Abstract
Background and aims
Arteriovenous fistula (AVF) maturation failure rates remain high in patients with end-stage renal disease (ESRD). Although preoperative morphological and functional assessment of blood vessels by duplex ultrasonography (DUS) has been shown to improve AVF maturation, there is no consensus regarding the optimal vein (VD) and artery (AD) diameters to be universally used for AVF creation. To improve patient selection, set out to investigate if there is a correlation between preoperative VD/AD and clinical covariates, and postoperative AVF outcome.
Methods
This was a prospective cohort study conducted during January–August 2014. ESRD patients referred to “Niculae Stăncioiu” Heart Institute Cluj-Napoca, who had a VD ≥1.9 mm and AD ≥1.5 mm, as measured by DUS, and underwent AVF creation were enrolled. We assessed whether preoperative VD/AD and clinical covariates were associated with AVF maturation rate and primary patency at 2 years after AVF creation.
Results
Of 115 patients referred for AVF creation, 93 were included in the study. Mean (± standard deviation) VD was 3.3 ± 1.1 mm and VDs were distributed in quartile Q1 <2.55 mm, Q2: 2.56–3.10 mm, Q3: 3.11–3.70 mm and Q4: >3.71 mm. Mean AD was 3.3 ± 1.4 mm and ADs were distributed in Q1 <2.55 mm, Q2: 2.56–3.10 mm, Q3: 3.11–3.70 mm, and Q4, >3.71 mm. AVF maturation rate increased proportionally with VD from Q1 (62%) to Q2 (70%), Q3 (82%) to Q4 (96%) (p=0.03). Based on AD, a higher AVF maturation rate was observed in Q3 (86%), Q4 (83%) vs Q1 (71%) and Q2 (67%). Long-term primary patency of AVFs seemed not to be influenced by VD and AD. In older patients and those with peripheral arterial disease, AVF maturation failure tended to be higher.
Conclusions
Our findings suggest that a preoperative VD ≥1.9 mm and AD ≥1.5 mm have a successful maturation rate of AVF greater than 60% in ESRD patients. The maturation rate of surgical AVF increases proportionally with the size of VD used for AVF creation.
Keywords: end-stage renal disease, arteriovenous fistula, duplex ultrasonography, vein diameter, artery diameter
Introduction
The incidence of chronic kidney disease (CKD) is increasing worldwide [1]. One of its major implications is the risk of end stage renal disease (ESRD) [2]. In Europe, according to a survey conducted in 16 countries, the incidence of ESRD varies between 81 and 195 per million and the prevalence between 309 and 1670 per million population [3]. The increasing prevalence of ESRD leads to a growing need for renal replacement therapies (RRTs) for survival [1]. Of the RRTs, arteriovenous fistulas (AVFs) are the preferred option for hemodialysis according to The National Kidney Foundation - Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) guidelines and the Fistula First Breakthrough Initiative (FFBI) [4].
However, AVF maturation and failure still represent significant obstacles for successful use of fistula [1]. AVF maturation failure ranges between 20% and 60% [5] and was associated with clinical predictors such as patient age, peripheral vascular disease, coronary artery disease, diabetes, non-white race, gender etc. [6].
Preoperative assessment of the morphological and functional parameters of peripheral blood vessels by duplex ultrasonography (DUS) has been shown to improve fistula maturation [7–9]. Because AVFs are created by a direct anastomosis between a native artery and vein, and successful maturation implies an increase in the blood flow rate and diameter of the inflow artery and the draining vein that can support the high blood flow [10], it is important to assess the preoperative vein (VD) and artery (AD) diameters, as well as their morphological characteristics. Even though DUS is routinely used to evaluate veins and arteries suitable for AVF creation, the maturation failure rate remains high [11]. There is no consensus regarding the optimal preoperative VD and AD threshold to be universally used for AVF creation, which could predict AVF maturation rate [12,13]. The VD threshold reported in different studies between fistula failure and success ranges from 1.6 to 4 mm [8,14,15]. Similarly, the AD threshold reported in literature ranges from 1.5 to 4.1 mm [16–19].
To improve patient selection for AVF creation, we conducted a prospective cohort study in patients with ESRD in the Department of Cardiovascular Surgery, “Niculae Stăncioiu” Heart Institute, Cluj-Napoca, Romania. Our aim was to evaluate whether preoperative characteristics of the blood vessels were associated with the AVF maturation rate and long-term primary patency. The first epoch study results were previously published and showed that the decrease of digital pressure at 1 and 2 months after AVF creation was not influenced by the arterial remodelling degree, VD or fistula flow in a subset of ESRD patients with preoperative cephalic and basilic VDs >1.7 mm and brachial AD >2 mm [20]. We also showed that vein morphology was correlated with AVF outcome [21].
End-of-study results are reported here. We assessed if there was a correlation between preoperative VD and AD, as measured by DUS, and the postoperative AVF maturation rate and long-term primary patency, and tried to identify a threshold to be used during pre-operative evaluation. We also assessed if patient clinical status correlated with postoperative AVF outcome.
Material and methods
Study design and participants
This was a prospective cohort study conducted in the Department of Cardiovascular Surgery, “Niculae Stăncioiu” Heart Institute, Cluj-Napoca, Romania, during January 2014 – August 2014. The study was approved by the ethical committee of the hospital and of the “Iuliu Haţieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania, and was conducted in accordance with the principles of the Declaration of Helsinki. Informed consent was obtained from all patients before inclusion in the study.
