Abstract
Background:
Smokers are highly susceptible to lung and cardiovascular disease that can reduce their survival. Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) is a protein in the circulation that may suppress vascular and pulmonary inflammation. Therefore, we hypothesized that diminished circulating TRAIL levels would be associated with poor survival in smokers with lung and cardiovascular disease.
Methods:
Serum TRAIL level was measured by immunoassay in 474 smokers. Coronary atherosclerosis was assessed by coronary artery calcium scoring along with emphysema, lung function, and survival.
Results:
The 474 smokers were 65.7 ± 6.3 years old and 52.2% male with 55.3 ± 31.5 pack-years of tobacco-exposure. 83 of them died during 3588.2 person-years of follow up. At baseline, lower TRAIL level was associated with more coronary artery calcium (OR=1.2 per SD, 95%CI 1.1–1.5, p=0.02), and with history of myocardial infarction (OR=2.3 per SD, 95%CI 1.2–4.5, p=0.02), angina (OR=1.6 per SD, 95%CI 1.1–2.6, p=0.03), and angioplasty (OR=1.8 per SD, 95%CI 1.0–3.1, p=0.04) in models adjusted for cardiovascular risk-factors, FEV1, and emphysema. Also, lower TRAIL level was associated with emphysema severity independent of demographics and tobacco exposure (β=0.11 sq. root units, 95% CI 0.01–0.22, p=0.03). Further, TRAIL level was lowest in smokers with comorbid emphysema and coronary artery calcification rather than either condition alone. Finally, lower TRAIL level was independently associated with increased mortality in smokers particularly in those with comorbid emphysema and coronary artery calcification (HR=1.38, 95% CI 1.01–1.90).
Conclusions:
TRAIL level is reduced in smokers with comorbid emphysema and coronary artery disease, and is associated with reduced survival.
Keywords: COPD, mortality, coronary artery calcium, emphysema, TRAIL
Introduction
Smoking is responsible for 1 in 5 deaths among adults in the US [1]. Smokers commonly manifest cardiovascular and lung disease that can reduce their survival. Early identification of smokers with lung and cardiovascular disease and those who are at risk for premature death is important because these individuals are likely to benefit the most from preventative measures. However, we have limited means to identify smokers with these multisystem comorbidities who are likely to experience poor outcomes.
TNF-related apoptosis inducing ligand (TRAIL) is a trans-membrane protein with an extracellular domain that can be proteolytically cleaved and detected in the circulation as soluble TRAIL [2]. The soluble form of TRAIL (henceforth referred to as TRAIL) can ligate receptors on the cell membrane and induce downstream signaling [3]. In vitro and animal studies suggest that TRAIL can reduce inflammation in the vasculature and the and lungs. Specifically, TRAIL deficient mice have accelerated vascular inflammation, vascular calcification, and atherosclerosis [4, 5]. Also, inhalation of recombinant TRAIL results in apoptosis of airway inflammatory cells and reduction in lung inflammation, while anti-TRAIL treatment blocked apoptosis and increased infiltration of inflammatory cells into the airway [6]. Therefore, the studies suggest that TRAIL levels may be inversely associated with vascular and pulmonary inflammation.
Given the importance of increased inflammation in the pathogenesis of coronary artery disease (CAD) and COPD, we hypothesized that TRAIL levels would be suppressed in smokers with CAD and COPD, and would predict reduced survival. We tested our hypothesis by examining TRAIL, CAD, COPD, and survival in a cohort of 474 smokers.
Methods
Study Population
The Pittsburgh SCCOR (Specialized Centers for Clinically Oriented Research) study is a longitudinal cohort study that enrolled 751 individuals. These individuals were derived from two sources: 544 participants were enrolled from the community as part of the Pittsburgh Lung Screening Study (PLuSS) study [7], while 207 were recruited from patients referred for lung transplantation due to advanced COPD. The 207 pre-transplant participants were excluded from the current analysis because transplantation would confound their survival assessment and they had not undergone complete assessment of cardiovascular risk factors. Of the remaining 544 participants, 474 with complete data on coronary artery calcium score, serum TRAIL level, spirometry, and emphysema assessments were included in the current study. These 474 participants were similar to the remaining 70 of 544 community-based individuals in terms of age, gender, and FEV1 % predicted (all p>0.05)
Enrollment criteria for the SCCOR cohort have been described previously [8]. In brief, all participants were 40–79 years in age with >10 pack-years of current or prior smoking, and without prior thoracic surgery, vascular or cardiac events in the preceding year, a restrictive pattern on spirometry, active malignancy or any other comorbidity expected to reduce survival below five years.
Because the presence of airflow obstruction (i.e. FEV1/FVC<0.70) was not required for enrollment, the cohort included smokers with and without COPD. The study was approved by the Institutional Review Board at the University of Pittsburgh and written informed consent was obtained from each participant.
TRAIL
Blood samples for TRAIL measurement were collected at enrollment when CT scans, spirometry, questionnaire, and other data used in this analysis were collected. Serum TRAIL level was measured by electrochemiluminescence immunoassay, per the manufacturer’s instructions (Meso Scale Discovery, Gaithersburg, Maryland). The sensitivity of the assay was 2.86 pg/ml with an intra- and inter-assay coefficient of variation of 3.9% and 6% respectively per the manufacturer.
Coronary artery disease (CAD)
CAD was assessed by quantifying coronary artery calcium using non-EKG gated chest CT. Calcium scores from non-EKG gated CT have excellent correlation with scores from EKG gated CT, and have been independently validated as predictors of coronary vascular events and mortality by multiple investigators [9–12]. Coronary artery calcium was scored visually using the Weston method as described previously [10]. This scoring system has been validated by independent investigators, including our group [10, 13]. Inter and intra-observer agreement on Weston scores is excellent (both κ>0.90) [13].
