Abstract
The transmembrane glycoprotein N-cadherin (NCad) mediates cell–cell interactions found during mesenchymal condensation and chondrogenesis. Here, NCad-derived peptides (i.e., HAV) were incorporated into hyaluronic acid (HA) hydrogels with encapsulated mesenchymal stem cells (MSCs). Since the dose and timing of NCad signaling are dynamic, the presentation of HAV peptide presentation was tuned via alterations in peptide concentration and incorporation of an ADAM10-cleavable domain between the hydrogel and the HAV motif, respectively. HA hydrogels functionalized with HAV resulted in dose-dependent increases in early chondrogenesis of encapsulated MSCs and in resultant cartilage matrix production. For example, type II collagen and glycosaminoglycan production increased ~9- and 2-fold with the highest dose of HAV (i.e., 2 mM), respectively, when compared to unmodified hydrogels, while incorporation of an efficient ADAM10-cleavable domain between the HAV peptide and hydrogel abolished increases in chondrogenesis and matrix production. Treatment with a small-molecule ADAM10 inhibitor restored the functional effect of the HAV peptide, indicating that timing and duration of HAV peptide presentation is crucial for robust chondrogenesis. This study demonstrates a nuanced approach to the biofunctionalization of hydrogels to better emulate the complex cell microenvironment during embryogenesis towards stem cell-based cartilage production.
Keywords: cartilage, hydrogel, tissue engineering, N-cadherin, chondrogenesis
Graphical Abstract
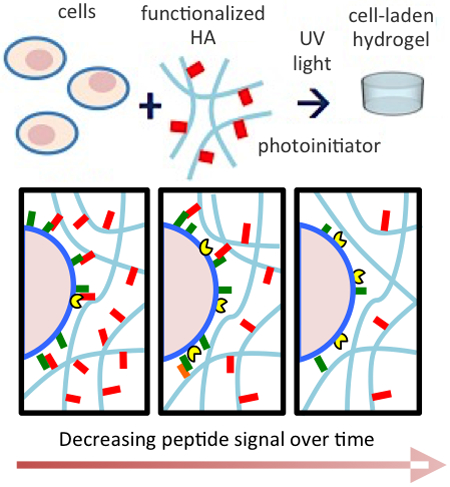
The dose and timing of presentation of an N-cadherin mimetic peptide that represents a critical and dynamic cue in cartilage development can be tuned in 3D hydrogels to modulate and enhance chondrogenesis of human mesenchymal stem cells. This work presents a nuanced approach to incorporate biochemical signals that are tailored to the complex cell microenvironment found during tissue development.
1. Introduction
Human mesenchymal stem cells (MSCs) have gained widespread use as a cell source for tissue engineering applications due to their clinical relevance and multipotency.[1] As our understanding of the native cell microenvironment expands, this information can be incorporated into the design of tissue-engineered constructs to control MSC differentiation. To this end, a variety of soluble, tethered, and physical considerations have been incorporated into the design of hydrogels, and prior works have explored the effects of combinations of these factors on MSC chondrogenesis; however, although the physical nature of hydrogels has become more dynamic, e.g. with cell-mediated degradation of crosslinkers to match matrix deposition, investigating the dynamic nature of the biological aspects of hydrogels remain a challenge. [2, 3]
Hyaluronic acid (HA) has emerged as one of the most studied biomaterial components for cartilage tissue engineering.[3, 4] HA is a linear polysaccharide (a non-sulfated glycosaminoglycan, or GAG) that is part of the nascent extracellular matrix (ECM) in tissues such as cartilage and is present during the condensation phase of limb bud development in the embryo.[5] Cellular interactions with HA are mediated via its principal cell-surface receptor CD44 along with others such as CD168, which induces chondrogenic differentiation via nuclear Smad translocation.[6] HA hydrogels are widely known to support improved matrix synthesis by chondrocytes and chondrogenic differentiation of MSCs over typical inert materials.[7] Importantly, HA disaccharide repeat units can be easily functionalized, and this has been exploited for not only crosslinking of HA, but for the tethering of peptides that mimic components of the native ECM.[8, 9]
Beyond cell-ECM interactions, cell-cell interactions are known to be important during mesenchymal condensation and the regulation of chondrogenesis.[10] Cell-cell interactions during condensation are widely understood to be achieved via homotypic binding of NCad, a cell-surface protein that binds the transcription factor β-catenin with its cytosolic domain, regulating the localization of this transcription factor for proper signaling downstream of cell-cell interactions.[11–13] In fact, deletion of the extracellular or intracellular domains, or blocking NCad interactions via antibody treatment inhibits condensation and subsequent chondrogenesis.[12, 14] However, the encapsulation of cells within hydrogels typically occurs as single cells, which then limits recapitulation of these cell-cell interactions; thus, strategies to introduce NCad signaling into these environments are needed. Full-length NCad has been successfully incorporated into hydrogels, such as the surface functionalization of polyacrylamide and alginate hydrogels;[15] however, the sheer size of NCad (135 kDa) presents challenges when considering incorporation throughout a 3D hydrogel.
Within the structure of NCad, a conserved three-amino acid sequence, His-Ala-Val (HAV), exists on the adhesive interface, and synthetic peptides containing this sequence along with a flanking aspartic acid residue are known to exhibit NCad-like binding activity.[16] The incorporation of HAV into an HA hydrogel did indeed promote early chondrogenic gene expression and subsequent matrix production of MSCs in culture and when implanted in vivo.[17] Furthermore, incorporation of this peptide into hydrogels resulted in increased β-catenin recruitment to the membrane and subsequent translocation to the nucleus, as is observed with NCad signaling.[18]
Despite these findings, the dose and timing of the HAV motif presentation, two critical aspects of this essential cell-cell interaction and downstream signaling, have not been explored. In the developing limb, NCad signaling varies greatly over space and time, with a biphasic response to the number of NCad interactions either increasing or reducing collagen and proteoglycan synthesis.[12] In the mesenchyme, cell-surface metalloprotease ADAM10 temporally regulates NCad interactions during development by cleaving the extracellular domain of NCad, thereby exerting control over the β-catenin membrane-bound and cytoplasmic pool levels.[11, 19, 20] Mutants of NCad that lack the extracellular domain exhibit altered localization of β-catenin and expression of chondrogenic markers.[21] Meanwhile, mutants of NCad whose extracellular domains cannot be cleaved by ADAM10 prevented cartilage aggregate formation, proteoglycan synthesis, and expression of both chondrogenic and hypertrophic markers, likely due to alterations in β-catenin localization and, importantly, chondrogenic gene expression.[20, 21] With this in mind, we functionalized HA hydrogels with peptides containing the HAV motif at different concentrations to control the dose, and with an efficient ADAM10-cleavable domain to control the timing, to investigate how these parameters regulate chondrogenesis and neocartilage production of MSCs in HA hydrogels.
