Abstract
Longitudinal studies have shown that clinical precursors of antisocial personality disorder (ASPD) include attention-deficit/hyperactivity disorder (ADHD) and more notably comorbid ADHD and conduct disorder (CD). Despite existing evidence for the purported role of abnormal serotonergic function in aggressive youth and adults, little evidence exists on the role of serotonin in the progression from childhood disruptive behavior disorders to adult psychopathology, including ASPD. This study examined the relation between serotonergic function in children diagnosed with ADHD and the development of ASPD in early adulthood. We hypothesized that low serotonin response to a pharmacological probe in childhood would predict the development of adult ASPD. Towards this goal we divided 40 adults (M=37,F=3), ages 23–26 (m-24.57,sd-2.33) diagnosed with childhood ADHD into 2 groups: participants with (n=21) and without (n=19) ASPD. We used logistic regression to assess whether serotonergic measures in childhood assessed via prolactin and cortisol responses to a fenfluramine challenge, would selectively predict the development of ASPD in early adulthood. Logistic regression models showed that low central serotonergic response in childhood indexed by cortisol response significantly predicted adult ASPD (Wald=4.427,p=.035) but not ADHD diagnosis in adulthood. Adults without ASPD had the highest serotonergic response whereas adults with adolescent ASPD (i.e. early onset ASPD) had the lowest response. Thus we provide new evidence of the link between low serotonergic function in childhood and the development of ASPD in adulthood, particularly for boys with adolescent onset of ASPD. These findings are relevant for understanding the contribution of childhood neurobiology to risk for later ASPD.
Introduction
Adult psychopathology characterized by impulsivity and/or aggression is frequently preceded by childhood disruptive behavior disorders, including attention deficit/hyperactivity disorder (ADHD), oppositional defiant disorder (ODD) and conduct disorder (CD). In prior iterations of DSM, the diagnosis of antisocial personality disorder (ASPD) required evidence of prior CD. Although this stipulation was removed in DSM-5, the link between childhood disruptive behavior disorders and ASPD has been well documented. Longitudinal studies have consistently shown that clinical precursors of ASPD include ADHD and more notably comorbid ADHD and CD (Retz et al., 2009; von Polier et al., 2012; Storebø & Simonsen 2016). Further, children with severe ADHD symptomatology and comorbid antisocial behavior are at heightened risk for the development of antisocial behavior and substance use in adolescence (Marshal & Molina, 2006).
Less is known, however, about the biological factors that underlie the progression from childhood disruptive behaviors to antisocial behaviors in adolescence and adulthood. Multiple animal studies have suggested a link between abnormalities in serotonergic function and impulsive and/or aggressive behaviors (Sijbesman et al., 1991; Holmes et al., 2002; Veenema et al., 2005; de Boer et al., 2005). Accordingly, serotonergic dysregulation has been documented in human studies of impulsive aggression, with findings pointing to negative correlations of serotonergic activity in patients with borderline (Soloff et al., 2003) and antisocial personality disorders (Moore et al., 2002), intermittent explosive disorder (Coccaro et al., 2010), as well as in individuals with impulsive aggression and suicidal behaviors (New et al., 2004) or with comorbid substance use disorders (Sher et al., 2008). Thus serotonergic abnormalities have been linked to both categorical (e.g. personality disorder diagnoses) and dimensional (e.g. aggression, impulsivity, hostility) features of adult psychopathology, even in nonpatient samples (Manuck et al., 1998). These findings raise the question of whether aggression in childhood might be associated with similar serotonergic findings, or whether low serotonergic reactivity in childhood might be a precursor to later aggression. However, the relation between serotonergic dysregulation in children and aggression has remained uncertain, with studies showing both positive (Halperin et al., 1994; Pine et al., 1997) and negative (Kruesi et al., 1990, 1992; Halperin et al., 1997a, 1997b) associations between measures of serotonergic function and aggression in youth with disruptive behavior disorders. Yet, the number of studies to date is few, and almost all are cross-sectional in nature. Consequently, the question of whether the presence of serotonergic dysfunction in early life is associated with the development of later psychopathology remains largely unanswered. This premise can only be addressed by early in life assessment of serotonergic function and subsequent longitudinal follow-up.
Two reports from our group provide initial evidence for a longitudinal relation between childhood serotonergic function and adolescent outcomes. We used the fenfluramine (FEN) challenge test, which has been shown to produce reliable patterns of prolactin (PRL) and cortisol (CORT) release, as an indirect measure of central serotonin reactivity (Stoff et al., 1992a). In addition to findings of associations between serotonin response and aggression measures in cross-sectional studies (Halperin et al., 1994, 1997a, 1997b) there also were associations with longitudinal course. Halperin et al (2006) reported that elevated PRL response to FEN (i.e. elevated serotonin function) in childhood (e.g. ages 7–11) may be a protective factor for adolescent aggression. Subsequently, Flory et al. (2007) found that adolescents ages 16–21 who met criteria for ASPD had significantly lower PRL response to FEN challenge in childhood as compared to those without ASPD at outcome. In contrast, a multicenter study of 448 adult ADHD patients and 580 controls from Norway failed to demonstrate support for any major role of serotonin function, measured by variances of the serotonin transporter gene, in persistent ADHD (Landaas et al., 2010). Taken together, these reports provide a possible link between low serotonergic reactivity in childhood and aggressive or psychopathology in adolescents and young adults.
In this study, we evaluated the longitudinal relationships between serotonergic function in children with ADHD and ASPD diagnosis in early adulthood. We hypothesized that lower serotonergic responsivity in childhood would predict the development of ASPD at 15-year follow-up, but it would not predict the persistence of ADHD (Landaas et al., 2010) – indicating the relative independence of the two conditions. We also aimed to evaluate if the purported association between low serotonergic responsivity in childhood (in a cohort of youth with ADHD and/or disruptive behavior) and adult ASPD may exclusively be accounted for by those children with comorbid ADHD + CD, and to examine for possible moderating effects of these conditions for the development of ASPD. Lastly, we examined patterns of central serotonin function in childhood between individuals with adolescent vs. adult onset ASPD.
