Summary
calculated based on prevalence data of a complex interplay of the sex chromosomes, sex hormones, the microbiota, and additional environmental and sociological factors.
Keywords: sex bias, autoimmunity, sex-dependent gene regulation, X chromosome, VGLL3
Introduction
The immune system functions to defend against infection. Responses must be robust and specific enough to ward off or overcome infection without causing undue harm to the organism. Autoimmune disease arises when an exaggerated or misdirected immune response damages native tissues or organs. While individual autoimmune diseases are rare, they are collectively among the most prevalent diseases in Western society (1). Despite intensive investigation, our understanding of the pathogenesis of autoimmune disease is incomplete. A growing body of evidence supports a model wherein environmental and lifestyle factors precipitate development of autoimmunity in genetically susceptible hosts. Cures have been elusive, and lifetime treatment is often required.
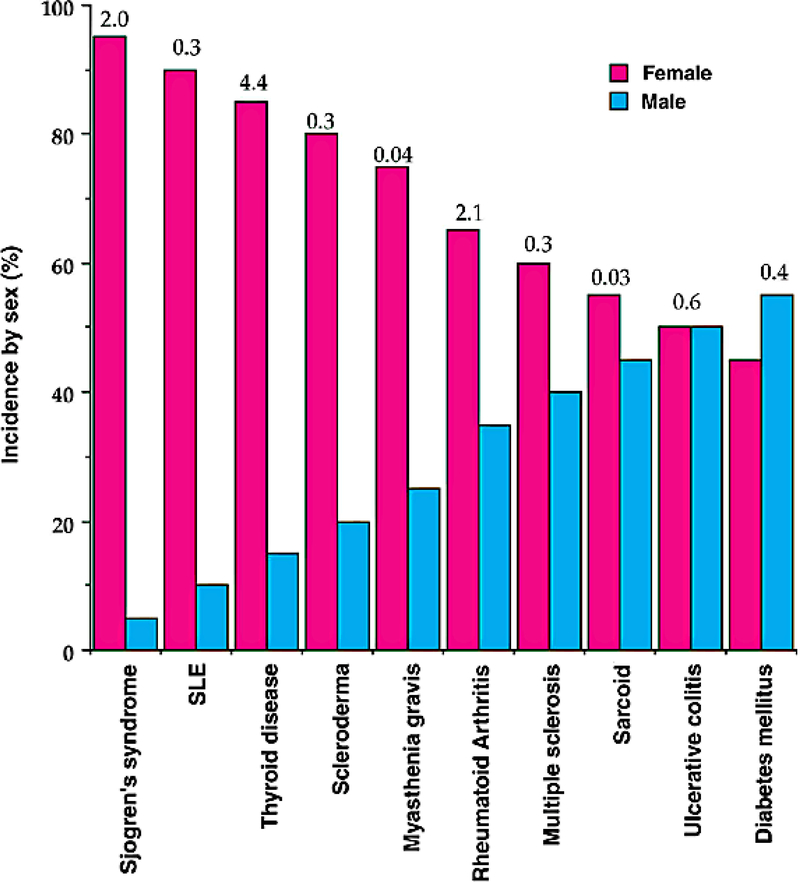
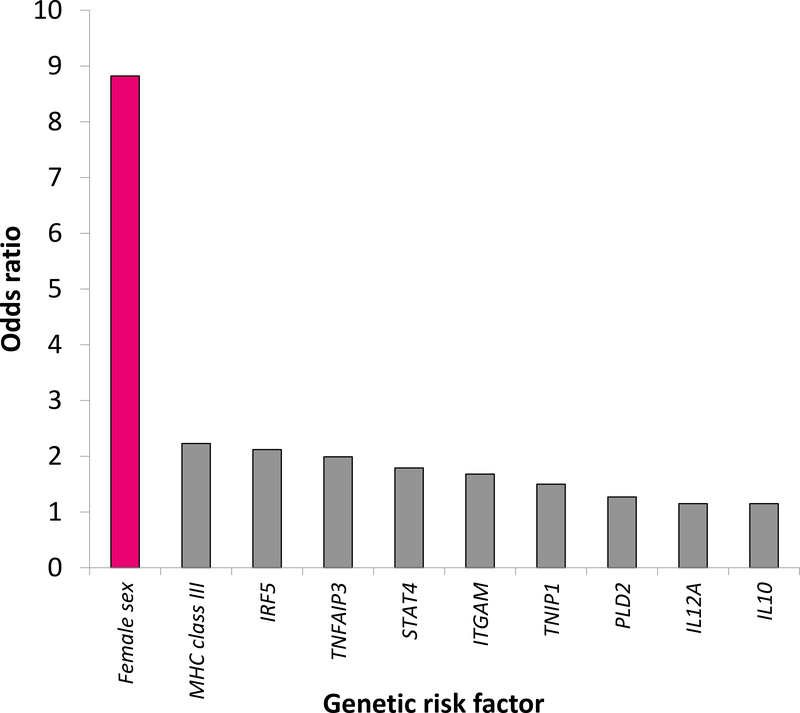
Cellular and humoral immunity are generally stronger in women; women have higher levels of circulating antibodies, more circulating CD4 T cells, more robust cytokine production in response to infection, and enhanced rejection of tumors and allografts (2). Many autoimmune diseases show a striking female sex bias (Figure 1) (3). Systemic lupus erythematosus (SLE), Sjogren’s syndrome, Grave’s disease, and Hashimoto’s thyroiditis are seven to ten times more common in women than men; multiple sclerosis (MS), rheumatoid arthritis (RA), and scleroderma are two to three times more common (4). Overall, it is estimated that 78% of people affected with autoimmune diseases are women (5). For many diseases such as SLE, genome-wide association studies and meta-analyses have identified numerous risk variants, yet female sex carries a risk of autoimmunity that dwarfs that of any susceptibility locus noted to date (Figure 2) (6, 7). The biological mechanisms underlying this bias are incompletely understood. Previous inquiry centered on the influence of sex hormones, yet female sex bias is frequently observed even in autoimmune diseases with onset in childhood, when estrogen levels do not differ between the sexes, or in post-menopausal women. More recent work suggests that the sex chromosomes themselves and sex-specific environmental factors such as sexually dimorphic microbiota are also important drivers of sex bias in autoimmunity. In this review, we discuss current and foundational studies addressing mechanisms of sex bias in autoimmunity.
Figure 1. The sex distribution of the major autoimmune diseases.
The numbers above the bars refer to the total number of disease cases (×1,000,000) in the USA (3). SLE: systemic lupus erythematosus.
Figure 2. Female sex alone carries a risk of autoimmunity four times greater than any other known genetic risk variant for SLE.
Odds ratio (OR) for female sex was calculated based on prevalence data from the Georgia Lupus Registry from 2002 (6). ORs are shown for allelic associations at SLE susceptibility loci from a genome-wide association replication study (7).