Patients with ESRD referred to our department were considered eligible for study inclusion if they had: vessels suitable for AVF creation as established by clinical examination and ultrasonography, with a VD ≥1.9 mm, and AD ≥1.5 mm, and if written informed consent was given prior to enrolment. Regardless of whether they had or not a previous arteriovenous access, patients were included in the study. Any of the following were considered reason for exclusion: previous diagnosis of stenosis or thrombosis in the draining veins to be used for AVF creation, concomitant diagnosis of other disease and/or ongoing treatment that may influence AVF maturation, and physician decision that patients may not comply with study procedures. Prior to AVF creation, patients were clinically examined and baseline patient demographic data, clinical comorbidities, and dialysis status were recorded. DUS of artery and veins was performed by the same vascular surgeon and the arteriovenous access type was decided based on the KDOQI and Society for Vascular Surgery guidelines [22]. Patient-specific procedure was determined by the operating surgeon based on each patient’s history, clinical status and preoperative DUS evaluation of the vessels to be used for AVF creation. The surgical interventions consisted in radiocephalic, brachiocephalic or brachiobasilic fistula, and were performed using standard techniques. We evaluated if a correlation between preoperative diameter of the inflow artery and of the cephalic vein on the postoperative AVF maturation rate and primary patency at 2 years after AVF creation could be observed.
DUS evaluation of the blood vessels used for AVF creation
Patients were examined in sitting position, after a tourniquet was applied on the upper third of the forearm or arm, while the arm was resting on an adjustable supporting stand. Before DUS examination, the radial artery was palpated at wrist level, the brachial artery at the anterior side of the elbow and veins along the trajectory, to identify their direction and exact position. The ultrasound examination was performed using the NextGen LOGIQTM doppler ultrasound machine. A 2-D broad-spectrum linear probe of 7.0 to 15.0 MHz was used. The size of the arteries was assessed in real time, grey scale, and on longitudinal sections at the level of wrist for the radial artery, and antecubital fossa for the brachial artery. The ADs selected for AVF location were recorded. Vein evaluation was performed through the forearm and upper-arm. VDs were measured in cross-section. When the vein image was frozen on the screen, the cross-sectional area was calculated by an automatic function. The risk of underestimating the vein size due to compression exercised by the transducer was minimized by using big amounts of gel. Assuming that the narrowest portion of the vein is a good indicator of pre-existing stenosis, decreased AVF flow or risk for AVF failure, the minimum VD in the location selected for AVF creation was recorded. DUS was performed by the same vascular surgeon to ensure consistency of measurements. Depending on the vessels size, radiocephalic AVF type was preferred first, followed by brachiocephalic and brachiobasilic AVF types, as also proposed by KDOQI and Society for Vascular Surgery guidelines [22].
Surgical procedure for AVF creation
Based on preoperative findings, radial-cephalic, brachial-cephalic or transposed brachial-basilic AVFs were created by the same vascular surgeon.
Postoperative AVF assessment
AVF was considered mature if permitted cannulation with 2 needles for 2/3 dialysis runs within 1 month and up to 6 months after its creation, at an average blood flow rate of 300 to 450 ml/minute for 3.5 to 4.0 hours [23]. In this analysis, AVF maturation and patency were assessed at 2 years after AVF creation.
Statistical analysis
Statistical analyses were performed with Epi InfoTM 7 software (CDC, USA). For descriptive statistics, continuous data were tabulated as prevalence and percentages, and categorical variables as mean values with standard deviation (± SD) or 95% confidence intervals (CIs). To find the optimal minimum VD and AD to be used for preoperative evaluation, the frequency distribution of VD and AD in the entire study population was assessed. The 25th, 50th and 75th percentile rank values were used to define VD and AD quartiles. VD and AD recorded in each patient were included either within the 1st, 2nd, 3rd, or 4th quartile (Q1, Q2, Q3, or Q4).
To compare the continuous variables, ANOVA Parametric Test for Inequality of Population Means was used. To assess the association between categorical variables, chi-square test was used; to determine if there was an association between ≥2 categorical variables, the values were automatically compared between one another in all possible combinations and a mid p value was generated. P-value was considered statistically significant if less than 0.05. Correlation between two variables was assessed by Pierson correlation test: if r=1, a strong (positive/negative) correlation was considered; if r=−/+0.5 was considered a medium (positive/negative correlation); and if r =−/+0.25 was considered a weak (positive/negative) correlation.
Results
Demographic characteristics and AVF type
Of 115 patients referred to our department for AVF creation during the study period, 93 were eligible for study inclusion. Of the 22 patients excluded, 19 refused to be included in the study, 2 died and 1 underwent kidney transplant. Baseline demographic characteristics of study participants are showed in Table I. The number of male patients was around two times higher than the numbers of females.
Table I.
Demographic characteristics and clinical data at baseline.
| Category | Overall population (N=93) | |
|---|---|---|
| n | mean ± SD or % (95% CI) | |
| Age (years) | 93 | 60.5 ± 11.8 |
| Female | 35 | 37.6 (27.8–48.3) |
| Male | 58 | 62.4 (51.7–72.2) |
| Urban area | 55 | 59.1 (48.5–69.2) |
| Rural area | 38 | 40.9 (30.8–51.5) |
| Smoker | 20 | 21.5 (13.7–31.2) |
| DM | 32 | 34.4 (24.9–45.0) |
| AHT | 81 | 87.1 (78.6–93.2) |
| IC | 31 | 33.3 (56.1–76.1) |
| PAD | 18 | 19.4 (11.9–28.9) |
| Before dialysis start | 34 | 36.6 (26.8–47.2) |
N, total number of patients included in the analysis; n, number of patients in a given category; SD, standard deviation; CI, confidence interval; DM, diabetes mellitus; AHT, arterial hypertension; IC, ischemic cardiomyopathy; PAD, peripheral arterial disease.
Overall AVF maturation rate and long-term functional patency
The distribution of AVF types was as follows: 47 patients (50.5%; 95% CI: 40.0–61.1) had radiocephalic AVF placed, 37 patients (39.8%; 95% CI: 29.8–50.5) had brachiocephalic AVF, and 9 patients (9.7%; 95% CI: 4.5–17.6) had brachiobasilic AVF. Of the 93 access procedures, 71 AVFs (76.3%; 95% CI: 66.40–84.54) matured and were further used. No difference in AVF maturation rate was recorded in patients with radiocephalic (32 of 47 [68.1%]), brachiocephalic (31 of 37 [83.8%]) and brachiobasilic (8 of 9 [88.9%]) AVFs (Table II). The total number of AVFs that failed to mature was 22 (22.3%; 95% CI: 15.5–33.6) and were not further used or followed-up.