2. Results and Discussion
Cell-cell contact among progenitor cells in the limb bud during condensation is widely understood to require homotypic binding of NCad, a cell-surface adhesion protein that binds the transcription factor β-catenin with its cytosolic domain, regulating localization of this transcription factor for proper signaling downstream of NCad binding.[11–13] Cell-cell interactions and subsequent downstream signaling via NCad are well established as critical features of chondrogenesis and development, but nuanced control of NCad signaling is desirable for the design of inductive materials for cartilage tissue engineering. Prior work has established that tethered NCad mimetic HAV peptides in HA hydrogels exhibit NCad agonistic activity due to one end being conjugated to the HA backbone, resulting in the expected nuclear β-catenin localization in MSCs when encapsulated within this material.[17, 18] Furthermore, blocking studies using antibody treatment have confirmed that the observed enhancement of chondrogenic gene expression in this system was indeed due to NCad interactions with these peptides.[17]
Here, we drew inspiration from two key features of our understanding of development: (i) variations in the amount of NCad interactions present in the limb bud mesenchyme[12] and (ii) the possibility of regulating NCad interactions over time by the cell-surface metalloprotease ADAM10.[11, 19, 20] To mimic both the dose and timing of NCad signals in hydrogels, we designed photocrosslinkable macromers that allowed us to titrate in varying amounts of tethered stable or transient NCad signal into hydrogels via HAV peptide presentation while keeping the hydrogel properties constant.
2.1. Cadherin mimetic peptides enhance early chondrogenesis in a dose-dependent manner
Hyaluronic acid (HA) was modified with methacrylate groups on ~37% of disaccharide repeat units to generate a photocrosslinkable macromer that could also be modified with peptides using a Michael addition reaction between the methacrylates and thiols (via cysteine residues) on the peptides (Figure S1). Specifically, peptides containing either the HAV motif of NCad or a non-active scrambled sequence (Figure 1a) were incorporated into hydrogels at various ratios at a total concentration of 2 mM. The incorporation of peptides at 2 mM did not significantly alter either the mass swelling behavior (Figure 1b) or compressive modulus (Figure 1c) of hydrogels as compared to unmodified hydrogel controls, confirming that cellular outcomes are not related to changes in network structure or mechanical properties. Additionally, MSCs used in these studies were verified to express cell-surface NCad (Figure S3) and were encapsulated in the HA hydrogels that were either unmodified or modified with various peptide compositions. MSC viability was high in all hydrogel formulations for up to 14 days of culture, the time period used to assess gene expression (>90%, Figure S4). There were no macroscopic changes in construct size or opacity during this 14 day period (Figure S5).
Figure 1.
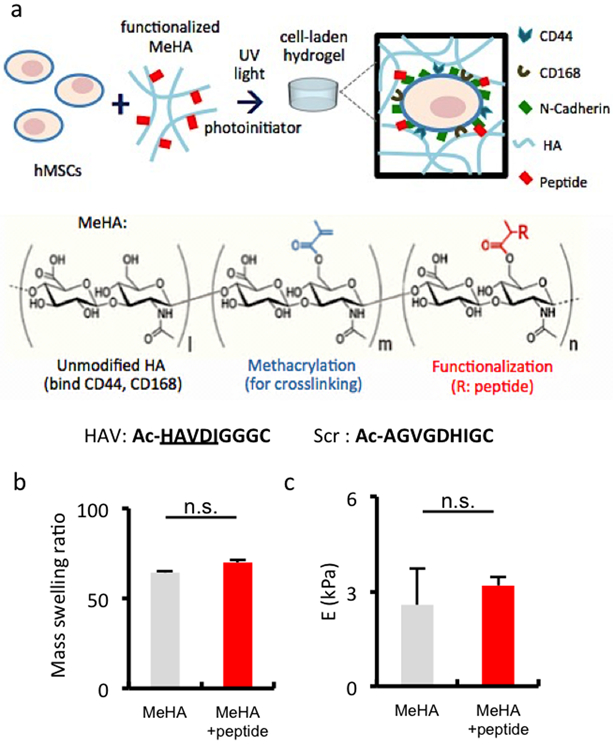
Fabrication of HA hydrogels that incorporate peptides that mimic cell-cell interactions. (a) Schematic of MSC encapsulation in HA hydrogels from macromers modified with methacrylates for crosslinking and a peptide that contains the HAV motif to enable interaction with NCad, or a respective scrambled control (Scr). (b) Mass swelling ratio and (c) elastic compressive modulus (E) of MeHA hydrogels without (grey) or with (red) HAV peptide incorporated at 2 mM. (n ≥ 3 hydrogels per group, error bars represent s.e.m.); n.s. denotes no significant differences between groups.
To control the HAV dose in MSC-laden hydrogels, three different ratios of HAV and scrambled peptide were incorporated onto the HA macromer for a final hydrogel concentration of 2 mM: 100% scramble (HAV 0), 50%:50% HAV/scramble (HAV 50), and 100% HAV (HAV100) (Figure 2a). After 3 days in culture, a dose-dependent type II collagen gene expression response was observed, with the HAV-free (HAV 0) condition similar to hydrogels without peptide (Figure 2b). The dose-dependent increases in gene expression persisted for up to 7 days; however, there were no significant differences between groups at 14 days. Aggrecan gene expression was greatest for the highest HAV concentration at 3 days; however, these differences diminished by 7 days and 14 days, although there were some modest improvements over the hydrogels without peptides (Figure 2c). No significant changes in type I collagen expression were observed over the course of the study (Figure S6). This is consistent with our previous observations on HAV peptide modification, where early markers of gene expression towards chondrogenesis were altered by HAV incorporation and that these early changes resulted in long-term differences in matrix production.[17] These trends also agree with what is known about cadherin regulation in development: NCad expression increases at the onset of condensation and then diminishes with cartilage maturation as cell-cell contacts are lost; thus, although the HAV peptide presentation is stable, its effects are most prominent at earlier times.[12, 17, 22]
Figure 2.
