Introduction
Cerebrovascular disease is a major global health concern in the United States, with approximately 795,000 new or recurrent strokes occurring every year.1 Stroke is one of the leading causes of disability in the U.S., and individuals that experience a stroke are likely to have residual deficits even years after stroke onset.1,2 Due to the frequency of strokes occurring in the middle cerebral artery,3 commonly affected tracts are the corticospinal and corticobulbar pathways controlling the upper extremity. For this reason, even though the lower extremity can also be very impaired, the upper extremity tends to be more affected after a stroke in a large number of individuals.4 Indeed, approximately 50–75% of individuals who suffer a stroke experience impairments in the arm that substantially affect or impede normal daily function.1,5
A widespread neural impairment observed following stroke is decreased voluntary motor drive, which manifests as significant weakness of the affected upper (or lower) extremity.6 Other neural impairments include hyperactive stretch reflexes,7 referred to as spasticity, and a loss of independent joint control.8–10 Loss of independent joint control is clinically referred to as abnormal synergies,11 with the flexion synergy reported to be the most prevalent abnormal synergy observed in the upper limb.8,11 The flexion synergy manifests as an involuntary coupling of elbow, wrist, and finger flexor muscles as an individual attempts to lift their paretic arm at the shoulder. Neural impairments are significant enough as to substantially restrict any useful function in the arm in individuals with moderate to severe impairment. The decrease in voluntary use of the arm, combined with the involuntary drive to the flexors, can result in a flexion bias, where an individual exhibits a flexed posture of the paretic arm even during rest.12,13 In addition, after a stroke, individuals can experience secondary symptoms such as pain, increased stiffness, and joint contractures, all of which further limit an individual’s quality of life and functional use of the paretic arm.14,15
Because a stroke is an injury to the brain, much research has justifiably been focused on the resulting neural deficits. However, the impaired neural drive combined with the limited use and the consistently flexed posture of the impaired arm likely contributes to secondary changes of the muscles within the limb, which can be expected to exacerbate the motor deficits seen in the chronic stroke population and further limit function. One important musculoskeletal parameter that may be affected is fascicle length, which has an important influence on a muscle’s operating range (excursion capability) and its absolute shortening velocity.16 Fascicle length, which affects force production in muscle,16,17 has been shown to be highly adaptable.18–20
A literature search reveals at least 11 studies specifically quantifying fascicle length in paretic limbs after stroke.21,22,31,23–30 Notably, despite the significant prevalence of impairment in the upper limb in the stroke population,1,2,5 just one of these studies24 investigated fascicle length changes between the paretic and nonparetic upper extremities, likely due to the technical challenges with using conventional ultrasound on the longer fascicles of the upper extremity32,33 combined with a large focus on gait in rehabilitation interventions. The only known study in the upper extremity after stroke reported shorter fascicles in the paretic brachialis muscle24 (an elbow flexor). Similarly, the studies that investigate adaptation of fascicle length in the lower extremity overwhelmingly report shorter fascicles in the plantarflexor muscles of the paretic ankle22,23,29 perhaps due to the higher incidence of abnormal neural drive and muscle tone to the antigravity muscles of the lower extremity34,35. Interestingly, the antagonist to the abnormal synergy is rarely studied, even in the lower limb. For example, to our knowledge, only two studies have investigated changes in fascicle length in the dorsiflexor muscles of the paretic lower extremity; both of these found no difference in the dorsiflexors with those of the nonparetic ankle.26,30
Despite the evidence for secondary musculoskeletal changes after stroke in the lower limb and clinical perception of secondary changes in the upper limb, current clinical interventions rarely target musculoskeletal changes individually, and those that attempt to have limited or unknown effectiveness in long-term improvement.36,37 It is important to quantify muscle adaptation in the paretic upper extremity in order to move the field toward an improved understanding of what is contributing to musculoskeletal changes. Ultimately, such research could aid in the design of more targeted rehabilitation interventions to directly address these changes and limit their progression starting in the early stages after a stroke. Thus, the objective of our study was to quantify muscle fascicle lengths in the affected (paretic) and unaffected (nonparetic) arms after chronic stroke. We hypothesized that, due to the effects of hemiparesis resulting in a decreased ability to actively flex or extend the elbow through its full range, as well as the prevalence of a flexed posture of the paretic upper extremity, biceps brachii fascicle lengths would be shorter compared to the nonparetic limb. Because muscles function about a joint in agonist-antagonist pairs, we also measured fascicle lengths in the triceps brachii.
Materials and Methods
Participants
Eleven individuals with chronic hemiparetic stroke (>1 year post-stroke; 6 female, 5 male) volunteered to participate in the study. Participants were recruited through the Clinical Neuroscience Research Registry, housed at the former Rehabilitation Institute of Chicago (now Shirley Ryan AbilityLab). Participants ranged from 21–69 years of age, and were on average 12.0 years post-stroke (see Table 1). All participants were classified as moderately to severely impaired based on the upper limb score of the Fugl-Meyer Motor Assessment38, with scores ranging from 12–31 (mean of 22.5, maximum score of 66 representing no impairment). To provide context, we compare the data collected from both limbs of these 11 stroke subjects to previously published interlimb norms we established by implementing the same methods in a group of 11 healthy, age-matched control subjects.33 Northwestern University Institutional Review Board approved the human subject protocol, and all participants provided informed consent prior to participating in the study.
Table 1.