Table II.
Overall maturation rate based on baseline characteristics and DUS evaluation.
| Category | Mature AVF (N=71) | Non-mature AVF (N=22) | p-value | |||
|---|---|---|---|---|---|---|
| n | mean ± SD or % (95% CI) | n | mean ± SD or % (95% CI) | |||
| Age (years) | 71 | 59 ± 11.1 | 22 | 65 ± 13.1 | 0.054 | |
| Gender | Female | 26 | 74.3 (56.7–87.5) | 9 | 25.7 (12.5–43.3) | 0.35 |
| Male | 45 | 77.6 (64.7–87.5) | 13 | 22.4 (12.5–35.3) | ||
| Area | Urban | 42 | 76.4 (63.00–86.8) | 13 | 23.64 (13.2–37.0) | 0.49 |
| Rural | 29 | 76.3 (59.8–88.6) | 9 | 23.7 (11.4–40.2) | ||
| Smoker | Yes | 15 | 75.0 (50.9–91.3) | 5 | 25 (8.7–49.1) | 0.43 |
| No | 56 | 76.5 (65.4–85.8) | 17 | 23.3 (14.2–34.7) | ||
| DM | Yes | 25 | 78.1 (60.0–90.7) | 7 | 21.9 (9.3–40.0) | 0.39 |
| No | 46 | 75.4 (62.7–85.5) | 15 | 24.6 (14.5–37.3) | ||
| AHT | Yes | 61 | 75.3 (64.5–84.2) | 20 | 24.6 (15.8–35.5) | 0.29 |
| No | 10 | 83.3 (51.6–97.9) | 2 | 16.7 (2.1–48.4) | ||
| IC | Yes | 24 | 72.4 (58.9–90.4) | 7 | 22.6 (9.6–41.1) | 0.43 |
| No | 47 | 75.8 (63.3–85.8) | 15 | 24.2 (14.2–36.7) | ||
| PAD | Yes | 11 | 61.1 (35.8–82.7) | 7 | 38.9 (17.3–64.3) | 0.056 |
| No | 60 | 80.0 (69.2–88.4) | 15 | 20 (11.7–30.8) | ||
| Before dialysis | Yes | 27 | 79.4 (62.1–91.3) | 7 | 20.6 (8.7–37.9) | 0.31 |
| No | 44 | 74.6 (61.6–85.0) | 15 | 25.4 (15.0–38.4) | ||
| AVF type | Radiocephalic | 32 | 68.1 (52.9–80.9) | 15 | 31.9 (9.1–47.1) | 0.15 |
| Brachiocephalic | 31 | 83.8 (68.0–93.8) | 6 | 16.2 (6.2–32.0) | ||
| Brachiobasilic | 8 | 88.9 (51.8–99.7) | 1 | 11.1 (0.3–48.3) | ||
Note: statistical analysis performed by ANOVA chi-square test and ANOVA Parametric Test for Inequality of Population Means (p-value was considered statistically significant if ≤0.05). DUS, duplex ultrasonography; N, total number of patients; n, number of patients in a given category; SD, standard deviation; CI, confidence interval; DM, diabetes mellitus; AHT, arterial hypertension; IC, ischemic cardiomyopathy; PAD, peripheral arterial disease; AVF; arteriovenous fistula.
Of the 71 mature AVFs, 45 (81.8%; 95% CI: 69.1–90.9) achieved functional patency at 2 years after AVF creation and 10 (18.18%; 9.1–30.9) were non-functional. 16 AVFs that matured were lost to follow-up at 2 years after AVF creation, 9 patients died, 5 patients underwent renal transplant, and 2 patients did not start dialysis.
The highest number of functional AVF at 2 years after AVF creation was recorded in patients with radiocephalic AVF (23 of 26 [88.5%]) and brachiocephalic AVF (20 of 23 [87.0%]) (Table III). The percentage of non-functional AVFs was increased in patients with brachiobasilic AVF (4 of 6 [66.7%]), the difference being statistically significant compared to the percentage of non-functional radiocephalic (3 of 26 [11.5%]) and brachiocephalic (3 of 23 [13.0%]) AVFs (p=0.002).
Table III.
Functional patency at 2 years after AVF creation based on baseline characteristics and DUS evaluation.
| Category | Functional AVF (N=45) | Non-functional AVF (N=10) | p-value | |||
|---|---|---|---|---|---|---|
| n | mean ± SD or % (95% CI) | n | mean ± SD or % (95% CI) | |||
| Age (years) | 45 | 59 ± 11.8 | 10 | 65 ± 11.3 | 0.19 | |
| Gender | Female | 15 | 75.0 (50.9–91.3) | 5 | 25.0 (8.7–49.1) | 0.17 |
| Male | 30 | 85.7 (69.7–95.2) | 5 | 14.3 (4.8–30.3) | ||
| Area | Urban | 24 | 80.0 (61.4–92.3) | 6 | 20.0 (7.7–38.6) | 0.36 |
| Rural | 21 | 84.0 (63.9–95.5) | 4 | 16.0 (4.5–36.1) | ||
| Smoker | Yes | 7 | 77.8 (40.0–97.2) | 2 | 22.2 (2.8–60.0) | 0.36 |
| No | 38 | 82.6 (68.6–92.2) | 8 | 17.4 (7.8–31.4) | ||
| DM | Yes | 15 | 93.8 (69.8–99.8) | 1 | 6.3 (0.2–30.2) | 0.07 |
| No | 30 | 76.9 (60.7–88.9) | 9 | 23.1 (11.1–39.3) | ||
| AHT | Yes | 38 | 82.6 (68.6–92.2) | 8 | 17.4 (7.8–31.4) | 0.36 |
| No | 7 | 77.8 (40.0–97.2) | 2 | 22.2 (2.8–60.0) | ||
| IC | Yes | 16 | 80.0 (56.3–94.3) | 4 | 20.0 (5.7–43.7) | 0.39 |
| No | 29 | 83.0 (66.4–93.4) | 6 | 17.1 (6.6–33.6) | ||
| PAD | Yes | 5 | 71.4 (29.0–96.3) | 2 | 28.6 (3.7–71.0) | 0.23 |
| No | 40 | 83.3 (69.8–92.5) | 8 | 16.7 (7.5–30.2) | ||
| Before dialysis | Yes | 17 | 89.5 (66.9–98.7) | 2 | 10.5 (1.3–33.1) | 0.16 |
| No | 28 | 77.3 (60.9–89.9) | 8 | 22.2 (10.1–39.2) | ||
| AVF type | Radiocephalic | 23 | 88.5 (69.9–97.6) | 3 | 11.5 (2.5–30.2) | 0.002 |
| Brachiocephalic | 20 | 87.0 (66.4–97.2) | 3 | 13.0 (2.8–33.6) | ||
| Brachiobasilic | 2 | 33. 3 (4.3–77.7) | 4 | 66.7 (22.3–95.7) | ||
Note: statistical analysis performed by ANOVA chi-square test and ANOVA Parametric Test for Inequality of Population Means (p-value was considered statistically significant if ≤ 0.05). DUS, duplex ultrasonography; N, total number of patients; n, number of patients in a given category; SD, standard deviation; CI, confidence interval; BMI, body mass index; DM, diabetes mellitus; AHT, arterial hypertension; IC, ischemic cardiomyopathy; PAD, peripheral arterial disease; AVF; arteriovenous fistula.