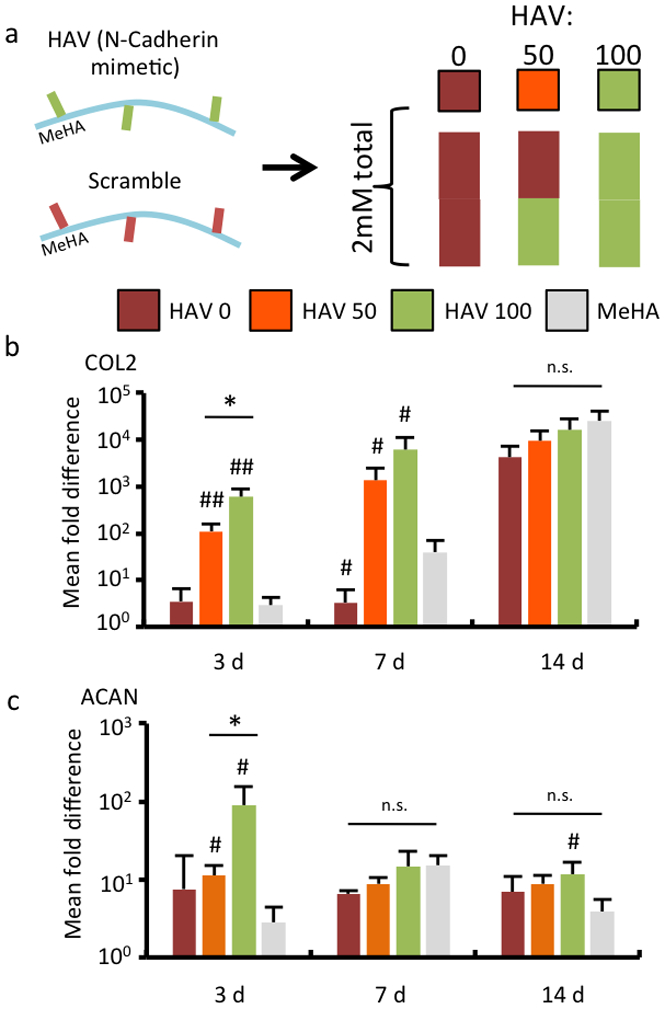
HA hydrogels functionalized with NCad mimetic peptides enhance early chondrogenesis in a dose-dependent manner. (a) MSCs were encapsulated in hydrogels from MeHA macromers with either HAV or Scrambled peptides incorporated, at a dose of 2 mM of peptide. The groups included either only the Scrambled peptide (HAV 0), a 50:50 mixture of the two peptides (HAV 50), or only the HAV peptide (HAV 100). A control without peptide (MeHA) was also investigated. Gene expression for (b) type II collagen (COL2) and (c) aggrecan (ACAN) assessed over a 14 day culture period in chondrogenic media. (n = 4, error bars represent s.e.m.). *: P< 0.05 between groups, #: P< 0.05 compared to no peptide (MeHA) control. n.s. denotes no significant differences between groups.
2.2. Cadherin mimetic peptides enhance long-term neocartilage formation in a dose-dependent manner
To evaluate the effects of the HAV peptides on long-term cartilage formation, we cultured MSC-laden constructs prepared with different ratios of macromers functionalized with either the HAV and scramble peptides for 56 days. Quantification of matrix components present in cartilage showed a concentration-dependent increase in sGAG and collagen (Figure 3a). HAV-free (HAV 0) and no peptide control (MeHA) groups had similar sGAG and collagen levels, whereas matrix deposition increased up to 2-fold in sGAG and 9-fold in collagen content for the 2mM HAV group after 56 days of culture when compared to these controls. Unconfined mechanical testing also showed that the inclusion of HAV increased the compressive elastic modulus of the constructs when compared to the no HAV peptide and no peptide hydrogels, which exhibited a similar modulus (~13 kPa) (Figure 3b). When compared to the initial hydrogel modulus (Figure 1C), there was an increase in elastic compressive modulus in all formulations after 56 days; however, the increase (~340%) was greatest for the highest HAV concentration when compared to MeHA gels (Figure 3b).
Figure 3.
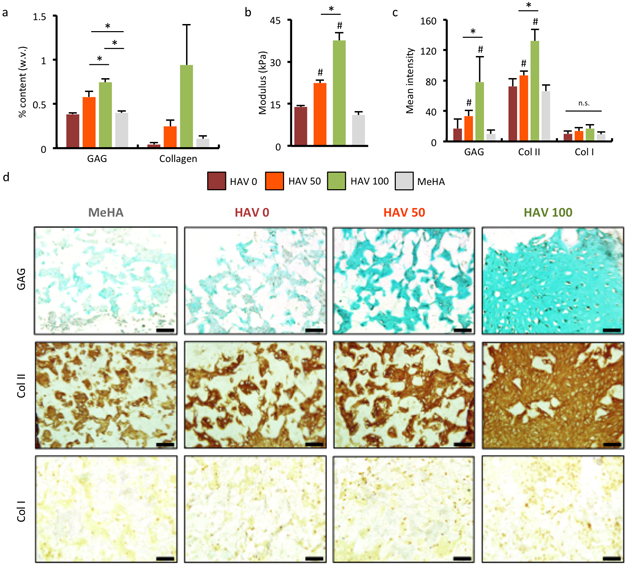
NCad interactions promote long-term neocartilage formation in a dose-dependent manner. (a) Quantification of sGAG and collagen content and (b) elastic compressive modulus of MSC-encapsulated constructs after 8 weeks of culture. Histology and immunohistochemistry (c) quantification and (d) images of sGAG and type I and type II collagens after 8 weeks of culture. (n = 5 hydrogels per group, error bars represent s.e.m.). Scale bar 500 μm. *: P< 0.05 between groups, #: P< 0.05 compared to no peptide (MeHA) control. n.s. denotes no significant differences between groups.
Quantification of staining for sGAG and type II collagen showed a dependence on HAV concentration (Figure 3c). In addition to differences between the HAV-free (HAV 0) and low HAV (HAV 50) groups, the presence of HAV peptide (HAV 50, HAV 100) increased the staining intensity compared to the MeHA control without peptide for both markers, with significantly greater values for the highest HAV group. Representative images of immunohistochemistry staining for sGAG and type II collagen revealed more intense and uniformly distributed staining with greater doses of HAV, suggesting more cartilage matrix elaboration in these constructs compared to scrambled and no peptide controls (Figure 3d). Quantification of type I collagen sections showed low levels and no significant differences in staining intensity between groups investigated (Figure 3c,d). Thus, the changes in early gene expression appear to influence matrix production over the long term at the various HAV concentrations investigated. The homogeneity of sGAG and collagen distribution also appears to be different in MeHA and peptide-modified hydrogels, and this may be due in part to the inherent heterogeneity in the MSC population and differences in ECM synthesis and deposition.