Demographics and clinical characteristics of N = 11 participants after stroke.
| Subject | Age | Sex | Years post-stroke | Side of paresis | FMA score | Maximal Isometric Voluntary Flexion Torque (N-m) | Maximal Isometric Voluntary Extension Torque (N-m) | ||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Paretic | Non-paretic | Paretic | Non-paretic | ||||||
| 1 | 55 | Male | 3 | Right | 24 | 27.7 | 67 | 18.4 | 49.4 |
| 2 | 61 | Male | 7 | Right | 18 | 25.08 | 53.6 | 21.76 | 45.2 |
| 3 | 57 | Female | 8 | Right | 26 | 17.46 | 35.14 | 19.76 | 27.75 |
| 4 | 48 | Female | 4 | Left | 22 | 27.62 | 43.03 | 16.36 | 42.95 |
| 5 | 49 | Male | 26 | Right | 31 | 57.14 | 103.11 | 37.77 | 51.7 |
| 6 | 21 | Female | 4 | Right | 25 | 27.2 | 45.05 | 21.78 | 43.6 |
| 7 | 44 | Male | 6 | Left | 20 | 10.98 | 72.31 | 13.64 | 59.53 |
| 8 | 62 | Female | 28 | Right | 16 | 10.8 | 35.25 | 8.74 | 27.94 |
| 9 | 59 | Male | 20 | Left | 12 | 22.3 | 61.2 | 18.6 | 48.16 |
| 10 | 66 | Female | 11 | Right | 24 | 18.92 | 33.8 | 6.51 | 25.2 |
| 11 | 69 | Female | 15 | Left | 29 | 19.15 | 27.7 | 8.78 | 18.46 |
|
| |||||||||
| MEAN ± SD: | 53.7 ± 13.3 | 12.0 ± 9.0 | 22.5 ± 5.6 | 24.0 ± 12.6 | 52.5 ± 22.2 | 17.5 ± 8.6 | 40.0 ± 13.0 | ||
Abbreviations: FMA, Fugl-Meyer Assessment
Experimental Setup
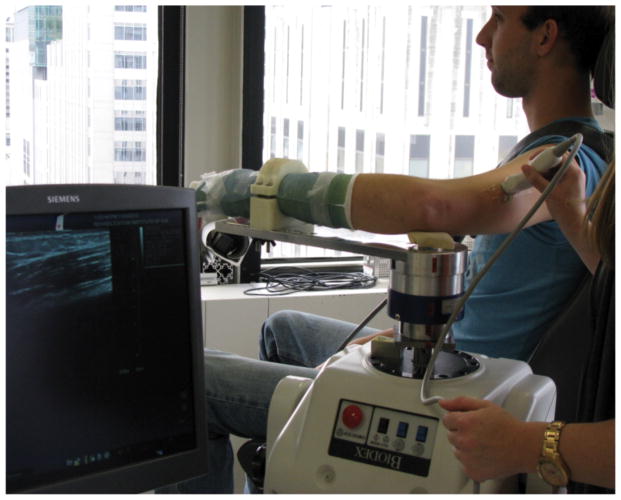
Ultrasound images of the biceps brachii (long head) and triceps brachii (lateral head) were obtained from both limbs of each subject under passive conditions in three elbow postures, and during active muscle contraction in a single elbow posture. Separate sessions were conducted for each arm, using the same protocol, with the paretic arm being the first tested in all participants. Participants were seated in a Biodex™ chair, secured at the trunk, with the shoulder approximately in the horizontal plane (Fig. 1). The forearm and wrist were casted and secured to a metal manipulandum, with the elbow centered over a 6-degree of freedom load cell (JR3, Inc. Model 45E15A4, Woodland, CA), which enabled measurement of the elbow torque produced during active muscle contraction. EMGs of the biceps brachii, triceps brachii, and brachioradialis were monitored via surface electrodes (Delsys Bagnoli™ 16-channel desktop EMG system, Delsys, Inc., Natick, MA) during image acquisition to ensure the muscles were not active for the passive condition. Trials with any observed increases in EMG activity (determined via visual inspection after each trial) were discarded and repeated to ensure passive conditions during data collection. Off-line analysis of EMG activity demonstrated the data we analyzed from the passive trials were collected in the presence of minimal EMG activity. For biceps brachii trials, mean EMG values for the paretic limb were .007 mV (95% CI: .003–.010), and nonparetic = .006 mV (95% CI: .002–.010). For the triceps trials, the mean EMG values for the paretic limb were .005 mV (95% CI: .003–.007) and nonparetic = .007 mV (95% CI: .003–.010). No statistical difference in mean EMG value was observed for any factor (arm, elbow position, and muscle), suggesting paretic hypertonicity did not contribute to fascicle differences between limbs.
Figure 1.
Experimental setup, with subject seated in Biodex chair and elbow, forearm, and wrist secured to an armpiece connected to the Biodex dynamometer.
Ultrasound image acquisition
B-mode, extended field-of-view ultrasound images (Siemens Antares™ Siescape v.5 software, Siemens Medical Solutions USA, Inc., Mountain View, CA) were acquired using an 11.43-MHz linear probe (45 mm width). During image acquisition, the probe was continuously moved along the path of the desired muscle, maintaining a probe alignment determined to provide clarity of fascicle viewing.33 Under passive conditions (verified via EMG), images were acquired at three elbow angles: extended (25°), neutral (55°), and flexed (80°). Images were also collected during active muscle contraction while the elbow was flexed (80°); in these trials, each participant maintained a constant isometric contraction during image acquisition. Visual feedback was given during the isometric contraction to ensure the desired level of torque was reached and maintained throughout image acquisition.
