze the trajectories of prenatal depressive and anxiety symptoms and the associations of these trajectories with sociodemographic factors in both mothers and fathers in a population-based pregnancy cohort. In addition, the aim was to evaluate the congruence of the anxiety and depression trajectories and the congruence between paternal and maternal symptom trajectories inside the families. To our knowledge, this is the first study evaluating the symptom trajectories across trimesters in both mothers and fathers. The study is part of the large FinnBrain Birth Cohort Study that prospectively investigates the long-term effects of early life stress exposures on e.g. brain development.
Methods
Sample
Recruitment for the FinnBrain Birth Cohort Study took place at maternal welfare clinics between December 2011 and April 2015 in the South-Western Hospital District and the Åland Islands in Finland. The study subjects gave their written informed consent. the Ethics Committee of the Hospital District of Southwest Finland approved the study protocol 14th June 2011. The study population (N = 3808 families) comprises of women that attended the free-of-charge ultrasound at the gestational week 12, their children-to-be-born and fathers of the children/partners of the mothers [48]. Verified pregnancy and sufficient knowledge of either Finnish or Swedish (the official languages in Finland) were required for participation. Of those informed about the study (N = 5790), a total of N = 3808 mothers and N = 2623 fathers or other partners of the mothers decided to participate. In this study, only reported fathers of the children participating were included, and other partners of mothers in the study were excluded. Mothers (N = 3202) and fathers of the children (N = 2076) responding to at least one symptom questionnaire during pregnancy were included.
Procedure and measures
After recruitment, the participants filled in a set of self-report questionnaires three times during pregnancy; at gestational weeks (gwks) 14, 24, and 34. The questionnaires comprised of standardized and internationally validated measures and they could be filled in online or sent via postal mail. Prenatal depressive symptoms were evaluated in both parents using the Edinburgh Postnatal Depression Scale (EPDS, 34) at gwks 14, 24 and 34. EPDS is a self-report questionnaire consisting of 10 items. The parent is asked whether she or he has experienced the proposed symptoms during the last two weeks using a 4-point Likert scale. The EPDS has been studied extensively and it is thought to be a valid screen for both pre- and postnatal depression [49–53], also in fathers [54,55]. Prenatal anxiety of both parents was evaluated using the anxiety scale of Symptom Checklist 90 (SCL-90) at gwks 14, 24 and 34. The anxiety subscale of SCL-90 is a reliable and valid measure of anxiety symptoms in both clinical and research settings [56] and consists of 10 items rated from 0 to 5.
The sum scores of the EPDS and SCL-90 anxiety scales were used as continuous variables in the data analyses and they demonstrated good internal consistency (Cronbach’s α’s ranging from .82 to .84 in mothers and .79 to .81 in fathers for EPDS and .83 to .85 in mothers and .83 to .86 in fathers for SCL-90). The background variables i.e. relationship status, educational level, age, parity, and paternal use of serotonin reuptake inhibitors (SSRIs) during the index pregnancy were drawn from the questionnaires filled in at gwk 14. Educational level was categorized into four classes: secondary school (less than 9 years of schooling), high school/vocational education, university/polytechnics degree, or higher (doctoral/licenciate). No family income variable was available. Therefore, only variables describing maternal and paternal education were used as indicators of socioeconomic status. Other background variables i.e. maternal prenatal smoking, the household variable (living together) and maternal use of serotonin reuptake inhibitors (SSRIs) during the index pregnancy, were drawn from the questionnaires filled in at 34 gwks. In all, 80% of the mothers using SSRI at 34 gwks had already used SSRI-medication at 14 gwks. Father’s use of SSRI was measured only at 14 gwks.
The validity of the measures
The measurement validities of EPDS and SCL-90 anxiety subscale were first examined using confirmatory analysis (CFA) that tests the adequacy of the specified relations, whereby indicators are linked to their underlying latent constructs [57]. The following indices indicating fit of the models were used: the Chi-square test, the Root Mean Square Error of Approximation (RMSEA, values close to .06 indicating a good fit), the comparative fit index (CFI, values close to .90 indicating a suitable model), and the standardized root mean square residual (SRMR, values below .08 reflecting good fit) (see [58,59]). Concerning EPDS, the fit indices were acceptable i.e. χ2 (df) = 1781.46 (366), p < .001, CFI = .94, RMSEA = .04, SRMR = .04 for maternal data and χ2 (df) = 894.23 (366), p < .001, CFI = .95, RMSEA = .03, SRMR = .04 for paternal data. Concerning SCL-90 models, the errors of the verbally similar items 5 and 10; 7 and 10 and consecutive items 9 and 10 were allowed to correlate. After this, the fits of the models were acceptable: χ2 (df) = 1667.42 (366), p < .001, CFI = .93, RMSEA = .03, SRMR = .04 for maternal data and χ2 (df) = 1291.005 (366), p < .001, CFI = .90, RMSEA = .04, SRMR = .05 for paternal data.
Statistical analyses
The associations between the means of depressive and anxiety symptom scales at each time point in mothers and in fathers were investigated with zero-order Spearman correlations. As measures of effect sizes, Cohen’s d and partial ƞ2 were used. Structural Equation Modeling in Mplus 6, and latent growth mixture modeling, were conducted to examine the trajectories of maternal and paternal EPDS and SCL-90 symptoms [60]. Latent growth mixture modeling (LGMM) is a method for identifying multiple unobserved sub-populations (latent classes), describing the longitudinal change within each latent class and examining differences in the change among the latent classes [61]. The numbers of latent classes consisting of mothers’ and fathers’ EPDS and SCL-90 scores were determined by increasing the numbers of classes in separate analyses and observing the change in indices. The indices that were used in decision-making were Bayesian Information Criterion (BIC), Akaike Information Criterion (AIC) [60,62], Entropy value (with values closer to 1.0 indicating higher confidence of classification; [63] and posterior probabilities of class membership. Furthermore, the theoretical and clinical interpretability of the class solutions were used as a help in choosing the best model, a step considered necessary in the decision-making in LGMM [64]. Missing values were treated with the Expectation Maximization (EM) method in Mplus. The reliabilities of the final solutions were checked by replicating the analyses with non-imputed datasets (consisting complete data from 2157 mothers and 1475 fathers). The resultant model solutions (with and without EM) were alike. Next, the associations between trajectories and parental age, education, parity and SSRI medication use were investigated using ANOVA (age) and cross-tabulation with χ2 test (education, parity and SSRIs).
Results
Demographics and attrition
Parents with missing questionnaire data over the course of pregnancy had lower educational levels (χ2 (3) = 38.76, p < .001) and were more often single or divorced (χ2 (1) = 22.26, p < .001) than those who filled in the questionnaires at each of the three assessment points. No differences were seen in age, parity or in the level of depressive or anxiety symptoms at 14 gwks between those parents who filled in all questionnaires and the parents with missing questionnaire data. In the study sample, the mothers had higher educational levels and they were younger than the fathers. In addition, mothers reported more depressive and anxiety symptoms at each assessment point compared to fathers. Descriptive statistics and comparisons between mothers and fathers are displayed in Table 1. Maternal depressive (continuous variables) and anxiety symptoms (continuous variables) were intercorrelated at all assessment points during pregnancy (r = .58-.62, p < .001). Similarly, paternal depressive and anxiety symptoms were intercorrelated at all assessment points (r = .58-.59, p < .001).
Table 1. The descriptive statistics on background and study variables in the subgroups of mothers and fathers.