Experimental Procedures
Overview
The sample was originally recruited between 1990 – 1997 for studies funded by the National Institute of Mental Health (Halperin et al., 2003) to examine relations between central serotonergic function and aggression in children with ADHD. Children were classified into either of two groups: Aggressive and Non-aggressive ADHD (Based upon parent and teacher ratings of physical aggression), and all children received the fenfluramine challenge to assess central serotonergic function. All participants were 7–11 years-old at the time of recruitment and the sample was racially and ethnically diverse. The group was primarily of lower to low-middle SES, with a large portion at the poverty level. All participants were English-speaking. Children were primarily referred by school personnel and mental health providers, and as part of the study, children obtained a comprehensive, multi-method, multi -informant assessment. The sample was rated as having significant behavior problems by both parents and teachers. Those recruited prior to 1994 were diagnosed using DSM-III-R criteria and tested using the Wechsler Intelligence Scale for Children-Revised edition (WISC-R; Wechsler, 1974); those recruited after 1994 were evaluated using DSM-IV criteria and the Wechsler Intelligence Scale for Children-Third edition (WISC-III; Wechsler, 1991). Diagnoses were determined using the Diagnostic Interview Schedule for Children (DISC; Shaffer et al., 1996), which was administered to parents. A diagnosis of schizophrenia, pervasive developmental disorder, Tourette’s syndrome, or Full Scale IQ (FSIQ) below 70 was exclusionary.
This sample was re-evaluated 9.4 years (s.d.=1.9) after the baseline assessment (ages 16 – 21 years; Halperin et al., 2006) and then again as adults 15.58 years (s.d.=2.17) after the baseline assessment (ages 21 – 25 years), at which time they received a clinical evaluation along with fMRI (Clerkin et al., 2013). Fifty eight participants took part in the second wave assessment, which included interviews from participants and other informants (e.g. parents) for the identification of Axis II psychopathology. The third wave follow-up was conducted between 2006 and 2011 and included interviews with participants to identify adult DSM-IV Axis I and Axis II psychopathology. The third wave of assessments was conducted only in a subset of adult participants from the original sample who were available for assessment and eligible to participate in a magnetic resonance imaging study (Clerkin et al., 2013). The adult evaluation provided the outcome measures for the current study. This current study examined the relation between measures from the adult assessment and the childhood measures of central serotonin response and is the first study to do so.
The current report is therefore based on data from a subsample of 40 adults who completed the FEN challenge in childhood and were assessed at the third follow-up. This third follow-up sample represents 63% of the number of participants who completed the second wave follow-up by February 2005 and 36% of the original sample.
Demographics
Participants (n=40) were recruited at ages 7–11 (m- 9.36; sd-1.36), had a mean Full Scale IQ of 97.07 (SD = 12.54), were generally of lower - to lower - middle socioeconomic status (SES; Nakao & Treas, 1994), and predominantly male (M-37, F-3) aged 23–26 years (m-24.57, sd-2.33). This sample was divided into 2 groups based on adult status: 21 with and 19 without ASPD. Seven participants who completed the second wave adolescent follow-up and were diagnosed with ASPD at that time were not available for assessment because they were either incarcerated (n=4) or deceased (n=3). The 4 individuals who were incarcerated were included in the analyses since, based on prior assessments and their current situation, they likely would have met the diagnostic criteria for ASPD.
Clinical evaluation
During the baseline evaluation children were screened using teacher reports on the IOWA Conners’ Teacher Rating Scale (Loney & Milich, 1982) and parent reports on the Child Behavior Checklist (CBCL; Achenbach, 1991); parents were also interviewed with the Diagnostic Interview Schedule for Children (DISC). Aggression scores were derived from the IOWA Conners’ Teacher Rating Scale. All participants in the current sample received a diagnosis of ADHD in childhood as per DSM-III-R; additionally, 20 participants were diagnosed with ODD and 12 with CD. During the adult follow-up, all participants were administered the Structured Clinical Interview for DSM–IV Axis I (SCID-I; First et al., 1996) and Axis II Personality Disorders (SCID–II; First et al., 1997). In addition, ADHD diagnosis was assessed using a semi-structured interview comprised of the 18 DSM-IV ADHD symptoms. Prompts for the interview were adapted from the Schedule for Affective Disorders and Schizophrenia for School-Age Children (Kaufman et al., 1997 and the Conners’ Adult ADHD Diagnostic Interview for DSM-IV (Epstein et al., 2001). The adapted interview demonstrated strong internal consistency in our sample (alpha=0.92). In addition, Conners’ Adult ADHD rating Scale (CAARS; Conners et al., 1999) was administered to both the proband and another informant. In the case of ASPD, a prior diagnosis of CD was a requirement at the time of the assessment; in our study interviewers were blind to baseline data, including whether children had been given a diagnosis of CD during the baseline study. Participants were asked about prior diagnosis if CD and that information was used to established diagnoses at the time of the third follow up. Signed informed consent was obtained from all participants or parents at all three time points. The protocols were approved by the Institutional Review Boards at City University of New York and the Mount Sinai School of Medicine.
Serotonergic Function.
FEN is a known sympathomimetic agent that releases and blocks the re-uptake of central stores of 5-HT (Rowland & Carlton, 1986). The administration of FEN leads to an increase in plasma CORT and PRL (Muhbauer & Muller-Oerlinghausen, 1985; Stoff et al., 1992b), and the magnitude of the CORT and PRL responses is considered to reflect overall 5-HT responsivity in the hypothalamic-pituitary axis (Fuller, 1992; Coccaro, 1989). Central serotonergic function was assessed by measuring the CORT and PRL responses to an acute dose of FEN. Details about the FEN test are presented elsewhere (Flory et al., 2007). In short, participants did not take medication for 7 days and followed a low monoamine diet for 3 days prior to the challenge; they reported to the laboratory at 08.00 h after fasting overnight. We collected two baseline blood samples drawn 10 min apart at 09.45h and 09.55h from an indwelling catheter inserted into a forearm vein. At 10.00h, a 1mg/kg dose of d,l-fenfluramine hydrochloride was administered orally. Blood samples were drawn 60, 120, 180, 240 and 300min later for determination of plasma PRL, CORT, FEN and norfenfluramine (NORFEN) concentrations. Participants remained awake and fasting during the entire procedure, reclining in a bed and watching videotapes. Videos with violent and/or emotionally-charged content were excluded. We allowed the participants to select a few videos since the procedure was lengthy (7 hrs total) and extended the length of a single film. The procedure of collecting the blood sample for indirect measures of central serotonin function was conducted at baseline when participants were aged 7–11 years old.