The Weston score ranges from 0 to 12 with higher scores representing increased amount of coronary artery calcium. Scores were divided into 4 categories due to their skewed distribution (0 = none, 1–3 = mild, 4–7 = moderate, 8–12 = severe) that correspond to the 4 categories of Agatston scores (0, 1–100, 101–400, >400) with sensitivity of 100% and specificity >85% [10].
Vascular Events
Data on prior myocardial infarction, angina, stroke, and angioplasty was selfreported via questionnaire.
Emphysema
Chest CT images were acquired at full inspiration. Emphysema severity was assessed by visual interpretation as well as density mask analysis [14].
Visual scoring was performed using the method of the National Emphysema Treatment Trial (NETT) by an expert radiologist (a NETT co-investigator) blinded to subject identities. Emphysema was scored using a 6-point scale: no emphysema = 0, 110% emphysema = 1 point, 11% to 25% emphysema = 2 points, 26% to 50% emphysema = 3 points, 51% to 75% emphysema = 4 points, and >75% emphysema = 5 points. These visual scores are associated with clinically important disease manifestations and outcomes in smokers [8, 15], and have been validated previously [16].
Emphysema was also assessed by density mask analyses as the percent of total lung volume less than −950 Hounsfield units (HU) in density, i.e. % low attenuation area (%LAA) as described previously [17].
Pulmonary function
Post-bronchodilator spirometry was performed and adjusted to standard population-derived predicted values [18]. Diffusing capacity was assessed as the singlebreath diffusing capacity for carbon monoxide (DLco) per standard guidelines [19]. Lung hyperinflation (i.e. ratio of residual volume to total lung capacity or RV/TLC) was assessed by full body plethysmography.
Unscheduled visits
All participants had to be free from exacerbations for at least 30 days prior to onset of our study. At enrollment, we collected data on self-reported unscheduled visits to an emergency room or physician for respiratory difficulty in the six months prior.
Cancer
Patients with cancers that had been treated and in remission were eligible for inclusion, while those with active malignancies with expected survival <5 years were not enrolled. At baseline, 1 participant with a history of lung cancer, and 62 participants with histories of other types of cancers were enrolled. Diagnoses of new cancers was tracked prospectively via periodic phone interview by the Pittsburgh Lung Screening Study (PLuSS) study from which these patients were enrolled.
Mortality
Vital status was collected from the Social Security Death Index until March 31st 2014. From April 2014-Dec 2016, vital status was ascertained during biannual telephone interview with participants/their families.
Covariate ascertainment
Demographics, medication use, smoking status, and pack-years of smoking were self-reported. Hypertension was ascertained by use of anti-hypertensive medication or systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg measured during the study visit. Hyperlipidemia and diabetes were defined by use of lipid-lowering or diabetes medication respectively, or self-report of the diagnosis. High sensitivity C-reactive protein (hs-CRP) was measured in plasma samples using an ultrasensitive electrochemiluminescence immunoassay, according to the manufacturer’s protocol (Meso Scale Discovery, Gaithersburg, Maryland). Interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α) levels were measured using ELISA kits according to the manufacturer’s instructions (R and D Systems, Minneapolis, MN). Exercise capacity was assessed by measuring the shuttle walk distance as described previously [20].
Statistical Analyses
Retrospective analysis of prospectively collected data was performed. Descriptive analyses of baseline characteristics was performed across quartiles of TRAIL to facilitate tabular display. For the remaining analyses, TRAIL was analyzed as a continuous variable and effect estimates are presented per standard deviation difference in TRAIL levels. The association between TRAIL and various outcomes of interest was assessed using logistic regression if the outcome was a yes/no variable (history of MI, angina, angioplasty, and stroke), ordinal logistic regression if the outcome was in multiple ordinal categories (coronary artery calcium score and visual emphysema score), linear regression if the outcome was normally distributed (square root of LAA% −950), and using Cox proportional hazard models for time to event outcome (survival time). All regression models were adjusted for relevant covariates listed in the results. Use of ordinal models was supported by non-significant p for likelihood ratio tests for the proportional odds assumption. The proportionality assumption for Cox models was tested using Schoenfeld residuals. Interactions were tested in all models and considered statistically significant if p<0.15, per convention.
Analyses were performed using STATA MP version 14.0 (StataCorp, College Station, TX). Statistical significance was defined as two-tailed p<0.05
Results
Study population
The 474 participants were 65.7 ± 6.3 years old, predominantly Caucasian (95% white), and had an almost equal gender distribution (52.2% men and 47.7% women). There was a high prevalence of hypertension (53%) and hyperlipidemia (57%), while diabetes was less frequent (10%).
Participants had smoked for an average of 55.3 ± 31.5 pack years (46% were active smokers) and 50.8% had spirometric airflow obstruction. The median %LAA −950 was 0.8% (IQR 0.4–3.0 %), median emphysema score was 1 (IQR 0–2), and 58.0% of participants had some emphysema by visual scoring. 15.8% of participants had no coronary artery calcium, while 25.1% had mild, 33.1% had moderate, and 25.9% had severe coronary artery calcification.
Serum TRAIL
Mean TRAIL level was 92.6 ± 38.2 pg/ml. Baseline characteristics stratified by TRAIL level are summarized in Table 1. Lower serum TRAIL level was associated with older age, female gender, greater pack-years of smoking, and higher systolic blood pressure.
Table 1.