Because of the significant variation in paretic strength and muscle endurance across limbs and subjects (see Table 1), the contraction level for both arms for a given subject was chosen based on the capabilities of the paretic arm. Prior to image acquisition, the maximum voluntary torques the subject could produce in elbow flexion and extension were determined. In addition, the proportion of the maximum torque (%MVT) that a subject could sustain in the intended direction (elbow flexion/extension) for the entire duration of image acquisition (~10 sec for biceps brachii, ~7–8 sec for triceps brachii) was determined. The resulting torque magnitude was matched on the nonparetic arm to investigate fascicle length changes during active contraction between arms at an identical torque output. In addition, because the torque that could be maintained by the paretic limb was much less compared to the nonparetic limb, we repeated image acquisition during active contraction in the nonparetic limb to match the proportion of MVT that the paretic arm was required to hold. For example, if the absolute torque magnitude matched between arms was 50% MVT for the paretic but only 20% MVT for the nonparetic, the second active condition for the nonparetic limb would require the subject to hold the level of torque that was equivalent to 50% of MVT for the nonparetic arm. Individuals were monitored for any compensatory motions at the trunk and/or shoulder complex, and were given verbal instructions to decrease these unwanted movements if they did occur. Subject 11 experienced difficulty performing any level of active contraction for the triceps, and was therefore not included in analysis of the active condition for the triceps only. EMG and torque profiles were inspected visually after each trial, and the trial was discarded if there were any observed abnormalities (such as co-contraction of the biceps and triceps).
Data Analysis
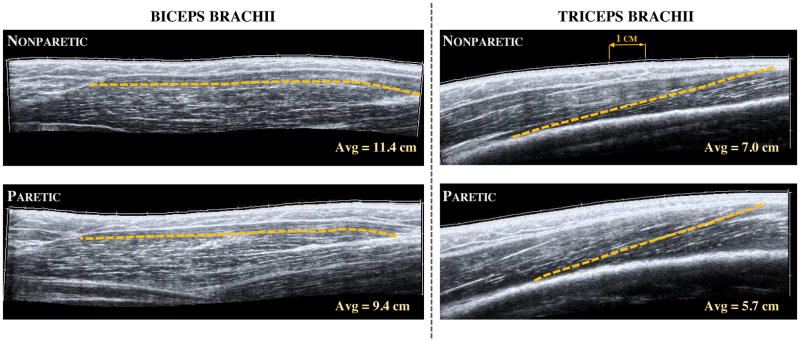
Images were exported in DICOM format and converted to jpeg format using custom Matlab code. The three best images for each position and condition were chosen qualitatively, based on the images with the best fascicle visualization throughout the entire muscle. An open-source software (Image J 2.0.0, National Institutes of Health, Bethesda, MD) was used to manually digitize 4 fascicles in each image; fascicle lengths were calculated by the software from the digitized fascicles. This resulted in a total of 12 repeated measurements for each position/condition. As implemented in our previous work,33 all measurements in the biceps brachii were made in the anterior portion of the muscle belly because of the consistently high quality images of fascicles in this region (Fig. 2). For the triceps brachii (distal portion of lateral head), fascicle length measurements were made in the section of the image where fascicles could be consistently viewed from origin to insertion (Fig. 2).
Figure 2.
Extended field-of-view ultrasound images of the long head of biceps brachii (left) and the distal portion of the lateral head of triceps brachii (right) in a single subject with chronic hemiplegia. Images were taken in the same neutral elbow posture for both muscles and in both the nonparetic (top) and paretic (bottom) limb. The dashed line in each image represent a single fascicle measurement as detailed in the methods, while the hash marks in each image represent 1 cm. The average values noted here were calculated over the 12 repeated measurements (3 images, 4 fascicle measurements each) in each arm and muscle for this participant. Schematic representation of muscle architecture can be viewed in previous work.33
Statistical Analysis
To quantify differences in fascicle lengths between arms for an individual subject in a given position or condition, we report the normalized difference in fascicle length:
| (1) |
A positive percentage indicates shorter fascicles in the paretic extremity. Thus, we define a positive, normalized, interlimb difference in fascicle lengths as a reduction in paretic fascicle length.
To test our hypothesis that, in the passive condition, fascicle length would depend on arm (paretic, nonparetic) and elbow posture, separate two-factor repeated measures ANOVAs were conducted for each muscle. To test if the normalized difference in fascicle length between arms in the passive condition was different than what we observed in a population of age-matched control participants described previously,33 we used a two-factor mixed ANOVA with group (control, stroke) and elbow position as factors. A two-factor, repeated measures ANOVA was also conducted to test if the normalized, interlimb difference in fascicle lengths observed in the stroke subjects depended on muscle or elbow position. To investigate effects of arm on the % change in fascicle length during an isometric contraction, a paired t-test was used for each muscle with Bonferroni correction for multiple comparisons. All statistical analysis was completed using IBM SPSS Statistics version 22 (SPSS, Inc.). Significance for all tests was set at α<.05.
Results
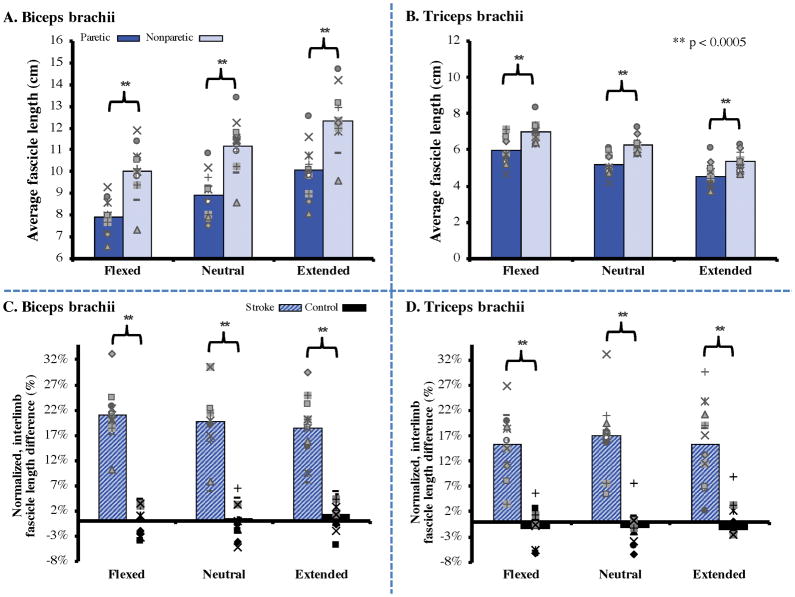
In each of the 11 stroke participants, fascicle lengths measured from both the biceps brachii (long head) and triceps brachii (distal portion of lateral head) under passive conditions were significantly shorter in the paretic arm compared to the nonparetic arm (Fig. 3A & 3B). Across three elbow positions, fascicle lengths of the paretic biceps brachii were an average of 2.23 cm (19.7%) shorter than those in the nonparetic limb (95% CI of mean difference: 1.70–2.76 cm, p<.0005). Fascicle lengths of the paretic triceps brachii were an average of 0.98 cm (15.9%) shorter than those in the nonparetic triceps (95% CI of mean difference: .77–1.19 cm, p<.0005). For context, the normalized differences in fascicle lengths between limbs in an age-matched control population33 were 0.54% and −1.35% for biceps and triceps, respectively (Fig. 3C & 3D).