Impact of preoperative VD and AD on the AVF maturation and long-term functional patency
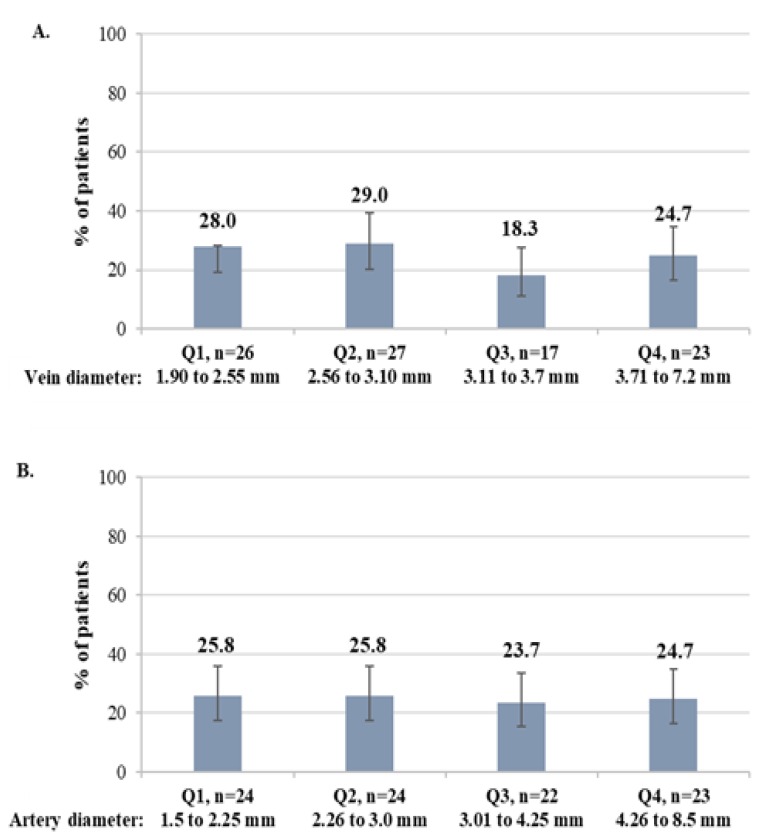
The mean VD of the overall population included in this analysis was 3.3 ± 1.1 mm. To find the optimal minimum diameters to be used for preoperative evaluation, VD were distributed in the following quartiles: Q1, <2.55 mm; Q2, 2.56 to 3.10 mm; Q3, 3.11 to 3.70 mm; Q4, >3.71 mm. The mean AD was 3.3 ± 1.4. VD quartiles were defined as follows: Q1, <2.55 mm; Q2, 2.56 to 3.10 mm; Q3, 3.11 to 3.70 mm; Q4, >3.71 mm. The percentage of patients with VD and AD included within a quartile was similar between quartiles (Figure 1A and 1B, respectively).
Figure 1.
Distribution of patients in each quartile by preoperative vein diameter (A) and artery diameter (B).
Q, quartile; n, number of patients in a given category.
AVF maturation rate increased proportionally with VD, the prevalence was statistically significant, being increased from Q1 to Q4 (p=0.03) (Table IV). Moreover, a positive correlation between VD increase and maturation rate increase was also observed by Pierson correlation test (r=0.3).
Table IV.
AVF maturation and long-term primary patency by vein and artery diameter quartiles.
| Category | Q1 | Q2 | Q3 | Q4 | p-value |
|---|---|---|---|---|---|
| Vein diameter |
1.90–2.55 mm N=26 |
2.56–3.10 mm N=27 |
3.11–3.70 mm N=17 |
3.71–7.20 mm N=23 |
|
| Fistula maturation, n (%) | 16 (61.5 %) | 19 (70.4%) | 14 (82.3) | 22 (95.7) | 0.03 |
| Patency at 2 years, n (%) | 10 (76.9%) | 13 (86.7%) | 7 (77.8) | 15 (83.3) | 0.47 |
| Artery diameter |
1.50–2.25 mm N=24 |
2.26–3.00 mm N=24 |
3.01–4.25 mm N=22 |
4.26–8.50 mm N=23 |
|
| Fistula maturation, n (%) | 16 (66.7) | 17 (70.8) | 19 (86.4) | 19 (82.6) | 0.33 |
| Patency at 2 years, n (%) | 12 (92.3) | 10 (83.3) | 12 (75.0) | 11 (78.6) | 0.66 |
Note: statistical analysis performed by ANOVA chi-square test (p-value was considered statistically significant if ≤ 0.05). Q, quartile; N, number of patients in a quartile; n (%), number (percentage) of patients in a given category.