Participant characteristics by quartile of serum TRAIL level. Continuous variables are summarized as median (IQR).
| 1st/L006Fwest Quartile | 2nd Quartile | 3rd Quartile | 4th/Highest Quartile | p | |
|---|---|---|---|---|---|
| n (%) | 119 (25.1) | 118 (24.9) | 119 (25.1) | 118 (24.9) | |
| Serum TRAIL, pg/ml | 54.0 (45.3; 59.5) | 77.6 (73.2; 81.9) | 98.7 (91.6; 104.6) | 130.6 (122.6; 153.2) | |
| Age, years | 68.0 (62.0; 72.0) | 64.0 (60.0; 69.0) | 64.0 (60.0; 69.0) | 64.5 (61.0; 70.0) | 0.005 |
| Male (%) | 49 (41.2) | 51 (43.2) | 55 (46.2) | 72 (61.0) | 0.002 |
| Caucasian (%) | 115 (96.6) | 110 (93.2) | 113 (95.0) | 110 (93.2) | 0.37 |
| BMI, kg/m2 | 28.0 (25.1; 30.0) | 27.8 (24.9; 31.4) | 28.0 (24.8; 31.4) | 27.6 (25.1; 31.6) | 0.22 |
| Current smoking (%) | 45 (37.8) | 57 (48.3) | 52 (43.7) | 62 (52.5) | 0.053 |
| Pack years of smoking | 52.0 (40.0; 75.0) | 45.0 (30.0; 60.0) | 45.0 (33.8; 63.0) | 48.0 (35.0; 75.2) | 0.04 |
| FEV1, % predicted | 79.1 (22.4) | 86.1 (18.3) | 85.7 (19.7) | 82.6 (19.6) | 0.20 |
| FEV1/FVC | 63.8 (13.3) | 68.8 (11.5) | 68.5 (12.1) | 67.7 (12.4) | 0.10 |
| Shuttle walk distance, ft | 403.3 (144.8) | 446.8 (137.4) | 450.3 (139.6) | 409.3 (138.6) | 0.70 |
| Unscheduled visits (%)* | 4.2 | 7.6 | 8.4 | 9.3 | 0.46 |
| Systolic BP, mm Hg | 132.0 (125.0; 144.0) | 134.0 (121.0; 145.0) | 130.0 (120.0; 144.0) | 129.0 (118.0; 143.0) | 0.05 |
| Diastolic BP, mm Hg | 76.0 (69.0; 82.0) | 77.0 (69.0; 82.0) | 74.0 (69.0; 80.0) | 75.0 (68.0; 81.0) | 0.21 |
| Hypertension (%) | 64 (54.2) | 62 (53.9) | 62 (52.5) | 56 (47.9) | 0.32 |
| Diabetes mellitus (%) | 14 (11.9) | 13 (11.4) | 10 (8.5) | 8 (6.9) | 0.15 |
| Hyperlipidemia (%) | 74 (63.2) | 63 (54.8) | 59 (49.6) | 65 (56.0) | 0.19 |
| hs-CRP, mg/L | 1.0 (0.4; 3.7) | 1.1 (0.4; 2.1) | 1.2 (0.5; 2.4) | 1.3 (0.5; 2.6) | 0.62 |
| IL-6, pg/mL | 1.4 (0.9; 2.0) | 1.1 (0.8; 1.7) | 1.2 (0.9; 1.9) | 1.3 (0.8; 2.3) | 0.29 |
| TNF-α, pg/mL | 1.6 (1.4; 2.1) | 1.7 (1.4; 2.4) | 1.8 (1.3; 2.4) | 1.9 (1.4; 2.6) | 0.24 |
Defined as an unscheduled visit to a physician or emergency room for respiratory difficulty in the six months prior to enrollment.
TRAIL and coronary artery disease
Lower TRAIL level was associated with greater coronary artery calcium, history of myocardial infarction and angioplasty, and showed a trend for history of angina. Mean TRAIL level was 21.6 pg/ml lower in those with a history of myocardial infarction (p = 0.006), 8.2 pg/ml lower in those with a history of angina (p = 0.12), 17.6 pg/mL lower in those with history of angioplasty (p = 0.02) than in those without these conditions (Figure 1). Adjustment for age, gender, race, body mass index, hypertension, hyperlipidemia, diabetes, smoking status, pack-years of smoking, FEV1 % predicted, and visual emphysema score did not substantially alter these associations (Figure 2). Also, limiting the analysis to patients with subclinical disease only (i.e. removing those with history of MI, angina, or angioplasty) had no impact on the association between lower TRAIL and increased coronary artery calcium (OR = 1.23, 95% CI 1.01–1.50, p = 0.04 adjusted for the same covariates as above). Unlike coronary artery disease, TRAIL level was not associated with history of stroke (p = 0.40).
Figure 1:
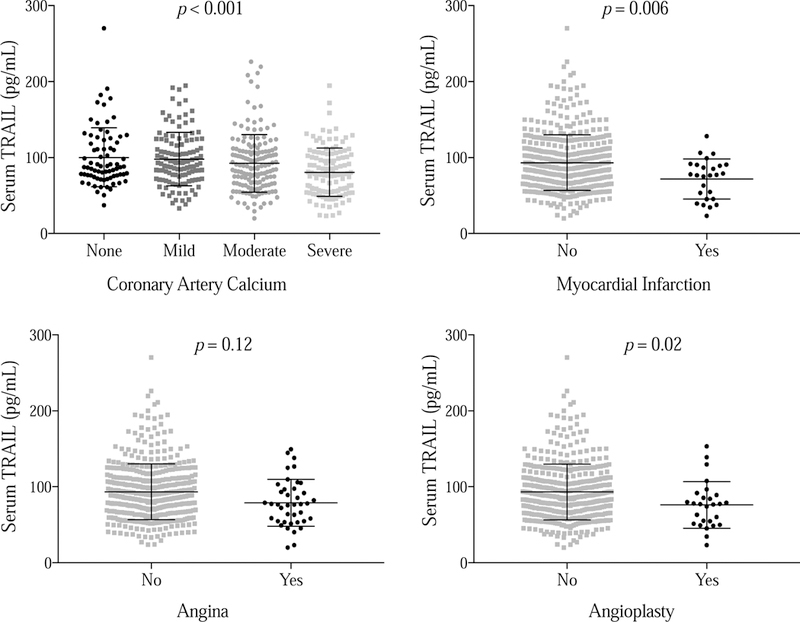
Lower serum TRAIL level was associated with increased coronary artery calcium, history of myocardial infarction and angioplasty, and demonstrated a trend for an association with history of angina.
