Abstract
The influenza A virus protein PA-X comprises an N-terminal PA region and a C-terminal PA-X-specific region. PA-X suppresses host gene expression, termed shutoff, via mRNA cleavage. Although the endonuclease active site in the N-terminal PA region of PA-X and basic amino acids in the C-terminal PA-X-specific region are known to be important for PA-X shutoff activity, other amino acids may also play a role. Here, we used yeast to identify novel amino acids of PA-X that are important for PA-X shutoff activity. Unlike wild-type PA-X, most PA-X mutants predominantly localized in the cytoplasm, indicating that these mutations decreased the shutoff activity of PA-X by affecting PA-X translocation to the nucleus. Mapping of the identified amino acids onto the N-terminal structure of PA revealed that some of them likely contribute to the formation of the endonuclease active site of PA.
Introduction
Influenza A virus suppresses host gene expression via its NS1 (Nemeroff et al., 1998), viral polymerase complex (Garfinkel and Katze, 1992), NP (Khaperskyy et al., 2014), and PA-X (Khaperskyy et al., 2014) proteins to prevent anti-viral host protein expression from infected cells (Gao et al., 2015; Hayashi et al., 2015). PA-X, which is expressed via ribosomal frameshifting of the PA mRNA (Jagger et al., 2012; Shi et al., 2012), suppresses protein expression by using its endonuclease activity to digest mRNA (Desmet et al., 2013; Jagger et al., 2012). During influenza A virus infection, PA-X inhibits the production of IFN-β and anti-viral antibodies, which results in the modulation of viral growth and pathogenicity (Gao et al., 2015; Hayashi et al., 2015; Jagger et al., 2012). In vitro assays using recombinant PA-X have revealed that PA-X digests both single- and double-stranded RNAs and requires a bivalent cation as a cofactor for its shutoff activity (Bavagnoli et al., 2015). In virus-infected cells, PA-X selectively targets the products of cellular RNA polymerase II (Khaperskyy et al., 2016).
PA-X comprises the N-terminal 191 amino acids of PA fused to the C-terminal 41 or 61 amino acids that result from the frameshift. Since the bivalent cation-binding residue D108 and the catalytic residue K134 are important for both the endonuclease activity of PA (Yuan et al., 2009) and the shutoff activity of PA-X (Jagger et al., 2012), the endonuclease domain of PA-X likely forms a similar structure to that of PA. At the C-terminal PA-X-specific region, the basic amino acid residues spanning the N-terminus of this region are also important for PA-X shutoff activity (Oishi et al., 2015). Thus, although some residues important for PA-X shutoff activity have been identified, other amino acid residues may also be involved in the shutoff activity of PA-X. Here we identified novel amino acid residues involved in the shutoff activity of PA-X by using yeast.
Results
Emergence of unintentional PA-X mutants in yeast.
It remained unclear whether PA-X suppressed protein expression in eukaryotes other than mammals. To this end, we expressed PA-X in Saccharomyces cerevisiae (yeast) and found that yeast that were transformed with a plasmid expressing wild-type PA-X formed very few colonies, indicating that PA-X has shutoff activity even in yeast. Based on this finding, we attempted to identify host genes involved in the shutoff activity of PA-X by screening a yeast knockout library (manuscript submitted). Briefly, we transformed ~4,700 knockout yeast with a plasmid encoding the C-terminally FLAG-tagged authentic PA-X (PA-X C-FLAG) and observed colony formation. From this screen, we obtained 29 colonies, which harbored plasmids encoding unintended PA-X mutants. Of these 29 clones, a single nucleotide mutation was detected in 27, whereas two nucleotide mutations were detected in the other two clones (Table). Three mutations, g322a, g372t, and c436t, were found in two clones and the a80g mutation was found in three clones. Since all of these identified nucleotide mutations led to amino acid changed, 24 PA-X mutants were ultimately identified. We assumed that these amino acid substitutions affected the shutoff activity of PA-X.
Table.