Sex hormones
In the search for drivers of sex-biased autoimmunity, sex hormones represent obvious culprits. Indeed, sex hormone regulation of immunity is extensive, with interconnections to the other mechanisms discussed in this review. Sex hormones act primarily through associating with their respective intracellular receptors and binding their cognate response elements in target genes (8–10). Sex hormone receptors are widely expressed in immune cells, and estrogen and androgen response elements are found in the promoters of several innate immunity genes (11). Variations in autoimmune phenotype across puberty, pregnancy, and menopause demonstrate the complex regulation of immunity by sex hormones. The ease of antagonizing and supplementing these hormones renders them attractive as therapeutic targets and agents, but results have been inconsistent, and exposure to non-physiologic sex hormone levels carries other intrinsic risks (12–14). Thus, identifying and targeting the downstream immunological effectors of sex hormones may hold more therapeutic promise.
Progesterone, which is present at high levels during the luteal phase of the menstrual cycle and in pregnancy, is likely a key promoter of immune tolerance during pregnancy (15). Progesterone is generally immune suppressive, decreasing pro-inflammatory mediators and inhibiting immune cell activation (reviewed in (16)). Progesterone signaling occurs primarily through progesterone receptors, which are expressed in many immune cell types including NK cells, macrophages, dendritic cells, and T cells (17). At high levels, signaling may also occur through glucocorticoid receptors (18). Progesterone decreases activation of NK cells (19), macrophages, and dendritic cells (20, 21) and promotes skewing from Th1 to Th2 type T cell responses (22), which may account for the amelioration of Th1-associated autoimmune diseases such as MS and RA during pregnancy. Studies of human cord blood have shown that progesterone has strong regulatory T cell (Treg) induction activity and suppresses Th17 cell differentiation (15). Some of the effects of progesterone may be mediated by NF-κB inhibition (23).
Regulation of immunity by estrogens is more nuanced. Estrogen levels are high in pregnancy, low in menopause, and variable across the menstrual cycle. Estrogen receptor (ER) subtypes show differential expression in immune cells: ERα is highly expressed in T cells and ERβ in B cells (24). In addition to binding estrogen response elements (EREs) in target genes, ERs also interact with ERE-independent transcription factors in immune cells (25). Estrogens upregulate a number of key immunity factors including interferon (IFN) regulatory factor 5 (IRF5) (26), and IFN-γ (27), as well as the intracellular TLR trafficking protein UNC93B1 (28). Estrogens also function through ERα to downregulate the autoimmune regulator (AIRE), a critical factor in central tolerance, through promoter methylation(29); AIRE expression is also downregulated by progesterone and upregulated by the androgen dihydrotestosterone (29, 30). The effects of sex hormones on sex-biased DNA methylation and autoimmunity are otherwise as yet unclear (31). Additionally, estrogens regulate miRNAs (32), who in turn regulate estrogen-dependent signaling (33). Estrogens increase neutrophil numbers (34, 35) but overall inhibit their activation and trafficking via multiple mechanisms, some mediated by NF-κB inhibition (reviewed in (36)). Estrogens enhance NK cell cytotoxicity and IFN-γ production (37) but downregulate NK cell granzyme B secretion and cell surface activation markers (38). Effects of estrogen on monocytes and macrophages vary by dose: production of pro-inflammatory cytokines is enhanced at low concentrations and suppressed at high (39). The response of dendritic cells to estrogens is mixed, inducing production of both anti-inflammatory and Th1-type pro-inflammatory cytokines (40–42). At lower concentrations, estrogens have immunostimulatory effects, promoting a Th1 response through enhancing the secretion of IFN-γ (27, 43, 44); in contrast, at high concentrations, estrogens promote a Th2 response (45–47). In pregnant SLE patients, this Th2 shift and consequent increased production of autoantibodies often exacerbates disease (48). Effects on Th17 cells are less clear (36). Treg cells increase with estrogens (49, 50). Estrogens promote B cell survival, maturation, class switching, and antibody production (51–53) and interfere with peripheral negative selection of autoreactive B cells (54, 55). Oophorectomy has been reported to be protective in lupus-prone mice (56); however, recent data suggest that this protection may not rely solely on estrogen-mediated effects, as complete absence of ERα does not delay lupus in prone mice (57).
Androgens predominantly downregulate the immune response (58), decreasing pro-inflammatory mediators and inhibiting the proliferation and activation of a number of immune cell populations (reviewed in (36)). Accordingly, androgens have been shown to exert a protective effect in multiple autoimmune rodent models (30, 59, 60). This appears to be mediated in part by androgen-induced upregulation of AIRE in the male thymus: androgens increase AIRE levels by binding the androgen receptor (AR) and targeting the AIRE promoter, and the protective effects of androgens and male sex are lost in AIRE-deficient mice in a model of experimental autoimmune encephalitis (30). Additionally, ARs are broadly expressed in neutrophil-lineage cells, with no difference in male and female expression patterns (61), and act to increase the number and trafficking of neutrophils (62); however, androgens decrease pro-inflammatory responses of neutrophils (63), natural killer (NK) cells (64), and macrophages (65). ARs are not expressed in peripheral lymphocytes but are found in lymphoid and non-lymphoid thymic and bone marrow cells (66), where they limit the number of immature thymocytes and restrain active cell cycling (67). While androgens decrease the activation of Th1 and Th2 cells (68), Th17 cell responses are enhanced (69). Treatment to reduce testosterone decreases Treg count (70). Androgens have also been found to limit the number of immature type 2 innate lymphoid cells in the lung (71). In epidermal cells, androgens were found to modulate the expression of PRDM1, a transcriptional repressor involved in thymic T cell apoptosis and other immune cell processes that is implicated in female-dependent autoimmune risk (72). B cell number is negatively regulated by androgens (62).
Some studies support a role for psychosocial stress in initiation or exacerbation of autoimmune disease, although causation has been challenging to establish (73). Response to stress occurs through the hypothalamus-pituitary-adrenal (HPA) axis, which also exhibits sexual dimorphism in cortisol response to psychosocial stressors (reviewed in (31)). Stress triggers release of glucocorticoids, which generally inhibit immune responses through decreasing production of pro-inflammatory cytokines and inhibiting activation and proliferation of multiple immune cell types. The HPA axis also produces prolactin, another hormone with immunological effects. The prolactin receptor is widely expressed in immune cells (74), and prolactin signaling is largely immunostimulatory (75). In particular, prolactin may promote autoimmunity by inhibiting negative selection of autoreactive B cells, augmenting autoantibody production (76–78). Positive correlations of prolactin levels and disease activity have been identified in SLE patients, but the causality remains to be determined (79).