P values and Cohen’s d’s are given for the differences between mothers and fathers.
| Mothers | Fathers | |||
|---|---|---|---|---|
| N = 3202 | N = 2076 | |||
| N (total) = 5278 | Mean (SD) | Mean (SD) | p< | Cohen's d **** |
| Age | 30.92 (4.56) | 32.60 (5.33) | .001* | |
| Marital status (%) | ||||
| In relationship | 90.9 | 89.9 | ||
| Missing | 7.6 | 9.7 | ||
| Parents living together | 93.0 | |||
| Education (%) | ||||
| Primary school | 2.9 | 4.6 | ||
| Secondary | 33.1 | 41.2 | ||
| University | 55.2 | 46.2 | ||
| Higher | 4.1 | 2.6 | ||
| Missing | 4.7 | 5.4 | ||
| Parity (%) | ||||
| Nulliparous | 49.1 | NA | ||
| Multiparous | 45.9 | NA | ||
| Missing | 5.1 | NA | ||
| SSRIs medication (%) | 3.1# | 2.1## | ||
| Smoking | 14.2# | |||
| Anxiety (SCL) | ||||
| 14 gwks | 3.31 (3.91) | 2.45 (3.48) | .001*** | 0.22 |
| 24 gwks | 3.92 (4.26) | 2.66 (3.81) | .001*** | 0.31 |
| 34 gwks | 3.24 (4.00) | 1.94 (3.20) | .001*** | 0.36 |
| Depression (EPDS) | ||||
| 14 gwks | 5.17 (4.02) | 3.74 (3.42) | .001*** | 0.38 |
| 24 gwks | 5.00 (4.12) | 3.50 (3.47) | .001*** | 0.40 |
| 34 gwks | 4.91 (4.10) | 3.13 (3.38) | .001*** | 0.47 |
* t-test
** χ2 -test
***logistic regression
**** , #3rd pregnancy trimester
## 1st pregnancy trimester
Cohen`s d effect size d > .20 = small; d > .50 = medium; d > .80 = large.
The trajectories of maternal and paternal prenatal depressive and anxiety symptoms
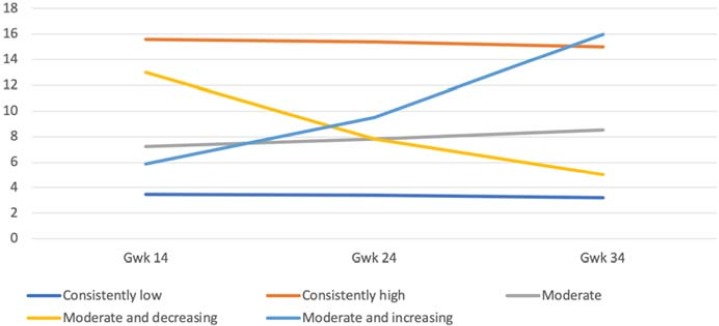
Different class solutions were examined to obtain the best fit of the model for maternal and paternal prenatal depressive and anxiety symptoms. Regarding maternal depressive symptoms the four- and five-class-solutions resembled each other in terms of statistical indices, but the five-class solution had a better fit with the assumption of trajectories and it was thus chosen (Fig 1). The resultant fit indices are presented in Table 2. The five-class solution yielded a large group of mothers (N = 2157, 67%) with consistently low and only slightly decreasing symptoms throughout pregnancy (M = 3.40; Estimate = –0.31, p < .001). Estimate refers to the change in symptoms in each group over the course of pregnancy. Two smaller groups were extracted: mothers (N = 67; 2%) who had consistently high (M = 15.86; Estimate = –0.81, p = .11) symptom levels and a group of mothers (N = 72, 2%) with moderate and increasing symptoms (M = 5.67; Estimate = 4.92, p < .001). The fourth group (N = 773, 24%) had moderate and relatively stable symptoms (M = 7.37; Estimate = 0.67, p < .01). The fifth group (N = 131, 4%) had an initially high symptom score indicating a potentially clinically relevant level of symptoms (M = 12.44) in early pregnancy, but the level of symptoms decreased to a subthreshold level (Estimate = –3.96, p < .001) towards the end of pregnancy.
Fig 1. The five-class solution of the trajectories of maternal depressive symptoms during pregnancy.
Table 2. The growth mixture model indices: Trajectories of maternal depressive symptoms during pregnancy.
| AIC | BIC | Entropy | Class Proportions | Average Latent Class Posterior Probabilities | |
|---|---|---|---|---|---|
| EPDS | |||||
| 1 Class | 44516.29 | 44564.85 | 1.000 | 1.000 | 1.000 |
| 2 Class | 44161.04 | 44227.82 | .73 | .16/.84 | .83/.94 |
| 3 Class | 43908.77 | 43993.76 | .77 | .83/.08/.09 | .93/.78/.78 |
| 4 Class | 43765.96 | 43869.15 | .79 | .74/.05/.02/.20 | .91/.80/.87/.80 |
| 5 Class | 43626.62 | 43748.04 | .79 | .67/.02/.24/.04/.02 | .91/.79/.78/.74/.83 |
| 6 Class | 43555.34 | 43694.97 | .77 | .02/.01/.13/.69/.03/.12 | .84/.77/.68/.89/.79/.70 |
BIC = Bayesian Information Criterion, AIC = Akaike Information Criterion
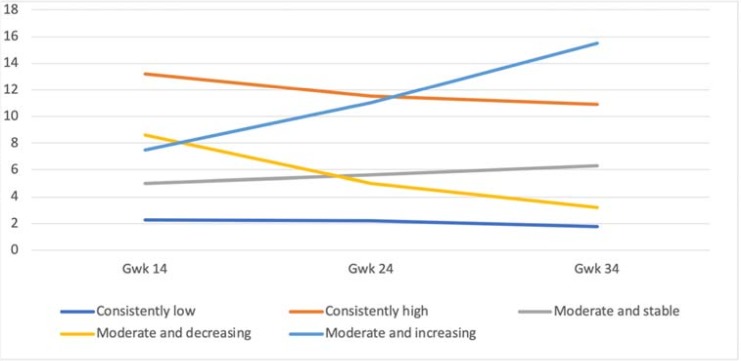
In fathers, the five-group solution yielded similar results as with mothers (Fig 2). The resultant fit indices are presented in Table 3. A large group of fathers with consistently low depressive symptom scores (N = 1475, 71%, M = 2.24; Estimate = –0.30, p < .001) was identified, and a smaller group with consistently high symptom scores (N = 66, 3%, M = 13.18; Estimate = –1.43, p < .001). Three other groups were divided into those with moderate and stable (N = 307, 15%, M = 5.03; Estimate = 0.90, p < .001), moderate and decreasing (N = 185; 9%; M = 8.61; Estimate = –0.29, p < .001), and moderate and increasing symptom scores (N = 42; 2%; M = 7.50; Estimate = 3.85, p < .001). Although this solution did not reach as optimal a fit based on statistical indices as the other solutions, it was chosen because it was replicated as completely similar with the non-imputed data.
Fig 2. The five-class solution of the trajectories of paternal depressive symptom during pregnancy.
Table 3. The growth mixture model indices: Trajectories of paternal depressive symptoms during pregnancy.
| AIC | BIC | Entropy | Class Proportions | Average Latent Class Posterior Probabilities | |
|---|---|---|---|---|---|
| EPDS | |||||
| 1 Class | 24975.14 | 25020.24 | 1.000 | 1.000 | 1.000 |
| 2 Class | 24511.83 | 24573.84 | .88 | .92/.09 | .98/.88 |
| 3 Class | 24304.56 | 24383.51 | .87 | .88/.04/.08 | .96/.89/.83 |
| 4 Class | 24161.38 | 24257.22 | .84 | .02/.79/.05/.14 | .88/.94/.82/.80 |
| 5 Class | 24013.63 | 24126.38 | .81 | .15/.71/.03/.02/.09 | .76/.92/.84/.90/.74 |
| 6 Class | 23926.68 | 24056.34 | .81 | .17/.68/.03/.01/.08/.03 | .76/.91/.83/.95/.73/.87 |
BIC = Bayesian Information Criterion, AIC = Akaike Information Criterion
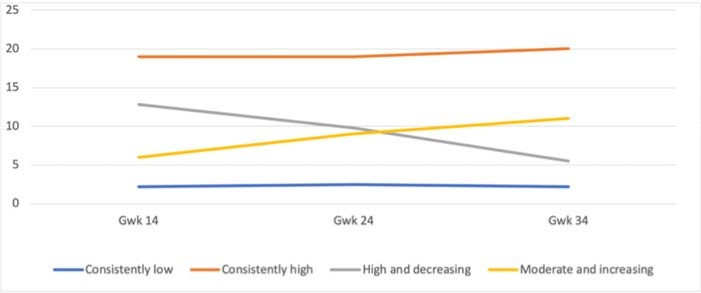
For the maternal anxiety symptom trajectories, the four-class solution was chosen, as the five-class solution did not provide any additional information on the course of symptoms (Fig 3). The resultant fit indices are presented in Table 4. The solution comprised one large group with consistently low symptoms (N = 2718, 85%; M = 2.20; Estimate– 0.08, p = .06) and three smaller groups with high or fluctuating symptoms. The smaller groups included mothers with consistently high symptom scores throughout the pregnancy (N = 27; 1%; M = 19.25, Estimate = 0.36, p = 0.64), mothers with high and decreasing symptom scores (N = 213; 7%, M = 12.53; Estimate = –3.49, p < .001) and mothers with moderate and increasing symptom scores (N = 243; 7%, M = 6.37; Estimate = 2.72, p < .001).