Unintended mutations in PA-X found in yeast colonies.
| Amino acid mutations (Nucleotide mutation) | Number of clones obtained |
|---|---|
| F4S (t11c) | 1 |
| F9L (c27a) | 1 |
| Y24S (a71c) | 1 |
| D27G (a80g) | 3 |
| C39Y (g116a) | 1 |
| C45W (c135g) | 1 |
| A87V (c260t) | 1 |
| I94N (t281a) | 1 |
| L106P/S184I (t317c/g551t) | 1 |
| P107S (c319t) | 1 |
| D108N (g322a) | 2 |
| D108E (t324a) | 1 |
| E119N (g355a/a357t) | 1 |
| I120F (a358t) | 1 |
| T123I (c368t) | 1 |
| R124S (g372t) | 2 |
| R125K (g374a) | 1 |
| H146Y (c436t) | 2 |
| E154K (g460a) | 1 |
| E154A (a461c) | 1 |
| D160Y (g478t) | 1 |
| L163R (t488g) | 1 |
| R168M (g503t) | 1 |
| I171M (c513g) | 1 |
Identified mutations reduce the shutoff activity of PA-X in mammalian cells.
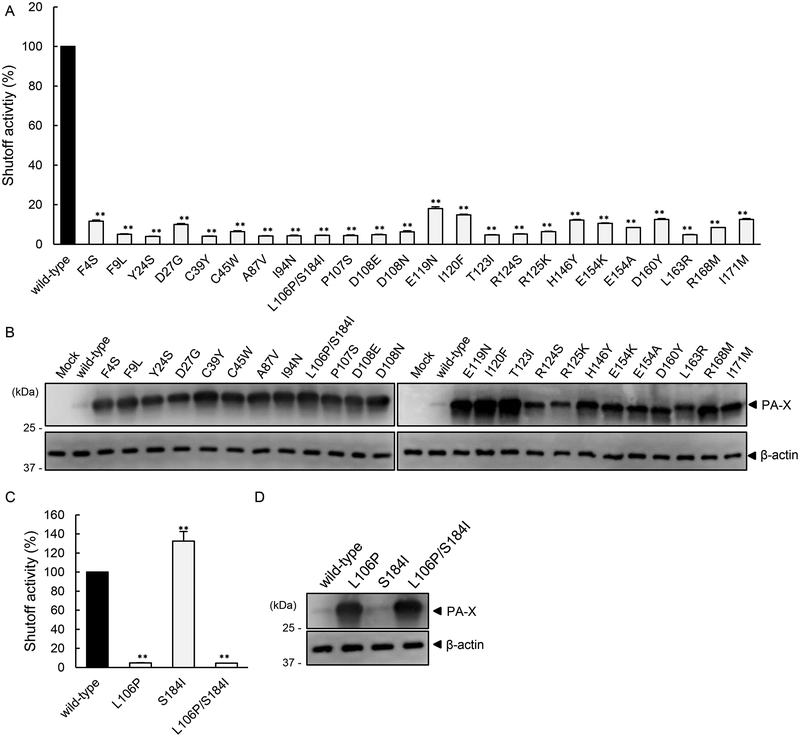
To evaluate the shutoff activity of the PA-X mutants in mammalian cells, we constructed plasmids encoding each mutant PA-X and conducted the shutoff assay using the luciferase system. The shutoff activities of all of the PA-X mutants were significantly lower than that of wild-type PA-X (Fig. 1A). Since PA-X suppresses its own expression via its shutoff activity (Oishi et al., 2015), the expression of each PA-X mutant was assessed by western blotting to confirm findings of the shutoff assay. The expression of all of the PA-X mutants was higher than that of wild-type PA-X (Fig. 1B). These results demonstrate that these amino acid mutations reduced the shutoff activity of PA-X. Of note, although the shutoff activities of PA-X R124S and PA-X R125K were comparable to those of the other PA-X mutants (Fig. 1A), the expression of these two mutants was somewhat lower than that of the other mutants (Fig. 1B). This observation could indicate that these mutations may decrease the stability of PA-X as well as its shutoff activity.
Figure 1. Identified mutations that reduced the shutoff activity of PA-X in mammalian cells.
Shutoff activity (A and C) and expression (B and D) of wild-type PA-X or the indicated PA-X mutants in 293 cells were compared. (A and C) The shutoff activity of wild-type PA-X was set to 100%. The shutoff activities are presented as the mean values ± SD (n=3 technical replicates). Representative data from three individual experiments are shown. **, P < 0.01 (one-way ANOVA followed by Dunnett’s test). (B and D) Expression of wild-type or mutant PA-X was analyzed by western blotting using a anti-FLAG antibody; β-actin served as a loading control.