Sex chromosomes
The presence of two X chromosomes in the female also contributes to sex bias of autoimmunity. While canonically one of the X chromosomes is inactivated in early development, this process is imperfect, with approximately 15% of genes escaping X chromosome inactivation (XCI) (80). A majority of the genes that escape XCI show female expression bias (81–83), and there is variation among individuals in which genes escape (84). Males with Klinefelter syndrome (karyotype XXY) have an increased risk of SLE commensurate with that of females (85), and one male patient with severe pre-pubertal SLE was found to have an XX karyotype due to an X-Y translocation (86), demonstrating the influence of X dosage. In contrast, females with Turner syndrome, who have complete (XO) or partial X chromosome monosomy, are at increased risk of developing autoimmune disease relative even to XX females, but the excess risk may be greater for male-predominant autoimmune conditions such as ankylosing spondylitis (87, 88). In contrast, females with Turner syndrome (karyotype X0) are at increased risk of developing autoimmune disease, but the risk is strongest for male-predominant conditions (87). In most females, XCI is random, resulting in half of cells expressing the maternal and half the paternal X chromosome; however, some females show non-random silencing leading to an 80% or more predominance of one X chromosome. This skewed inactivation is more common in patients with autoimmune diseases (89, 90), although it may be a consequence of autoimmunity rather than a cause (91).
Many genes with established roles in autoimmunity are located on the X chromosome. Several of these have been found to be overexpressed or hypomethylated in female but not male SLE patients (92, 93), and dosage of the X-linked TLR7 and TLR8 genes has been shown to influence development of SLE in humans and lupus-prone mice (94–98). Recently, escape of TLR7 inactivation in a substantial number proportion of immune cells has been described in females and men with Klinefelter syndrome; this biallelic expression of TLR7 primes for increased class switching in activated B cells and increased TLR7 reactivity (99). The X chromosome is also highly enriched in microRNAs (miRNAs) (100). MicroRNAs, including some located on the X chromosome, are essential for maintenance of immune tolerance (reviewed in (101)), and a subset of X-linked miRNAs was found to be overexpressed in females, but not males, with SLE (93). Finally, the X chromosome can become partially reactivated in lymphocytes in women, resulting in overexpression of immunity genes and possibly contributing to sex bias in SLE (102).
The Y chromosome has garnered much less attention as a driver of sex bias in autoimmunity, but evidence is accumulating. In a mouse model of autoimmune disease, Y chromosome polymorphisms, including gene copy number variation, correlate with disease susceptibility and severity (103–105), although the observed effects may also reflect impaired balancer function in mismatched X and Y chromosomes that evolved in different strains (106). However, data from men with MS suggest the influence of the Y chromosome on autoimmunity may extend to humans (105). Further investigation of male mice with specific Y-linked defects in immunity (107, 108) and examples of human Y-linked immune variation (109) is ongoing and may shed additional light.
Gut immunology and the microbiota
The gut microbiota plays a critical role in maturation and modulation of innate and adaptive immunity (110) yet is itself shaped by the immune system. Both the gut immune system and microbiota exhibit sexual dimorphism. Immune tissues in the gut of male and female rodents differ in representation of immune cells, with an overall trend toward enhanced innate immunity and attenuated adaptive immunity in the male gut relative to the female (111, 112), and many immune genes show sex-biased expression in mouse gut (113, 114). Although some studies have observed no differences in the diversity or composition of male and female gut microbiota, sex differences in the human gut microbiota have been extensively documented (115), raising interest in the gut microbiota as a potential driver of sex bias in autoimmune disease. Additionally, microbiome aberrations have been observed in the vast majority of immune-mediated diseases, although demonstrating causation has proven a significant obstacle thus far (116), with many resorting to animal models for further investigation.
Female mice show increased microbiota diversity relative to male, and many bacterial species show sex-biased enrichment that occasionally varies with strain, age, and diet (reviewed in (115)). Sex hormones likely also play a role. In human twin studies, the microbiota of opposite-sex twins becomes more divergent after puberty relative to same-sex twins (117). Similarly, in the nonobese diabetic (NOD) mouse model of spontaneous autoimmune type I diabetes (T1D), the gut microbiota does not differ in prepubertal male and female mice; however, microbial diversity decreases in intact postpubertal males, while this does not occur in females and castrated males (118). Transfer of male microbiota into germ-free female mice and female microbiota into germ-free male mice revealed that some manifestations of immunological sexual dimorphism appear to depend on sex-specific gut microbiota: regardless of the sex of the recipient, RORγt+Foxp3+ cells are increased in gut immune tissues of mice who received male microbiota, and T cell precursors are increased in mice who received female microbiota (113).
There is mounting evidence of a direct role for the gut microbiota in driving sex-biased autoimmunity. Female NOD mice develop spontaneous autoimmune T1D at twice the rate of male mice. Under germ-free conditions, however, incidence of T1D is equal in both sexes (119), indicating that male microbiota may confer protection. Gavage of female NOD weanlings with male NOD intestinal microbiota results in elevated serum testosterone and protects against development of T1D. This effect is abrogated in recipient female mice treated with androgen receptor antagonist, suggesting protection is conferred by a testosterone-dependent mechanism (119). Female MRL/Mp-Faslpr mice, who develop lupus at far higher rates than males, show significantly higher gut microbiota diversity but lower abundance of Lactobacillales species and increased intestinal permeability (120). In female and castrated male Faslpr mice, Lactobacillales gavage restores gut mucosal barrier function, promoting an anti-inflammatory cytokine environment in which autoantibody production decreases and renal disease improves, with increased renal Treg cells and suppression of renal Th17 cells (120). The same benefits were not observed in intact male Faslpr mice, suggesting that Lactobacillus species in the gut attenuate renal disease in lupus-prone mice in a sex hormone-dependent manner. In aggregate, the animal data suggest that sex and androgens appear to regulate gut microbiota composition and function, which reciprocally influences the immune response and development of autoimmunity.
Other factors
Recently, we identified the transcription factor VGLL3 to be critical in orchestrating sex-biased expression of key autoimmune genes in a sex hormone-independent fashion (83). VGLL3 is required for robust expression of genes implicated in autoimmunity and for mounting a full IFN-I response. In healthy skin, VGLL3 shows nuclear localization that is more prominent in women than in men. In lesional skin of patients with cutaneous lupus, however, VGLL3 shows nuclear localization in both sexes, indicating disease-dependent regulation. This suggests VGLL3 governs an autoimmunity pathway that is constitutively active in women but must be triggered by other means in men (83). VGLL3 is located on chromosome 3 and appears to be epigenetically regulated (unpublished observation), and its exact role in driving autoimmune diseases is being actively explored.