Notably, while too much cell-cell adhesion by overexpression of NCad can result in failure to differentiate (a 2-fold increase in NCad enhances chondrogenesis, while a 4-fold increase inhibits chondrogenesis)[23], the doses used in this study stimulated the greatest chondrogenesis at the highest concentration. With the peptides designed for this study and the hydrogel used, solubility limited the investigation of higher concentrations where perhaps inhibited chondrogenesis would be observed. Also, the presentation of the peptide or its accessability may be inherently altered as the cells lay down their own matrix throughout the hydrogel, in a sense to self-regulate HAV levels. The dose dependence in the range probed here, while it does not demonstrate this biphasic response, agrees with this well-known dose-dependence of native NCad-mediated cell-cell interactions in embryogenesis.[11] Importantly, the mean increases in type II collagen and sGAG synthesis in constructs with the HAV peptide being 9- and 2-fold, respectively, with collagen content beginning to overtake sGAG content, is favorable in light of the fact that the composition of native articular cartilage—where there is more type II collagen than sGAGs--mediates final tissue properties; here, the increases in type II collagen and sGAG content led to an almost 3-fold enhancement of the hydrogel modulus.[24]
2.3. Transient presentation of NCad mimetic peptide alters the influence on MSC chondrogenesis
To assess how the transient presentation of the NCad mimetic HAV motif affects early chondrogenesis of MSCs, MeHA macromers were functionalized with HAV or scrambled peptides containing an additional ADAM10-cleavable domain. Presentation of this transient peptide relies on endogenous ADAM10 on MSCs to cleave the peptides and thus reduce presentation in a time-dependent fashion (Figure 4a). The MSCs used were verified to express cell-surface ADAM10 (Figure S3). To validate the peptide design, acellular hydrogels functionalized with a FITC-tagged form of this cleavable peptide were incubated in PBS in the presence or absence of exogenous ADAM10. Peptide cleavage was measured as FITC signal in the supernatant and observed over 9 days with ADAM10 present, with minimal cleavage without exogenous ADAM10 present (Figure 4b). To ensure that the larger peptides did not change the properties of the hydrogels formed, we verified that the incorporation of these peptides did not significantly alter their modulus (Figure 4c) or mass swelling ratio (Figure 4d). Again, cell viability was high in these hydrogels over a 14 day culture period (>90%, Figure S7) and macroscopic differences in construct size and opacity were not observed (Figure S5).
Figure 4.
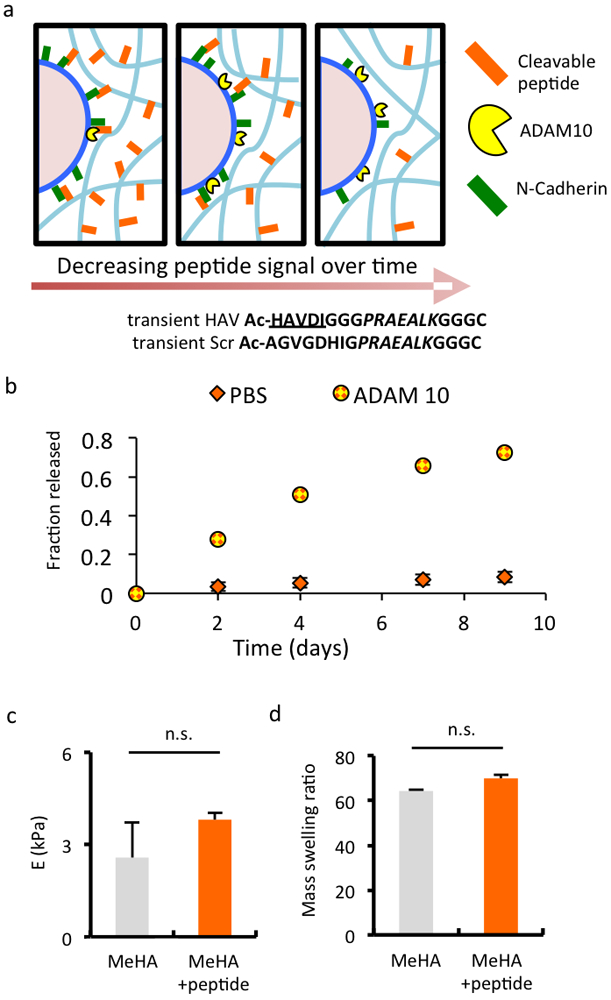
Fabrication of hydrogels with transient presentation of NCad mimetic peptides by metalloprotease ADAM10. (a) Schematic of MSCs within hydrogels containing transient peptides regulated by ADAM10, and transient NCad mimetic HAV peptide and control scrambled peptide sequences. (b) Release of FITC-labeled transient peptide coupled to the MeHA macromer from bulk hydrogels by ADAM10 and compared to control buffer without ADAM10. (c) Elastic modulus (E) and (d) mass swelling ratio of MeHA hydrogels without (grey) or with (orange) transient HAV peptide incorporated at 2 mM. (n ≥ 3 hydrogels per group, error bars represent s.e.m.); n.s. denotes no significant differences between groups.
To control the dose of transient HAV (tHAV), three different ratios of tHAV and transient scrambled peptide were incorporated into MeHA macromers: 100% transient scramble (tHAV 0), 50%:50% transient HAV/transient scramble (tHAV 50), and 100% transient HAV (tHAV 100) (Figure 5a). In contrast to our observations in the stable (non-transient) groups, after 3 days in culture there were no significant differences in expression of chondrogenic gene markers type II collagen (Figure 5b) and aggrecan (Figure 5c). There was a significant increase in the expression of type II collagen and aggrecan in all peptide-containing groups by 7 days, and subsequently no temporal differences in expression between 7 and 14 days. As observed with the stable HAV peptides, however, no significant changes in type I collagen expression were observed in these groups for the duration of the study (Figure S8). These observations are consistent with the understanding that, in development, premature or induced shedding of N-Cadherin-mediated interactions results in impaired chondrogenesis.[22] To verify that the length of the peptide sequence did not influence these findings, MeHA was functionalized with a peptide of the same length as the transient peptide that contained the HAV motif but now incorporating a scrambled cleavage domain (length-matched HAV that is not ADAM10-cleavable, or LHAV) (Figure S9). When MSCs were encapsulated in this hydrogel, we observed increases in early type II collagen and aggrecan gene expression, and subsequent sGAG and collagen deposition, similar to the earlier stable HAV studies (Figure S9, S10). Thus, it appears that the ADAM10 cleavage of the designed peptide occurs rapidly and fails to influence chondrogenesis in the hydrogels, confirming that the duration of peptide presentation is important.
Figure 5.