To determine the contribution of each mutation in PA-X L106P/S184I to the shutoff activity, we prepared two PA-X mutants possessing single amino acid mutations and compared their shutoff activities. The shutoff activity of PA-X L106P was lower than that of wild-type PA-X and comparable to that of PA-X L106P/S184I. PA-X S184I showed comparable shutoff activity to wild-type PA-X (Fig. 1C). The expression level of PA-X L106P was similar to that of PA-X L106P/S184I, whereas that of PA-X S184I was similar to that of wild-type PA-X (Fig. 1D). These data indicate that L106 is required for the shutoff activity of PA-X.
Intracellular localization of the PA-X mutants.
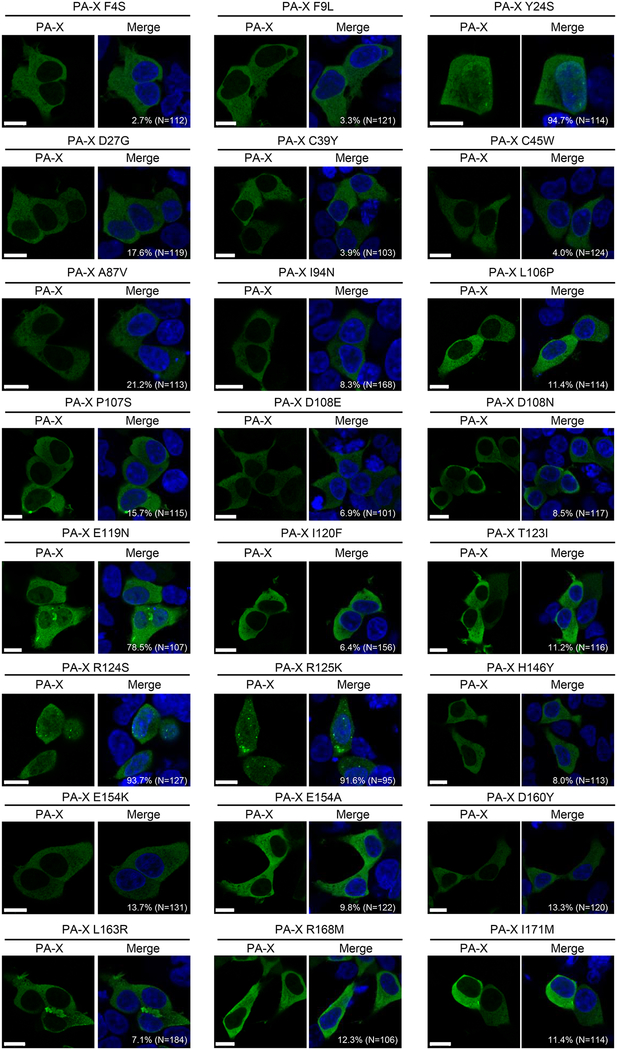
To examine whether the mutations that reduced the shutoff activity of PA-X affect its intracellular localization, the PA-X mutants were expressed in 293 cells by means of plasmid transfection. At 24 h post-transfection, we performed immunostaining with an anti-FLAG antibody to observe the intracellular localization of the PA-X mutants using confocal microscopy. Since wild-type PA-X robustly suppresses its own expression, we could not detect wild-type PA-X even in transfected cells, as described previously (Oishi et al., 2015). PA-X Y24S, PA-X R124S, and PA-X R125K localized both in the nucleus and the cytoplasm, as did PA-X E119N albeit at lower levels than these other three PA-X mutants; the remaining PA-X mutants predominantly localized in the cytoplasm (Fig. 2). All of the PA-X mutants were detected as a punctual staining pattern in a limited number of cells. Since nuclear localization of PA-X is important for its shutoff activity (Hayashi et al., 2016; Khaperskyy et al., 2016), we conclude that most of the identified mutations likely decrease the shutoff activity of PA-X by inhibiting its translocation to the nucleus.
Figure 2. Intracellular localization of mutant PA-X.
293 cells were transfected with a plasmid encoding the mutant PA-X and fixed 24 h later. These cells were then stained with an anti-DYKDDDDK (FLAG) tag antibody (green). All images were obtained using confocal microscopy. The nuclei are stained with Hoechst 33342 (blue). The percentage of cells in which PA-X was localized both in the nucleus and the cytoplasm is indicated in each panel. The total number of counted cells is indicated in parentheses. Bars, 10 μm.