Figure 3.
(Top) Average fascicle lengths measured under passive conditions in the biceps brachii (A) and triceps brachii (B) for N=11 individuals with chronic stroke in three elbow postures. Across all subjects, paretic fascicles were significantly shorter (p<.0005) in all positions for both muscles. Individual data (markers representing individual subjects) are overlaid on top of each bar graph for representation of the variability across subjects.
(Bottom) The normalized interlimb differences seen between the paretic and nonparetic limb in our study were significantly greater (p<.0005) than the small differences observed between arms in N=11 healthy, age-matched control participants, described in a previous study.33 Individual data points represent each individual subject’s normalized difference for both stroke (blue hashed bars) and control (black bars) groups.
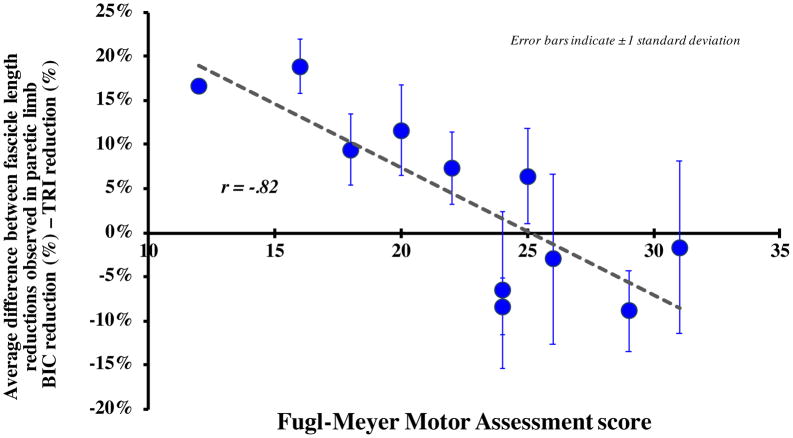
The average fascicle length reduction observed in the paretic limb for the biceps (19.7%) was not significantly different (p = 0.234) than that for the triceps (15.9%). However, as impairment level increased across these subjects, the fascicle length reduction observed in the paretic limb for the biceps increased relative to the triceps. Specifically, we observed a significant negative correlation (r = −0.82, p = 0.002) between each subject’s impairment level, as measured by the Fugl-Meyer Assessment, and the difference between the fascicle length reductions observed in the paretic biceps and triceps (Fig. 4).
Figure 4.
The average difference between the fascicle length % reductions observed in the paretic limb for the biceps and the triceps (average % biceps reduction – average % triceps reduction) in each subject plotted as a function of Fugl-Meyer Assessment score, a clinical assessment of impairment level. Each data point indicates the average difference between the biceps and triceps paretic limb reduction for each individual subject, observed under passive conditions across the three tested elbow positions; error bars indicate ± 1 standard deviation. A positive difference indicates that there was a greater average decrease in fascicle lengths in the paretic biceps brachii than that seen in the triceps; a negative difference indicates a greater average decrease in paretic triceps brachii fascicle lengths. Pearson correlation analysis indicates a significant correlation between the difference in paretic % reduction between the two muscles and impairment level across all positions (r=−.82, p=.002).