Sex bias is prominent in DNA methylation, affecting chromatin accessibility of immune genes (121). The X and Y chromosome may influence DNA methylation, as was shown for an autosomal locus in human cells (122). Prenatal environmental exposures also show sex-specific epigenetic effects on DNA methylation (reviewed in (123)), although the underlying mechanisms remain unknown. Fetal microchimerism, wherein circulating fetal cells travel to the mother and persist for years after pregnancy (124), may predispose to development of autoimmune disease; however, studies disagree on whether autoimmune diseases are more common in women with prior pregnancies, and a definitive connection has not been established (125). Additionally, men and women differ in exposure to environmental endocrine-disrupting chemicals, with estrogenic and anti-estrogenic properties that may affect genetic and epigenetic regulation of immunity (reviewed in (126, 127)). Finally, sociological differences between the sexes, such as rates of smoking in men versus women, may influence the development and manifestations of autoimmune disease (128).
Conclusion
In treating severe autoimmune disease, physicians often must turn to broad immunosuppressive therapies with high side effect burden and inherent risks of infection and malignancy due to decreased immune surveillance activity. The trend toward stratifying immunological research studies by gender and continuous improvements in high-throughput technologies have enabled increasingly sophisticated characterization of the differences between the male and female autoimmune phenotypes; this is evidenced by work such as the recent description of sex bias in the influence of HLA associations on T cell selection and expansion as revealed by TCR immunosequencing of large cohorts (129). These nuanced descriptions are helping to explain observed sex differences in infection susceptibility and autoimmunity, but we must continue to interrogate the mechanistic underpinnings of sex-biased autoimmunity to pave the way toward development of highly specific therapeutics that spare patients the dangers of broad immunosuppression. Targeting the precise pathways that drive the female autoimmune disease burden above the baseline male prevalence will be an immense boon to human welfare, particularly if accompanied only by the relatively modest increased risk of infection and malignancy native to the male immune system.
Key points.
The mechanisms underlying sex bias in autoimmunity remain incompletely understood.
The effects of sex hormones on autoimmune disease are mediated in part by direct regulation of key immunity factors such as AIRE, IRF5, and IFN-γ and the intracellular TLR trafficking protein UNC93B1.
Newly discovered non-hormonally–regulated immune modulators such as VGLL3 may contribute to female-biased autoimmunity.
The gut microbiota influences the immune response and development of autoimmunity and is itself shaped by androgens.
These and future investigations may yield targets for more selective and therefore less toxic therapies for autoimmune disease.
Acknowledgement
Financial support and sponsorship
This work was in part supported by the University of Michigan Babcock Endowment Fund (JEG); the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health (NIH) under Award Numbers 5T32AR007197–40 (ACB), R01-AR071384 (JMK), and R01-AR069071 (JEG); the A. Alfred Taubman Medical Research Institute Parfet Emerging Scholar Award (JMK) and Kenneth and Frances Eisenberg Emerging Scholar Award (JEG); a Rheumatology Research Foundation Career Development K Supplement Award (JMK); and a Doris Duke Physician Scientist Development Award (to JMK).
Recent findings
Recent studies investigating the origins of sex bias in autoimmune disease have revealed an extensive and interconnected network of genetic, hormonal, microbial, and environmental influences. Investigation of sex hormones has moved beyond profiling the effects of hormones on activity and prevalence of immune cell types to defining the specific immunity-related genes driving these changes. Deeper examination of the genetic content of the X and Y chromosomes and genetic escapees of X chromosome interaction has revealed some key drivers of female-biased autoimmunity. Animal studies are offering further insights into the connections between microbiota, particularly that of the gut, and the immune system.
Footnotes
Purpose of review
To give an overview of recently published articles addressing the mechanisms underlying sex bias in autoimmune disease.
Conflicts of interest
The authors have no conflicts of interest.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
- 1.El-Gabalawy H, Guenther LC, Bernstein CN. Epidemiology of immune-mediated inflammatory diseases: incidence, prevalence, natural history, and comorbidities. J Rheumatol Suppl. 2010;85:2–10. [DOI] [PubMed] [Google Scholar]
- 2.Nalbandian G, Kovats S. Understanding sex biases in immunity: effects of estrogen on the differentiation and function of antigen-presenting cells. Immunol Res. 2005;31(2):91–106. [DOI] [PubMed] [Google Scholar]
- 3.Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2(9):777–80. [DOI] [PubMed] [Google Scholar]
- 4.Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol. 2008;8(9):737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84(3):223–43. [DOI] [PubMed] [Google Scholar]
- 6.Lim SS, Bayakly AR, Helmick CG, Gordon C, Easley KA, Drenkard C. The incidence and prevalence of systemic lupus erythematosus, 2002–2004: The Georgia Lupus Registry. Arthritis Rheumatol. 2014;66(2):357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bentham J, Morris DL, Graham DSC, Pinder CL, Tombleson P, Behrens TW, et al. Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nat Genet. 2015;47(12):1457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, et al. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87(3):905–31. [DOI] [PubMed] [Google Scholar]
- 9.Hewitt SC, Winuthayanon W, Korach KS. What’s new in estrogen receptor action in the female reproductive tract. J Mol Endocrinol. 2016;56(2):R55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang CS, Kokontis J, Liao ST. Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science. 1988;240(4850):324–6. [DOI] [PubMed] [Google Scholar]
- 11.Hannah MF, Bajic VB, Klein SL. Sex differences in the recognition of and innate antiviral responses to Seoul virus in Norway rats. Brain Behav Immun. 2008;22(4):503–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gubbels Bupp MR, Jorgensen TN. Androgen-Induced Immunosuppression. Front Immunol. 2018;9:794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lateef A, Petri M. Hormone replacement and contraceptive therapy in autoimmune diseases. J Autoimmun. 2012;38(2–3):J170–6. [DOI] [PubMed] [Google Scholar]