Discussion
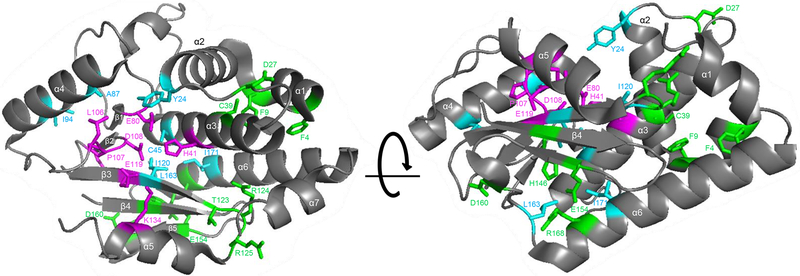
Here, we identified 22 amino acids that were important for the shutoff activity of PA-X. Since PA-X shares its N-terminal 191 amino acids with PA (Jagger et al., 2012), we mapped the identified 22 amino acids onto the N-terminal structure of PA, which includes a nuclease motif for mRNA cleavage (Fig. 3). Amino acid residues H41, E80, L106, P107, D108, E119, and K134 (Fig. 3; magenta) are known to be important for the nuclease activity of PA (Dias et al., 2009; Yuan et al., 2009). The former four residues bind to a bivalent cation, whereas K134 is the catalytic amino acid (Crepin et al., 2010). These findings thus suggest that these residues are also important for the shutoff activity of PA-X. Of the seven residues, our study demonstrated the importance of L106, P107, D108, and E119 (Fig. 3, shown in magenta) to the shutoff activity of PA-X. Therefore, some of the other amino acids identified as important for the shutoff activity of PA-X may also be important for the nuclease activity of PA in the cap-snatching process. Around the nuclease active site, the side chains of Y24, C45, A87, I94, I120, L163, and I171 (Fig. 3; cyan) extend toward the endonuclease active site, suggesting that these residues structurally support the formation of the nuclease active site. In contrast, F4, F9, D27, C39, T123, R124, and R125 (Fig. 3; green) are located far from the endonuclease active site. Therefore, these amino acids are likely involved in other ways. Although amino acid residues in the C-terminal unique region of PA-X, especially the six basic amino acids, are important for the shutoff activity of PA-X (Oishi et al., 2015), we did not obtain a PA-X mutant possessing a mutation in its C-terminal unique region.
Figure 3. Structural positions of the identified amino acids that reduced the shutoff activity of PA-X.
The identified amino acids are mapped onto a ribbon diagram of the structure of the N-terminal region of PA (2W69). Amino acid residues that are important for the endonuclease activity of PA are shown in magenta. Identified amino acid residues that locate near the endonuclease active site are shown in cyan. Other identified amino acids are shown in green.
PA-X possessing a mutation at Y24, E119, R124 or R125 localized both in the nucleus and the cytoplasm. This localization pattern is similar to that of wild-type PA-X (Hayashi et al., 2016; Khaperskyy et al., 2016). Y24 and E119 are located near the endonuclease active site, whereas R124 and R125 are not. Therefore, there was no correlation between the position of the mutation and PA-X localization.
In summary, here we identified novel amino acid residues that are important for the shutoff activity of PA-X by expressing PA-X in yeast. Since some of these amino acids have been shown to be important for the nuclease activity of PA, these residues in PA-X are likely involved in mRNA cleavage. The roles of the other amino acid residues that we identified in this study remain unknown; structure–activity correlation analyses will be required as part of future studies to further characterize these residues.
Materials and Methods
Cells.
Human embryonic kidney 293 cells (ATCC: CRL 1573) were maintained in DMEM (Sigma) supplemented with 10% FCS and penicillin-streptomycin.
Plasmids.
Nucleotide sequences of PA-X derived from A/WSN/33 (H1N1) were cloned into a mammalian expression plasmid, pCAGGS/MCS, or a yeast protein expression plasmid, pKT10 (Ura) (Tomita et al., 2011), with a C-terminal FLAG tag (C-FLAG). Mutant PA-X with the C-FLAG was amplified with primers possessing the desired substitution, and then cloned into pCAGGS/MCS. Primer sequences are available upon request. All constructs were sequenced to confirm the absence of unwanted mutations.