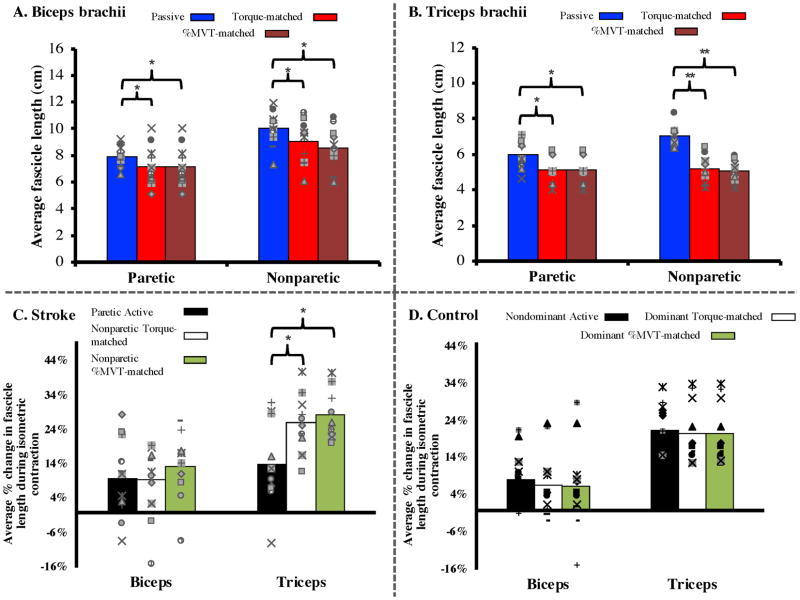
The shorter fascicle lengths observed in the paretic arm under passive conditions remained observable during active muscle contraction for the biceps brachii but not for the triceps brachii. As expected, fascicles in both muscles significantly shortened during active contraction (cf., Fig. 5A & 5B; red and maroon bars are significantly shorter than blue bars; 95% CI: .36–1.36 cm difference between passive and torque-matched conditions, .57–1.6 cm between passive and %MVT-matched condition, p=.001 for main effect of condition for biceps brachii, simple main effect for triceps brachii: p=.007 for paretic limb; 95% CI: 0.32–1.46 cm between passive and active conditions, p<.0005 for nonparetic; 95% CI: 1.37–2.29 for passive to torque-matched condition, with 95% CI: 1.57–2.46 cm for passive to %MVT-matched condition). Despite this, for the triceps, the fascicle lengths measured during active contraction in the paretic limb (average = 5.12 cm) were comparable to those in the nonparetic limb in both the torque-matched (average = 5.19 cm) and %MVT-matched (average = 5.07 cm) active conditions, respectively. In contrast, fascicle lengths measured during active contraction in the paretic limb for biceps (average = 7.14 cm) remained significantly shorter than those in the nonparetic limb (average = 9.08 cm, 8.56 cm for each condition; p= .001). In other words, normalized % decreases in fascicle length observed during active contraction for the biceps were comparable in both limbs, ranging between 10–15% of the fascicle lengths observed in the passive condition. For the triceps, significantly larger changes in fascicle lengths (25–30% decreases) were observed during active contraction in the nonparetic limb compared to the paretic limb (Fig. 5C; 95% CI: 3.27%–20.6%, p=.012 and 95% CI: 7.68%–15.9%, p<.0005 for torque-matched and %MVT-matched conditions, respectively). For context, normalized fascicle length decreases in both limbs during active contraction in control subjects are presented (Fig. 5D).
Figure 5.
(Top) Average fascicle length during all three conditions (passive, torque-matched, and %MVT) for both limbs of N=11 (biceps) and N=10 (triceps) subjects in our study. For the paretic limb, the torque-matched and %MVT categories are identical and are repeated for ease of viewing. Across both muscles and both limbs, there was a significant decrease in fascicle length during an isometric contraction (p=.001 main effect of condition for biceps, p<.0005 simple main effect of condition for triceps). The difference in fascicle length between the paretic and nonparetic limbs seen in the passive condition (Figure 3) was also observed in the biceps brachii during an isometric contraction (p=.001 main effect of arm), but not in the triceps brachii (p=.619, .723 for simple main effect of arm for torque-matched and %MVT conditions, respectively). Individual subject data are again represented with separate markers, similar to Figure 3.
(Bottom) Average % decrease in fascicle length from the passive condition during an isometric contraction for N=11 and N=10 (biceps and triceps, respectively) subjects with chronic stroke and N=11 age-matched control subjects from a previous study (unpublished data). The % decrease in fascicle length during an isometric contraction did not significantly differ between limbs in either stroke or control subjects for the biceps brachii. However, although the % decrease in fascicle length in the control subjects was not significantly different between limbs or conditions for the triceps, there was a significant decrease in the amount of triceps fascicle length decrease seen during an isometric contraction in the paretic limb when compared to that in the nonparetic limb (p=.012 for torque-matched condition, p<.0005 for %MVT-matched condition).
Discussion
We utilized extended field-of-view ultrasound to quantify fascicle lengths in the biceps and triceps brachii in individuals following chronic hemiparetic stroke and observed shorter fascicles in the paretic arm when compared to the nonparetic arm under passive conditions for both muscles. Average fascicle lengths for both biceps brachii (long head), and triceps brachii (distal portion of lateral head) were significantly shorter in the paretic arm across all elbow positions in the relaxed condition; the relative interlimb reduction in fascicle length between the biceps and triceps was strongly correlated to impairment levels. Fascicle length differences observed between arms in stroke subjects were significantly larger than those observed in age-matched control participants in our previous study.33 Fascicle lengths significantly shortened from the passive to active conditions for both muscles in both limbs. However, the shorter fascicle length observed under passive conditions in the paretic triceps was no longer observed under active conditions. Rather, we observed much larger fascicle length decreases in the nonparetic triceps during active force-generating conditions compared to the paretic limb.
Shorter fascicle lengths observed in the paretic versus nonparetic elbow muscles under passive conditions
Biceps brachii fascicle lengths were significantly shorter in the paretic arm compared to those in the nonparetic at all positions during the passive condition. Fascicle adaptation is hypothesized to occur due to the lack of normal use of the paretic extremity,24,39 with the paretic joint often held in a more flexed posture compared to the nonparetic upper extremity. The paretic elbow is relatively immobilized, with the paretic biceps brachii and other elbow flexors in a shortened position when compared to the nonparetic elbow.14 Previous animal work has shown that when skeletal muscle is immobilized in a shortened position, muscle fibers shorten and length-tension curves are shifted to shorter fiber lengths corresponding to the joint position of immobilization.18,19 While we were not able to assess the length-tension curve of the biceps, our results are consistent with both the animal immobilization studies and in vivo results in human subjects following chronic stroke for other muscles. Specifically, a previous study reported shorter fascicle lengths in the paretic brachialis muscle.24 Similarly, shorter fascicles in plantarflexor muscles of the ankle have been observed in both passive21,23 and active22,29 conditions in adults with decreased dorsiflexion range of motion following stroke.
Fascicle lengths for the distal portion of the triceps brachii lateral head were also shorter in the paretic vs. the nonparetic arm. Although the paretic arm is kept in a slightly flexed position,12 it is rarely flexed more than 90° where the triceps brachii would be substantially lengthened. Concurrently, both flexor and extensor muscles are subjected to much less excursion of the musculotendon unit during daily activities as compared to the nonparetic elbow. Decreased excursion of the muscle due to a limited range of elbow motion, consisting of postures where both the flexors and extensors stay at relatively shortened lengths, combined with the overall disuse, may explain why we observed both shortened biceps and triceps fascicles in the paretic arm.