- 14.Zarkavelis G, Kollas A, Kampletsas E, Vasiliou V, Kaltsonoudis E, Drosos A, et al. Aromatase inhibitors induced autoimmune disorders in patients with breast cancer: A review. Journal of advanced research. 2016;7(5):719–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JH, Lydon JP, Kim CH. Progesterone suppresses the mTOR pathway and promotes generation of induced regulatory T cells with increased stability. Eur J Immunol. 2012;42(10):2683–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–38. [DOI] [PubMed] [Google Scholar]
- 17.Teilmann SC, Clement CA, Thorup J, Byskov AG, Christensen ST. Expression and localization of the progesterone receptor in mouse and human reproductive organs. J Endocrinol. 2006;191(3):525–35. [DOI] [PubMed] [Google Scholar]
- 18.Druckmann R, Druckmann MA. Progesterone and the immunology of pregnancy. J Steroid Biochem Mol Biol. 2005;97(5):389–96. [DOI] [PubMed] [Google Scholar]
- 19.Arruvito L, Giulianelli S, Flores AC, Paladino N, Barboza M, Lanari C, et al. NK cells expressing a progesterone receptor are susceptible to progesterone-induced apoptosis. J Immunol. 2008;180(8):5746–53. [DOI] [PubMed] [Google Scholar]
- 20.Butts CL, Shukair SA, Duncan KM, Bowers E, Horn C, Belyavskaya E, et al. Progesterone inhibits mature rat dendritic cells in a receptor-mediated fashion. Int Immunol. 2007;19(3):287–96. [DOI] [PubMed] [Google Scholar]
- 21.Jones LA, Kreem S, Shweash M, Paul A, Alexander J, Roberts CW. Differential modulation of TLR3- and TLR4-mediated dendritic cell maturation and function by progesterone. J Immunol. 2010;185(8):4525–34. [DOI] [PubMed] [Google Scholar]
- 22.Piccinni MP, Giudizi MG, Biagiotti R, Beloni L, Giannarini L, Sampognaro S, et al. Progesterone favors the development of human T helper cells producing Th2-type cytokines and promotes both IL-4 production and membrane CD30 expression in established Th1 cell clones. J Immunol. 1995;155(1):128–33. [PubMed] [Google Scholar]
- 23.Hardy DB, Janowski BA, Corey DR, Mendelson CR. Progesterone receptor plays a major antiinflammatory role in human myometrial cells by antagonism of nuclear factor-kappaB activation of cyclooxygenase 2 expression. Mol Endocrinol. 2006;20(11):2724–33. [DOI] [PubMed] [Google Scholar]
- 24.Phiel KL, Henderson RA, Adelman SJ, Elloso MM. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol Lett. 2005;97(1):107–13. [DOI] [PubMed] [Google Scholar]
- 25.Kovats S Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015;294(2):63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen H, Panchanathan R, Rajavelu P, Duan X, Gould KA, Choubey D. Gender-dependent expression of murine Irf5 gene: implications for sex bias in autoimmunity. J Mol Cell Biol. 2010;2(5):284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fox HS, Bond BL, Parslow TG. Estrogen regulates the IFN-gamma promoter. J Immunol. 1991;146(12):4362–7. [PubMed] [Google Scholar]
- 28.Panchanathan R, Liu H, Choubey D. Expression of murine Unc93b1 is up-regulated by interferon and estrogen signaling: implications for sex bias in the development of autoimmunity. Int Immunol. 2013;25(9):521–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. •.Dragin N, Bismuth J, Cizeron-Clairac G, Biferi MG, Berthault C, Serraf A, et al. Estrogen-mediated downregulation of AIRE influences sexual dimorphism in autoimmune diseases. J Clin Invest. 2016;126(4):1525–37.Estrogen downregulates expression of AIRE in the thymus of female humans and mice by inducing epigenetic changes at the AIRE promoter through ERα receptors. In a mouse model of experimental autoimmune thyroiditis, the protective effects of male sex are abrogated by downregulation of Aire, suggesting that estrogen-mediated regulation of AIRE likely contributes to female-biased autoimmunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. •.Zhu ML, Bakhru P, Conley B, Nelson JS, Free M, Martin A, et al. Sex bias in CNS autoimmune disease mediated by androgen control of autoimmune regulator. Nat Commun. 2016;7:11350.Androgens bind AR and target the AIRE promoter to drive higher AIRE expression in the thymus of male humans and mice. In a mouse model of experimental autoimmune encephalitis, androgens and male sex are protective against disease in an Aire-dependent manner, suggesting that androgen-mediated regulation of AIRE likely contributes to female-biased autoimmunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edwards M, Dai R, Ahmed SA. Our Environment Shapes Us: The Importance of Environment and Sex Differences in Regulation of Autoantibody Production. Front Immunol. 2018;9:478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klinge CM. Estrogen Regulation of MicroRNA Expression. Curr Genomics. 2009;10(3):169–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan D, Dai R, Ansar Ahmed S. Sex differences and estrogen regulation of miRNAs in lupus, a prototypical autoimmune disease. Cell Immunol. 2015;294(2):70–9. [DOI] [PubMed] [Google Scholar]
- 34.Jilma B, Eichler HG, Breiteneder H, Wolzt M, Aringer M, Graninger W, et al. Effects of 17 beta-estradiol on circulating adhesion molecules. J Clin Endocrinol Metab. 1994;79(6):1619–24. [DOI] [PubMed] [Google Scholar]
- 35.Robinson DP, Hall OJ, Nilles TL, Bream JH, Klein SL. 17beta-estradiol protects females against influenza by recruiting neutrophils and increasing virus-specific CD8 T cell responses in the lungs. J Virol. 2014;88(9):4711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bereshchenko O, Bruscoli S, Riccardi C. Glucocorticoids, Sex Hormones, and Immunity. Front Immunol. 2018;9:1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakaya M, Tachibana H, Yamada K. Effect of estrogens on the interferon-gamma producing cell population of mouse splenocytes. Biosci Biotechnol Biochem. 2006;70(1):47–53. [DOI] [PubMed] [Google Scholar]
- 38.Hao S, Zhao J, Zhou J, Zhao S, Hu Y, Hou Y. Modulation of 17beta-estradiol on the number and cytotoxicity of NK cells in vivo related to MCM and activating receptors. Int Immunopharmacol. 2007;7(13):1765–75. [DOI] [PubMed] [Google Scholar]
- 39.Karpuzoglu E, Phillips RA, Gogal RM Jr., Ansar Ahmed S. IFN-gamma-inducing transcription factor, T-bet is upregulated by estrogen in murine splenocytes: role of IL-27 but not IL-12. Mol Immunol. 2007;44(7):1808–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu HY, Buenafe AC, Matejuk A, Ito A, Zamora A, Dwyer J, et al. Estrogen inhibition of EAE involves effects on dendritic cell function. J Neurosci Res. 2002;70(2):238–48. [DOI] [PubMed] [Google Scholar]
- 41.Delpy L, Douin-Echinard V, Garidou L, Bruand C, Saoudi A, Guery JC. Estrogen enhances susceptibility to experimental autoimmune myasthenia gravis by promoting type 1-polarized immune responses. J Immunol. 2005;175(8):5050–7. [DOI] [PubMed] [Google Scholar]