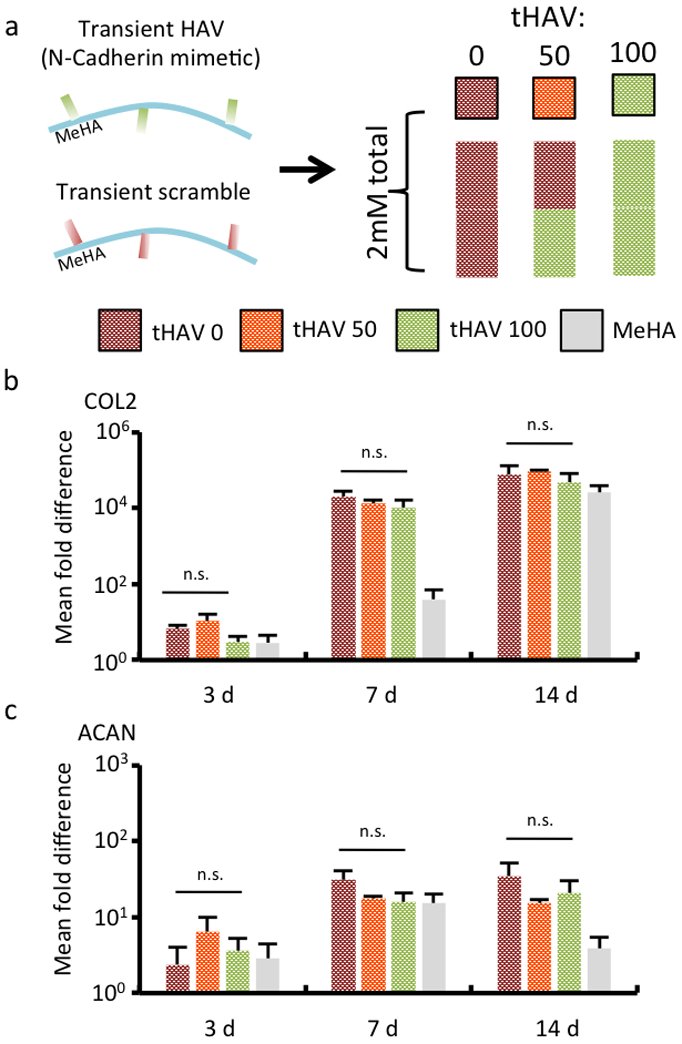
Transient NCad mimetic peptides do not enhance early chondrogenesis. (a) MSCs were encapsulated in hydrogels from MeHA macromers with either transient tHAV or Scrambled peptides incorporated, at a dose of 2 mM of peptide. The groups included either entirely the Scrambled peptide (tHAV 0), a 50:50 mixture of the two peptides (tHAV 50), or entirely the transient HAV peptide (tHAV 100). A control without peptide (MeHA) was also investigated. Gene expression for (b) type II collagen (COL2) and (c) aggrecan (ACAN) assessed over a 14 day culture period in chondrogenic media. (n = 4, error bars represent s.e.m.). *: P< 0.05 between groups, #: P< 0.05 compared to no peptide (MeHA) control. n.s. denotes no significant differences between groups.
To further explore the role that HAV timing plays on MSC chondrogenesis, constructs were treated with the small-molecule ADAM10 inhibitor GI254023X to further stabilize the presentation of HAV to encapsulated MSCs, as well as cell-surface NCad (Figure 6a). Gene expression at an early culture time of 3 days was used, since changes in chondrogenesis were clearly different with the presence of the stable HAV peptide in this time frame. With respect to type II collagen expression (Figure 6b), the addition of the ADAM10 inhibitor did not alter expression in hydrogels without peptide (MeHA) or those with the incorporation of 2mM of the stable HAV peptide (HAV 100); however, there was elevated expression of type II collagen in the transient HAV group (tHAV 100), indicating that expression is rescued when peptide cleavage is blocked. With respect to aggrecan expression (Figure 6c), the addition of the ADAM10 inhibitor resulted in decreased aggrecan expression in hydrogels without peptide (MeHA) or those with the incorporation of 2mM of the stable HAV peptide (HAV 100), likely due to some specific alteration in the transcriptional program for aggrecan production in the presence of the inhibitor. However, this decrease in aggrecan expression was rescued in the transient HAV group, as the decreased expression was counteracted by enhanced chondrogenesis when peptide cleavage was blocked (and thus HAV presentation is maintained).
Figure 6.
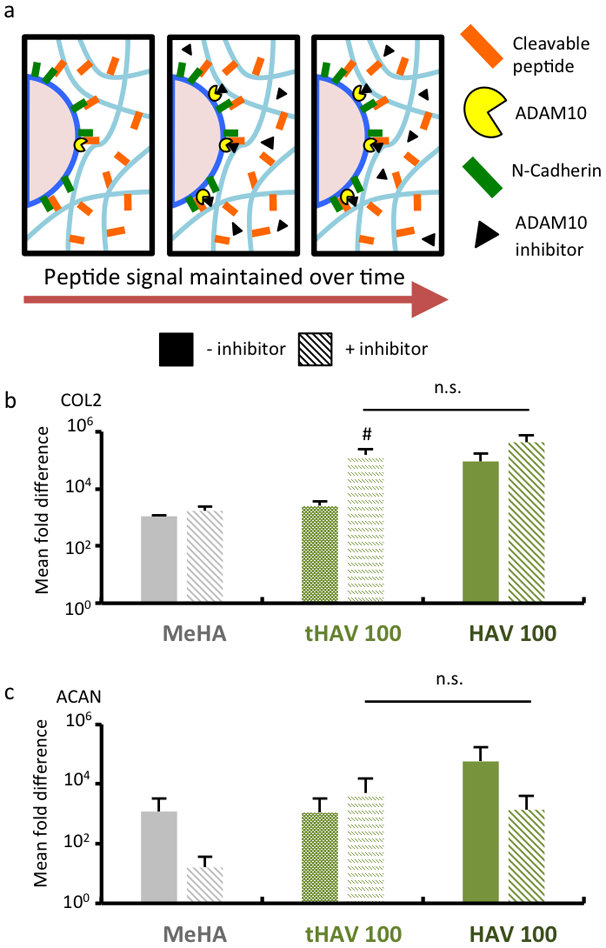
Inhibition of ADAM10 recovers early chondrogenic gene expression in cell-laden hydrogels with cleavable peptides. (a) Schematic of MSCs within hydrogels containing transient peptides regulated by ADAM10 and in the presence of ADAM10 inhibitor GI254023X. Gene expression of (b) type II collagen (COL2) and (c) aggrecan (ACAN) after 3 days of culture in chondrogenic media for control hydrogels without peptides (MeHA) or 2mM of either transient HAV (tHAV 100) or stable HAV peptides (HAV 100), alone (solid bars) or with inhibitor (dashed bars). (n = 4 hydrogels per group) #: P< 0.05 compared to respective untreated control; other comparisons were consistent with prior studies. n.s. denotes no significant differences between groups.
Taken together, inhibition of ADAM10-mediated cleavage of peptide did enhance chondrogenesis in the form of both type II collagen and aggrecan expression. Interestingly, there was no statistical difference in the expression of either aggrecan or type II collagen between the stable and transient HAV groups in the presence of the inhibitor. Thus, these results indicate that cellular control of HAV presentation, here via the protease activity of ADAM10, can mediate MSC chondrogenesis. Due to technical limitations, we were unable to quantify the exact timing of HAV presentation to encapsulated cells.