Figure 2:
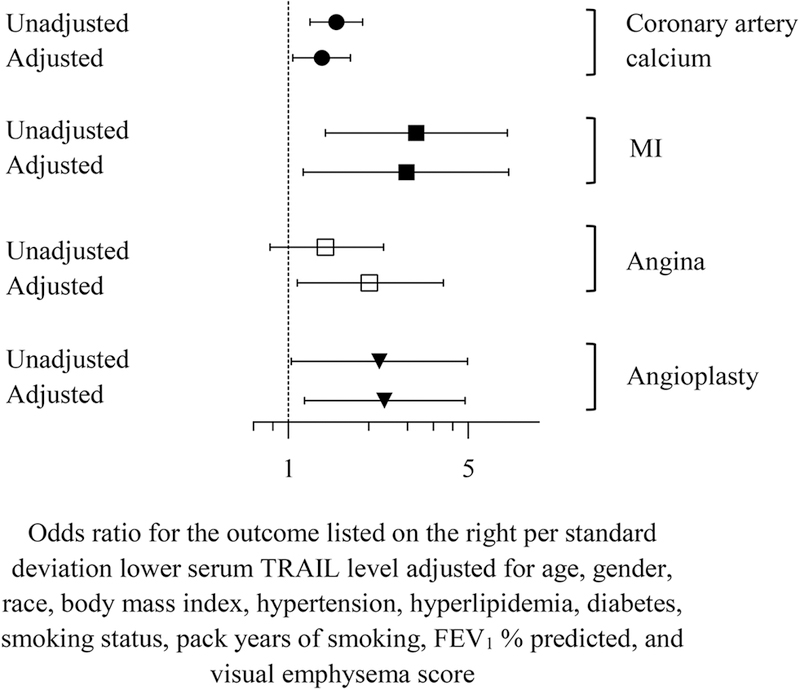
The unadjusted and adjusted association between lower circulating TRAIL level and sub-clinical and clinical coronary artery disease.
Lung disease
Lower TRAIL level was associated with more emphysema. Each standard deviation lower TRAIL level was associated with 20.2% increase in the odds of being in one higher category of visual emphysema score (OR = 1.20, 95% CI 1.02–1.42, p = 0.03) and with increased LAA% −950 (β = 0.13 sq. root units, 95% CI 0.03–0.24, p = 0.01, Figure 3). Adjustment for age, gender, race, smoking status, pack years of smoking, and coronary artery calcium score had minimal impact on these associations: each standard deviation lower TRAIL was associated with 23% increase in the odds of being in one higher category of visual emphysema score (OR = 1.23, 95% CI 1.03–1.46, p = 0.02) and with LAA% −950 (β = 0.11 sq. root units, 95% CI 0.01–0.22, p = 0.03) in adjusted models. In contrast, there was no association between TRAIL and FEV1 % predicted (p = 0.51), DLco % predicted (p = 0.25), or RV/TLC (p = 0.99).
Figure 3:
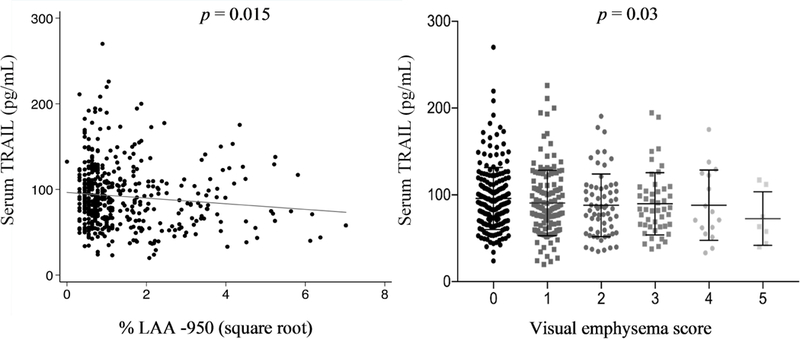
Lower serum TRAIL level was associated with more emphysema.
Comorbid coronary artery disease and emphysema
Because lower TRAIL level was associated with both increased coronary artery calcium and emphysema, we divided our cohort into 4 subgroups based on the presence (or absence) of visually assessed CAD and emphysema: (1) healthy smokers - negative for coronary artery calcium and no emphysema, (2) emphysema only - negative for coronary artery calcium and positive for emphysema, (3) CAD only - positive for coronary artery calcium and negative for emphysema, and (4) comorbid emphysema and CAD - positive for both coronary artery calcium and emphysema. The characteristics of these 4 subgroups are summarized in Table 2. There were significant differences in age, gender, race, body mass index, pack-years of smoking, systolic blood pressure, and hypertension between the subgroups.
Table 2:
Participant characteristics by presence/absence of emphysema and coronary artery calcium. Continuous variables are summarized as median (IQR).