- 42.Subramanian S, Yates M, Vandenbark AA, Offner H. Oestrogen-mediated protection of experimental autoimmune encephalomyelitis in the absence of Foxp3+ regulatory T cells implicates compensatory pathways including regulatory B cells. Immunology. 2011;132(3):340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grasso G, Muscettola M. The influence of beta-estradiol and progesterone on interferon gamma production in vitro. Int J Neurosci. 1990;51(3–4):315–7. [DOI] [PubMed] [Google Scholar]
- 44.Karpuzoglu-Sahin E, Hissong BD, Ansar Ahmed S. Interferon-gamma levels are upregulated by 17-beta-estradiol and diethylstilbestrol. J Reprod Immunol. 2001;52(1–2):113–27. [DOI] [PubMed] [Google Scholar]
- 45.Marzi M, Vigano A, Trabattoni D, Villa ML, Salvaggio A, Clerici E, et al. Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clin Exp Immunol. 1996;106(1):127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matalka KZ. The effect of estradiol, but not progesterone, on the production of cytokines in stimulated whole blood, is concentration-dependent. Neuro Endocrinol Lett. 2003;24(3–4):185–91. [PubMed] [Google Scholar]
- 47.Sabahi F, Rola-Plesczcynski M, O’Connell S, Frenkel LD. Qualitative and quantitative analysis of T lymphocytes during normal human pregnancy. Am J Reprod Immunol. 1995;33(5):381–93. [DOI] [PubMed] [Google Scholar]
- 48.Eudy AM, Siega-Riz AM, Engel SM, Franceschini N, Howard AG, Clowse MEB, et al. Effect of pregnancy on disease flares in patients with systemic lupus erythematosus. Ann Rheum Dis. 2018;77(6):855–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Polanczyk MJ, Hopke C, Huan J, Vandenbark AA, Offner H. Enhanced FoxP3 expression and Treg cell function in pregnant and estrogen-treated mice. J Neuroimmunol. 2005;170(1–2):85–92. [DOI] [PubMed] [Google Scholar]
- 50.Tai P, Wang J, Jin H, Song X, Yan J, Kang Y, et al. Induction of regulatory T cells by physiological level estrogen. J Cell Physiol. 2008;214(2):456–64. [DOI] [PubMed] [Google Scholar]
- 51.Asaba J, Bandyopadhyay M, Kindy M, Dasgupta S. Estrogen receptor signal in regulation of B cell activation during diverse immune responses. Int J Biochem Cell Biol. 2015;68:42–7. [DOI] [PubMed] [Google Scholar]
- 52.Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28(5):521–74. [DOI] [PubMed] [Google Scholar]
- 53.Herblot S, Aplan PD, Hoang T. Gradient of E2A activity in B-cell development. Mol Cell Biol. 2002;22(3):886–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bynoe MS, Grimaldi CM, Diamond B. Estrogen up-regulates Bcl-2 and blocks tolerance induction of naive B cells. Proc Natl Acad Sci U S A. 2000;97(6):2703–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grimaldi CM, Michael DJ, Diamond B. Cutting edge: expansion and activation of a population of autoreactive marginal zone B cells in a model of estrogen-induced lupus. J Immunol. 2001;167(4):1886–90. [DOI] [PubMed] [Google Scholar]
- 56.Cunningham MA, Wirth JR, Scott JL, Eudaly J, Collins EL, Gilkeson GS. Early Ovariectomy Results in Reduced Numbers of CD11c+/CD11b+ Spleen Cells and Impacts Disease Expression in Murine Lupus. Front Immunol. 2016;7:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. •.Scott JL, Wirth JR, Eudaly J, Ruiz P, Cunningham MA. Complete knockout of estrogen receptor alpha is not directly protective in murine lupus. Clin Immunol. 2017;183:132–41.Lupus prone mice with complete absence of ERα are protected from lupus disease expression only if their ovaries are intact, suggesting another protective factor, such as hypogonadism with resultant androgen elevation, may be involved. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Foo YZ, Nakagawa S, Rhodes G, Simmons LW. The effects of sex hormones on immune function: a meta-analysis. Biol Rev Camb Philos Soc. 2017;92(1):551–71. [DOI] [PubMed] [Google Scholar]
- 59.Roubinian JR, Talal N, Greenspan JS, Goodman JR, Siiteri PK. Effect of castration and sex hormone treatment on survival, anti-nucleic acid antibodies, and glomerulonephritis in NZB/NZW F1 mice. J Exp Med. 1978;147(6):1568–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahmed SA, Penhale WJ. The influence of testosterone on the development of autoimmune thyroiditis in thymectomized and irradiated rats. Clin Exp Immunol. 1982;48(2):367–74. [PMC free article] [PubMed] [Google Scholar]
- 61.Mantalaris A, Panoskaltsis N, Sakai Y, Bourne P, Chang C, Messing EM, et al. Localization of androgen receptor expression in human bone marrow. J Pathol. 2001;193(3):361–6. [DOI] [PubMed] [Google Scholar]
- 62.Lai JJ, Lai KP, Zeng W, Chuang KH, Altuwaijri S, Chang C. Androgen receptor influences on body defense system via modulation of innate and adaptive immune systems: lessons from conditional AR knockout mice. Am J Pathol. 2012;181(5):1504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pergola C, Dodt G, Rossi A, Neunhoeffer E, Lawrenz B, Northoff H, et al. ERK-mediated regulation of leukotriene biosynthesis by androgens: a molecular basis for gender differences in inflammation and asthma. Proc Natl Acad Sci U S A. 2008;105(50):19881–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hou J, Zheng WF. Effect of sex hormones on NK and ADCC activity of mice. Int J Immunopharmacol. 1988;10(1):15–22. [DOI] [PubMed] [Google Scholar]
- 65.D’Agostino P, Milano S, Barbera C, Di Bella G, La Rosa M, Ferlazzo V, et al. Sex hormones modulate inflammatory mediators produced by macrophages. Ann N Y Acad Sci. 1999;876:426–9. [DOI] [PubMed] [Google Scholar]
- 66.Olsen NJ, Kovacs WJ. Effects of androgens on T and B lymphocyte development. Immunol Res. 2001;23(2–3):281–8. [DOI] [PubMed] [Google Scholar]
- 67.Olsen NJ, Viselli SM, Shults K, Stelzer G, Kovacs WJ. Induction of immature thymocyte proliferation after castration of normal male mice. Endocrinology. 1994;134(1):107–13. [DOI] [PubMed] [Google Scholar]
- 68.Liva SM, Voskuhl RR. Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J Immunol. 2001;167(4):2060–7. [DOI] [PubMed] [Google Scholar]
- 69.Zhang MA, Rego D, Moshkova M, Kebir H, Chruscinski A, Nguyen H, et al. Peroxisome proliferator-activated receptor (PPAR)alpha and -gamma regulate IFNgamma and IL-17A production by human T cells in a sex-specific way. Proc Natl Acad Sci U S A. 2012;109(24):9505–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Page ST, Plymate SR, Bremner WJ, Matsumoto AM, Hess DL, Lin DW, et al. Effect of medical castration on CD4+ CD25+ T cells, CD8+ T cell IFN-gamma expression, and NK cells: a physiological role for testosterone and/or its metabolites. Am J Physiol Endocrinol Metab. 2006;290(5):E856–63. [DOI] [PubMed] [Google Scholar]