Clinical correlates
In these subjects, individuals who were more impaired (lower FMA scores) had larger, interlimb fascicle length decreases in the paretic biceps than triceps. A potential hypothesis for the correlation seen in our study is that individuals with more severe impairment potentially have an increased expression of flexor hypertonicity and the abnormal flexion synergy, leading to an imbalance in elbow activity. As a result, flexors have an increased involuntary drive vs. the relatively inactive triceps, bringing the arm into a more flexed position throughout the day. This could potentially lead to greater adaptive changes to the biceps brachii vs. triceps brachii over time and greater elbow flexor contractures in more impaired individuals.12 Other investigators have demonstrated that contracture formation in the paretic wrist flexors is associated with increased impairment level in the year after stroke.39 Future research is needed to elucidate the timing of the musculoskeletal changes reported here and to determine how they may impact recovery of function into the subacute and chronic stages.
Changes in fascicle length during active conditions in the paretic versus nonparetic elbow muscles
During an isometric contraction, fascicle lengths in both muscles and both arms shortened as would be expected from normal muscle mechanics. However, while fascicles shortened approximately the same normalized amount in both the paretic and nonparetic biceps (relative to starting length in the passive condition), this was not the case for the triceps brachii. At an equivalent elbow torque, the paretic triceps fascicles shortened significantly less than seen in the nonparetic limb. This was also true when the %MVT was matched between limbs.
A potential reason for this difference could be a change in tendon properties in the paretic triceps. During an isometric contraction, muscle-tendon length remains constant, with a decrease in fascicle length from the passive condition yielding a corresponding increase in overall tendon length. Therefore, if fascicle length shortening during an isometric contraction is decreased, so too is the lengthening in the paretic tendon. Assuming that the other two heads of the triceps demonstrated similar changes as the lateral head and were comparably active across limbs, the torque-matched condition in our study would place the same amount of force through the muscle-tendon unit in each limb. Consequently, the fact that the paretic triceps brachii fascicles shorten less during an isometric, torque-matched contraction than they do in the nonparetic limb could indicate that the paretic tendon is stiffer compared to the nonparetic extremity, contributing to altered muscle dynamics during an isometric contraction. Clearly, this is an area that needs further investigation to elucidate contributing factors to impairments after stroke.
Limitations
Although interlimb fascicle lengths were compared at equivalent elbow postures, the possibility exists that even at the same elbow position, the amount of passive tension through the fascicle was not comparable between the paretic and nonparetic arms. Normalization of fascicle length by sarcomere length would be required to ensure muscle fascicle lengths are compared at equivalent portions of the force-length curve. Muscle sarcomere lengths are regularly measured for this purpose in cadaveric studies,40 but much less frequently in vivo, due to the invasive nature of the measurements41 and the fact that newer technology enabling these data to be collected is not yet commonly available.42 For these reasons, we were unable to determine sarcomere length for these muscles in this study. Despite this limitation (common to most ultrasound measures of fascicle lengths), our data in Figure 3 clearly demonstrate a substantial difference between the paretic and nonparetic limbs that is not seen in age-matched control subjects, using identical methods.
Another limitation of the current study is the potential for under or over-estimation of fascicle length due to errors in the estimation of the fascicle plane. Previous research has shown that error in fascicle length can occur when the probe orientation is tilted away from the true fascicular plane.43,44 However, we previously demonstrated33 that the variability in fascicle measurements between images, which provides an estimate of the intersession variability due to small changes in probe orientation between images, was very small. Similarly, a subsequent study provides evidence that implementation of extended field-of-view ultrasound is both valid and reliable in a different upper limb muscle.45 Therefore, any error in measurement as a result of small changes in probe orientation was assumed to be small. Furthermore, any changes in fascicle length between arms in the stroke population was much larger than any changes seen in control participants using identical methods.33
Conclusion
Adaptive changes in the musculoskeletal system have the potential to exacerbate existing neural deficits such as weakness, since an increase in joint stiffness or reduced range of motion would make it even harder for an individual with deficits in voluntary drive to generate enough joint torque to overcome the shorter fascicles’ resistance to movement, especially at end ranges of motion. This could have implications on functional movement: even if an individual could activate their muscles at near-normal conditions, the changes in muscle fascicle length measured here may place the muscle at a sub-optimal operating range, thereby restricting its force production even if normal independent joint control and muscle activation levels were to return.
The correlation between impairment level and the relative reduction in fascicle lengths between the biceps brachii and the triceps brachii may have implications that emphasize the need to identify individuals with severe impairment early on for the implementation of rehabilitation interventions aimed at reducing musculoskeletal adaptations. This also points to the need for further research investigating the rate of musculoskeletal adaptation during recovery from stroke, using the imaging approaches utilized in this study.
Acknowledgments
We would like to acknowledge funding from the American Heart Association (#14PRE20240022), Northwestern University terminal year fellowship, NIH-NIBIB (#T32EB009406), NIH R01 to Drs. Dewald and Murray (#1R01HD084009-01A1), Northwestern University Department of Physical Therapy and Human Movement Sciences, and the Searle Funds of the Chicago Community Trust. We would also like to acknowledge Ana Maria Acosta for her help with development of the experimental GUI, and Paul Krueger and Vikram Darbhe for their assistance during experiments.