Samples were processed by well-established procedures (Halperin yet al., 1994). In brief, samples were placed on ice prior to centrifugation (within 2 h); then CORT and PRL samples were frozen at −80C until assayed by radioimmunoassay (Kahn et al., 1994). Post-medication samples of plasma FEN and NORFEN were obtained hourly and were stored at −20°C until assayed by gas chromatography with electrical detection (Krebs et al., 1984).
Statistical analyses
Baseline CORT and PRL levels were calculated as the mean of two blood samples collected prior to the administration of FEN, and area under the curve (AUC) for both PRL and CORT was calculated using trapezoidal integration (Pruessner et al., 2003). Thus the calculated AUC for PRL and CORT (e.g. PRLAUC and CORTAUC) served as indicators of serotonin responsiveness. Both PRLAUC and CORTAUC were log-transformed prior to analyses to normalize the distributions. We used median split of PRLAUC and CORTAUC to define Low and High responsiveness groups (e.g. LowPRLAUC = 1; HighPRLAUC = 0 and LowCORTAUC = 1, HighCORTAUC = 0). These groups were used as predictors in a logistic regression model. We chose to include IOWA aggression score in childhood instead of childhood diagnosis of CD since CD was a requirement for later ASPD, as described above. Additional variables included maximum drug levels for FEN (MAXFEN) and NFEN(MAXNFEN) and socio-economic status (i.e. a known predictor of ASPD (Dohrenwend et al, 1992; Piotrowska et al., 2015). Baseline measures for both PRL and CORT were not included in the regressions since these were not significantly different between participants with and without ASPD. The 3 female participants, all of whom were in the NO-ASPD group were excluded from the analyses.
Post hoc analyses included independent sample t-tests to compare differences in PRL and CORT response at different time points of the experiment for participants with no ASPD, those with adolescent + adult ASPD, and those with adult ASPD only. Correlational analyses included Pearson’s correlations between serotonin responsiveness, childhood measures of aggression, delinquency and externalizing problems (CBCL scores), and adult ADHD symptoms and point-biserial correlations between those measures and ASPD diagnosis. Due to the finding of significantly higher PRL response in females, post hoc analyses were conducted in males only.
The hypothesis that lower serotonergic responsiveness would predict the development of ASPD was evaluated using logistic regression. We also performed linear regression analyses to investigate if serotonergic response in childhood may predict the persistence of ADHD in early adulthood, as indexed by scores on the CAARS.
Results
Descriptive and clinical features.
Twenty one of the 40 participants (52.5%) met criteria for ASPD; of these, 11 were diagnosed with ASPD during the second follow up and had the diagnosis confirmed at the third follow-up; another 10 participants were newly diagnosed with ASPD for the first time as adults. Fourteen participants met criteria for persistent ADHD in adulthood, 4 of these were in the No ASPD group (21%) and 10 were in the ASPD group (47%). Twenty six participants did not meet criteria for ADHD in adulthood. Similar to the original sample, the cohort was ethnically diverse; 9 participants self-identified as African-American, 11 as Caucasian, 17 as Hispanic and 3 as mixed ethnic background (Table 1). In addition, we compared the participants in the current study (n=40) to the rest of the participants from the original sample (n=70) on number of characteristics including age, FSIQ, sex, comorbid ODD/CD, as well as scores of inattention, delinquency, aggression, internalizing and externalizing problems. The two samples showed no significant differences on any of these measures (all p<0.05) thus suggesting that the follow up sample was fairly representative of the original cohort despite attritions.
Table 1.
Demographics
| Variables | ASPD n=21 | No ASPD n=19 | P |
|---|---|---|---|
| Childhood | |||
| ▓FSIQ | 92.14 (±12.92) | 102.52 (±9.77) | 0.007 |
| ▓SES | 29.85 (±14.08) | 36.84 (±18.25) | 0.18 |
| ▓Dx ODD | 9 | 12 | 0.17 |
| ▓Dx CD | 10 | 2 | 0.012 |
| Adulthood | |||
| CAARS-total | 59.06 (±13.08) | 52.47 (± 13.37) | 0.19 |
| ADHD persist | 10 | 4 | |
| ADHD remit | 11 | 15 | |
| ASPD – adolesc | 11 | 0 | |
| ASPD- adult | 10 | 0 | |
| ▓Sex | M=21/F=0 | M=16/ F=3 | |
Abbreviations: FSIQ – full scale IQ; SES – socio-economic status; ODD – oppositional defiant disorder; CD – conduct disorder;
CAARS – Connors’ adult ADHD rating scale; ADHD – attention deficit hyperactivity disorder; ASPD – antisocial personality disorder;
The ASPD group was comprised of males only; the No ASPD group had 3 females. Female participants without ASPD showed a significantly higher prolactin response during the FEN challenge compared to male participants without ASPD (F=27.86, df - 1/17, p=.001) and all male participants (F=38.23, df −1/38, p=.001); there were no differences in the CORT measures between male and female participants.
Relation to ASPD, aggression and ADHD with measures of serotonergic reactivity.