| Heathy Smokers | Emphysema only | CAD only | Emphysema and CAD | p | |
|---|---|---|---|---|---|
| n (%) | 38 (8.0) | 37 (7.8) | 161 (34.0) | 238 (50.2) | |
| Age, years | 60.0 (58.0; 63.0) | 62.0 (60.0; 64.0) | 66.0 (62.0; 69.0) | 66.0 (61.0; 72.0) | <0.001 |
| Male (%) | 28 (73.7) | 25 (67.6) | 68 (42.2) | 106 (44.5) | <0.001 |
| Caucasian (%) | 35 (92.1) | 31 (83.8) | 154 (95.7) | 228 (95.8) | 0.02 |
| BMI, kg/m2 | 29.6 (24.2; 32.2) | 26.7 (24.5; 28.8) | 29.0 (26.7; 31.9) | 27.1 (23.8; 30.1) | <0.001 |
| Current smoking (%) | 15 (39.5) | 21 (56.8) | 74 (46.0) | 106 (44.5) | 0.47 |
| Pack years of smoking | 36.5 (30.0; 50.0) | 40.0 (25.0; 60.0) | 44.0 (30.0; 60.0) | 55.5 (40.0; 80.0) | <0.001 |
| Systolic BP, mm Hg | 122.5 (113.0; 134.0) | 124.0 (119.0; 138.0) | 133.0 (124.0; 145.0) | 132.0 (122.0; 145.0) | 0.001 |
| Diastolic BP, mm Hg | 73.0 (68.0; 80.0) | 76.0 (70.0; 82.0) | 77.0 (69.0; 82.0) | 75.0 (68.0; 81.0) | 0.30 |
| Hypertension (%) | 13 (34.2) | 13 (35.1) | 88 (55.3) | 130 (55.6) | 0.01 |
| Diabetes mellitus (%) | 0 (0.0) | 1 (2.8) | 19 (11.9) | 25 (10.7) | 0.06 |
| Hyperlipidemia (%) | 17 (44.7) | 17 (47.2) | 94 (58.8) | 133 (57.1) | 0.30 |
| hs-CRP, mg/L | 0.9 (0.3; 2.6) | 1.4 (0.7; 2.5) | 1.1 (0.5; 2.3) | 1.3 (0.4; 2.8) | 0.43 |
TRAIL level was significantly reduced only in the subgroup with comorbid emphysema and CAD (Figure 4). In these individuals, mean TRAIL levels were lower by 9.2 pg/mL compared to the rest of the cohort (95% CI 2.4 – 16.1, p = 0.008). Adjustment for age, gender, race, body mass index, pack-years of smoking, systolic blood pressure, and hypertension had little impact (mean difference 8.0 pg/mL, 95% CI 0.8 – 15.2, p = 0.03).
Figure 4:
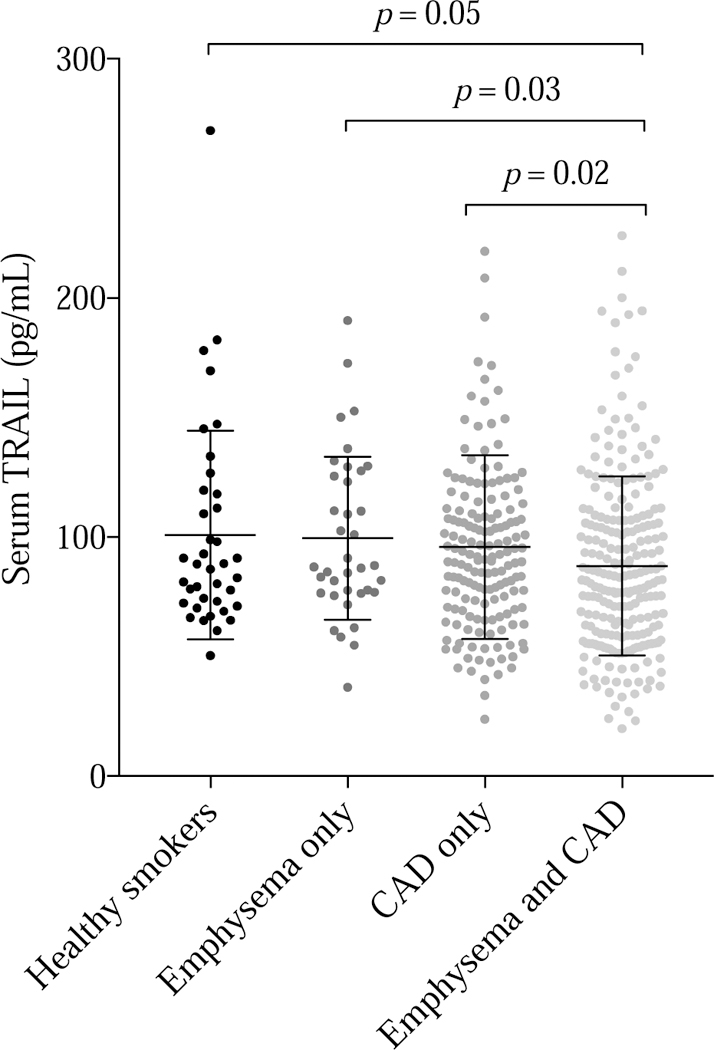
Serum TRAIL level was lower in those with comorbid emphysema and coronary artery calcium.
Mortality
The 474 participants were followed for an aggregate of 3588.2 person-years (mean 7.6 ± 1.5 years) during which 83 individuals died. Each standard deviation lower TRAIL level was associated with 42% higher risk of death (HR = 1.42, 95% CI 1.101.85, p = 0.004). The association remained significant despite adjustment for age, gender, race, body mass index, pack-years of smoking, current smoking status, and FEV1 percent predicted (HR = 1.28, 95% CI 1.00–1.63, p = 0.05). The association was strongest in those with comorbid emphysema and CAD (HR = 1.38, 95% CI 1.01–1.90, p = 0.04 adjusted for same covariates as above) and not statistically significant in the other subgroups (Figure 5, p for interaction due to comorbid emphysema and CAD = 0.09).
Figure 5:
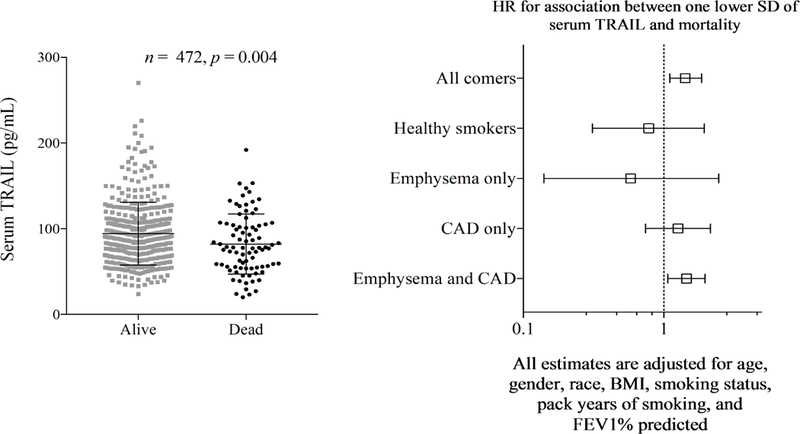
Lower serum TRAIL level was associated with reduced survival
Cancer
At baseline among the 474 participants 1 was included with history of lung cancer, while 62 patients with histories of other types of cancers were enrolled. At baseline, there was no relationship between serum TRAIL level and history of any type of cancer (OR=0.97, 95% CI 0.74–1.27, p=0.84). During follow up, 31 new cancers were diagnosed (7 lung cancers and 24 other cancers). Again there was no relationship between serum TRAIL level and the occurrence of new cancers (OR=0.75, 95% CI 0.49–1.15, p=0.18), including lung cancer (OR=8.65, 95% CI 0.10–716.8, p=0.34), or other cancers (OR=0.63, 95% CI 0.34–1.14, p=0.13).