- 71.Laffont S, Blanquart E, Savignac M, Cenac C, Laverny G, Metzger D, et al. Androgen signaling negatively controls group 2 innate lymphoid cells. J Exp Med. 2017;214(6):1581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kretzschmar K, Cottle DL, Donati G, Chiang MF, Quist SR, Gollnick HP, et al. BLIMP1 is required for postnatal epidermal homeostasis but does not define a sebaceous gland progenitor under steady-state conditions. Stem Cell Reports. 2014;3(4):620–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Porcelli B, Pozza A, Bizzaro N, Fagiolini A, Costantini MC, Terzuoli L, et al. Association between stressful life events and autoimmune diseases: A systematic review and meta-analysis of retrospective case-control studies. Autoimmun Rev. 2016;15(4):325–34. [DOI] [PubMed] [Google Scholar]
- 74.Orbach H, Zandman-Goddard G, Amital H, Barak V, Szekanecz Z, Szucs G, et al. Novel biomarkers in autoimmune diseases: prolactin, ferritin, vitamin D, and TPA levels in autoimmune diseases. Ann N Y Acad Sci. 2007;1109:385–400. [DOI] [PubMed] [Google Scholar]
- 75.Borba VV, Zandman-Goddard G, Shoenfeld Y. Prolactin and Autoimmunity. Front Immunol. 2018;9:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Buckley AR. Prolactin, a lymphocyte growth and survival factor. Lupus. 2001;10(10):684–90. [DOI] [PubMed] [Google Scholar]
- 77.Kochendoerfer SK, Krishnan N, Buckley DJ, Buckley AR. Prolactin regulation of Bcl-2 family members: increased expression of bcl-xL but not mcl-1 or bad in Nb2-T cells. J Endocrinol. 2003;178(2):265–73. [DOI] [PubMed] [Google Scholar]
- 78.Saha S, Gonzalez J, Rosenfeld G, Keiser H, Peeva E. Prolactin alters the mechanisms of B cell tolerance induction. Arthritis Rheum. 2009;60(6):1743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Song GG, Lee YH. Circulating prolactin level in systemic lupus erythematosus and its correlation with disease activity: a meta-analysis. Lupus. 2017;26(12):1260–8. [DOI] [PubMed] [Google Scholar]
- 80.Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434(7031):400–4. [DOI] [PubMed] [Google Scholar]
- 81.Cotton AM, Ge B, Light N, Adoue V, Pastinen T, Brown CJ. Analysis of expressed SNPs identifies variable extents of expression from the human inactive X chromosome. Genome Biol. 2013;14(11):R122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shen JJ, Wang TY, Yang W. Regulatory and evolutionary signatures of sex-biased genes on both the X chromosome and the autosomes. Biol Sex Differ. 2017;8(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. •.Liang Y, Tsoi LC, Xing X, Beamer MA, Swindell WR, Sarkar MK, et al. A gene network regulated by the transcription factor VGLL3 as a promoter of sex-biased autoimmune diseases. Nat Immunol. 2017;18(2):152–60.The transcription factor VGLL3 is upregulated in women and functions independently of sex hormones to regulate a novel inflammatory pathway promoting female-biased autoimmunity. In male skin affected by lupus, this pathway appears to be activated, suggesting that VGLL3 contributes to female sex bias in autoimmunity but is also relevant to autoimmune disease in men. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang Y, Castillo-Morales A, Jiang M, Zhu Y, Hu L, Urrutia AO, et al. Genes that escape X-inactivation in humans have high intraspecific variability in expression, are associated with mental impairment but are not slow evolving. Mol Biol Evol. 2013;30(12):2588–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Scofield RH, Bruner GR, Namjou B, Kimberly RP, Ramsey-Goldman R, Petri M, et al. Klinefelter’s syndrome (47,XXY) in male systemic lupus erythematosus patients: support for the notion of a gene-dose effect from the X chromosome. Arthritis Rheum. 2008;58(8):2511–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chagnon P, Schneider R, Hebert J, Fortin PR, Provost S, Belisle C, et al. Identification and characterization of an Xp22.33;Yp11.2 translocation causing a triplication of several genes of the pseudoautosomal region 1 in an XX male patient with severe systemic lupus erythematosus. Arthritis Rheum. 2006;54(4):1270–8. [DOI] [PubMed] [Google Scholar]
- 87.Jorgensen KT, Rostgaard K, Bache I, Biggar RJ, Nielsen NM, Tommerup N, et al. Autoimmune diseases in women with Turner’s syndrome. Arthritis Rheum. 2010;62(3):658–66. [DOI] [PubMed] [Google Scholar]
- 88.Goldacre MJ, Seminog OO. Turner syndrome and autoimmune diseases: record-linkage study. Arch Dis Child. 2014;99(1):71–3. [DOI] [PubMed] [Google Scholar]
- 89.Uz E, Mustafa C, Topaloglu R, Bilginer Y, Dursun A, Kasapcopur O, et al. Increased frequency of extremely skewed X chromosome inactivation in juvenile idiopathic arthritis. Arthritis Rheum. 2009;60(11):3410–2. [DOI] [PubMed] [Google Scholar]
- 90.Rubtsova K, Marrack P, Rubtsov AV. Sexual dimorphism in autoimmunity. J Clin Invest. 2015;125(6):2187–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kanaan SB, Onat OE, Balandraud N, Martin GV, Nelson JL, Azzouz DF, et al. Evaluation of X Chromosome Inactivation with Respect to HLA Genetic Susceptibility in Rheumatoid Arthritis and Systemic Sclerosis. PLoS One. 2016;11(6):e0158550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lu Q, Wu A, Tesmer L, Ray D, Yousif N, Richardson B. Demethylation of CD40LG on the inactive X in T cells from women with lupus. J Immunol. 2007;179(9):6352–8. [DOI] [PubMed] [Google Scholar]
- 93.Hewagama A, Gorelik G, Patel D, Liyanarachchi P, McCune WJ, Somers E, et al. Overexpression of X-linked genes in T cells from women with lupus. J Autoimmun. 2013;41:60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Umiker BR, Andersson S, Fernandez L, Korgaokar P, Larbi A, Pilichowska M, et al. Dosage of X-linked Toll-like receptor 8 determines gender differences in the development of systemic lupus erythematosus. Eur J Immunol. 2014;44(5):1503–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Garcia-Ortiz H, Velazquez-Cruz R, Espinosa-Rosales F, Jimenez-Morales S, Baca V, Orozco L. Association of TLR7 copy number variation with susceptibility to childhood-onset systemic lupus erythematosus in Mexican population. Ann Rheum Dis. 2010;69(10):1861–5. [DOI] [PubMed] [Google Scholar]
- 96. •.Shen N, Fu Q, Deng Y, Qian X, Zhao J, Kaufman KM, et al. Sex-specific association of X-linked Toll-like receptor 7 (TLR7) with male systemic lupus erythematosus. Proc Natl Acad Sci U S A. 2010;107(36):15838–43.TLR7 escapes X inactivation in some B cells and myeloid cells in females and men with Klinefelter syndrome. These biallelic B cells show enhanced TLR7-driven responses and greater propensity to class switch toward immunoglobulin G that may contribute to increased risk of SLE in women and men with Klinefelter syndrome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Subramanian S, Tus K, Li QZ, Wang A, Tian XH, Zhou J, et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci U S A. 2006;103(26):9970–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McDonald G, Cabal N, Vannier A, Umiker B, Yin RH, Orjalo AV Jr., et al. Female Bias in Systemic Lupus Erythematosus is Associated with the Differential Expression of X-Linked Toll-Like Receptor 8. Front Immunol. 2015;6:457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Souyris M, Cenac C, Azar P, Daviaud D, Canivet A, Grunenwald S, et al. TLR7 escapes X chromosome inactivation in immune cells. Sci Immunol. 2018;3(19). [DOI] [PubMed] [Google Scholar]