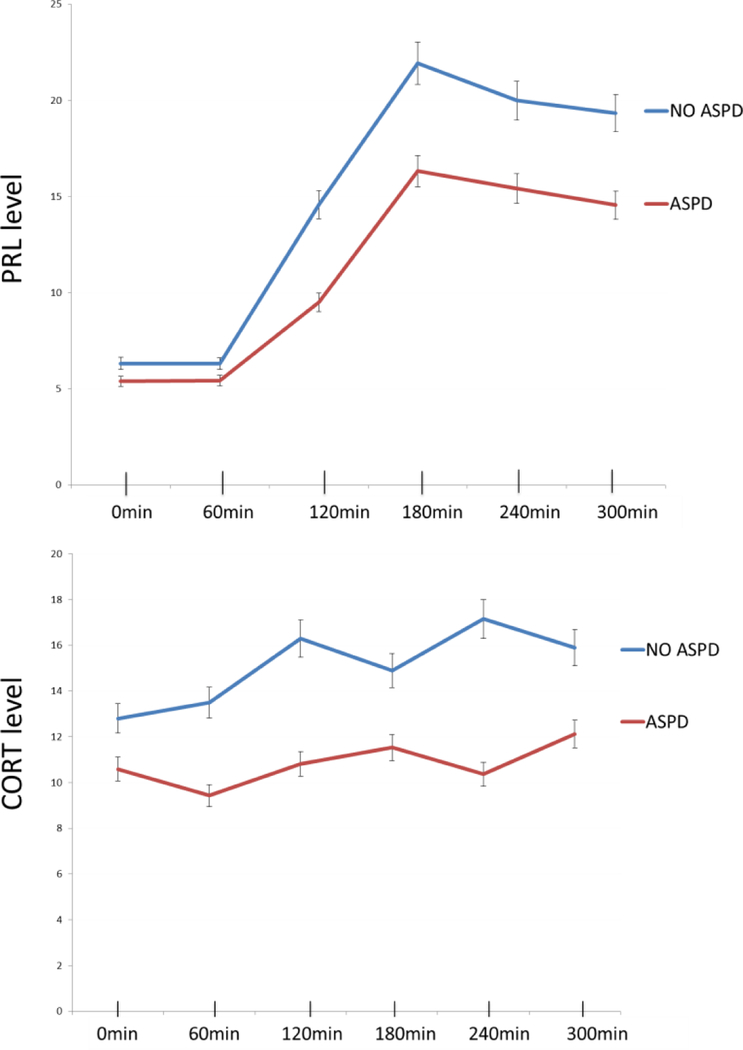
Two sets of step-wise logistic regressions, with ASPD as the dependent variable, were conducted. Regression 1, which included the variables for Low and High CORTAUC group as predictors showed that the participants who were in the Low CORTAUC group (i.e. participants with low serotonergic response) were significantly more likely to develop ASPD in early adulthood (Wald=4.427, p=.035; OR = 6.746 (CI 95% = 1.140; 39.933)). SES approached significance as an additional predictor (Wald=3.059, p=.080). Regression 2, which included Low and High PRLAUC group as a predictor, showed that none of predictor variables was significantly correlated with adult ASPD (Table 2). Unadjusted PRL and CORT levels throughout the 5h challenge are shown in Fig. 1. One way ANOVA with post hoc t-tests, conducted in male participants only, who were divided into No ASPD, adolescent+ adult ASPD and adult ASPD only, showed that the No ASPD group had the highest CORT and PRL response and the adolescent + adult ASPD group had the lowest response. This difference was significant for PRL response at 240min (t=2.155, df=28, p=0.04). For CORT response there were significant differences at 60min (t=2.065, df=28, p=0.048) and 240 min (t=2.171, df=28, p=0.039). The patterns of response are illustrated in Figure 2.
Table 2.
Comparison of demographic variables between the third wave sample and the rest of the original sample
ny downing on
| Variables | Original sample Mean /SD (N=72) | Follow up sample Mean/SD (N= 40) | P values |
|---|---|---|---|
| Age | 8.9/1.2 | 9.3/1.4 | .080 |
| CBCL Aggression | 71.1/13.3 | 72.9/ 13.5 | .497 |
| CBCL Delinquency | 68.9 / 8.6 | 67.1 / 9.7 | .366 |
| CBCL Attention | 73.3 / 9.8 | 72.2 / 9.1 | .538 |
| CBCL Externalizing | 69.7/ 10.4 | 70.2/10.3 | .802 |
| CBCL Internalizing | 65.9/ 10.3 | 64.4/13.2 | .578 |
| IOWA Aggression | 7.9/ 4.7 | 8.2/4.5 | .747 |
| FSIQ | 93.8/ 13.3 | 97.7/12.6 | .135 |
| SES | 32.5/ 15.1 | 33.1/16.3 | .838 |
| Sex | M=62, F= 10 | M=37, F=3 | .245 |
| ODD | Yes=60, No=12 | Yes=32,No=8 | .421 |
| CD | Yes=27, No= 45 | Yes=12, No=28 | .279 |
| Race/Ethnicity | AA-23, W- 14, H-27, Other - 8 | AA-9, W-11, H −17, Other-3 | .258 |
Abbreviations: CBCL – child behavioral check list; FSIQ – full scale IQ; SES – socio-economic status; ODD – oppositional defiant disorder; CD – conduct disorder;
Figure 1.
CORT and PRL response for participants with (red line) and without (blue line) ASPD
Legend: CORT – cortisol; PRL – prolactin; ASPD – antisocial personality disorder
Fig 2.
Prolactin response in male participants with No ASPD (blue line, n=19) Adult ASPD only (red line, n=10) and Adolescent ASPD (green line, n=11).
*indicate significant differences in CORT and PRL levels between No ASPD and Adolescent ASPD participants at p< .05.
Legend: CORT – cortisol; PRL – prolactin; ASPD – antisocial personality disorder
Adult ASPD diagnosis was positively correlated with childhood CBCL scores for delinquency (r=.46, p =.013) and externalizing behaviors (r=.39, p=.037), suggesting that youth with higher scores on scales measuring disruptive behavior were more likely to develop ASPD. ASPD diagnosis showed significant inverse correlations with CORTAUC (r=−.34, p=.041), which supports the contention that low serotonergic function in childhood, assessed by CORT response, is linked to adult ASPD.
Logistic regressions, which included ADHD diagnosis as the dependent variable, showed that neither PRL (Wald =.364, p=.546) nor CORT (Wald =.189, p=.663) significantly predicted ADHD diagnosis in early adulthood, further suggesting that serotonergic function may be more relevant to aggression in the context of antisocial behavior and less so in relation to persistence of ADHD (Table 3). No significant correlations were detected between ADHD symptoms in adulthood and ASPD diagnosis.
Table 3.
Results from logistic regression showing that low serotonergic response in childhood (i.e. indexed by dividing participants in Low and High CORT groups via median split) was a significant predictor of ASPD diagnosis in adulthood.