In Q4, the majority of AVFs were brachiocephalic, while in Q1–Q3 most of AVFs were radiocephalic. The VD means by AVF type (± SD) was 4.08 ± 1.59 mm for brachiobasilic AVFs, 3.70 ± 1.30 mm for brachiocephalic AVFs and 2.83 ± 0.59 for radiocephalic AVFs. Even if the difference in diameter means by AVF type was statistically significant (p=0.0001), maturation rate by AVF type was not statistically significant (p=0.15) due to the similar maturation rate between brachiobasilic and brachiocephalic AVFs (89% brachiobasilic, 84% brachiocephalic and 68% radiocephalic).
Long-term patency of AVFs seemed not to be influenced by VD within different quartiles. No statistically significant difference was observed (Table IV).
A higher AVF maturation rate was observed in patients with AD within Q3 (86%), closely followed by patients with AD within (Q4), compared to the patients with AD within Q1 and Q2 (71% and 67%, respectively). The difference in maturation rate by quartiles was not statistically significant due to similarities between Q3 and Q4 (p=0.33) (Table IV). A very weak positive correlation between AVF maturation rate and VD was also observed by Pierson correlation test (r=0.29).
In Q4 and Q3, majority of AVFs were brachiocephalic, while in Q2, most of AVFs were radiocephalic, and in Q1, all were radiocephalic. The AD means by AVF type (± SD) was 4.50 ± 1.21 mm for brachiocephalic AVFs, 3.82 ± 0.76 mm for brachiobasilic AVFs and 2.31 ± 0.47 for radiocephalic AVFs (p=0.0).
Long-term patency of AVFs seemed not to be influenced by AD distribution within different quartiles (p=0.66) (Table IV).
Impact of preoperative baseline characteristics and co-morbidities on the AVF maturation and long-term functional patency
AFV maturation failure occurred most likely in older patients (border line statistical significance, p=0.054). Patients with peripheral arterial disease (PAD) had lower AVF maturation rate (11 of 18 [61.1%]) compared to those without diagnosed PAD (maturation rate: 60 of 75 [80%]), the difference almost reaching a statistical significance (p=0.056). Gender, living area, tobacco use, diabetes, arterial hypertension (AHT), ischemic cardiomyopathy (IC), AVF creation before or after dialysis start, and AVF type had no effect on the AVF maturation rate (Table II).
Age, gender, living area, tobacco use, BMI, diabetes, AHT, IC, AVF creation before or after dialysis start seemed not to influence long-term patency (Table III).
Discussion
In our study, we observed no statistically significant difference in maturation rate between radiocephalic, brachiocephalic and brachiobasilic AVFs. Our results are in line with different studies suggesting that the fistula site had no effect on maturation rate [8,24,25], while other studies suggest that forearm fistula are at higher risk of failure [26,27]. Of note, in our study, the overall maturation failure rate was 22.3%, which is lower compared to other studies, where a AVF maturation failure up to 60% was reported [5]. The relatively increased failure rates highlight that the general recommendations provided for AVF placement are not always applicable at individual patient level [22,28].
Regarding the long-term primary patency, our results showed that AVF type seems to influence AVF patency at 2 years after fistula creation. The percentage of patients with non-functional AVF was significantly higher following brachiobasilic AVF placement compared to radiocephalic and brachiocephalic AVFs. However, these findings should be interpreted with caution due to low number of patients with this fistula type included in our study. Since 2004, when FBBI recommended first a target of 40% AVF utilization among the prevalent haemodialysis and subsequently increased the target to 66% based on NKF-KDOQI guidelines [29], placement of native AVFs had a significant success in the clinical practice [30]. However, long-term follow-up of AVF patency revealed high rates of failure with up to 51% by 2 years [31]. Other published data suggest a failure rate varying from 9% to 53% [7,24,25,32,33]. Compared to these studies, we recorded a relatively low overall failure rate (18.1%).
To improve patient selection for AVF creation and to increase AVF maturation rates, NKF-KDOQI guidelines recommended preoperative DUS. Even if DUS is routinely used, and has increased fistula placement and maturation rates [8, 34–36] the maturation failure rate remains high [11]. There is an imperative need to improve patient selection at individual level, but there is no consensus regarding the optimal blood vessel characteristics to be used when taking the decision of AVF placement. Because VD and AD thresholds related with a successful fistula are unclear, in our study we did not attempt to define a minimum VD or AD when comparing mature versus non-mature AVFs or functional versus non-functional AVFs, but to assess if a threshold related to AVF maturation failure and long-term primary patency loss can be identified in our population. In patients with a VD ≥1.9 mm, we observed that only AVF maturation rate increased proportionally with VD, from Q1 to Q2 and Q4. Compared to other studies suggesting that a VD ranging between 2 and 3 mm, with basilic VD ≥2.5 mm is optimal for AVF creation [11, 13, 25]; while other studies suggest that a cephalic VD ≥4 mm is a predictor for successful AVF maturation [19, 30], we suggest that a VD ≥1.9 mm is optimal for AVF use, regardless of vein type. It is to be noted that the American and European surgeons allow AVF creation with a cephalic VD of 1.5–1.9 mm, and European surgeons also with a basilic VD of 2–2.4 mm [13]. Most studies used mean or maximum VD [30], while we used minimum VD when deciding AVF location. This is the smallest vein size that can be recorded when a patient is vasoconstricted during DUS evaluation. Despite the possible vein vasoconstriction, minimum VD was correlated with fistula maturation rate. Similar findings were reported by Dageforde et al. [11]. In terms of long-term primary patency, no statistically significant difference was observed between patients from Q1, Q2, Q3 and Q4. Our observations are comparable with previous studies demonstrating that VD as measured by DUS is associated with fistula outcomes [30,37]. Vein size seems to be an important predictor of fistula maturation, but it should also be associated with arterial factors and blood flow [30]. Some studies have demonstrated a correlation between large vein sizes and improved AVF maturation and patency [11, 38], but the association between artery size and maturation rate is controversial [19]. In our study, we observed a higher AVF maturation rate in Q3 and Q4 compared to Q1 and Q2. The lowest AD included in our analysis was 1.5 mm. Different studies suggest that a preoperative AD <1.5 mm or <1.6 mm is associated with AVF early failure [16,17,39], while in other studies, when using a minimum AD of 2 mm, no association between AD and AVF outcome was found [19,40,41]. No statistically significant difference was observed between patients with AD within Q1, Q2, Q3 and Q4, and long-term primary patency outcome. In some studies, the AD considered optimal for AVF creation was ≥ 1.5 mm [16, 25] or ≥2 mm [42], while other studies suggest poor AVF functionality when radial AD is smaller than 1.5–2.3 mm [16–18]. The use of preoperative vessel mapping, in addition to diameter assessment, also excluded the small and diseased veins and arteries, which may lead to AVF failure.