Fig 3. The four-class solution of the trajectories of maternal anxiety symptoms during pregnancy.
Table 4. The growth mixture model indices: Trajectories of maternal anxiety symptoms during pregnancy.
| AIC | BIC | Entropy | Class Proportions | Average Latent Class Posterior Probabilities | |
|---|---|---|---|---|---|
| SCL | |||||
| 1 Class | 43839.20 | 43887.77 | 1.000 | 1.000 | 1.000 |
| 2 Class | 42779.04 | 42845.82 | .93 | .08/.92 | .90/.99 |
| 3 Class | 42102.32 | 42187.31 | .92 | .06/.07/.88 | .88/.87/.98 |
| 4 Class | 41809.45 | 41912.66 | .92 | .85/.01/.07/.07 | .97/.96/.86/.88 |
| 5 Class | 41518.84 | 41640.26 | .91 | .82/.01/.02/.09/.07 | .97/.96/.87/.85/.83 |
BIC = Bayesian Information Criterion, AIC = Akaike Information Criterion
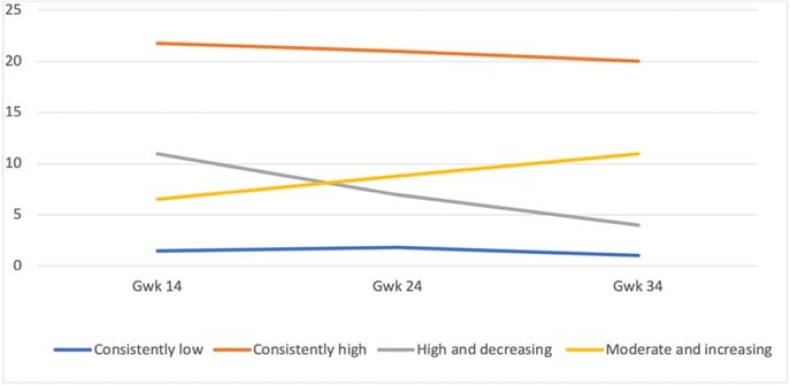
Similarly, to mothers, the five-class solution for the fathers did not provide any additional information on the course of anxiety symptoms, and the four-class solution was thus chosen (Fig 4). The resultant fit indices are presented in Table 5. One large group emerged with a consistently low symptom level (N = 1843; 89%, M = 1.57; Estimate = –0.17, p < .001). Further, a very small group with consistently high symptom counts throughout the pregnancy (N = 16; 1%; M = 22.06; Estimate = –1.30, p = .086) was identified. However, this group consisted of only 16 fathers. Two other small groups had either high and decreasing (N = 108; 5%; M = 10.58, Estimate = –3.45, p < .001) or moderate and increasing symptom levels (N = 109; 5%; M = 6.19, Estimate = 2.64, p < .001).
Fig 4. The four-class solution of the trajectories of paternal anxiety symptoms during pregnancy.
Table 5. The growth mixture model indices: Trajectories of paternal anxiety symptoms during pregnancy.
| AIC | BIC | Entropy | Class Proportions | Average Latent Class Posterior Probabilities | |
|---|---|---|---|---|---|
| SCL | |||||
| 1 Class | 25045.32 | 25090.42 | 1.000 | 1.000 | 1.000 |
| 2 Class | 24101.10 | 24163.12 | .96 | .95/.05 | .99/.93 |
| 3 Class | 23685.54 | 23764.48 | .95 | .91/.04/.05 | .99/.88/.88 |
| 4 Class | 23296.80 | 23392.65 | .94 | .01/.89/.05/.05 | 1.00/.98/.85/.91 |
| 5 Class | 23055.91 | 23168.67 | .91 | .03/.04/.01/.84/.09 | .83/.91/.99/.97/.80 |
BIC = Bayesian Information Criterion, AIC = Akaike Information Criterion
The associations between symptom trajectories and sociodemographic factors
The trajectories of maternal prenatal depressive symptoms differed in parity, household (living together) and smoking during pregnancy, and in educational level as well as in the use of SSRI medication at 34 gestational weeks in both parents. The mothers in the group of consistently low depressive symptoms included a lower proportion of mothers with lower education compared with the other depressive symptom groups mentioned above (χ2 (12) = 60.58, p < .001). In addition, the fathers who had consistently high depressive symptoms had more often a lower educational level than the fathers in the other groups (χ2 (12) = 23.36, p = .03). In addition, higher numbers of both mothers (3rd trimester) and fathers (1st trimester) with SSRI medication were observed in the groups of consistently high depressive symptoms and in the group of moderate and increasing depressive symptoms (Mothers: χ2 (3) = 59.1, p < .001, Fathers: χ2 (3) = 35.8, p < .001) compared to parents in other groups. In addition, mothers in the group of consistently low depressive symptoms were more often primiparous (χ2 (4) = 12.91, p = .012), non-smoking at the third trimester (χ2 (12) = 30.31, p = .001), and were more often living together with the father of the child (χ2 (4) = 34.51, p< .001) compared to the other trajectory groups.
The trajectory groups of prenatal anxiety symptoms differed in parity, household (living together) and smoking during pregnancy in mothers and in age, educational level, and in the use of SSRI medication at 34 gestational weeks in both parents. Mothers with consistently low levels of anxiety were, on average, older (F (3, 3197) = 11.28, p < .001), were living more often with the father of their child (χ2 (3) = 30.22, p < .001) and smoked less often during the third pregnancy trimester (χ2 (3) = 58.9, p = < .001) than the mothers in all the other groups. In addition, mothers in the groups with increasing or consistently low symptoms had higher educational levels than mothers in both the groups of consistently high as well as moderate and decreasing anxiety (χ2 (9) = 52.58, p < .001). Moreover, mothers with consistently high symptom levels were more often multiparous (χ2 (3) = 12.24, p < .01) compared to the mothers with increasing and decreasing symptoms. Fathers with decreasing anxiety symptoms were younger than the fathers in the other groups (F (3, 2066) = 5.02, p < .01). In addition, the groups with either consistently high, increasing or decreasing symptoms comprised of more fathers with lower education than the group with consistently low symptoms (χ2 (9) = 45.60, p < .001). Furthermore, higher numbers of both mothers (26%, χ2 (3) = 85.3, p < .001) and fathers (21%, χ2 (3) = 35.1, p < .001) using SSRIs during pregnancy were seen in the group of consistently high symptoms compared to parents in all the other trajectory groups of anxiety. Descriptive values of the associations between trajectories and all background variables are shown in Table 6.