| Variables | B | Wald | P | OR | CI 95% Upper/Lower |
|---|---|---|---|---|---|
| Regression 1 | |||||
| MaxFEN | −.028 | .811 | .368 | .972 | .914, 1.034 |
| MaxNFEN | .047 | .606 | .436 | 1.048 | .932, 1.178 |
| SES | −.053 | 3.059 | .080 | .948 | .893, 1.006 |
| Sex | −20.280 | .000 | .999 | .000 | .000 |
| IOWA AGG | .085 | .739 | .390 | 1.088 | .897, 1.321 |
| L/H CORT | −1.909 | 4.427 | .035 | 6.746 | .025, .877 |
| Regression 2 | |||||
| MaxFEN | −.003 | .006 | .937 | .997 | .936, 1.062 |
| MaxNFEN | .009 | .023 | .880 | 1.009 | .903, 1.127 |
| SES | −.038 | 1.838 | .175 | .963 | .912, 1.017 |
| Sex | −20.613 | .000 | .999 | .000 | .000 |
| IOWA AGG | .155 | 2.184 | .139 | 1.167 | .951, 1.433 |
| L/H PRL | −1.395 | 1.823 | .177 | 4.034 | .033, 1.878 |
Dependent variable – ASPD diagnosis in adulthood
Abbreviations: MaxFEN – maximal fenfluramin level: MaxNORFEN – maximal norfenfluramin level; SES – socioeconomic status; IOWA AGG – scores on the IOWA Aggression scale; L/H CORT – low/ high cortisol groups; L/H PRL – low/high prolactin groups; CORTAUC – cortisol area under the curve; PRLAUC – prolactin area under the curve
Discussion
The main finding from this study is that lower serotonergic function measured by CORT response to FEN challenge in childhood predicted the development of ASPD in adults with a history of childhood ADHD. In contrast, PRL response to FEN, as an alternative measure of central serotonin response, failed to significantly predict the development of adult ASPD – possibly because it was confounded by a strong sex effect. Serotonergic response in childhood did not predict persistence of ADHD symptoms in adulthood.
The relation of disruptive behavior disorders in children and subsequent ASPD has been evaluated in a number of longitudinal studies. A recent review (Storebo & Simonsen, 2016) of 18 prospective studies (n=5,501) reported that childhood ADHD with and without CD is a strong risk factor for later ASPD, and that impulsivity in ADHD may be an independent predictor for the later development of ASPD. One prospective study, in a cohort of 135 Caucasian males and 136 matched controls, showed that children diagnosed with ADHD at a mean age of 8 years had a significantly higher prevalence of ASPD (16% vs. 0% for controls) in adulthood 33 years later, despite the fact that CD was an exclusionary criterion for the original childhood study (Klein et al., 2012). Another longitudinal report (i.e. follow up from ages 7 to 22) in a cohort of 101 participants with ADHD documented personality disorder of any kind in 33% (i.e. ASPD being most commonly diagnosed) compared to 7% in their control group (Rasmussen & Gillberg, 2000). Of importance is that the recruitment of our original sample was focused on children with ADHD and was oversampled for individuals who were physically aggressive. As a result childhood CD was present in 34.8% (39/112) of the original sample and in 33.3% (e.g. 12/40) of the current sample. Some have considered that the persistence of ADHD symptoms might be a contributing factor for the development of adult ASPD – however, that was not the case in the Klein et al. study. In our sample, only about half of the participants with ASPD had persistent ADHD in adulthood (e.g. 10/21). Together, the findings indicate that ADHD and CD in childhood are both linked to increased risk for ASPD in late adolescence and early adulthood, and that the persistence of ADHD symptoms may contribute to the development of ASPD, but this is not a necessary factor for this trajectory. The high rates of ASPD in our sample seem also to reflect characteristics of the initial recruitment
While serotonergic response to FEN challenge has been well examined independently in both aggressive youths (Kriesi et al., 1900, 1992; Halperin et al., 1994, 1997a, 1997b, 2003; Pine et al., 1997; Soloff et al., 2000) and in adults with impulsive aggression and personality disorders (Coccaro et al., 1989a, 1989b; Dolan net al., 2002, 2003; Best et al., 2002) in cross sectional studies there is dearth of evidence regarding any possible link between serotonergic response in childhood and subsequent psychopathology in adolescence and/or adulthood. One report from our group shows evidence suggesting that low serotonergic adolescents with ASPD exhibited significantly lower serotonergic response to FEN in childhood in comparison to adolescents without ASPD (Flory et al. 2007). Consistent with those results, here we demonstrate that low serotonergic response in childhood indexed by CORT release significantly predicted the development of ASPD in early adulthood and that individuals who developed ASPD in adolescence tended to exhibit lower serotonergic responsivity compared to both individuals who developed ASPD in young adulthood and individuals who did not develop ASPD (Fig 2). Further we found significant positive correlations between both childhood delinquency and ratings of externalizing behaviors and adult ASPD, as well as significant negative correlations between serotonergic response measured by CORT response to FEN and adult ASPD. The findings from the current report add to our previous work by providing evidence of the relation between serotonergic function in childhood and adult psychopathology and support the assertion that individuals with the lowest serotonergic response in childhood might be the ones at greatest risk to develop early onset of ASPD symptoms (e.g. adolescence vs. adulthood).
Our finding of a link between a childhood biomarker and the development of ASPD 15 years later in youth with ADHD has considerable heuristic value and potentially important clinical implications as well. Compelling evidence suggests that a high level of childhood aggression (Lussier et al., 2012) that is stable over development, particularly in boys (Lopez-Romero et al., 2015)6, predicts adult antisocial personality traits. Others have shown that a measure of cognitive control might moderate the development of antisocial trait behavior, with high cognitive control serving as a protective factor (Hawes et al., 2016). Therefore, it stands to reason that a group at high risk for ASPD will consist of young boys with ADHD and comorbid CD (alternatively, with high aggression) who also have low serotonergic function and perhaps deficits in cognitive control. Although the use of FEN challenge is not clinically feasible, new technologies offer the potential for less invasive and possibly equally accurate ways to measure serotonergic activity using genetic markers such as serotonin transporter, serotonin receptor 2A and tryptophan hydroxylase genes (Huang et al., 2015). As such, early identification of children at greatest risk for antisocial outcomes could facilitate the development of and placements in early intervention programs. Further, emerging reports show that individuals with different genetic polymorphisms may respond differently to agents that affect serotonergic activity in the central nervous system, and this might be a direction for future research. For instance, one report suggests that patients with personality disorders and impulsive aggression, who also carry the l/l allele of the 5-HTTLPR gene, responded better to fluoxetine than patients carrying an s allele (Silva et al., 2010). Similarly, others have documented preferential antidepressant response to citalopram in individuals with polymorphism of the tryptophan hydroxylase-1 gene A218C (Ham et al., 2007). This emerging evidence further suggests that genotyping markers of serotonergic function might be potentially useful to identify individuals who are 1) at high risk to exhibit impulsive aggression and possibly develop ASPD and 2) are likely to respond to serotonergic agents.