Footnotes
Conflict of Interest Statement:
I declare that none of the authors of this work have had any financial or personal relationships with other individuals or organizations that could inappropriately influence or bias this work.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics—2015 Update. Circulation. 2015;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Raghavan P. Upper Limb Motor Impairment After Stroke. Phys Med Rehabil Clin N Am. 2015;26(4):599–610. doi: 10.1016/j.pmr.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng YS, Stein J, Ning M, Black-Schaffer RM. Comparison of Clinical Characteristics and Functional Outcomes of Ischemic Stroke in Different Vascular Territories. Stroke. 2007;38(8):2309–2314. doi: 10.1161/STROKEAHA.106.475483. [DOI] [PubMed] [Google Scholar]
- 4.Blumenfeld H. Neuroanatomy Through Clinical Cases. 2. Sinauer Associates Incorporated; 2010. [Google Scholar]
- 5.Faria-Fortini I, Michaelsen SM, Cassiano JG, Teixeira-Salmela LF. Upper Extremity Function in Stroke Subjects: Relationships between the International Classification of Functioning, Disability, and Health Domains. J Hand Ther. 2011;24(3):257–265. doi: 10.1016/j.jht.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Carin-Levy G, Greig C, Young A, Lewis S, Hannan J, Mead G. Longitudinal Changes in Muscle Strength and Mass after Acute Stroke. Cerebrovasc Dis. 2006;21(3):201–207. doi: 10.1159/000090792. [DOI] [PubMed] [Google Scholar]
- 7.Mirbagheri MM, Alibiglou L, Thajchayapong M, Rymer WZ. Muscle and reflex changes with varying joint angle in hemiparetic stroke. J Neuroeng Rehabil. 2008;5(1):6. doi: 10.1186/1743-0003-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dewald JPA, Beer RF. Abnormal joint torque patterns in the paretic upper limb of subjects with hemiparesis. Muscle Nerve. 2001;24(2):273–283. doi: 10.1002/1097-4598(200102)24:2<273::aid-mus130>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 9.Miller LC, Dewald JPA. Involuntary paretic wrist/finger flexion forces and EMG increase with shoulder abduction load in individuals with chronic stroke. Clin Neurophysiol. 2012;123(6):1216–1225. doi: 10.1016/j.clinph.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sukal TM, Ellis MD, Dewald JPA. Shoulder abduction-induced reductions in reaching work area following hemiparetic stroke: Neuroscientific implications. Exp Brain Res. 2007;183(2):215–223. doi: 10.1007/s00221-007-1029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunnstrom S. Movement Therapy in Hemiplegia - A Neurophysiological Approach. 1. Philadelphia: Harper & Row; 1970. [Google Scholar]
- 12.Bhadane MY, Gao F, Francisco GE, Zhou P, Li S. Correlation of Resting Elbow Angle with Spasticity in Chronic Stroke Survivors. Front Neurol. 2015 Aug;6:1–7. doi: 10.3389/fneur.2015.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kline TL, Schmit BD, Kamper DG. Exaggerated interlimb neural coupling following stroke. Brain. 2006;130(1):159–169. doi: 10.1093/brain/awl278. [DOI] [PubMed] [Google Scholar]
- 14.O’Dwyer NJ, Ada L, Neilson PD. Spasticity and muscle contracture following stroke. Brain. 1996;119:1737–1749. doi: 10.1093/brain/119.5.1737. [DOI] [PubMed] [Google Scholar]
- 15.Kwah LK, Harvey LA, Diong JHL, Herbert RD. Half of the adults who present to hospital with stroke develop at least one contracture within six months: an observational study. J Physiother. 2012;58(1):41–47. doi: 10.1016/S1836-9553(12)70071-1. [DOI] [PubMed] [Google Scholar]
- 16.Lieber RL, Fridén J. Functional and clinical significance of skeletal muscle architecture. Muscle Nerve. 2000;23(11):1647–1666. doi: 10.1002/1097-4598(200011)23:11<1647::aid-mus1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 17.Rassier DE, MacIntosh BR, Herzog W, et al. Length dependence of active force production in skeletal muscle. J Appl Physiol. 1999;86:1445–1457. doi: 10.1152/jappl.1999.86.5.1445. [DOI] [PubMed] [Google Scholar]
- 18.Williams PE, Goldspink G. Changes in sarcomere length and physiological properties in immobilized muscle. J Anat. 1978;127(3):459–468. [PMC free article] [PubMed] [Google Scholar]
- 19.Tabary JC, Tabary C, Tardieu C, Tardieu G, Goldspink G. Physiological and structural changes in the cat’s soleus muscle due to immobilization at different lengths by plaster casts*. J Physiol. 1972;224(1):231–244. doi: 10.1113/jphysiol.1972.sp009891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boakes JL, Foran J, Ward SR, Lieber RL. CASE REPORT: Muscle Adaptation by Serial Sarcomere Addition 1 Year after Femoral Lengthening. Clin Orthop Relat Res. 2007;456:250–253. doi: 10.1097/01.blo.0000246563.58091.af. [DOI] [PubMed] [Google Scholar]
- 21.Gao F, Grant TH, Roth EJ, Zhang L-Q. Changes in Passive Mechanical Properties of the Gastrocnemius Muscle at the Muscle Fascicle and Joint Levels in Stroke Survivors. Arch Phys Med Rehabil. 2009;90(5):819–826. doi: 10.1016/j.apmr.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Gao F, Zhang L-Q. Altered contractile properties of the gastrocnemius muscle poststroke. J Appl Physiol. 2008;105(6):1802–1808. doi: 10.1152/japplphysiol.90930.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwah LK, Herbert RD, Harvey LA, et al. Passive Mechanical Properties of Gastrocnemius Muscles of People With Ankle Contracture After Stroke. Arch Phys Med Rehabil. 2012;93(7):1185–1190. doi: 10.1016/j.apmr.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Li L, Tong KY, Hu X. The Effect of Poststroke Impairments on Brachialis Muscle Architecture as Measured by Ultrasound. Arch Phys Med Rehabil. 