Furthermore, we also assessed if patients baseline characteristics and co-morbidities were related with AVF outcome. We showed that older age increased the AVF maturation failure Similar results have been reported in literature [7,42–44]. This may be explained by the high prevalence of co-morbidities, poor quality of life and short life expectancy in elderly patients, which is associated with AVF maturation failure and decreased primary and cumulative AVF patency [45]. Regarding patient gender, we found no statistically significant difference between female and male patients. Similar results have been reported by Lauvao et al. [30]. However, conflicting data regarding the gender influence on the AVF failure are reported. The majority of studies suggest that maturation time is prolonged in women and patency is lower [14,46,47]. We also observed no statistical significant difference in maturation rate and long-term patency in patients with or without diabetes. In contrast with our findings, previous studies have shown that diabetes is directly related with AVF failure and patency loss [48,49]. In our study, the number of patients with diabetes was low, which may have led to this contradiction. Regarding smoking history and AHT, our results are in line with previous data [32]. No correlation between AVF maturation failure/long-term primary patency and smoking history and AHT was identified. IC and PAD are also considered predictive risk factors for AVF failure [6]. In our study, only patients with PAD had lower AVF maturation rate. The difference in maturation rate in patients with or without IC was not statistically significant.
We acknowledge that our study has some limitations. One of the limitations is the small size of the included population, which comes from the prospective nature of the study. The defined study duration permitted the inclusion of a limited number of patients. Secondly, we did not assess the calcification status of the inflow artery, which is also a risk factor for poor AVF outcome. Thirdly, due to the nature of follow-up, we were unable to calculate the mean number of days until AVF maturation.
Conclusions
Our findings suggest that a preoperative VD ≥1.9 mm and AD ≥ 1.5 mm, as measured by DUS, have a successful maturation rate over 60% and that the maturation rate increases with VD increase in patients with ESRD. However, we cannot claim that this can be used as a definitive minimal threshold, but we recommend using DUS to determine the minimal VD and AD. The individual age of the patients and PAD also had a negative influence on the maturation rate. Long-term primary patency loss seems to be higher in patients with brachiobasilic AVFs, however this hypothesis should be further investigated on a larger population size. To take a decision on what type of AVF to be used in ESRD patients, we suggest using a combination between preoperative DUS vessels mapping and clinical examination.
References
- 1.Hu H, Patel S, Hanisch JJ, Santana JM, Hashimoto T, Bai H, et al. Future research directions to improve fistula maturation and reduce access failure. Semin Vasc Surg. 2016;29(4):153–171. doi: 10.1053/j.semvascsurg.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Kidney Health Alliance. Recommendations for sustainable kidney care. Aug, 2015. Available from: http://ekha.eu/wp-content/uploads/2016/01/EKHA-Recs-for-Sustainable-Kidney-Care-25.08.2015.pdf.
- 3.International Society of Nephrology. Chronic kidney disease multinational inventory. International Society of Nephrology: International Society of Nephrology; 2014. Kidney Health for Life (KH4L) Available from: https://www.noexperiencenecessarybook.com/7WnWQv/statement-on-behalf-of-the-international-society-of-nephrology-isn-to-the-66th-session-of-the-who-regional-committee-for-europe-on-health-in-the-2030-agenda-for-sustainable-development-and-its-relation-to-health-2020-agenda-item-5a.html. [Google Scholar]
- 4.Vascular Access 2006 Work Group. Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006;48(Suppl 1):S176–S247. doi: 10.1053/j.ajkd.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 5.Kosa SD, Al-Jaishi AA, Moist L, Lok CE. Preoperative vascular access evaluation for haemodialysis patients. Cochrane Database Syst Rev. 2015 Sep 30;(9):CD007013. doi: 10.1002/14651858.CD007013.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lok CE, Allon M, Moist L, Oliver MJ, Shah H, Zimmerman D. Risk equation determining unsuccessful cannulation events and failure to maturation in arteriovenous fistulas (REDUCE FTM I) J Am Soc Nephrol. 2006;17(11):3204–3212. doi: 10.1681/ASN.2006030190. [DOI] [PubMed] [Google Scholar]
- 7.Malovrh M. Native arteriovenous fistula: preoperative evaluation. Am J Kidney Dis. 2002;39(6):1218–1225. doi: 10.1053/ajkd.2002.33394. [DOI] [PubMed] [Google Scholar]
- 8.Allon M, Lockhart ME, Lilly RZ, Gallichio MH, Young CJ, Barker J, et al. Effect of preoperative sonographic mapping on vascular access outcomes in hemodialysis patients. Kidney Int. 2001;60(5):2013–2020. doi: 10.1046/j.1523-1755.2001.00031.x. [DOI] [PubMed] [Google Scholar]
- 9.Mendes RR, Farber MA, Marston WA, Dinwiddie LC, Keagy BA, Burnham SJ. Prediction of wrist arteriovenous fistula maturation with preoperative vein mapping with ultrasonography. J Vasc Surg. 2002;36(3):460–463. doi: 10.1067/mva.2002.126544. [DOI] [PubMed] [Google Scholar]