Table 6. Associations between different trajectories and background factors.
| Mothers n = 3202 |
EPDS Low and stable (n = 2157) |
EPDS High and stable (n = 67) |
EPDS Moderate (n = 773) |
EPDS Decreasing (n = 131) |
EPDS Increasing (n = 72) |
SCL Low and stable (n = 2718) |
SCL High (n = 27) |
SCL High and decreasing (n = 243) |
SCL Moderate and increasing (n = 213) |
Whole sample |
| Age | 31.1 (4.4) | 30.4 (5.1) | 30.9 (4.6) | 30.7 (4.7) | 30.5 (4.9) | 31.10 (4.9) | 28.4 (5.3) | 30.2 (4.6) | 29.7 (5.0) | 30.9 (4.6) |
| Education | ||||||||||
| Secondary school or less | 2.3% | 11.7% | 3.1% | 3.7% | 6.2% | 2.3% | 12.5% | 6.2% | 6.9% | 3.0% |
| High school/vocational | 31.7% | 45.0% | 36.9% | 41.1% | 41.1% | 33.5% | 50.0% | 37.6% | 46.0% | 34.7% |
| University/Polytechnic | 61.3% | 38.3% | 52.3% | 48.8% | 48.8% | 59.8% | 33.3% | 52.2% | 44.1% | 58.0% |
| Doctoral/Licensiate | 4.6% | 5.0% | 7.7% | 3.9% | 3.9% | 4.4% | 4.2% | 4.0% | 3.0% | 4.3% |
| Nulliparity | 53.9% | 45.8% | 46.9% | 46.6% | 50.0% | 50.8% | 41.7% | 53.4% | 62.9% | 51.7% |
| SSRI-medication at 3rd tri | 1.6% | 14.6% | 4.8% | 5.1% | 11.3% | 1.9% | 26.1% | 8.3% | 8.9% | 3.1% |
| Smoking at 3rd tri | 3.98%% | 17.3% | 9.6% | 16.0% | 9.7% | 4.6% | 18.5% | 9.9% | 13.1% | 5.7% |
| Living together with father | 97% | 94% | 95% | 90% | 92% | 97% | 89% | 92% | 92% | 96% |
| Fathers n = 2076 |
EPDS Low and stable (n = 1475) |
EPDS High and stable (n = 66) |
EPDS Moderate (n = 307) |
EPDS Decreasing (n = 185) |
EPDS Increasing (n = 42) |
SCL Low and stable (n = 1470) |
SCL High (n = 16) |
SCL High and decreasing (n = 108) |
SCL Moderate and increasing (n = 109) |
Whole sample |
| Age | 32.6 (5.3) | 33.1 (5.3) | 32.6 (5.4) | 32.4(6.2) | 32.5(5.3) | 32.7 (5.3) | 31.6 (5.1) | 30.9 (5.0) | 32.0 (5.7) | 32.6 (5.3) |
| Education | ||||||||||
| Secondary school or less | 3.8% | 9.5% | 7.2% | 6.2% | 10.8% | 3.9% | 14.3% | 10.5% | 13.1% | 4.8% |
| High school/vocational | 42.6% | 50.8% | 46.8% | 44.9% | 40.5% | 42.8% | 64.3% | 44.8% | 52.3% | 43.6% |
| University/Polytechnic | 50.5% | 39.7% | 44.7% | 46.1% | 48.6% | 50.2% | 21.4% | 44.8% | 34.6% | 48.9% |
| Doctoral/Licensiate | 3.2% | 0.0% | 1.4% | 2.8% | 0.0% | 3.1% | 0.0% | 0.0% | 0.0% | 2.7% |
| SSRI -medication at 1st tri | 1.3% | 10.9% | 2.8% | 2.27% | 8.1% | 1.6% | 21.4% | 3.8% | 5.8% | 2.1% |
Congruence between depressive and anxiety symptom trajectories and between maternal and paternal symptom trajectories
The congruences between the trajectories of depressive and anxiety symptoms was studied using cross-tabulation, and the results are presented in Table 7. Most mothers and fathers with consistently low depressive symptoms belonged to the group of consistently low anxiety symptoms. Furthermore, the congruence between increasing depressive and anxiety symptom trajectories was relatively high. The most incongruent distributions found between the groups of consistently high levels of depressive and anxiety symptoms, both in mothers as well as in fathers.
Table 7. Congruence (%) between depressive and anxiety symptom trajectories.
| Mothers | |||
|---|---|---|---|
| EPDS consistently low | EPDS consistently low | ||
| SCL consistently low | 96 | SCL consistently low | 98 |
| SCL consistently high | 0 | SCL consistently high | 0 |
| SCL moderate, increasing | 2 | SCL moderate, increasing | 1 |
| SCL high, decreasing | 2 | SCL high, decreasing | 1 |
| EPDS consistently high | EPDS consistently high | ||
| SCL consistently low | 19 | SCL consistently low | 28 |
| SCL consistently high | 22 | SCL consistently high | 15 |
| SCL moderate, increasing | 24 | SCL moderate, increasing | 26 |
| SCL high, decreasing | 35 | SCL high, decreasing | 30 |
| EPDS moderate and stable | EPDS moderate and stable | ||
| SCL consistently low | 69 | SCL consistently low | 79 |
| SCL consistently high | 1 | SCL consistently high | 0.5 |
| SCL moderate, increasing | 18 | SCL moderate, increasing | 14 |
| SCL high, decreasing | 12 | SCL high, decreasing | 7 |
| EPDS moderate, decreasing | EPDS moderate, decreasing | ||
| SCL consistently low | 52 | SCL consistently low | 67 |
| SCL consistently high | 3 | SCL consistently high | 1 |
| SCL moderate, increasing | 8 | SCL moderate, increasing | 8 |
| SCL high, decreasing | 37 | SCL high, decreasing | 24 |
| EPDS moderate, increasing | EPDS moderate, increasing | ||
| SCL consistently low | 38 | SCL consistently low | 33 |
| SCL consistently high | 3 | SCL consistently high | 7 |
| SCL moderate, increasing | 57 | SCL moderate, increasing | 55 |
| SCL decreasing | 1 | SCL high, decreasing | 5 |
Mothers: χ2 (12) = 1346.36, p < .001, partial η2 = 0.44; Fathers: χ2 (12) = 885.22, p < .001, partial η2 = 0.43;
The overlap of maternal and paternal trajectories within families was examined using cross tabulation (Tables 8 & 9). Notable congruence between mothers and fathers was found only between the consistently low symptom trajectories, i.e. the most prevalent categories in this study population. In families where mothers reported consistently low levels of depressive or anxiety symptoms throughout the pregnancy, the fathers also reported low levels of depressive or anxiety symptoms throughout the pregnancy. The congruence between mothers and fathers in the other depressive or anxiety symptom trajectory groups was generally low.
Table 8. The congruence (%) between maternal and paternal trajectories of depressive symptoms.
| Depressive symptoms EPDS | Consistently low Father |
Consistently high Father | Moderate Father |
Moderate, decreasing Father |
Moderate, increasing Father |
|---|---|---|---|---|---|
| Consistently low Mother | 51 | 1.9 | 9.4 | 5.5 | 1.1 |
| Consistently high Mother | 1.0 | 0.2 | 0.3 | 0.2 | 0.2 |
| Moderate Mother |
15.6 | 0.8 | 4 | 2.3 | 0.5 |
| Moderate, decreasing Mother | 2.0 | 0.2 | 0.9 | 0.5 | 0.1 |
| Moderate, increasing Mother | 1.3 | 0.1 | 0.3 | 0.3 | 0 |
χ2 (16) = 55.81, p < .001, partial η2 = 0.10
Table 9. The congruence (%) between maternal and paternal trajectories of anxiety symptoms.
| Anxiety symptoms SCL |
Consistently low Father |
Consistently high Father |
Moderate, increasing Father |
High, decreasing, Father |
|---|---|---|---|---|
| Mother Consistently low |
77 | 0.7 | 4.0 | 3.9 |
| Mother Consistently high |
0.6 | 0 | 0 | 0.1 |
| Mother Moderate increasing |
5.9 | 0 | 0.7 | 0.5 |
| Mother High decreasing |
5.2 | 0 | 0.5 | 0.7 |
χ2 (9) = 31.80, p < .001, partial η2 = 0.10
Discussion
In the present study, we identified five different trajectories describing the course of prenatal depressive symptoms both in mothers and in fathers. Regarding the prenatal anxiety symptoms, four different trajectories were identified, equally in both parents. The five trajectories of prenatal depressive symptoms included the following groups: consistently low levels of symptoms, consistently high levels of symptoms, moderate levels of symptoms, moderate and decreasing levels of symptoms and moderate and increasing levels of symptoms. Regarding prenatal anxiety symptoms, the four trajectories were: consistently low levels of symptoms, consistently high levels of symptoms, high and decreasing levels of symptoms and moderate and increasing levels of symptoms.