2007;88(2):243–250. doi: 10.1016/j.apmr.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Ramsay JW, Buchanan TS, Higginson JS. Differences in Plantar Flexor Fascicle Length and Pennation Angle between Healthy and Poststroke Individuals and Implications for Poststroke Plantar Flexor Force Contributions. Stroke Res Treat. 2014;2014:1–6. doi: 10.1155/2014/919486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramsay JW, Wessel MA, Buchanan TS, Higginson JS. Poststroke Muscle Architectural Parameters of the Tibialis Anterior and the Potential Implications for Rehabilitation of Foot Drop. Stroke Res Treat. 2014;2014:1–5. doi: 10.1155/2014/948475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tok F, Özçakar L, Safaz İ, Alaca R. Effects of botulinum toxin-A on the muscle architecture of stroke patients: The First Ultrasonographic study. J Rehabil Med. 2011;43(11):1016–1019. doi: 10.2340/16501977-0876. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y-B, Zhang J, Leng Z-P, Chen X, Song W-Q. Evaluation of spasticity after stroke by using ultrasound to measure the muscle architecture parameters: a clinical study. Int J Clin Exp Med. 2014;7(9):2712–2717. [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao H, Ren Y, Roth EJ, Harvey RL, Zhang L-Q. Concurrent deficits of soleus and gastrocnemius muscle fascicles and Achilles tendon post stroke. J Appl Physiol. 2015;118(7):863–871. doi: 10.1152/japplphysiol.00226.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu P, Wang Y, Hu H, Mao Y, Huang D, Li L. Change of Muscle Architecture following Body Weight Support Treadmill Training for Persons after Subacute Stroke: Evidence from Ultrasonography. Biomed Res Int. 2014;2014:1–11. doi: 10.1155/2014/270676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dias CP, Freire B, Goulart NBA, et al. Muscle architecture and torque production in stroke survivors: an observational study. Top Stroke Rehabil. 2017;24(3):206–213. doi: 10.1080/10749357.2016.1210873. [DOI] [PubMed] [Google Scholar]
- 32.Noorkoiv M, Stavnsbo A, Aagaard P, Blazevich AJ. In vivo assessment of muscle fascicle length by extended field-of-view ultrasonography. J Appl Physiol. 2010;109(6):1974–1979. doi: 10.1152/japplphysiol.00657.2010. [DOI] [PubMed] [Google Scholar]
- 33.Nelson CM, Dewald JPA, Murray WM. In vivo measurements of biceps brachii and triceps brachii fascicle lengths using extended field-of-view ultrasound. J Biomech. 2016;49(9):1948–1952. doi: 10.1016/j.jbiomech.2016.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dietz V, Sinkjaer T. Spastic movement disorder: impaired reflex function and altered muscle mechanics. Lancet Neurol. 2007;6(8):725–733. doi: 10.1016/S1474-4422(07)70193-X. [DOI] [PubMed] [Google Scholar]
- 35.Welmer A-K, Widén Holmqvist L, Sommerfeld DK. Location and severity of spasticity in the first 1–2 weeks and at 3 and 18 months after stroke. Eur J Neurol. 2010;17(5):720–725. doi: 10.1111/j.1468-1331.2009.02915.x. [DOI] [PubMed] [Google Scholar]
- 36.Katalinic OM, Harvey LA, Herbert RD. Effectiveness of Stretch for the Treatment and Prevention of Contractures in People With Neurological Conditions: A Systematic Review. Phys Ther. 2011;91(1):11–24. doi: 10.2522/ptj.20100265. [DOI] [PubMed] [Google Scholar]
- 37.Leung J, Harvey LA, Moseley AM, et al. Electrical stimulation and splinting were not clearly more effective than splinting alone for contracture management after acquired brain injury: a randomised trial. J Physiother. 2012;58(4):231–240. doi: 10.1016/S1836-9553(12)70124-8. [DOI] [PubMed] [Google Scholar]
- 38.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The Post-Stroke Hemiplegic Patient. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 39.Malhotra S, Pandyan A, Rosewilliam S, Roffe C, Hermens H. Spasticity and contractures at the wrist after stroke: time course of development and their association with functional recovery of the upper limb. Clin Rehabil. 2011;25(2):184–191. doi: 10.1177/0269215510381620. [DOI] [PubMed] [Google Scholar]
- 40.Murray WM, Buchanan TS, Delp SL. The isometric functional capacity of muscles that cross the elbow. J Biomech. 2000;33(8):943–952. doi: 10.1016/S0021-9290(00)00051-8. [DOI] [PubMed] [Google Scholar]
- 41.Murray WM, Hentz VR, Fridén J, Lieber RL. Variability in surgical technique for brachioradialis tendon transfer: Evidence and implications. J Bone Jt Surg - Ser A. 2006;88(9):2009–2016. doi: 10.2106/JBJS.E.00973. [DOI] [PubMed] [Google Scholar]
- 42.Sanchez GN, Sinha S, Liske H, et al. In Vivo Imaging of Human Sarcomere Twitch Dynamics in Individual Motor Units. Neuron. 2015;88(6):1109–1120. doi: 10.1016/j.neuron.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bénard MR, Becher JG, Harlaar J, Huijing PA, Jaspers RT. Anatomical information is needed in ultrasound imaging of muscle to avoid potentially substantial errors in measurement of muscle geometry. Muscle Nerve. 2009;39(5):652–665. doi: 10.1002/mus.21287. [DOI] [PubMed] [Google Scholar]
- 44.Klimstra M, Dowling J, Durkin JL, MacDonald M. The effect of ultrasound probe orientation on muscle architecture measurement. J Electromyogr Kinesiol. 2007;17(4):504–514. doi: 10.1016/j.jelekin.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 45.Adkins AN, Franks PW, Murray WM. Demonstration of extended field-of-view ultrasound’s potential to increase the pool of muscles for which in vivo fascicle length is measurable. J Biomech. 2017;63:179–185. doi: 10.1016/j.jbiomech.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]