Discussion
We examined a cohort of 474 community-based smokers and found that TRAIL levels were lower in those with comorbid emphysema and coronary artery disease rather than those with either coronary artery disease or emphysema alone. Lower TRAIL level was also associated with increased mortality in smokers, particularly those with comorbid emphysema and coronary artery disease. Therefore, TRAIL maybe useful in identifying smokers with heart and lung disease associated with poor prognosis who may benefit from aggressive preventative measures.
TRAIL is regarded as an apoptosis inducing peptide that can enhance the resolution of inflammation [21]. Anti-inflammatory properties of TRAIL have been demonstrated in various diseases including arthritis, autoimmunity, nonalcoholic steatohepatitis, colitis, atherosclerosis, and airway inflammation [22–26].
TRAIL is being actively studied in vitro and in vivo as a regulator of vascular inflammation and atherosclerosis [27, 28]. Intraperitoneal administration of TRAIL or TRAIL overexpression by adenoviral transfection protected mice from atherosclerosis [4, 27, 29]. Also, TRAIL knockout mice exhibited increased vascular inflammation and lower insulin resistance. In vitro cell culture-based studies have reported both anti and pro-inflammatory actions of TRAIL, and variability in cell culture conditions is suspected to be the reason for these inconsistent results [4]. Human studies on serum TRAIL and cardiovascular disease have been consistent and found TRAIL levels to be reduced in those presenting with an acute vascular event [30–33]. Reduced TRAIL levels have also been associated with worse endothelial function [34, 35]. No prior study has reported an association between lower circulating TRAIL and stable coronary artery disease to our knowledge.
TRAIL has not been adequately examined in the context of human airway disease and early studies have produced inconsistent results. Inhalation of recombinant TRAIL by mice with chronic allergic airway inflammation resulted in apoptosis of inflammatory cells and reduction in inflammation, while anti-TRAIL treatment blocked apoptosis and increased infiltration of inflammatory cells into the airway [6]. In contrast, therapeutic neutralization of TRAIL reduced pulmonary inflammation, emphysema-like alveolar enlargement, and small airway changes in another study [36]. The only prior human study on circulating TRAIL level and lung disease included 57 smokers with COPD and 35 controls without COPD enrolled from the outpatient department of the West China Hospital [37]. Contrary to our findings, higher TRAIL level was associated with a diagnosis of COPD in univariate analysis, however, no analysis adjusted for age and gender differences between COPD and control patients was performed, limiting interpretation of results. Also, emphysema, coronary artery disease, and survival were not assessed.
Reduced circulating TRAIL levels have previously been associated with decreased survival. Specifically, reduce serum TRAIL was associated with increased serum C-reactive protein levels, reduced endothelial function, and decreased survival in patients with end stage renal disease who are also susceptible to premature atherosclerosis like patients with COPD [38].
The current study is the largest to examine serum TRAIL level in smokers. Prior animal studies suggesting that TRAIL is protective against lung injury are supported by our data, rather than those reporting that TRAIL is detrimental to the lungs. Also, our data suggests that TRAIL levels are reduced in those with stable and sub-clinical coronary artery disease not just in those presenting with acute vascular events. Further, our findings suggest that reduced serum trail levels may help identify smokers with comorbid coronary artery disease and emphysema at risk for poor outcomes.
The current study has limitations. First, history of MI, angina, and angioplasty was self-reported. However, results for these self-reported measures were similar to those observed with objectively measured coronary artery calcium scores suggesting that the association between TRAIL and coronary artery disease was not due to recall bias. Second, our cohort was predominantly Caucasian and generalizability to other race/ethnicities could not be demonstrated. Next, we have not demonstrated a clear cut off for using TRAIL as a clinical biomarker and there was significant overlap in TRAIL levels between subgroups in our cohort. However, it is frequently true that biomarkers for heterogenous and complex diseases such as COPD do not demonstrate large differences in a mixed group of individuals enrolled in research cohorts. TRAIL may prove to be useful prognostic biomarker for subgroups of individuals with lung and coronary artery disease there are, for example, at intermediate risk or have specific subphenotypes of COPD. Also, coronary artery calcium was measured using non-EKG gated CT, however, there is extensive evidence to support the validity of this approach. Further, we were not able to validate our results in an unrelated cohort as none of the COPD cohorts have performed measurement of serum TRAIL to our knowledge. Finally, all-cause rather than cause-specific mortality was assessed; therefore, we could not determine the cause of increased mortality in those with reduced TRAIL levels.
In conclusion, our results suggest that TRAIL levels are diminished in smokers with comorbid emphysema and coronary artery disease and are predictive of poor survival in these patients. Therefore, TRAIL may be useful in identifying smokers with heart and lung disease associated with poor prognosis who may benefit from aggressive preventative measures. TRAIL levels should be assessed in lung disease cohorts to further investigate it as a potential biomarker for comorbid emphysema and coronary artery disease and poor survival.
Reduced serum TRAIL is associated with more coronary artery disease (CAD).
Reduced serum TRAIL is associated with increased emphysema.
TRAIL level is lowest in smokers with both emphysema and CAD.
Reduced serum TRAIL predicts mortality in smokers, particularly those with emphysema and CAD.