- 10.Dixon BS. Why don’t fistulas mature? Kidney Int. 2006;70(8):1413–1422. doi: 10.1038/sj.ki.5001747. [DOI] [PubMed] [Google Scholar]
- 11.Dageforde LA, Harms KA, Feurer ID, Shaffer D. Increased minimum vein diameter on preoperative mapping with duplex ultrasound is associated with arteriovenous fistula maturation and secondary patency. J Vasc Surg. 2015;61(1):170–176. doi: 10.1016/j.jvs.2014.06.092. [DOI] [PubMed] [Google Scholar]
- 12.Pussell BA, Bendorf A, Kerridge IH. Access to the kidney transplant waiting list: a time for reflection. Intern Med J. 2012;42(4):360–363. doi: 10.1111/j.1445-5994.2012.02730.x. [DOI] [PubMed] [Google Scholar]
- 13.Nica A, Lok CE, Harris J, Lee TC, Mokrzycki MH, Maya ID, et al. Understanding surgical preference and practice in hemodialysis vascular access creation. Semin Dial. 2013;26(4):520–526. doi: 10.1111/sdi.12046. [DOI] [PubMed] [Google Scholar]
- 14.Dunn J, Herscu G, Woo K. Factors influencing maturation time of native arteriovenous fistulas. Ann Vasc Surg. 2015;29(4):704–707. doi: 10.1016/j.avsg.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 15.Patel ST, Hughes J, Mills JL., Sr Failure of arteriovenous fistula maturation: an unintended consequence of exceeding dialysis outcome quality Initiative guidelines for hemodialysis access. J Vasc Surg. 2003;38(3):439–445. doi: 10.1016/s0741-5214(03)00732-8. discussion 445. [DOI] [PubMed] [Google Scholar]
- 16.Parmar J, Aslam M, Standfield N. Pre-operative radial arterial diameter predicts early failure of arteriovenous fistula (AVF) for haemodialysis. Eur J Vasc Endovasc Surg. 2007;33(1):113–115. doi: 10.1016/j.ejvs.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Jemcov TK. Morphologic and functional vessels characteristics assessed by ultrasonography for prediction of radiocephalic fistula maturation. J Vasc Access. 2013;14(4):356–363. doi: 10.5301/jva.5000163. [DOI] [PubMed] [Google Scholar]
- 18.Usta E, Elkrinawi R, Salehi-Gilani S, Adili S, Sonnentag T, Alscher M, et al. Risk factors predicting the successful function and use of autogenous arteriovenous fistulae for hemodialysis. Thorac Cardiovasc Surg. 2013;61(5):438–444. doi: 10.1055/s-0032-1321953. [DOI] [PubMed] [Google Scholar]
- 19.Kakkos SK, Kaplanis N, Papachristou EC, Papadoulas SI, Lampropoulos GC, Tsolakis IA, et al. The Significance of Inflow Artery and Tourniquet Derived Cephalic Vein Diameters on Predicting Successful Use and Patency of Arteriovenous Fistulas for Haemodialysis. Eur J Vasc Endovasc Surg. 2017;53(6):870–878. doi: 10.1016/j.ejvs.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Oprea A, Molnar A, Scridon T, Mircea PA. Digital pressure in haemodialysis patients with brachial arteriovenous fistula. Indian J Med Sci. 2018 doi: 10.4103/ijmr.IJMR_415_17. accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oprea A, Molnar A, Encică S, Vlăduţiu DŞ, Scridon GT, Săcui DM, et al. Effect of the veins histopathological characteristics and preexisting medical conditions on arteriovenous fistula maturation and primary patency in patients with end-stage renal disease: an observational, prospective study. Rom J Morphol Embryol. 2017;58(3):871–880. [PubMed] [Google Scholar]
- 22.Sidawy AN, Spergel LM, Besarab A, Allon M, Jennings WC, Padberg FT, Jr, et al. The Society for Vascular Surgery: clinical practice guidelines for the surgical placement and maintenance of arteriovenous hemodialysis access. J Vasc Surg. 2008;48(5 Suppl):2S–25S. doi: 10.1016/j.jvs.2008.08.042. [DOI] [PubMed] [Google Scholar]
- 23.Lee T, Mokrzycki M, Moist L, Maya I, Vazquez M, Lok CE, et al. Standardized definitions for hemodialysis vascular access. Semin Dial. 2011;24(5):515–524. doi: 10.1111/j.1525-139X.2011.00969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tordoir JH, Rooyens P, Dammers R, van der Sande FM, de Haan M, Yo TI. Prospective evaluation of failure modes in autogenous radiocephalic wrist access for haemodialysis. Nephrol Dial Transplant. 2003;18(2):378–383. doi: 10.1093/ndt/18.2.378. [DOI] [PubMed] [Google Scholar]
- 25.Elsharawy MA. Prospective evaluation of factors associated with early failure of arteriovenous fistulae in hemodialysis patients. Vascular. 2006;14(2):70–74. doi: 10.2310/6670.2006.00018. [DOI] [PubMed] [Google Scholar]
- 26.Peterson WJ, Barker J, Allon M. Disparities in fistula maturation persist despite preoperative vascular mapping. Clin J Am Soc Nephrol. 2008;3(2):437–441. doi: 10.2215/CJN.03480807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ernandez T, Saudan P, Berney T, Merminod T, Bednarkiewicz M, Martin PY. Risk factors for early failure of native arteriovenous fistulas. Nephron Clin Pract. 2005;101(1):c39–c44. doi: 10.1159/000085710. [DOI] [PubMed] [Google Scholar]
- 28.III NKF-K/DOQI Clinical Practice Guidelines for Vascular Access: update 2000. Am J Kidney Dis. 2001;37(1 Suppl 1):S137–S181. doi: 10.1016/s0272-6386(01)70007-8. [DOI] [PubMed] [Google Scholar]
- 29.Neumann ME. “Fistula first” initiative pushes for new standards in access care. Nephrol News Issues. 2004;18(9):43, 47–48. [PubMed] [Google Scholar]