Limitations
There are several limitations that need to be considered. First, the third follow-up did not include all participants from the initial cohort, but only on those who were available and able to complete a neuroimaging protocol. The attrition rate negatively affected the power to detect differences in serotonergic response between those with and without ASPD. In addition we demonstrate that the follow up sample did not differ from the rest of the original sample in any of the key characteristics. Further, the sample was predominantly male and the low number of female participants prevented us from conducting more definitive analyses in relation to gender effects on serotonergic function. We did not collect DNA samples at baseline which would have allowed us to investigate additional hypothesis related to whether genetic markers of the serotonergic system may predict later in life ASPD. Lastly, FEN was withdrawn from the market in 1997 due to safety concerns when used together with phentermine, which precluded us from conducting similar physiological assessments at follow-up.
Conclusions
This report provides new evidence of the link between low serotonergic functioning in childhood and adult psychopathology, particularly regarding the development of ASPD. This link appears to be prominent in boys diagnosed with ADHD who may also have comorbid CD and/or high aggression. Assessing serotonergic function in youth with ADHD and disruptive behavior disorders may represent a useful approach to identifying those at highest risk for later ASPD.
Supplementary Material
Table 4.
Results from logistic regression showing that serotonergic response in childhood (i.e. indexed by dividing participants in Low and High CORT and Low and High PRL groups via median split) was NOT a significant predictor of ADHD diagnosis in adulthood.
| Variables | B | Wald | P | OR | CI 95% Upper/Lower | ||
|---|---|---|---|---|---|---|---|
| Regression 1 | |||||||
| MaxFEN | .033 | .385 | .535 | 1.033 | .932, 1.146 | ||
| MaxNFEN | −.194 | 1.973 | .160 | .824 | .629, 1.079 | ||
| SES | −.141 | 2.121 | .145 | .869 | .719, 1.050 | ||
| IOWA AGG | .422 | 3.128 | .077 | 1.525 | .955, 2.435 | ||
| L/H CORT | −.771 | .189 | .663 | .462 | .014, 14.914 | ||
| Regression 2 | |||||||
| MaxFEN | .048 | .525 | .469 | 1.050 | .921, 1.196 | ||
| MaxNFEN | −.162 | .1.943 | .163 | .850 | .677, 1.068 | ||
| SES | −.129 | 1.828 | .176 | .879 | .729, 1.060 | ||
| IOWA AGG | .397 | 3.636 | .057 | 1.487 | .989, 2.236 | ||
| L/H PRL | 1.081 | .364 | .546 | 2.949 | .088, 98.771 | ||
Abbreviations: MaxFEN – maximal fenfluramin level: MaxNORFEN – maximal norfenfluramin level; SES – socioeconomic status; IOWA AGG – scores on the IOWA Aggression scale; L/H CORT – low/ high cortisol groups; L/H PRL – low/high prolactin groups; CORTAUC – cortisol area under the curve; PRLAUC – prolactin area under the curve
Acknowledgments
Funding and Disclosure
All of the co-authors significantly contributed to this study/paper, reviewed this paper and given their final approval. As instructed, the manuscript includes a proper description of the study’s IRB approval. The study was supported by a KO1069979 grant to Dr. J. Flory and by a RO1MH046448 grant to Dr. J. Halperin. Drs. Ivanov and Newcorn have not received any funding for this project. None of the authors have any competing financial interests in relation to their work on this project. At the time when the study began fenfluramine was commercially available in the US and was subsequently withdrawn from the market.
Role of Funding source
Funding for this study was provided by NIMH KO1069979 grant to Dr. J. Flory and by a RO1MH046448 grant to Dr. J. Halperin. Drs. Ivanov and Newcorn have not received any funding for this project. The NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict
Author J. Newcorn is a recipient of research grants from Enzymotec, Lundbeck and Shire; he has been an advisor/consultant for Akili Interactive, Alcobra, Arbor, KemPharm, Ironshore, Medice, Neos, NLS, Shire, Sunovion and Supernus. Author I. Ivanov is member of Data Monitoring Committee for Lundbeck. All other authors declare that they have no conflicts of interest.
References
- Achenbach T Manual for the Child Behavioral Cchecklist/4–18 and 1991 profile Burlington, University of Vermont, 1991b. [Google Scholar]
- Best M, Williams JM, Coccaro EF. Evidence for a dysfunctional prefrontal circuit in patients with an impulsive aggressive disorder. Proc Natl Acad Sci U S A 2002. June 11;99(12):8448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerkin SM, Schulz KP, Berwid OG, Fan J, Newcorn JH, Tang CY, Halperin JM. Thalamo-cortical activation and connectivity during response preparation in adults with persistent and remittent ADHD. Am J Psychitry 2013; 170 (9): 1011–9. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Siever LJ, Klar HM, Maurer G, Cochrane K, Cooper TB, Davis KL. Serotonergic studies in patients with affective and personality disorders: Correlates with suicidal and impulsive aggressive behavior. Archives of General Psychiatry 1989a; 46(7):587–599. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Siever LJ, Klar HM, Maurer G, Cochrane K,Cooper TB, Mohs RC, Davis KL. Serotonergic studies of personality disorder: Correlates with behavioral aggression and impulsivity. Arch Gen Psychiatry 1989b, 46:587–599. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Lee R, Kavoussi RJ. Aggression, suicidality, and intermittent explosive disorder: serotonergic correlates in personality disorder and healthy control subjects. Neuropsychopharmacology 2010;35(2):435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK, Erhardt D, Epstein JN, Parker JDA, Sitarenios G, & Sparrow E Self-ratings of ADHD symptoms in adults I: Factor structure and normative data. Journal of Attention Disorders, 1999; 3, 141–151. [Google Scholar]
- de Boer SF, Koolhaas JM. 5-HT1A and 5-HT1B receptor agonists and aggression: a pharmacological challenge of the serotonin deficiency hypothesis. Eur J Pharmacol 2005; 526(1–3):125–39. [DOI] [PubMed] [Google Scholar]