Acknowledgements:
Funding: Supported by NHLBI (K23HL126912, Chandra), the Samuel Winters Foundation (713263, Chandra), PA Tobacco Fund Health Research Grant SAP 4100062224 (Sciurba) and HL084948. The sponsors had no direct role in the design, conduct, or reporting of the results of this study.
Abbreviations:
- TRAIL
TNF-related apoptosis inducing ligand
- SCCOR
Specialized Centers for Clinically Oriented Research
- CAD
coronary artery disease
- HU
Hounsfield units
- LAA
low-attenuation area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Additional Information
Availability of data: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations of interest: none
References
- 1. CDC. Centers for Disease Control and Prevention Fast Facts.
- 2.Fulda S Tumor-necrosis-factor-related apoptosis-inducing ligand (TRAIL). Adv Exp Med Biol 2014;818:167–80. doi: 10.1007/978-1-4471-6458-6_8. [DOI] [PubMed] [Google Scholar]
- 3.Rowinsky EK. Targeted induction of apoptosis in cancer management: the emerging role of tumor necrosis factor-related apoptosis-inducing ligand receptor activating agents. J Clin Oncol 2005;23(36):9394–407. doi: 10.1200/JCO.2005.02.2889. [DOI] [PubMed] [Google Scholar]
- 4.Cartland SP, Harith HH, Genner SW, Dang L, Cogger VC, Vellozzi M, et al. Non-alcoholic fatty liver disease, vascular inflammation and insulin resistance are exacerbated by TRAIL deletion in mice. Sci Rep 2017;7(1):1898 Epub 2017/05/17. doi: 10.1038/s41598-017-01721-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Bartolo BA, Cartland SP, Harith HH, Bobryshev YV, Schoppet M, Kavurma MM. TRAIL-deficiency accelerates vascular calcification in atherosclerosis via modulation of RANKL. PLoS One 2013;8(9):e74211 Epub 2013/09/17. doi: 10.1371/journal.pone.0074211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faustino L, Fonseca DM, Florsheim EB, Resende RR, Lepique AP, FaquimMauro E, et al. Tumor necrosis factor-related apoptosis-inducing ligand mediates the resolution of allergic airway inflammation induced by chronic allergen inhalation. Mucosal Immunol 2014;7(5):1199–208. doi: 10.1038/mi.2014.9. [DOI] [PubMed] [Google Scholar]
- 7.Wilson DO, Weissfeld JL, Fuhrman CR, Fisher SN, Balogh P, Landreneau RJ, et al. The Pittsburgh Lung Screening Study (PLuSS): outcomes within 3 years of a first computed tomography scan. Am J Respir Crit Care Med 2008;178(9):956–61. doi: 10.1164/rccm.200802-336OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandra D, Stamm JA, Palevsky PM, Leader JK, Fuhrman CR, Zhang Y, et al. The relationship between pulmonary emphysema and kidney function in smokers. Chest 2012;142(3):655–62. doi: 10.1378/chest.11-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Budoff MJ, Nasir K, Kinney GL, Hokanson JE, Barr RG, Steiner R, et al. Coronary artery and thoracic calcium on noncontrast thoracic CT scans: comparison of ungated and gated examinations in patients from the COPD Gene cohort. J Cardiovasc Comput Tomogr 2011;5(2):113–8. doi: 10.1016/j.jcct.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirsch J, Buitrago I, Mohammed TL, Gao T, Asher CR, Novaro GM. Detection of coronary calcium during standard chest computed tomography correlates with multidetector computed tomography coronary artery calcium score. The international journal of cardiovascular imaging 2012;28(5):1249–56. Epub 2011/08/13. doi: 10.1007/s10554011-9928-9. [DOI] [PubMed] [Google Scholar]
- 11.Einstein AJ, Johnson LL, Bokhari S, Son J, Thompson RC, Bateman TM, et al. Agreement of visual estimation of coronary artery calcium from low-dose CT attenuation correction scans in hybrid PET/CT and SPECT/CT with standard Agatston score. Journal of the American College of Cardiology 2010;56(23):1914–21. doi: 10.1016/j.jacc.2010.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SM, Chung MJ, Lee KS, Choe YH, Yi CA, Choe BK. Coronary calcium screening using low-dose lung cancer screening: effectiveness of MDCT with retrospective reconstruction. AJR Am J Roentgenol 2008;190(4):917–22. doi: 10.2214/AJR.07.2979. [DOI] [PubMed] [Google Scholar]
- 13.Chandra D, Gupta A, Leader JK, Fitzpatrick M, Kingsley LA, Kleerup E, et al. Assessment of coronary artery calcium by chest CT compared with EKG-gated cardiac CT in the multicenter AIDS cohort study. PLoS One 2017;12(4):e0176557. doi: 10.1371/journal.pone.0176557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Group COCW, Barr RG, Berkowitz EA, Bigazzi F, Bode F, Bon J, et al. A combined pulmonary-radiology workshop for visual evaluation of COPD: study design, chest CT findings and concordance with quantitative evaluation. COPD 2012;9(2):151–9. doi: 10.3109/15412555.2012.654923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bon J, Fuhrman CR, Weissfeld JL, Duncan SR, Branch RA, Chang CC, et al. Radiographic emphysema predicts low bone mineral density in a tobacco-exposed cohort. American journal of respiratory and critical care medicine 2011;183(7):885–90. doi: 10.1164/rccm.201004-0666OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson DO, Weissfeld JL, Balkan A, Schragin JG, Fuhrman CR, Fisher SN, et al. Association of radiographic emphysema and airflow obstruction with lung cancer. American journal of respiratory and critical care medicine 2008;178(7):738–44. doi: 10.1164/rccm.200803-435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandra D, Gupta A, Strollo PJ Jr., Fuhrman CR, Leader JK, Bon J, et al. Airflow Limitation and Endothelial Dysfunction: Unrelated and Independent Predictors of Atherosclerosis. American journal of respiratory and critical care medicine 2016. doi: 10.1164/rccm.201510-2093OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. American journal of respiratory and critical care medicine 1999;159(1):179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 19.Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. The European respiratory journal 2005;26(4):720–35. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 20.Benzo RP, Sciurba FC. Oxygen consumption, shuttle walking test and the evaluation of lung resection. Respiration 2010;80(1):19–23. Epub 2009/08/13. doi: 10.1159/000235543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGrath EE, Marriott HM, Lawrie A, Francis SE, Sabroe I, Renshaw SA, et al. TNF-related apoptosis-inducing ligand (TRAIL) regulates inflammatory neutrophil apoptosis and enhances resolution of inflammation. J Leukoc Biol 2011;90(5):855–65. Epub 2011/05/13. doi: 10.1189/jlb.0211062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JY, Kim YM, Park JM, Han YM, Lee KC, Hahm KB, et al. Cancer preventive effect of recombinant TRAIL by ablation of oncogenic inflammation in colitis-associated cancer rather than anticancer effect. Oncotarget 2018;9(2):1705–16. Epub 2018/02/09. doi: 10.18632/oncotarget.23083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chyuan IT, Tsai HF, Wu CS, Sung CC, Hsu PN. TRAIL-Mediated Suppression of T Cell Receptor Signaling Inhibits T Cell Activation and Inflammation in Experimental Autoimmune Encephalomyelitis. Front Immunol 2018;9:15 Epub 2018/02/07. doi: 10.3389/fimmu.2018.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirsova P, Weng P, Salim W, Bronk SF, Griffith TS, Ibrahim SH, et al. TRAIL Deletion Prevents Liver, but Not Adipose Tissue, Inflammation during Murine Diet-Induced Obesity. Hepatol Commun 2017;1(7):648–62. Epub 2017/11/11. doi: 10.1002/hep4.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chyuan IT, Tsai HF, Liao HJ, Wu CS, Hsu PN. An apoptosis-independent role of TRAIL in suppressing joint inflammation and inhibiting T-cell activation in inflammatory arthritis. Cell Mol Immunol 2017. Epub 2017/04/11. doi: 10.1038/cmi.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song K, Chen Y, Goke R, Wilmen A, Seidel C, Goke A, et al. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is an inhibitor of autoimmune inflammation and cell cycle progression. J Exp Med 2000;191(7):1095–104. Epub 2000/04/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watt V, Chamberlain J, Steiner T, Francis S, Crossman D. TRAIL attenuates the development of atherosclerosis in apolipoprotein E deficient mice. Atherosclerosis 2011;215(2):348–54. doi: 10.1016/j.atherosclerosis.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forde H, Harper E, Davenport C, Rochfort KD, Wallace R, Murphy RP, et al. The beneficial pleiotropic effects of tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) within the vasculature: A review of the evidence. Atherosclerosis 2016;247:87–96. doi: 10.1016/j.atherosclerosis.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Secchiero P, Candido R, Corallini F, Zacchigna S, Toffoli B, Rimondi E, et al. Systemic tumor necrosis factor-related apoptosis-inducing ligand delivery shows antiatherosclerotic activity in apolipoprotein E-null diabetic mice. Circulation 2006;114(14):1522–30. doi: 10.1161/CIRCULATIONAHA.106.643841. [DOI] [PubMed] [Google Scholar]
- 30.Michowitz Y, Goldstein E, Roth A, Afek A, Abashidze A, Ben Gal Y, et al. The involvement of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in atherosclerosis. Journal of the American College of Cardiology 2005;45(7):1018–24. doi: 10.1016/j.jacc.2004.12.065. [DOI] [PubMed] [Google Scholar]
- 31.Mori K, Ikari Y, Jono S, Shioi A, Ishimura E, Emoto M, et al. Association of serum TRAIL level with coronary artery disease. Thromb Res 1252010. p. 322–5. [DOI] [PubMed]
- 32.Osmancik P, Teringova E, Tousek P, Paulu P, Widimsky P. Prognostic value of TNF-related apoptosis inducing ligand (TRAIL) in acute coronary syndrome patients. PLoS One 2013;8(2):e53860. doi: 10.1371/journal.pone.0053860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Secchiero P, Corallini F, Ceconi C, Parrinello G, Volpato S, Ferrari R, et al. Potential prognostic significance of decreased serum levels of TRAIL after acute myocardial infarction. PLoS One 2009;4(2):e4442. doi: 10.1371/journal.pone.0004442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiang G, Yue L, Zhang J, Xiang L, Dong J. The relationship between circulating TRAIL and endothelial dysfunction in subclinical hypothyroidism. Endocrine 2015;49(1):184–90. doi: 10.1007/s12020-014-0443-3. [DOI] [PubMed] [Google Scholar]
- 35.Malyszko J, Przybylowski P, Malyszko J, Koc-Zorawska E, Mysliwiec M. Tumor necrosis factor-related apoptosis-inducing ligand is a marker of kidney function and inflammation in heart and kidney transplant recipients. Transplant Proc 2011;43(5):1877–80. doi: 10.1016/j.transproceed.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 36.Haw TJ, Starkey MR, Nair PM, Pavlidis S, Liu G, Nguyen DH, et al. A pathogenic role for tumor necrosis factor-related apoptosis-inducing ligand in chronic obstructive pulmonary disease. Mucosal Immunol 2016;9(4):859–72. doi: 10.1038/mi.2015.111. [DOI] [PubMed] [Google Scholar]
- 37.Wu Y, Shen Y, Zhang J, Wan C, Wang T, Xu D, et al. Increased serum TRAIL and DR5 levels correlated with lung function and inflammation in stable COPD patients. Int J Chron Obstruct Pulmon Dis 2015;10:2405–12. doi: 10.2147/COPD.S92260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liabeuf S, Barreto DV, Barreto FC, Chasseraud M, Brazier M, Choukroun G, et al. The circulating soluble TRAIL is a negative marker for inflammation inversely associated with the mortality risk in chronic kidney disease patients. Nephrol Dial Transplant 2010;25(8):2596–602. doi: 10.1093/ndt/gfq042. [DOI] [PubMed] [Google Scholar]