Yeast strains.
Yeast strain BY4743 and Yeast Knockout Collection Homozygous Diploid (BY4743 background), which includes 4,653 knockout strains covering 96% of the yeast genome, were purchased from GE Healthcare Dharmacon.
Yeast transformation.
Each knockout yeast strain was transformed with pKT10 (Ura) encoding PA-X with the C-FLAG or with an empty plasmid by using the S. cerevisiae Direct Transformation Kit Wako (Wako). Transformed yeast were plated onto SD/-Ura plates containing 200 μg/ml G418 and incubated for 3–5 days at 30 °C.
Plasmid extraction from yeast.
Transformed yeast were picked from the SD/-Ura plate, grown in liquid SD/-Ura medium for 1–2 days at 30 °C, and then harvested by centrifugation. To disrupt the yeast, they were suspended in PBS and vigorously vortexed together with acid-washed glass beads (Sigma). Plasmids were extracted from the supernatant of the disrupted yeast by using Wizard Plus SV Minipreps DNA Purification Systems (Promega). By using the extracted plasmids as a template, an open reading frame of PA-X was amplified by PCR and directly sequenced. To exclude the possibility of PCR errors, the plasmids extracted from yeast were also used to transform E. coli (DH5α strain). Plasmids extracted from E. coli were then resequenced.
Shutoff assay.
293 cells in four wells of a 24-well plate were transfected with a plasmid encoding firefly luciferase together with an empty plasmid, or a plasmid encoding wild-type or mutant PA-X with a C-FLAG. The transfected cells in three of the wells were analyzed for firefly luciferase activity by using the Bright-Glo luciferase assay system (Promega) at 24 h post-transfection. The shutoff activity of PA-X was calculated by dividing the luminescence value of firefly luciferase co-transfected with an empty plasmid by that co-transfected with PA-X-expressing plasmids. Data are shown as the average of the relative shutoff activity ± standard deviation (n=3). To analyze the expression of PA-X, the transfected cells in the fourth well were lysed in SDS sample buffer at 24 h post-transfection. The cell lysates were sonicated, incubated for 10 min at 95 °C, and then loaded onto an Any KD Mini-PROTEAN TGX Gel (Bio-Rad). Separated proteins were transferred to Immobilon-P PVDF membrane (Millipore) and detected by using the anti-DYKDDDDK (FLAG) tag antibody clone 1E6 (Wako) or the anti-β-actin antibody clone AC-74 (Sigma), followed by a sheep anti-mouse IgG-HRP (GE Healthcare).
Immunofluorescent assay.
293 cells were transfected with a plasmid encoding the indicated PA-X mutant. At 24 h post-transfection, the cells were fixed with 4% paraformaldehyde and permeabilized with 0.2% Triton X-100. Cells were stained with the anti-DYKDDDDK (FLAG) tag antibody clone 1E6, followed by an Alexa Fluor 488-conjugated goat anti-mouse IgG (Invitrogen). Nuclei were stained with Hoechst 33342 (Invitrogen). Images were obtained by using a Zeiss LSM780 (Carl Zeiss).
Acknowledgements
We thank Susan Watson for editing the manuscript.
Funding
This work was supported by the Japan Initiative for Global Research Network on Infectious Diseases (J-GRID) from the Japan Agency for Medical Research and Development (AMED), by Leading Advanced Projects for medical innovation (LEAP) from AMED, e-ASIA Joint Research Program from AMED, by Grants-in-Aid for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Science, Sports, and Technology (MEXT) of Japan (Nos. 16H06429, 16K21723, and 16H06434), by the Center for Research on Influenza Pathogenesis (CRIP) funded by NIAID Contract HHSN272201400008C, and by the NIH Functional Genomics award, “Characterization of novel genes encoded by RNA and DNA viruses” (U19 AI 107810).
Footnotes
Conflicts of interest
Y.K. has received speaker’s honoraria from Toyama Chemical and Astellas Inc., has received grant support from Chugai Pharmaceuticals, Daiichi Sankyo Pharmaceutical, Toyama Chemical, Tauns Laboratories, Inc., Tsumura & CO., and Denka Seiken Co., Ltd., and is a co-founder of FluGen.