Our findings are in line with previous trajectory studies indicating 3 to 6 prenatal parental psychiatric symptom trajectories, including at least one trajectory of chronically high symptoms and one trajectory of consistently low symptom levels during the transition from pregnancy to early childhood [32,34,37]. Our findings suggest that potentially important variance exists in the timing and persistence of parent-reported depressive and anxiety symptoms, already during the prenatal period. This should be considered when prenatal exposures are studied, and when child outcomes related to both maternal and paternal symptoms are investigated. Our findings also indicate that higher educational level and older age may be associated with consistently low levels of prenatal psychiatric symptoms in both parents. In mothers, consistently low symptom levels were related to primiparity, not smoking at the 3rd trimester, and living together with the father of the child. Consistently elevated symptom levels were related to the use of SSRI medication in both parents. The congruence between the parents’ trajectories inside families as well as between the trajectories of depressive and anxiety symptoms were relatively low suggesting that the trajectories of depressive and anxiety symptoms have partially independent courses within child-expecting families. However, the congruence in consistently low symptom levels between mothers and fathers within families, as well as between anxiety and depressive symptoms, was notable. This could be explained by the preponderance of the low symptom category in this generally healthy population-based cohort sample.
Approximately 85–90% of the parents in the FinnBrain Cohort reported low levels of depressive or anxiety symptoms at any trimester. This is in line with several previous studies showing that most parents in the general population report only minimal or mild symptoms during pregnancy and the postpartum period [32,33,35,36]. Mothers with low levels of depressive or anxiety symptoms were more often living with the father of the child, smoked less frequently, and were expecting their first child more often than mothers with high or changing levels of depressive or anxiety symptoms. While it has been recognized earlier that parental psychiatric symptoms often co-occur with other risk factors, our notion of several known sociodemographic risk factors accumulating especially in certain symptom trajectories deserves further attention. Thus, it may be that not only the chronicity of symptoms but also the affiliated risk factors play an important role when the offspring outcomes are considered.
Only 1–2% of the parents in our sample experienced consistently high levels of depressive or anxiety symptoms throughout the pregnancy. In line with previous studies, these parents were also more likely to have lower education and more previous children, suggesting that, among other risk factors, socioeconomic burden and the demands of everyday life are associated with the emergence of parental psychiatric symptoms not only during the postnatal but also during the prenatal period [65,66]. These parents had also more often SSRI medication, which might reflect the primary severity of their symptoms. Clinically, it would be essential to identify these parents of consistently high levels of depressive or anxiety symptoms, as parents with chronically pronounced psychiatric symptoms reportedly form an evident risk for the offspring as well as for parenting problems and family adversity [5,67,68]. In addition, chronically elevated symptom levels during pregnancy may reflect prolonged (i.e. covering both prenatal and postnatal time periods) psychiatric problems and, based on previous research, strongly predict postpartum depression [69–72]. Further, 24% of mothers and 16% of fathers in our study reported moderate levels (i.e. differential trajectory to those with consistently low symptom levels) of depressive symptoms throughout pregnancy. In addition to identifying the chronically highly depressed and anxious parents, it would also be important to recognize the parents reporting subclinical levels of depressive or anxiety symptoms, especially if they appear as chronic. Recently, it has been emphasized that also parental subclinical, especially chronic, psychiatric symptoms might have negative associations with future child well-being [73–74].
Furthermore, a group of parents reporting decreasing symptom levels was identified, that presented high symptom scores during the first trimester but very low levels of depressive or anxiety symptoms during the third trimester. This decrease in symptoms may reflect a normative psychological adaptation to pregnancy and parenthood, as the first trimester often includes worries and stress due to the uncertainty of the pregnancy, whereas the second trimester is seen as a period of stability, and the third trimester as a period of positive expectations relating to the upcoming birth and parenthood [75]. By contrast, the groups with increasing levels of depressive or anxiety symptoms, including parents with a low level of symptoms in the first trimester but moderate or high levels of symptoms in the third trimester, may reflect a specific psychiatric symptomatology relating to the upcoming birth, parenthood or concerns about the health of the child. High levels of symptoms before birth are reported to have a negative effect on the child through a prenatal programming effect [76–78], or through postnatal factors including more vulnerable parenting behavior transferring from the antenatal period [76,77,79]. Still, only few studies, to date, have considered the longitudinal course of parental symptoms as a factor in pre- and postnatal programming. Further research is needed to explore the mechanisms underlying the different trajectories and to identify how variation in timing and chronicity affect specified child and parent outcomes.
Overall, the mothers in our sample reported more depressive and anxiety symptoms during pregnancy than the fathers, which is in line with previous studies reporting that in general, depressive and anxiety symptoms are more prevalent among females than males both in the prenatal period and overall [9,10]. On the other hand, within the groups of elevated anxiety symptom levels, mothers had lower symptom scores than fathers, suggesting that fathers experiencing psychological distress may represent a relatively severely anxious population. Interestingly, the trajectory solutions of depressive and anxiety symptoms were very similar between mothers and fathers, suggesting that the differences in trajectories are not sex-specific, but rather may depend on other factors that may be more related to the pathophysiologies and the courses of depression/anxiety per se [10,32,36].
The findings on the congruence of within-person depressive and anxiety symptom trajectories were only partly supportive of previous reports. Congruence was high between the trajectories of consistently low levels of depressive and anxiety symptoms (the same person is likely to report no symptoms at all), and moderate between the trajectories of increasing symptoms, both in mothers and in fathers. However, the congruence between depressive and anxiety symptoms was low in the groups of both consistently high symptoms and decreasing symptoms suggesting that these trajectories develop independently from one another, in both parents. This supports the previously presented notion that in the context of prenatal and early life stress, parental depressive and anxiety symptoms should be treated independently as stress exposures [47]. The congruence of maternal and paternal depressive and anxiety trajectories within families was also low, suggesting that the trajectories of depressive and anxiety symptoms during the prenatal period develop relatively independently inside the family. However, the congruence between maternal and paternal trajectories was high in the most prevalent categories of consistently low depressive and anxiety symptom levels, which suggests that in the majority of families, neither of the parents experience marked psychological distress. The differential trajectories within families, and the interactions between maternal and paternal symptom courses should be taken into consideration, when related offspring outcomes are investigated.
A few limitations should be pointed out in the present study. First, sample sizes in the groups of high depressive and/or anxiety symptoms levels were relatively low. Drop-outs may partially explain this small number of parents with very high symptoms levels in the study population. On the other hand, imputation in Mplus was used to diminish this bias. Another limitation was that the parents’ symptoms were assessed using self-report questionnaires as opposed to diagnostic interviews that may have yielded more detailed information on mood and anxiety disorder diagnoses. However, the questionnaires employed in this study are widely used and reliable, and have been shown to be robustly in line with clinical assessments [50–53,56]. In the current study, we were not able to examine the mechanisms behind each trajectory, but this remains to be investigated in the follow-up reports after this description of typical symptom courses and their within-family occurrence. Additionally, the necessary next step should be to study the identified trajectories, and more specifically, the significance of timing, intensity and persistence of both maternal and paternal depressive and anxiety symptoms across the antenatal period in relation to offspring well-being and development. Both the independent maternal and paternal effects as well as the interactive effects of spousal symptom levels on child developmental outcomes need further attention.
To the best of our knowledge, this is the first study to evaluate the trajectories of parental depressive and anxiety symptoms during pregnancy in both mothers and in fathers as well as within families, in a large population-based birth cohort setting. Our findings indicate that the longitudinal development of parental depressive and anxiety symptoms during the prenatal period may represent processes independent of parental sex, but more characteristic of the specific psychiatric symptom type. As depressive and anxiety symptoms do not always overlap, they both should be independently targeted in research as well as in clinical work. The simultaneous assessment of both parents during gestation seems warranted also in future research. Importantly, the trajectories found in the present study provide a basis for investigating possible parental and offspring follow-up outcomes in more detail and in a more stratified manner, and for evaluating long-term prognoses of prenatal exposure to either maternal and/or paternal symptoms of depression and anxiety.