2.4. Transient timing of NCad mimetic peptide presentation alters its influence on neocartilage formation
To evaluate the effects of transient NCad-mimetic HAV (tHAV) peptides on long-term cartilage formation, MSC-laden constructs were prepared with different ratios of macromers functionalized with either tHAV or transient scramble peptides and cultured for 56 days. No peptide control (MeHA) groups had similar sGAG content to all transient peptide groups, and similar Col II content to transient HAV-containing groups (Figure 7a). Thus, they did not show the concentration-dependent increase in cartilage matrix that was observed with hydrogels functionalized with stable HAV peptides. Unconfined mechanical testing also did not show any concentration-dependent increase in modulus (~ 16 kPa), with the no peptide control group exhibiting a slightly lower modulus than the transient peptide-containing groups (~ 12 kPa) (Figure 7b). Quantification and images of staining for sGAG and type II collagen showed that the HAV-concentration dependence observed with stable HAV peptides was mitigated, while again type I collagen exhibited no transient HAV-concentration dependence (Figure 7c,d). In agreement with our observations of early gene expression, MSC-laden hydrogels functionalized with the control LHAV mimetic peptide showed significant increases in sGAG and collagen content after long-term culture, as measured by biochemical assays, histology, and immunohistochemistry (Figure S10). Thus, this work suggests that the timing of the HAV peptide is indeed important to its effect on encapsulated MSCs, as rapid cleavage limits the influence on cell behavior.
Figure 7.
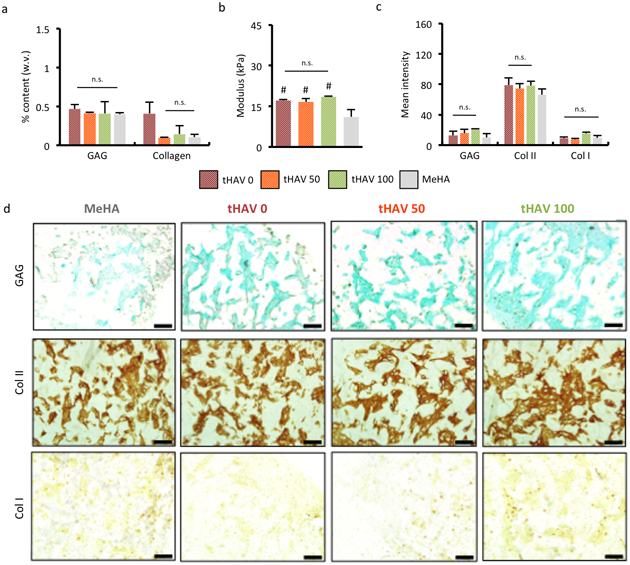
Transient NCad interactions do not enhance long-term neocartilage formation. (a) Quantification of sGAG and collagen content and (b) elastic modulus of constructs after 8 weeks of culture. Histology and immunohistochemistry (c) quantification and (d) images of sGAG and type I and type II collagen after 8 weeks of culture. (n = 5 hydrogels per group, error bars represent s.e.m.). Scale bar 500 μm. *: P< 0.05 between groups, #: P< 0.05 compared to no peptide (MeHA) control. n.s. denotes no significant differences between groups.
The findings of this study indicate that the inclusion of an HAV peptide into hydrogels enhances chondrogenesis in a dose dependent manner, as long as the peptide presentation is sustained to influence MSC behavior. Enhanced sGAG, collagen, and mechanics were observed, although the values did not reach those of native cartilage tissue.[25] Although the ADAM10 regulated presentation of the HAV peptide, here it did not enhance chondrogenesis, emphasizing the importance of timing on the MSC response. Potentially, self regulation in such systems—through the deposition of matrix that occurs in 3D as chondrogenesis and cartilage formation proceed—is sufficient, and this mimics the transition from cell-cell to cell-ECM interactions during development, which can be different than how mechanical signaling occurs in 2D HA cultures of MSCs.[26] One important consideration in these studies is that HAV peptide was shown as chondroinductive in the presence of TGF-β3; whether the peptide is chondroinductive alone, particularly in vivo, remains a consideration for future investigation. Additionally, further enhancing NCad signaling in hydrogels via variations in peptide design and presentation could be considered. For instance, NCad dimerization on the cell surface is important for proper signal transduction, and, accordingly, cyclic tandem repeat-containing peptides have been reported to exert greater agonist activity,[27] which was not included in the current study. The spatial clustering of mimetic peptides in the hydrogel microenvironment to encourage adherens junction formation and stability may also need to be optimized to enhance peptide-cell interaction on cell signaling, as this stable cluster formation is important in cadherin signaling in general. [28]
3. Conclusion
The findings in this study demonstrate that tethered NCad mimetic peptides that mimic cell-cell interaction via NCad binding both enhance the early expression of chondrogenic markers and promote long-term cartilage matrix production in a strong dose-dependent fashion. Furthermore, these results suggest that the timing and duration of presentation is critical: highly transient peptides with the same NCad mimetic moiety did not exert the same effects on MSC chondrogenesis and matrix production, whereas the blocking of this cleavage with protease inhibition enhanced outcomes. These findings underscore the potential of nuanced material design to guide differentiation by providing biochemical signals that capture the complex cell microenvironment found during tissue development.
4. Experimental Section
Materials were acquired from Sigma-Aldrich unless otherwise indicated.
MeHA synthesis:
HA (75 kDa; Lifecore) was modified with methacrylates as previously described.[8] Briefly, the primary hydroxyl groups of HA macromers were reacted with methacrylic anhydride (Sigma-Aldrich) in 20-fold excess under basic conditions. The resulting methacrylated HA (MeHA) was then dialyzed (MW cutoff 6–8 kDa) for 4 days and lyophilized for storage. The extent of MeHA methacrylation was assessed using 1H NMR spectroscopy and determined to be ~37% of repeat units (Figure S1).
Peptide design and coupling to MeHA:
Stable N-cad mimetic HAV peptide (HAV, Ac-HAVDIGGGC), stable scramble (Scr, Ac-AGVGDHIGC), ADAM10-cleavable transient HAV (tHAV, Ac-HAVDIGGGPRAEA↓LKGGGC), transient scramble (tScr, Ac-AGVGDHIGPRAEA↓LKGGGC), and stable length-matched HAV (LHAV, Ac-HAVDIGGGAAKREPLGGGC) peptides (stable peptides 827.9 g/mol, transient peptides 1765.0 g/mol) with a cysteine residue at the C-terminal end were obtained from GenScript. Purity was confirmed to be >95%, and MALDI analysis was performed to verify peptide mass (data not shown); “↓” indicates ADAM10 cleavage site.
MeHA (100 mg) was dissolved in triethanolamine-buffered saline (TEOA buffer, pH 8) and reacted at 37 °C overnight in the presence of either stable (9.28 mg) or transient (19.78 mg) peptides, followed by dialysis (4 days) and lyophilization (3 days). This coupling ratio was designed to obtain a final peptide concentration of 2 mM in a 1.5 wt% MeHA hydrogel (i.e., HA content held constant) for all functionalized macromer stocks while consuming no more than 20% of available methacrylate groups on the MeHA. The activity of ADAM10 on transient peptides coupled to MeHA was verified using 0.5 mM fluorescein-modified transient HAV peptide, in PBS containing 2 nM ADAM10.