- Dohrenwend BP, Levav I, Shrout PE, Schwartz S, Naveh G, Link BG, Skodol AE, Stueve A. Socioeconomic status and psychiatric disorders: the causation-selection issue. Science 1992. February 21;255(5047):946–52. [DOI] [PubMed] [Google Scholar]
- Dolan MC, Anderson IM. The relationship between serotonergic function and the Psychopathy Checklist: Screening Version. J Psychopharmacol 2003;17(2):216–22. [DOI] [PubMed] [Google Scholar]
- Dolan M, Deakin WJ, Roberts N, Anderson I. Serotonergic and cognitive impairment in impulsive aggressive personality disordered offenders: are there implications for treatment? Psychol Med 2002;32(1):105–17. [DOI] [PubMed] [Google Scholar]
- Epstein JN, Johnson DE, & Conners CK Conners’ adult ADHD diagnostic interview for DSM-IV North Tonawanda, NY: Multi-Health Systems; 2001. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW.: Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) Washington, D.C.: American Psychiatric Press, Inc, 1996. [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS.: Structured Clinical Interview for DSM- IV Axis II Personality Disorders, (SCID-II) Washington, D.C.: American Psychiatric Press, Inc, 1997. [Google Scholar]
- Flory JD, Newcorn JH, Miller C, Harty S, Halperin JM. Serotonergic function in children with attention-deficit hyperactivity disorder: relationship to later antisocial personality disorder. Br J Psychiatry 2007;190:410–4. [DOI] [PubMed] [Google Scholar]
- Fuller RW. The involvement of serotonin in regulation of pituitary-adrenoncortical function. Front Neuroendocrinol 1992; 13:250–270. [PubMed] [Google Scholar]
- Halperin JM, Sharma V, Siever LJ, Schwartz ST, Matier K, Wornell G, Newcorn JH. Serotonergic function in aggressive and nonaggressive boys with attention deficit hyperactivity disorder. Am J Psychiatry 1994;151(2):243–8. [DOI] [PubMed] [Google Scholar]
- Halperin JM, Newcorn JH, Kopstein I, McKay KE, Schwartz ST, Siever LJ, Sharma V. Serotonin, aggression, and parental psychopathology in children with attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 1997a; 36(10):1391–8. [DOI] [PubMed] [Google Scholar]
- Halperin JM, Newcorn JH, Schwartz ST, Sharma V, Siever LJ, Koda VH, Gabriel S. Age-related changes in the association between serotonergic function and aggression in boys with ADHD. Biol Psychiatry 1997b; 41(6):682–9. [DOI] [PubMed] [Google Scholar]
- Halperin JM, Schulz KP, McKay KE, Sharma V, Newcorn JH. Familial correlates of central serotonin function in children with disruptive behavior disorders. Psychiatry Research 2003; 119, 205–216. [DOI] [PubMed] [Google Scholar]
- Halperin JM, Kalmar JH, Schulz KP, Marks DJ, Sharma V, Newcorn JH. Elevated childhood serotonergic function protects against adolescent aggression in disruptive boys. J Am Acad Child Adolesc Psychiatry 2006; 45(7):833–40. [DOI] [PubMed] [Google Scholar]
- Ham BJ, Lee BC, Paik JW, Kang RH, Choi MJ, Choi IG, Lee MS. Association between the tryptophan hydroxylase-1 gene A218C polymorphism and citalopram antidepressant response in a Korean population. Prog Neuropsychopharmacol Biol Psychiatry 2007; 31(1):104–7. [DOI] [PubMed] [Google Scholar]
- Hawes SW, Perlman SB, Byrd AL, Raine A, Loeber R, Pardini DA. Chronic anger as a precursor to adult antisocial personality features: The moderating influence of cognitive control. J Abnorm Psychol 2016; 125(1):64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Murphy DL, Crawley JN. Reduced aggression in mice lacking the serotonin transporter. Psychopharmacology (Berl) 2002;161(2):160–7. [DOI] [PubMed] [Google Scholar]
- Huang TL, Lin CC. Chapter Seven – Advances in Biomarkers of Major Depressive Disorder. Advances in Clinical Chemistry 2015; 68, 177–204 [DOI] [PubMed] [Google Scholar]
- Kahn RS, Trestman R, Lawlor BA, Gabriel S, Davidson M, Siever L. Effects of ipsapirone in healthy subjects: A dose-response study. Psychopharmacology 1994;114:155–160. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; 36(7):980–8. [DOI] [PubMed] [Google Scholar]
- Klein RG, Mannuzza S, Olazagasti MA, Roizen E, Hutchison JA, Lashua EC, Castellanos FX. Clinical and functional outcome of childhood attention-deficit/hyperactivity disorder 33 years later. Arch Gen Psychiatry 2012; 69(12):1295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs HA, Chens LK, Wright GJ. Determination of fenfluramine and norfenfluramine in plasma using a nitrogen-sensitive detector. J Chromatogr 1984; 310:412–417. [DOI] [PubMed] [Google Scholar]
- Kruesi MJ, Rapoport JL, Hamburger S, Hibbs E, Potter WZ, Lenane M, Brown GL. Cerebrospinal fluid monoamine metabolites, aggression, and impulsivity in disruptive behaviordisorders of children and adolescents. Arch Gen Psychiatry 1990; 47(5):419–26. [DOI] [PubMed] [Google Scholar]
- Kruesi MJ, Hibbs ED, Zahn TP, Keysor CS, Hamburger SD, Bartko JJ, Rapoport JL. A 2-year prospective follow-up study of children and adolescents with disruptive behaviordisorders. Prediction by cerebrospinal fluid 5-hydroxyindoleacetic acid, homovanillic acid, and autonomic measures? Arch Gen Psychiatry 1992; 49(6):429–35. [DOI] [PubMed] [Google Scholar]
- Landaas ET, Johansson S, KJacobsen KK, Ribases M, Bosch R, Sanchez-Mora C, Jacob CP, Boreatti-Hummer A, Kreiker S, Lesch KP, Kiemeney LA, Kooij S, Kan C,. Buitelaar JK, Faraone SV, Halmøy A, Ramos-Quiroga JA, Cormand B, Reif A, Franke B, Mick E, Knappskog PM, Haavik J. An international multicenter association study of the serotonin transporter Loney J, Milich R. Hyperactivity, inattention, and aggression in clinical practice. In: Wolraich M, Routh DK, editors. Advances in developmental and behavioral pediatrics (3) 113–147. Greenwich, CT: JAI; 1982. [Google Scholar]
- López-Romero L, Romero E, Andershed H. Conduct Problems in Childhood and Adolescence: Developmental Trajectories, Predictors and Outcomes in a Six-Year Follow Up. Child Psychiatry Hum Dev 2015; 46(5):762–73. [DOI] [PubMed] [Google Scholar]