Data Availability
We have a strict legal rules in terms of data sharing in the medical faculty at the University of Turku. The anonymized dataset is available upon request. The requests of data can be pointed to statistician Juho Pelto (juho.pelto@utu.fi).
Funding Statement
This study is funded by the Academy of Finland (129839, 134950, 253270, 286829, 287908, 308176, 308252), Emil Aaltonen Foundation, Finnish Medical Association, Jane and Aatos Erkko Foundation, Signe and Ane Gyllenberg Foundation, State Grants for Clinical Research (ERVA) and Yrjö Jahnsson Foundation.
References
- 1.Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: An integrated review of human literature. Psychopharmacology (Berl). 2011;214(1):55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis EP, Snidman N, Wadhwa PD, Glynn LM, Schetter CD, Sandman C a. Prenatal Maternal Anxiety and Depression Predict Negative Behavioral Reactivity in Infancy. Infancy. 2004;Vol 6 (3)(3):319–31. [Google Scholar]
- 3.Nolvi S, Karlsson L, Bridgett DJ, Korja R, Huizink AC, Kataja EL, et al. Maternal prenatal stress and infant emotional reactivity six months postpartum. J Affect Disord [Internet]. 2016;199:163–70. Available from: 10.1016/j.jad.2016.04.020 [DOI] [PubMed] [Google Scholar]
- 4.Korja R, Nolvi S, Grant KA, McMahon C. The Relations Between Maternal Prenatal Anxiety or Stress and Child’s Early Negative Reactivity or Self-Regulation: A Systematic Review. Child Psychiatry Hum Dev [Internet]. 2017;0(0):0. Available from: http://link.springer.com/10.1007/s10578-017-0709-0 [DOI] [PubMed] [Google Scholar]
- 5.Pearson RM, Evans J, Kounali D, Lewis G, Heron J, Ramchandani PG, et al. Maternal Depression During Pregnancy and the Postnatal Period. JAMA Psychiatry [Internet]. 2013;70(12):1312 Available from: http://archpsyc.jamanetwork.com/article.aspx?doi=10.1001/jamapsychiatry.2013.2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramchandani P, Psychogiou L. Paternal psychiatric disorders and children’s psychosocial development. Lancet. 2009;374(9690):646–53. 10.1016/S0140-6736(09)60238-5 [DOI] [PubMed] [Google Scholar]
- 7.Ramchandani PG, Richter LM, Norris S a, Stein A. Maternal prenatal stress and later child behavioral problems in an urban South African setting. J Am Acad Child Adolesc Psychiatry [Internet]. 2010;49(3):239–47. Available from: 10.1016/j.jaac.2009.11.013 [DOI] [PubMed] [Google Scholar]
- 8.Ramchandani P., O’Connor T., Evans J., Heron J.,Murray L., and Stein S. The effects of pre- and postnatal depression in fathers: a natural experiment comparing the effects of exposure to depression on offspring. J Child Psychol Psychiatry [Internet]. 2008;49(10):1069–78. 10.1111/j.1469-7610.2008.02000.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Figueiredo B, Conde A. Anxiety and depression symptoms in women and men from early pregnancy to 3-months postpartum: Parity differences and effects. J Affect Disord. 2011;132(1–2):146–57. 10.1016/j.jad.2011.02.007 [DOI] [PubMed] [Google Scholar]
- 10.Teixeira C, Figueiredo B, Conde A, Pacheco A, Costa R. Anxiety and depression during pregnancy in women and men. J Affect Disord [Internet]. 2009;119(1–3):142–8. Available from: 10.1016/j.jad.2009.03.005 [DOI] [PubMed] [Google Scholar]
- 11.Escribe-Aguir V, Gonzalez-Galarzo MC, Barona-Vilar C, Artazcoz L. Factors related to depression during pregnancy: are there gender differences? J Epidemiol Community Heal. 2008;62(5):410–4. [DOI] [PubMed] [Google Scholar]
- 12.Parfitt Y, Ayers S. Transition to parenthood and mental health in first-time parents. Infant Ment Health J. 2014;35(3):263–73. 10.1002/imhj.21443 [DOI] [PubMed] [Google Scholar]
- 13.Ngai FW, Ngu SF. Predictors of maternal and paternal depressive symptoms at postpartum. J Psychosom Res. 2015;78(2):156–61. 10.1016/j.jpsychores.2014.12.003 [DOI] [PubMed] [Google Scholar]
- 14.Navaratne P, Foo XY, Kumar S. Impact of a high Edinburgh Postnatal Depression Scale score on obstetric and perinatal outcomes. Sci Rep [Internet]. 2016;6(October 2007):33544 Available from: http://www.nature.com/articles/srep33544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skouteris H, Wertheim EH, Rallis S, Milgrom J, Paxton SJ. Depression and anxiety through pregnancy and the early postpartum: An examination of prospective relationships. J Affect Disord [Internet]. 2009;113(3):303–8. Available from: 10.1016/j.jad.2008.06.002 [DOI] [PubMed] [Google Scholar]
- 16.Paulson JF, Bazemore SD. Prenatal and Postpartum Depression in Fathers and Its Association With Maternal Depression. JAMA [Internet]. 2010;303(19):1961 Available from: http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2010.605 [DOI] [PubMed] [Google Scholar]
- 17.Verreault N, Da Costa D, Marchand A, Ireland K, Dritsa M, Khalifé S. Rates and risk factors associated with depressive symptoms during pregnancy and with postpartum onset. J Psychosom Obstet Gynaecol [Internet]. 2014;35(3):84–91. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25123985 [DOI] [PubMed] [Google Scholar]
- 18.Heron J, O’Connor TG, Evans J, Golding J, Glover V. The course of anxiety and depression through pregnancy and the postpartum in a community sample. J Affect Disord. 2004;80(1):65–73. 10.1016/j.jad.2003.08.004 [DOI] [PubMed] [Google Scholar]
- 19.Ibanez G, Charles MA, Forhan A, Magnin G, Thiebaugeorges O, Kaminski M, et al. Depression and anxiety in women during pregnancy and neonatal outcome: Data from the EDEN mother-child cohort. Early Hum Dev [Internet]. 2012;88(8):643–9. Available from: 10.1016/j.earlhumdev.2012.01.014 [DOI] [PubMed] [Google Scholar]
- 20.Field T, Diego M, Hernandez-Reif M, Figueiredo B, Deeds O, Contogeorgos J, et al. Prenatal paternal depression. Infant Behav Dev. 2006;29(4):579–83. 10.1016/j.infbeh.2006.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Field T, Diego M, Hernandez-Reif M, Figueiredo B, Deeds O, Ascencio A, et al. Comorbid depression and anxiety effects on pregnancy and neonatal outcome. Infant Behav Dev. 2010;33(1):23–9. 10.1016/j.infbeh.2009.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Penninx BWJH, Nolen WA, Lamers F, Zitman FG, Smit JH, Spinhoven P, et al. Two-year course of depressive and anxiety disorders: Results from the Netherlands Study of Depression and Anxiety (NESDA). J Affect Disord [Internet]. 2011;133(1–2):76–85. Available from: 10.1016/j.jad.2011.03.027 [DOI] [PubMed] [Google Scholar]
- 23.Almeida CP de, Sá E, Cunha FF, Pires EP. Common mental disorders during pregnancy and baby’s development in the first year of life. J Reprod Infant Psychol [Internet]. 2012;30(4):341–51. Available from: http://www.scopus.com/inward/record.url?eid=2-s2.0-84869161025&partnerID=tZOtx3y1 [Google Scholar]
- 24.Lemche E, Surguladze SA, Brammer MJ, Phillips ML, Sierra M, David AS, et al. Dissociable brain correlates for depression, anxiety, dissociation, and somatization in depersonalization-derealization disorder. CNS Spectr. 2013;21(1):35–42. 10.1017/S1092852913000588 [DOI] [PubMed] [Google Scholar]