Hydrogel fabrication and characterization:
Macromers were sterilized using a germicidal lamp for 40 minutes and subsequently dissolved in sterile phosphate buffered saline (PBS) containing 0.05 wt% photoinitiator 2-methyl-1-[4-(hydroxyethoxy)phenyl]-2-methyl-1-propanone (I2959). This hydrogel precursor was pipetted into a 50 μL mold, covered with a glass coverslip, and exposed to an ultraviolet (UV) light source (Eiko, ~2.0 mW/cm2) for 10 minutes. The peptide concentration was maintained at 2 mM in all samples (except for hydrogel controls without any peptide coupling, denoted as MeHA) and the dose of HAV was controlled through the ratio of the HAV and scrambled peptides (e.g., HAV 0, HAV 50, and HAV 100 denotes samples of 2 mM scrambled, 1 mM HAV/1 mM scrambled, and 2 mM HAV, respectively).
Acellular hydrogels (~5mm Ø, ~2 mm thickness) were tested with a Dynamic Mechanical Analyzer (DMAQ800, TA Instruments) in unconfined compression. Hydrogels were compressed to a maximum of 70% of their initial thickness, and the elastic compressive modulus was determined as the slope of the stress versus strain curve at low strains (10 to 20%) using a sweep with a force ramp of 0.5 N/min to max 15 N (Figure S11). The mass swelling behavior of the hydrogels was characterized by the mass ratio of swollen hydrogels (after 24 h in PBS) to dry hydrogels.
Cell culture and encapsulation:
Human MSCs (Lonza) were expanded to passage 3 in growth media comprised of α-MEM with 16.7% (v/v) FBS (Gibco), 1% (v/v) l-glutamine (Invitrogen), and 1% (v/v) penicillin–streptomycin (Invitrogen). Cells were harvested by rinsing with PBS and treating with 0.05 wt% trypsin and then resuspended in 1.5 wt% macromer, 0.05 wt% I2959, and 20 M/mL MSCs. This hydrogel precursor was slowly pipetted into 50 μL molds, covered with glass coverslips, and exposed to a UV light source (Eiko, ~2.0 mW/cm2) for 10 minutes for crosslinking. Hydrogels were cultured in vitro in DMEM supplemented with 1% penicillin/streptomycin, 1mM sodium pyruvate, 40 mg/ml L-proline, 100 nM dexamethasone, 50 μg/ml ascorbic acid 2-phosphate, 1% ITS+, and 10 ng/mL TGF-β3 (chondrogenic media). For studies with small-molecule ADAM10 inhibitor GI254023X, inhibitor was added from stock solutions to a final concentration of 5 μM in chondrogenic media. The viability of MSCs in functionalized hydrogels was assessed using a live/dead cytotoxicity kit (Invitrogen) according to manufacturer instructions. Live/dead images were captured on a Leica SP5 confocal microscope.
Gene expression analysis:
Samples were disrupted in Trizol (Invitrogen) using a handheld tissue homogenizer; RNA was extracted according to manufacturer specifications and measured with an ND-1000 spectrophotometer (Nanodrop Technologies). 1 µg of RNA was taken from each sample for cDNA synthesis using reverse transcriptase (Superscript II, Invitrogen) and oligoDT as the primer (Invitrogen). Polymerase chain reaction (PCR) was then performed using the Applied Biosystems 7300 system for Real-Time PCR with a 25 μL reaction volume for Taqman (5′-nuclease) reactions (n=4). Primers and probes for releant targets glyceraldehyde 3-phosphate dehydrogenase (GAPDH, housekeeping gene), type I collagen (COL1), type II collagen (COL2), and aggrecan (ACAN) were selected as shown in Table S1. Relative gene expression was assessed with ΔΔCT method, where the fold difference is found by 2−ΔΔCt.
Biochemical and histological analysis:
After MSC-laden hydrogels were cultured for 8 weeks, they were digested using papain (1 mL/construct, 0.56 U/mL in 0.1 M sodium acetate, 10 M cysteine hydrochloric acid, 0.05 M ethylenediaminetetraacetic acid, pH 6.0) at 60°C for 16 hours. Samples were then analyzed for sulfated glycosaminoglycan (sGAG, using dimethylmethylene blue), DNA (using PicoGreen), and collagen (orthohydroxyproline, using dimethylaminobenzaldehyde and chloramine T reagents) content. [10, 29] For the histological analysis of constructs, all samples were fixed in 10% formalin (24 hours), embedded in paraffin and stabilized at 4°C (24 hours), and subsequently processed using standard histological protocols. Histological sections (5 μm) were stained using antibodies for type I collagen (Col I, mouse monoclonal anti-collagen type 1, Sigma), type II collagen (Col II, (mouse monoclonal anti-collagen type II, Developmental Studies Hybridoma Bank)), and sulfated glycosaminoglycan (sGAG). For quantification, images were first converted to 8-bit and then inverted. Mean staining intensity within randomly placed frames for each section was measured with ImageJ (Figure S2).
Statistical analysis:
Values in this work are reported as mean values ± the standard error of the mean (SEM). Unless otherwise specified, transformation and outlier removal were not performed. Normalization was performed using relevant control groups (e.g. gene expression was normalized to that of control cells plated in 2D). StatPlus:mac LE (AnalystSoft) was used to perform statistical analyses with one-way ANOVA (and Tukey’s honestly significant difference post hoc test of the means) to compare between groups (n≥4), where culture duration and experimental group were independent factors.
Supplementary Material
Acknowledgements
This work was supported by grants from the National Institutes of Health (R01 EB008722, T32 AR007132) and a National Science Foundation Graduate Research Fellowship to MYK.