- Lussier P, Corrado R, Tzoumakis S. Gender differences in physical aggression and associated developmental correlates in a sample of Canadian preschoolers. Behav Sci Law 2012; 30(5):643–71. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, McCaffery JM, Matthews KA, Mann JJ, Muldoon MF. Aggression, Impulsivity, and Central Nervous System Serotonergic Responsivity in a Nonpatient Sample. Neuropsychopharmacology 1998; 19, 287–299. [DOI] [PubMed] [Google Scholar]
- Marshal MP, Molina BS. Antisocial behaviors moderate the deviant peer pathway to substance use in children with ADHD. J Clin Child Adolesc Psychol 2006; 35(2):216–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T, Scarpa A, Raine A. A Meta-Analysis of Serotonin Metabolite 5-HIAA and Antisocial Behavior. Aggressive Behavior 2002; 28, 299–316. [Google Scholar]
- Muhlbauer HD, Muller-Oerlinghausen B. Fenfluramine stimulation of serum cortisol in patients with major affective disorders and healthy controls: Further evidence for a central serotonergic action of lithium in man. J Neural Transm 1985; 61:81–94. [DOI] [PubMed] [Google Scholar]
- Nakao K, Treas J. Updating occupational prestige and socioeconomic scores; how the new measures measure up. Sociological Methodology 1994;24, 1–72. [Google Scholar]
- New AS, Trestman RF, Mitropoulou V, Goodman M, Koenigsberg HH, Silverman J, Siever LJ. Low prolactin response to fenfluramine in impulsive aggression. J Psychiatr Res 2004; 38(3):223–30. [DOI] [PubMed] [Google Scholar]
- Pine DS, Coplan JD, Wasserman GA, Miller LS, Fried JE, Davies M, Cooper TB, Greenhill L, Shaffer D, Parsons B. Neuroendocrine response to fenfluramine challenge in boys. Associations with aggressive behavior and adverse rearing. Arch Gen Psychiatry 1997; 54(9):839–46. [DOI] [PubMed] [Google Scholar]
- Piotrowska PJ, Stride CB, Croft SE, Rowe R. Clin Psychol Rev. Socioeconomic status and antisocial behaviour among children and adolescents: a systematic review and meta-analysis 2015. February;35:47–55. doi: 10.1016/j.cpr.2014.11.003. Epub 2014 Nov 28. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, et al. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology, 2003; 28, 916–931. [DOI] [PubMed] [Google Scholar]
- Rasmussen P, Gillberg C. Natural outcomes of ADHD with developmental coordination disorder at age 22 years; a controlled, longitudinal, community- based study. J Am Acas Child Adolesc Psychiatry 2000; 39 (11): 1424–31. [DOI] [PubMed] [Google Scholar]
- Retz W1, Rösler M. The relation of ADHD and violent aggression: What can we learn from epidemiological and genetic studies? Int J Law Psychiatry 2009; 32(4):235–43. [DOI] [PubMed] [Google Scholar]
- Rowland NE, Carlton J. Neurobiology of an anoreticdrug: Fenfluramine. Prog Neurobiol 1986; 27:13–62. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Dulcan MK, Davies M, Piacentini J, Schwab-Stone ME, Lahey BB, Bourdon K, Jensen PS, Bird HR, Canino G, Regier DA. The NIMH Diagnostic Interview Schedule for Children Version 2.3 (DISC-2.3): description, acceptability, prevalence rates, and performance in the MECA Study. Methods for the Epidemiology of Child and Adolescent Mental Disorders Study. J Am Acad Child Adolesc Psychiatry 1996; 35(7):865–77. [DOI] [PubMed] [Google Scholar]
- Sher L, Stanley BH, Cooper TB, Malone KM, Mann JJ, Oquendo MA. Serotonergic responses in depressed patients with or without a history of alcohol use disorders and healthy controls. Eur Neuropsychopharmacol 2008; 18(9):692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijbesman H, Schipper J, de Kloet ER, Mos J, van Aken H, Olivier B. Postsynaptic 5-HT1 receptors and offensive aggression in rats: a combined behavioural and autoradiographic study with eltoprazine. Pharmacol Biochem Behav 1991; 38, (2), 447–458. [DOI] [PubMed] [Google Scholar]
- Silva H, Iturra P, Solari A, Villarroel J, Jerez S, Jiménez M, Galleguillos F, Bustamante ML. Fluoxetine response in impulsive-aggressive behavior and serotonin transporter polymorphism in personality disorder. Psychiatr Genet 2010; 20(1):25–30. [DOI] [PubMed] [Google Scholar]
- Soloff PH, Kelly TM, Strotmeyer SJ, Malone KM, Mann JJ. Impulsivity, gender, and response to fenfluramine challenge in borderline personality disorder. Psychiatry Res 2003;119(1–2):11–24. [DOI] [PubMed] [Google Scholar]
- Soloff PH, Lynch KG, Moss HB. Serotonin, impulsivity, and alcohol use disorders in the older adolescent: a psychobiological study. Alcohol Clin Exp Res 2000; 24(11):1609–19. [PubMed] [Google Scholar]
- Stoff DM, Pasatiempo AP, Yeung JH, Bridger WH, Rabinovich H. Test-retest reliability of the prolactin and cortisol responses to D,L-fenfluramine challenge in disruptive behavior disorders. Psychiatry Res 1992a; 42(1):65–72. [DOI] [PubMed] [Google Scholar]
- Stoff DM, Pasatiempo AP, Yeung J, Cooper TB, Bridger WH, Rabionovich H: Neuroendocrine responses to challenge with dl-fenfluramine and aggression in disruptive behavior disorders of children and adolescents. Psychiatry Res 1992b, 43:263–276. [DOI] [PubMed] [Google Scholar]
- Storebø OJ, Simonsen E. The Association Between ADHD and Antisocial Personality Disorder (ASPD): A Review. J Atten Disord 2016; 20(10):815–24. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Cremers TI, Jongsma ME, Steenbergen PJ, de Boer SF, Koolhaas JM. Differences in the effects of 5-HT(1A) receptor agonists on forced swimming behavior and brain 5-HT metabolism between low and high aggressive mice. Psychopharmacology (Berl) 2005;178(2–3):151–60. [DOI] [PubMed] [Google Scholar]
- von Polier GG1, Vloet TD, Herpertz-Dahlmann B. ADHD and delinquency-a developmental perspective. Behav Sci Law 2012; 30(2):121–39. [DOI] [PubMed] [Google Scholar]
- Wechsler D (1974). Wechsler intelligence scale for children—revised New York: Psychological Corporation. [Google Scholar]
- Wechsler D (1991). The Wechsler intelligence scale for children—third edition. San Antonio, TX: The Psychological Corporation. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.