- 25.Karlsson L, Nousiainen N, Scheinin NM, Maksimow M, Salmi M, Lehto SM, et al. Cytokine profile and maternal depression and anxiety symptoms in mid-pregnancy—the FinnBrain Birth Cohort Study. Arch Womens Ment Health [Internet]. 2017;20(1):39–48. Available from: 10.1007/s00737-016-0672-y [DOI] [PubMed] [Google Scholar]
- 26.Nolvi S, Karlsson L, Bridgett DJ, Korja R, Huizink AC, Kataja E-L, et al. Maternal prenatal stress and infant emotional reactivity six months postpartum. J Affect Disord. 2016;199:163–70. 10.1016/j.jad.2016.04.020 [DOI] [PubMed] [Google Scholar]
- 27.Pesonen AK, Lahti M, Kuusinen T, Tuovinen S, Villa P, Hämäläinen E, et al. Maternal prenatal positive affect, depressive and anxiety symptoms and birth outcomes: The PREDO study. PLoS One. 2016;11(2):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buist A, Morse CA, Durkin S. Men’s Adjustment to Fatherhood: Implications for Obstetric Health Care. J Obstet Gynecol Neonatal Nurs [Internet]. 2003;32(2):172–80. Available from: 10.1177/0884217503252127 [DOI] [PubMed] [Google Scholar]
- 29.Condon JT, Boyce P, Corkindale CJ. The First-Time Fathers Study: a prospective study of the mental health and wellbeing of men during the transition to parenthood. 2003; [DOI] [PubMed] [Google Scholar]
- 30.Cents R a M, Diamantopoulou S, Hudziak JJ, Jaddoe VW V, Hofman a, Verhulst FC, et al. Trajectories of maternal depressive symptoms predict child problem behaviour: the Generation R study. Psychol Med [Internet]. 2013;43(1):13–25. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22490169 10.1017/S0033291712000657 [DOI] [PubMed] [Google Scholar]
- 31.Hill J, Breen G, Quinn J, Tibu F, Sharp H, Pickles A. Evidence for interplay between genes and maternal stress in utero: Monoamine oxidase A polymorphism moderates effects of life events during pregnancy on infant negative emotionality at 5weeks. Genes, Brain Behav. 2013;12(4):388–96. [DOI] [PubMed] [Google Scholar]
- 32.Vanska M, Punamaki R-L, Tolvanen A, Lindblom J, Flykt M, Unkila-Kallio L, et al. Maternal pre- and postnatal mental health trajectories and child mental health and development: Prospective study in a normative and formerly infertile sample. Int J Behav Dev [Internet]. 2013;35(6):517–31. Available from: http://hpq.sagepub.com/cgi/doi/10.1177/1359105313484781 [Google Scholar]
- 33.Fredriksen E, Soest T Von, Smith L, Moe V, Soest T Von, Smith L. Patterns of Pregnancy and Postpartum Depressive Symptoms: Latent Class Trajectories and Predictors Patterns of Pregnancy and Postpartum Depressive Symptoms: Latent Class Trajectories and Predictors. 2017; Journal of Abnormal Psychology, 26(2):173–183. 10.1037/abn0000246 Epub 2016 Dec 1. [DOI] [PubMed] [Google Scholar]
- 34.Sutter-Dallay AL, Cosnefroy O, Glatigny-Dallay E, Verdoux H, Rascle N. Evolution of perinatal depressive symptoms from pregnancy to two years postpartum in a low-risk sample: The MATQUID cohort. J Affect Disord [Internet]. 2012;139(1):23–9. Available from: 10.1016/j.jad.2011.08.018 [DOI] [PubMed] [Google Scholar]
- 35.Mora PA, Bennett IM, Elo IT, Mathew L, Coyne JC, Culhane JF. Distinct trajectories of perinatal depressive symptomatology: Evidence from growth mixture modeling. Am J Epidemiol. 2009;169(1):24–32. 10.1093/aje/kwn283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vanska M, Punama R, Tolvanen A, Lindblom J, Flykt M, Unkila-kallio L, et al. Paternal mental health trajectory classes and early fathering experiences: Prospective study on a normative and formerly infertile sample. 2016; [Google Scholar]
- 37.Baron E, Bass J, Murray SM, Schneider M, Lund C. A systematic review of growth curve mixture modelling literature investigating trajectories of perinatal depressive symptoms and associated risk factors. J Affect Disord [Internet]. 2017;223(February):194–208. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0165032717303476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Donnell K, O’Connor TG, Glover V. Prenatal stress and neurodevelopment of the child: Focus on the HPA axis and role of the placenta. Dev Neurosci. 2009;31(4):285–92. 10.1159/000216539 [DOI] [PubMed] [Google Scholar]
- 39.Ramchandani P, Psychogiou L. Paternal psychiatric disorders and children’s psychosocial development. Lancet [Internet]. 2009;374(9690):646–53. Available from: 10.1016/S0140-6736(09)60238-5 [DOI] [PubMed] [Google Scholar]
- 40.Liu C, Cnattingius S, Bergström M, Östberg V, Hjern A. Prenatal parental depression and preterm birth: a national cohort study. BJOG An Int J Obstet Gynaecol. 2016;123(12):1973–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Batenburg-Eddes T, Brion MJ, Henrichs J, Jaddoe VWV, Hofman A, Verhulst FC, et al. Parental depressive and anxiety symptoms during pregnancy and attention problems in children: A cross-cohort consistency study. J Child Psychol Psychiatry Allied Discip. 2013;54(5):591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Egliston K-A, McMahon C, Austin M-P. Stress in pregnancy and infant HPA axis function: conceptual and methodological issues relating to the use of salivary cortisol as an outcome measure. Psychoneuroendocrinology. 2007;32(1):1–13. 10.1016/j.psyneuen.2006.10.003 [DOI] [PubMed] [Google Scholar]
- 43.Van den Bergh BRH, van den Heuvel MI, Lahti M, Braeken M, de Rooij SR, Entringer S, et al. Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy. Neurosci Biobehav Rev. 2017;(July). [DOI] [PubMed] [Google Scholar]
- 44.Mychasiuk R, Harker A, Ilnytskyy S, Gibb R. Paternal stress prior to conception alters dna methylation and behavior of developing rat offspring. Neuroscience [Internet]. 2013;241:100–5. Available from: 10.1016/j.neuroscience.2013.03.025 [DOI] [PubMed] [Google Scholar]
- 45.Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. Paternal Stress Exposure Alters Sperm MicroRNA Content and Reprograms Offspring HPA Stress Axis Regulation. 2013;33(21):9003–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glover V. Annual research review: Prenatal stress and the origins of psychopathology: An evolutionary perspective. J Child Psychol Psychiatry Allied Discip. 2011;52(4):356–67. [DOI] [PubMed] [Google Scholar]
- 47.O’Donnell K.J., Meaney MJ. Broader Focus Required to Understand the Effects of the Perinatal Environment on Child Neurodevelopment: Response to Bell and Chimata. Am J Psychiatry. 2017;174(10):999–1000. [DOI] [PubMed] [Google Scholar]
- 48.Karlsson L, Tolvanen M, Scheinin NM, Uusitupa H-M, Korja R, Ekholm E, et al. Cohort profile: The FinnBrain Birth Cohort Study (FinnBrain). Int J Epidemiol. 2017; [DOI] [PubMed] [Google Scholar]
- 49.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987. June;150:782–6. [DOI] [PubMed] [Google Scholar]
- 50.Cox J. Validation of the Edinburgh postnatal depression scale (EPDS) in non-postnatal women. J Affect Disord [Internet]. 1996;39(3):185–9. Available from: http://www.sciencedirect.com/science/article/pii/0165032796000080 [DOI] [PubMed] [Google Scholar]
- 51.Chaudron LH, Wisner KL. Perinatal depression screening: Let’s not throw the baby out with the bath water! J Psychosom Res [Internet]. 2014;76(6):489–91. Available from: 10.1016/j.jpsychores.2014.03.011 [DOI] [PubMed] [Google Scholar]