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Contributor Information
Mi. Y. Kwon, Department of Bioengineering, University of Pennsylvania, 240 Skirkanich Hall, 210 S. 33rd St, Philadelphia PA, 19104, USA
Dr. Sebastian. L. Vega, Department of Bioengineering, University of Pennsylvania, 240 Skirkanich Hall, 210 S. 33rd St, Philadelphia PA, 19104, USA
Dr. William. M. Gramlich, Department of Chemistry, University of Maine, Orono, ME, 04469, USA
Dr. Minwook. Kim, Department of Orthopedic Surgery, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, 19104, USA
Prof. Robert. L. Mauck, Department of Bioengineering, University of Pennsylvania, 240 Skirkanich Hall, 210 S. 33rd St, Philadelphia PA, 19104, USA; Department of Orthopedic Surgery, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, 19104, USA
Prof. Jason. A. Burdick, Department of Bioengineering, University of Pennsylvania, 240 Skirkanich Hall, 210 S. 33rd St, Philadelphia PA, 19104, USA, burdick2@seas.upenn.edu
References
- [1].Caplan AI, J. Orthop. Res 1991, 9, 641; [DOI] [PubMed] [Google Scholar]; Rogers JJ, The American Surgeon 1995, 61, 231. [PubMed] [Google Scholar]
- [2].Kim IL, Khetan S, Baker BM, Chen CS, Burdick JA, Biomaterials 2013, 34, 5571; [DOI] [PMC free article] [PubMed] [Google Scholar]; Guvendiren M, Burdick JA, Nat. Commun 2012, 3, 792; [DOI] [PubMed] [Google Scholar]; Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, Rivera-Feliciano J, Mooney DJ, Nat. Mater 2010, 9, 518; [DOI] [PMC free article] [PubMed] [Google Scholar]; Bahney CS, Hsu CW, Yoo JU, West JL, Johnstone B, FASEB Journal 2011, 25, 1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Connelly JT, Garcia AJ, Levenston ME, Biomaterials 2007, 28, 1071. [DOI] [PubMed] [Google Scholar]
- [4].Kim IL, Mauck RL, Burdick JA, Biomaterials 2011, 32, 8771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Knudson CB, Birth Defects Res. C Embryo Today 2003, 69, 174; [DOI] [PubMed] [Google Scholar]; Astachov L, Vago R, Aviv M, Nevo Z, Front. Biosci 2011, 16, 261; [DOI] [PubMed] [Google Scholar]; Maleski MP, Exp. Cell Res 1996, 225, 55. [DOI] [PubMed] [Google Scholar]
- [6].Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B, Cell 1990, 61, 1303; [DOI] [PubMed] [Google Scholar]; Andhare RA, Takahashi N, Knudson W, Knudson CB, Osteoarthritis Cartilage 2009, 17, 906; [DOI] [PMC free article] [PubMed] [Google Scholar]; Peterson RS, Andhare RA, Rousche KT, Knudson W, Wang W, Grossfield JB, Thomas RO, Hollingsworth RE, Knudson CB, J. Cell Biol 2004, 166, 1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chung C, Mesa J, Randolph MA, Yaremchuk M, Burdick JA, J. Biomed. Mater. Res. A 2006, 77, 518; [DOI] [PMC free article] [PubMed] [Google Scholar]; Chung C, Burdick JA, Tissue Eng. Part A 2009, 15, 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Burdick JA, Chung C, Jia X, Randolph MA, Langer R, Biomacromolecules 2005, 6, 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Smeds KA, Pfister-Serres A, Miki D, Dastgheib K, Inoue M, Hatchell DL, Grinstaff MW, J. Biomed. Mater. Res 2001, 54, 115; [DOI] [PubMed] [Google Scholar]; Khetan S, Burdick JA, Biomaterials 2010, 31, 8228; [DOI] [PubMed] [Google Scholar]; Khetan S, Katz J, Burdick J, Soft Matter 2009, 5, 1601. [Google Scholar]
- [10].Kim M, Erickson IE, Choudhury M, Pleshko N, Mauck RL, J. Mech. Behav. Biomed. Mater 2012, 11, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Oberlender SA, Tuan RS, Cell. Adhes. Commun 1994, 2, 521. [DOI] [PubMed] [Google Scholar]
- [12].DeLise AM, J. Cell. Biochem 2002, 87, 342. [DOI] [PubMed] [Google Scholar]
- [13].Tuan RS, J. Bone Joint Surg. Am 2003, 85, 137. [DOI] [PubMed] [Google Scholar]
- [14].Delise AM, Developmental Dynamics 2002, 225, 195. [DOI] [PubMed] [Google Scholar]
- [15].Vega L JCM, Lee MK, Jeong JH, Smith CE, Lee KY, Chung HJ, Leckband DE, Kong H, Biomacromolecules 2014, 15, 2172; [DOI] [PubMed] [Google Scholar]; J. C. M. V. L, Lee MK, Qin EC, Rich M, Lee KY, Kim DH, Chung HJ, Leckband DE, Kong H, J. Mater. Chem. B 2016, 4, 6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Williams E, Williams G, Gour GJ, Blaschuk OW, Doherty P, J. Biol. Chem 2000, 275, 4007; [DOI] [PubMed] [Google Scholar]; Shapiro LL, Nature 1995, 374, 327; [DOI] [PubMed] [Google Scholar]; Blaschuk OW, Developmental Biology 1990, 139, 227. [DOI] [PubMed] [Google Scholar]
- [17].Bian L, Guvendiren M, Mauck RL, Burdick JA, Proc. Natl. Acad. Sci. U.S.A 2013, 110, 10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Vega SL, Kwon M, Mauck RL, Burdick JA, Ann. Biomed. Eng 2016, 44, 1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Reiss K, Maretzky T, Ludwig A, Tousseyn T, de Strooper B, Hartmann D, Saftig P, EMBO Journal 2005, 24, 742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nakazora S, Matsumine A, Iino T, Hasegawa M, Kinoshita A, Uemura K, Niimi R, Uchida A, Sudo A, Biochem. and Biophys. Res. Comm 2010, 400, 493. [DOI] [PubMed] [Google Scholar]
- [21].Modarresi R, Lafond T, Roman-Blas JA, Danielson KG, Seghatoleslami TRS,MR, J. Cell Biochem 2005, 95, 53. [DOI] [PubMed] [Google Scholar]
- [22].Oberlender SA, Tuan RS, Development 1994, 120, 177; [DOI] [PubMed] [Google Scholar]; Jin EJ, Park KS, Kim D, Lee YS, Sonn JK, Jung JC, Bang OS, Kang SS, Mol. Cells 2010, 29, 425. [DOI] [PubMed] [Google Scholar]
- [23].Haas AR, Tuan RS, Differentiation 1999, 64, 77. [DOI] [PubMed] [Google Scholar]
- [24].Bayliss MT, Ali SY, Biochem. J 1978, 176, 683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Treppo S, Koepp H, Quan EC, Cole AA, Kuettner KE, Grodzinsky AJ, J. Orthop. Res 2000, 18, 739. [DOI] [PubMed] [Google Scholar]
- [26].Cosgrove BD, Mui KL, Driscoll TP, Caliari SR, Mehta KD, Assoian RK, Burdick JA, Mauck RL, Nat. Mater 2016, 15, 1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Williams G, Williams EJ, Doherty P, J. Biol. Chem 2002, 277, 4361. [DOI] [PubMed] [Google Scholar]
- [28].Hong S, Troyanovsky RB, Troyanovsky SM, J. Cell Biol 2013, 201, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Stegemann H, Stalder K, Clin. Chim. Acta 1967, 18, 267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