- 30.Lauvao LS, Ihnat DM, Goshima KR, Chavez L, Gruessner AC, Mills JL., Sr Vein diameter is the major predictor of fistula maturation. J Vasc Surg. 2009;49(6):1499–1504. doi: 10.1016/j.jvs.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 31.Al-Jaishi AA, Oliver MJ, Thomas SM, Lok CE, Zhang JC, Garg AX, et al. Patency rates of the arteriovenous fistula for hemodialysis: a systematic review and meta-analysis. Am J Kidney Dis. 2014;63(3):464–478. doi: 10.1053/j.ajkd.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 32.Schild AF, Prieto J, Glenn M, Livingstone J, Alfieri K, Raines J. Maturation and failure rates in a large series of arteriovenous dialysis access fistulas. Vasc Endovascular Surg. 2004;38(5):449–453. doi: 10.1177/153857440403800509. [DOI] [PubMed] [Google Scholar]
- 33.Feldman HI, Joffe M, Rosas SE, Burns JE, Knauss J, Brayman K. Predictors of successful arteriovenous fistula maturation. Am J Kidney Dis. 2003;42(5):1000–1012. doi: 10.1016/j.ajkd.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Ascher E, Gade P, Hingorani A, Mazzariol F, Gunduz Y, Fodera M, et al. Changes in the practice of angioaccess surgery: impact of dialysis outcome and quality initiative recommendations. J Vasc Surg. 2000;31(1 Pt 1):84–92. doi: 10.1016/s0741-5214(00)70070-x. [DOI] [PubMed] [Google Scholar]
- 35.Gibson KD, Caps MT, Kohler TR, Hatsukami TS, Gillen DL, Aldassy M, et al. Assessment of a policy to reduce placement of prosthetic hemodialysis access. Kidney Int. 2001;59(6):2335–2345. doi: 10.1046/j.1523-1755.2001.00751.x. [DOI] [PubMed] [Google Scholar]
- 36.Wong CS, McNicholas N, Healy D, Clarke-Moloney M, Coffey JC, Grace PA, et al. A systematic review of preoperative duplex ultrasonography and arteriovenous fistula formation. J Vasc Surg. 2013;57(4):1129–1133. doi: 10.1016/j.jvs.2012.11.094. [DOI] [PubMed] [Google Scholar]
- 37.Khavanin Zadeh M, Gholipour F, Naderpour Z, Porfakharan M. Relationship between Vessel Diameter and Time to Maturation of Arteriovenous Fistula for Hemodialysis Access. Int J Nephrol. 2012;2012:942950. doi: 10.1155/2012/942950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farber A, Imrey PB, Huber TS, Kaufman JM, Kraiss LW, Larive B, et al. Multiple preoperative and intraoperative factors predict early fistula thrombosis in the Hemodialysis Fistula Maturation Study. J Vasc Surg. 2016;63(1):163–170.e6. doi: 10.1016/j.jvs.2015.07.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong V, Ward R, Taylor J, Selvakumar S, How TV, Bakran A. Reprinted article “Factors associated with early failure of arteriovenous fistulae for haemodialysis access”. Eur J Vasc Endovasc Surg. 2011;42(Suppl 1):S48–S54. doi: 10.1016/j.ejvs.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 40.Korten E, Toonder IM, Schrama YC, Hop WC, van der Ham AC, Wittens CH. Dialysis fistulae patency and preoperative diameter ultrasound measurements. Eur J Vasc Endovasc Surg. 2007;33(4):467–471. doi: 10.1016/j.ejvs.2006.10.035. [DOI] [PubMed] [Google Scholar]
- 41.Ives CL, Akoh JA, George J, Vaughan-Huxley E, Lawson H. Pre-operative vessel mapping and early post-operative surveillance duplex scanning of arteriovenous fistulae. J Vasc Access. 2009;10(1):37–42. doi: 10.1177/112972980901000107. [DOI] [PubMed] [Google Scholar]
- 42.Woo K, Ulloa J, Allon M, Carsten CG, 3rd, Chemla ES, Henry ML, et al. Establishing patient-specific criteria for selecting the optimal upper extremity vascular access procedure. J Vasc Surg. 2017;65(4):1089–1103.e1. doi: 10.1016/j.jvs.2016.10.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rao RK, Azin GD, Hood DB, Rowe VL, Kohl RD, Katz SG, et al. Basilic vein transposition fistula: a good option for maintaining hemodialysis access site options? J Vasc Surg. 2004;39(5):1043–1047. doi: 10.1016/j.jvs.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 44.Allon M. Arteriovenous Grafts: Much Maligned But in Need of Reconsideration? Semin Dial. 2017;30(2):125–133. doi: 10.1111/sdi.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tordoir JHM, Bode AS, van Loon MM. Preferred strategy for hemodialysis access creation in elderly patients. Eur J Vasc Endovasc Surg. 2015;49(6):738–743. doi: 10.1016/j.ejvs.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 46.Almasri J, Alsawas M, Mainou M, Mustafa RA, Wang Z, Woo K, et al. Outcomes of vascular access for hemodialysis: A systematic review and meta-analysis. J Vasc Surg. 2016;64(1):236–243. doi: 10.1016/j.jvs.2016.01.053. [DOI] [PubMed] [Google Scholar]
- 47.Miller CD, Robbin ML, Allon M. Gender differences in outcomes of arteriovenous fistulas in hemodialysis patients. Kidney Int. 2003;63(1):346–352. doi: 10.1046/j.1523-1755.2003.00740.x. [DOI] [PubMed] [Google Scholar]
- 48.Huijbregts HJ, Bots ML, Moll FL, Blankestijn PJ CIMINO members. Hospital specific aspects predominantly determine primary failure of hemodialysis arteriovenous fistulas. J Vasc Surg. 2007;45(5):962–967. doi: 10.1016/j.jvs.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 49.Monroy-Cuadros M, Yilmaz S, Salazar-Bañuelos A, Doig C. Risk factors associated with patency loss of hemodialysis vascular access within 6 months. Clin J Am Soc Nephrol. 2010;5(10):1787–1792. doi: 10.2215/CJN.09441209. [DOI] [PMC free article] [PubMed] [Google Scholar]