- 52.Boyce P, Stubbs J, Todd A. The Edinburgh Postnatal Depression Scale: Validation for an Australian Sample. Aust New Zeal J Psychiatry [Internet]. 1993;27(3):472–6. Available from: http://journals.sagepub.com/doi/10.3109/00048679309075805 [DOI] [PubMed] [Google Scholar]
- 53.Eberhard-Gran M, Eskild A, Tambs K, Opjordsmoen S, Samuelsen SO. Review of validation studies of the Edinburgh Postnatal Depression Scale. Acta Psychiatr Scand. 2001;104(4):243–9. [DOI] [PubMed] [Google Scholar]
- 54.Matthey S, Barnett B, Kavanagh DJ, Howie P. Validation of the Edinburgh Postnatal Depression Scale for men, and comparison of item endorsement with their partners. J Affect Disord. 2001;64(2–3):175–84. [DOI] [PubMed] [Google Scholar]
- 55.Massoudi P, Hwang CP, Wickberg B. How well does the Edinburgh Postnatal Depression Scale identify depression and anxiety in fathers? A validation study in a population based Swedish sample. J Affect Disord [Internet]. 2013;149(1–3):67–74. Available from: 10.1016/j.jad.2013.01.005 [DOI] [PubMed] [Google Scholar]
- 56.Derogatis LR, Lipman RS, Covi L. SCL-90: an outpatient psychiatric rating scale—preliminary report. Psychopharmacol Bull. 1973. January;9(1):13–28. [PubMed] [Google Scholar]
- 57.Kline RB. Principles and practice of structural equation modeling. The Guilford Press: New York; 1998. [Google Scholar]
- 58.Hu L, Bentler P. Cutoff criteria for fit indices in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Model. 1999;6:1–55. [Google Scholar]
- 59.Steiger JH. Structural model evaluation and modification: An interval estimation approach. Multivariate Behav Res. 1990;25:173–80. 10.1207/s15327906mbr2502_4 [DOI] [PubMed] [Google Scholar]
- 60.Muthén LK, Muthén BO. Mplus User’s Guide. 1998–2012. Sixth Edit Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- 61.Bryk A.S., Raudenbush SW. Application of hierarchical linear models to assessing change. Psychol Bull. 1987;101:147–158. [Google Scholar]
- 62.Nylund KL, Asparouhov T, Muthén BO. Deciding on the Number of Classes in Latent Class Analysis and Growth Mixture Modeling: A Monte Carlo Simulation Study. Struct Equ Model A Multidiscip J. 2007. October;14(4):535–69. [Google Scholar]
- 63.Jedidi K, Ramaswamy V, Desarbo WS. A maximum likelihood method for latent class regression involving a censored dependent variable. Psychometrika. 1993. September;58(3):375–94. [Google Scholar]
- 64.Ram N. Grimm K. Growth Mixture Modeling: A Method for Identifying Differences in Longitudinal Change Among Unobserved Groups. Int J Behav Dev. 2009;33(6):565–576. 10.1177/0165025409343765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leinonen JA, Solantaus TS, Punamäki R-L. The specific mediating paths between economic hardship and the quality of parenting. Int J Behav Dev [Internet]. 2002;26(5):423–35. Available from: http://www.tandf.co.uk/journals/pp/01650254.html [Google Scholar]
- 66.Elovainio M, Pulkki-Råback L, Jokela M, Kivimäki M, Hintsanen M, Hintsa T, et al. Socioeconomic status and the development of depressive symptoms from childhood to adulthood: a longitudinal analysis across 27 years of follow-up in the Young Finns study. Soc Sci Med [Internet]. 2012;74(6):923–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22305468 [DOI] [PubMed] [Google Scholar]
- 67.Murray L, Arteche A, Fearon P, Halligan S, Goodyer I, Cooper P. Maternal postnatal depression and the development of depression in offspring Up to 16 years of age. J Am Acad Child Adolesc Psychiatry [Internet]. 2011;50(5):460–70. Available from: 10.1016/j.jaac.2011.02.001 [DOI] [PubMed] [Google Scholar]
- 68.Prenoveau JM, Craske MG, West V, Giannakakis A, Zioga M, Lehtonen A, et al. Maternal postnatal depression and anxiety and their association with child emotional negativity and behavior problems at two years. Dev Psychol [Internet]. 2017;53(1):50–62. Available from: http://doi.apa.org/getdoi.cfm?doi=10.1037/dev0000221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vliegen N, Casalin S, Luyten P. The Course of Postpartum Depression. Harv Rev Psychiatry [Internet]. 2014;22(1):1–22. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00023727-201401000-00001 10.1097/HRP.0000000000000013 [DOI] [PubMed] [Google Scholar]
- 70.O’Hara MW, Wisner K. Perinatal mental illness: definition, description and aetiology. Best Pr Res Clin Obs Gynaecol. 2014;28(1):3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morse CA, Buist A, Durkin S. First-time parenthood: influences on pre- and postnatal adjustment in fathers and mothers. J Psychosom Obstet Gynaecol. 2000;21(2):109–20. [DOI] [PubMed] [Google Scholar]
- 72.Matthey S, Barnett B, et al. Paternal and maternal depressed mood during the transition to parenthood. J Affect Disord [Internet]. 2000;60(2):75–85. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS%7B&%7DPAGE=reference%7B&%7DD=med4%7B&%7DNEWS=N%7B&%7DAN=10967366%5Cnhttp://ovidsp.ovid.com/ovidweb.cgi?T=JS%7B&%7DPAGE=reference%7B&%7DD=emed8%7B&%7DNEWS=N%7B&%7DAN=30680124 [DOI] [PubMed] [Google Scholar]
- 73.Sohr-Preston S.L., & Scaramella LV. Implications of timing of maternal depressive symptoms for early cognitive and language development. Clin Child Fam Psychol Rev. 2006;9(65–66). [DOI] [PubMed] [Google Scholar]
- 74.van der Waerden J, Bernard JY, De Agostini M, Saurel-Cubizolles MJ, Peyre H, Heude B, et al. Persistent maternal depressive symptoms trajectories influence children’s IQ: The EDEN mother-child cohort. Depress Anxiety. 2016;(August 2016):105–17. 10.1002/da.22552 [DOI] [PubMed] [Google Scholar]
- 75.Slade Arietta; Cohen Lisa; Sadler Lois; Miller M. The psychology and psychopathology of pregnancy: Reorganization and transformation. In: Handbook of infant mental health. Guilford Press; 2009. p. 22–39. [Google Scholar]
- 76.de Weerth C, Buitelaar JK, Beijers R. Infant cortisol and behavioral habituation to weekly maternal separations: Links with maternal prenatal cortisol and psychosocial stress. Psychoneuroendocrinology [Internet]. 2013;38(12):2863–74. Available from: 10.1016/j.psyneuen.2013.07.014 [DOI] [PubMed] [Google Scholar]
- 77.McMahon CA, Boivin J, Gibson FL, Hammarberg K, Wynter K, Saunders D, et al. Pregnancy-specific anxiety, ART conception and infant temperament at 4 months post-partum. Hum Reprod. 2013;28(4):997–1005. 10.1093/humrep/det029 [DOI] [PubMed] [Google Scholar]
- 78.Austin MP, Hadzi-Pavlovic D, Leader L, Saint K, Parker G. Maternal trait anxiety, depression and life event stress in pregnancy: Relationships with infant temperament. Early Hum Dev. 2005;81(2):183–90. 10.1016/j.earlhumdev.2004.07.001 [DOI] [PubMed] [Google Scholar]
- 79.Grant KA, McMahon C, Reilly N, Austin MP. Maternal sensitivity moderates the impact of prenatal anxiety disorder on infant responses to the still-face procedure. Infant Behav Dev [Internet]. 2010;33(4):453–62. Available from: 10.1016/j.infbeh.2010.05.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We have a strict legal rules in terms of data sharing in the medical faculty at the University of Turku. The anonymized dataset is available upon request. The requests of data can be pointed to statistician Juho Pelto (juho.pelto@utu.fi).