- 100.Pinheiro I, Dejager L, Libert C. X-chromosome-located microRNAs in immunity: might they explain male/female differences? The X chromosome-genomic context may affect X-located miRNAs and downstream signaling, thereby contributing to the enhanced immune response of females. Bioessays. 2011;33(11):791–802. [DOI] [PubMed] [Google Scholar]
- 101.Lam IKY, Chow JX, Lau CS, Chan VSF. MicroRNA-mediated immune regulation in rheumatic diseases. Cancer Lett. 2018;431:201–12. [DOI] [PubMed] [Google Scholar]
- 102.Wang J, Syrett CM, Kramer MC, Basu A, Atchison ML, Anguera MC. Unusual maintenance of X chromosome inactivation predisposes female lymphocytes for increased expression from the inactive X. Proc Natl Acad Sci U S A. 2016;113(14):E2029–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Teuscher C, Noubade R, Spach K, McElvany B, Bunn JY, Fillmore PD, et al. Evidence that the Y chromosome influences autoimmune disease in male and female mice. Proc Natl Acad Sci U S A. 2006;103(21):8024–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Spach KM, Blake M, Bunn JY, McElvany B, Noubade R, Blankenhorn EP, et al. Cutting edge: the Y chromosome controls the age-dependent experimental allergic encephalomyelitis sexual dimorphism in SJL/J mice. J Immunol. 2009;182(4):1789–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Case LK, Wall EH, Dragon JA, Saligrama N, Krementsov DN, Moussawi M, et al. The Y chromosome as a regulatory element shaping immune cell transcriptomes and susceptibility to autoimmune disease. Genome Res. 2013;23(9):1474–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Arnold AP. Y chromosome’s roles in sex differences in disease. Proc Natl Acad Sci U S A. 2017;114(15):3787–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Krementsov DN, Case LK, Dienz O, Raza A, Fang Q, Ather JL, et al. Genetic variation in chromosome Y regulates susceptibility to influenza A virus infection. Proc Natl Acad Sci U S A. 2017;114(13):3491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Case LK, Teuscher C. Y genetic variation and phenotypic diversity in health and disease. Biol Sex Differ. 2015;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maan AA, Eales J, Akbarov A, Rowland J, Xu X, Jobling MA, et al. The Y chromosome: a blueprint for men’s health? Eur J Hum Genet. 2017;25(11):1181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pickard JM, Zeng MY, Caruso R, Nunez G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol Rev. 2017;279(1):70–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Elderman M, van Beek A, Brandsma E, de Haan B, Savelkoul H, de Vos P, et al. Sex impacts Th1 cells, Tregs, and DCs in both intestinal and systemic immunity in a mouse strain and location-dependent manner. Biol Sex Differ. 2016;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shastri P, McCarville J, Kalmokoff M, Brooks SP, Green-Johnson JM. Sex differences in gut fermentation and immune parameters in rats fed an oligofructose-supplemented diet. Biol Sex Differ. 2015;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fransen F, van Beek AA, Borghuis T, Meijer B, Hugenholtz F, van der Gaast-de Jongh C, et al. The Impact of Gut Microbiota on Gender-Specific Differences in Immunity. Front Immunol. 2017;8:754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Steegenga WT, Mischke M, Lute C, Boekschoten MV, Pruis MG, Lendvai A, et al. Sexually dimorphic characteristics of the small intestine and colon of prepubescent C57BL/6 mice. Biol Sex Differ. 2014;5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Elderman M, de Vos P, Faas M. Role of Microbiota in Sexually Dimorphic Immunity. Front Immunol. 2018;9:1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Round JL, Palm NW. Causal effects of the microbiota on immune-mediated diseases. Sci Immunol. 2018;3(20). [DOI] [PubMed] [Google Scholar]
- 117.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yurkovetskiy L, Burrows M, Khan AA, Graham L, Volchkov P, Becker L, et al. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;39(2):400–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339(6123):1084–8. [DOI] [PubMed] [Google Scholar]
- 120.Mu Q, Zhang H, Liao X, Lin K, Liu H, Edwards MR, et al. Control of lupus nephritis by changes of gut microbiota. Microbiome. 2017;5(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Qu K, Zaba LC, Giresi PG, Li R, Longmire M, Kim YH, et al. Individuality and variation of personal regulomes in primary human T cells. Cell Syst. 2015;1(1):51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ho B, Greenlaw K, Al Tuwaijri A, Moussette S, Martinez F, Giorgio E, et al. X chromosome dosage and presence of SRY shape sex-specific differences in DNA methylation at an autosomal region in human cells. Biol Sex Differ. 2018;9(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chiaroni-Clarke RC, Munro JE, Ellis JA. Sex bias in paediatric autoimmune disease - Not just about sex hormones? J Autoimmun. 2016;69:12–23. [DOI] [PubMed] [Google Scholar]
- 124.Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, DeMaria MA. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci U S A. 1996;93(2):705–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ngo ST, Steyn FJ, McCombe PA. Gender differences in autoimmune disease. Front Neuroendocrinol. 2014;35(3):347–69. [DOI] [PubMed] [Google Scholar]
- 126.Pellegrini M, Bulzomi P, Lecis M, Leone S, Campesi I, Franconi F, et al. Endocrine disruptors differently influence estrogen receptor beta and androgen receptor in male and female rat VSMC. J Cell Physiol. 2014;229(8):1061–8. [DOI] [PubMed] [Google Scholar]
- 127.Rogers JA, Metz L, Yong VW. Review: Endocrine disrupting chemicals and immune responses: a focus on bisphenol-A and its potential mechanisms. Mol Immunol. 2013;53(4):421–30. [DOI] [PubMed] [Google Scholar]
- 128.Borchers AT, Gershwin ME. Sociological differences between women and men: implications for autoimmunity. Autoimmun Rev. 2012;11(6–7):A413–21. [DOI] [PubMed] [Google Scholar]
- 129.Schneider-Hohendorf T, Gorlich D, Savola P, Kelkka T, Mustjoki S, Gross CC, et al. Sex bias in MHC I-associated shaping of the adaptive immune system. Proc Natl Acad Sci U S A. 2018;115(9):2168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]