References
- Bavagnoli L, Cucuzza S, Campanini G, Rovida F, Paolucci S, Baldanti F, Maga G, 2015. The novel influenza A virus protein PA-X and its naturally deleted variant show different enzymatic properties in comparison to the viral endonuclease PA. Nucleic acids research 43, 9405–9417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepin T, Dias A, Palencia A, Swale C, Cusack S, Ruigrok RW, 2010. Mutational and metal binding analysis of the endonuclease domain of the influenza virus polymerase PA subunit. Journal of virology 84, 9096–9104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmet EA, Bussey KA, Stone R, Takimoto T, 2013. Identification of the N-terminal domain of the influenza virus PA responsible for the suppression of host protein synthesis. Journal of virology 87, 3108–3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias A, Bouvier D, Crepin T, McCarthy AA, Hart DJ, Baudin F, Cusack S, Ruigrok RW, 2009. The cap-snatching endonuclease of influenza virus polymerase resides in the PA subunit. Nature 458, 914–918. [DOI] [PubMed] [Google Scholar]
- Gao H, Sun Y, Hu J, Qi L, Wang J, Xiong X, Wang Y, He Q, Lin Y, Kong W, Seng LG, Sun H, Pu J, Chang KC, Liu X, Liu J, 2015. The contribution of PA-X to the virulence of pandemic 2009 H1N1 and highly pathogenic H5N1 avian influenza viruses. Scientific reports 5, 8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel MS, Katze MG, 1992. Translational control by influenza virus. Selective and cap-dependent translation of viral mRNAs in infected cells. The Journal of biological chemistry 267, 9383–9390. [PubMed] [Google Scholar]
- Hayashi T, Chaimayo C, McGuinness J, Takimoto T, 2016. Critical Role of the PA-X C-Terminal Domain of Influenza A Virus in Its Subcellular Localization and Shutoff Activity. Journal of virology 90, 7131–7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, MacDonald LA, Takimoto T, 2015. Influenza A Virus Protein PA-X Contributes to Viral Growth and Suppression of the Host Antiviral and Immune Responses. Journal of virology 89, 6442–6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagger BW, Wise HM, Kash JC, Walters KA, Wills NM, Xiao YL, Dunfee RL, Schwartzman LM, Ozinsky A, Bell GL, Dalton RM, Lo A, Efstathiou S, Atkins JF, Firth AE, Taubenberger JK, Digard P, 2012. An overlapping protein-coding region in influenza A virus segment 3 modulates the host response. Science (New York, N.Y.) 337, 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaperskyy DA, Emara MM, Johnston BP, Anderson P, Hatchette TF, McCormick C, 2014. Influenza a virus host shutoff disables antiviral stress-induced translation arrest. PLoS pathogens 10, e1004217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaperskyy DA, Schmaling S, Larkins-Ford J, McCormick C, Gaglia MM, 2016. Selective Degradation of Host RNA Polymerase II Transcripts by Influenza A Virus PA-X Host Shutoff Protein. PLoS pathogens 12, e1005427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff ME, Barabino SM, Li Y, Keller W, Krug RM, 1998. Influenza virus NS1 protein interacts with the cellular 30 kDa subunit of CPSF and inhibits 3’end formation of cellular pre-mRNAs. Molecular cell 1, 991–1000. [DOI] [PubMed] [Google Scholar]
- Oishi K, Yamayoshi S, Kawaoka Y, 2015. Mapping of a Region of the PA-X Protein of Influenza A Virus That Is Important for Its Shutoff Activity. Journal of virology 89, 8661–8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Jagger BW, Wise HM, Digard P, Holmes EC, Taubenberger JK, 2012. Evolutionary conservation of the PA-X open reading frame in segment 3 of influenza A virus. Journal of virology 86, 12411–12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita Y, Noda T, Fujii K, Watanabe T, Morikawa Y, Kawaoka Y, 2011. The cellular factors Vps18 and Mon2 are required for efficient production of infectious HIV-1 particles. Journal of virology 85, 5618–5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P, Bartlam M, Lou Z, Chen S, Zhou J, He X, Lv Z, Ge R, Li X, Deng T, Fodor E, Rao Z, Liu Y, 2009. Crystal structure of an avian influenza polymerase PA(N) reveals an endonuclease active site. Nature 458, 909–913. [DOI] [PubMed] [Google Scholar]