Abstract
Coronary microvascular disease (CMD) refers to the subset of disorders affecting the structure and function of the coronary microcirculation, is prevalent in patients across a broad spectrum of cardiovascular risk factors, and is associated with increased risk of adverse events. Contemporary evidence supports that most patients with CMD also have macrovessel atherosclerosis, which has important implications for their prognosis and management. In this state-of-the-art review, we summarize the pathophysiology of CMD, provide an update of diagnostic testing strategies, and classify CMD into phenotypes according to severity and coexistence with atherosclerosis. We examine emerging data highlighting the significance of CMD in specific populations, including obesity and insulin resistance, myocardial injury and heart failure with preserved ejection fraction, and nonobstructive and obstructive coronary artery disease. Finally, we discuss the role of CMD as a potential target for novel interventions beyond conventional approaches, representing a new frontier in cardiovascular disease reduction.
Keywords: coronary flow reserve, coronary microvascular dysfunction, heart failure with preserved ejection fraction, ischemic heart disease, nonobstructive coronary artery disease
CONDENSED ABSTRACT:
Coronary microvascular disease (CMD) refers to the subset of disorders affecting the structure and function of the coronary microcirculation, is prevalent in patients across a broad spectrum of cardiovascular risk factors and associated with increased risk of adverse events. Contemporary evidence supports that most patients with CMD also have macrovessel atherosclerosis. In this state-of-the-art review, the authors summarize what is known about the pathophysiology of CMD, provide an update of diagnostic testing strategies, classify CMD into clinical phenotypes according to severity and coexistence with atherosclerosis, and discuss its role as a potential target for novel interventions.
Since the early 1900s, cardiovascular disease (CVD) has accounted for the highest number of disease-related deaths in the developed world (1), but hidden in this stable statistic are indications that CVD is changing. Angina and dyspnea remain common presenting complaints, but for a high proportion of cardiac patients now seeking an explanation for their symptoms in the catheterization laboratory, no significantly obstructing coronary lesion is found (2–4). Despite this, their prognosis is not necessarily benign (2,3). Over the last 2 decades, diagnostic yields have fallen not only for invasive coronary angiography (2,3), but also for noninvasive stress testing (5). Meanwhile, the incidence of acute presentations of atherothrombotic plaque rupture causing myocardial infarction (MI), particularly with ST-segment elevation, has decreased (6), whereas the rates of hospitalizations with a secondary MI diagnosis and heart failure with preserved ejection fraction (HFpEF) (7) have risen sharply. These observations follow other epidemiological shifts in the prevalence of cardiovascular risk factors in the population, including the growth of obesity, glucose intolerance, and older age (1).
SMALL VESSELS, LARGE IMPACT
Often synonymous with coronary artery disease (CAD), heart disease has been conventionally defined anatomically as obstructive atherosclerosis involving the epicardial coronary arteries (Figure 1A). There is now greater recognition that structural and functional disorders affecting the whole coronary circulation, including the microcirculation (Figure 1B), serve as key mediators of patient symptoms and prognosis in what may be more aptly termed ischemic heart disease (IHD). The subset of disorders affecting the coronary microcirculation itself is termed coronary microvascular disease (CMD). Consequently, symptomatic patients without identifiable obstructive plaque may still harbor significant nonobstructive coronary atherosclerosis and microvascular ischemia with resultant increased rates of major adverse cardiovascular events (MACE). How should these patients be optimally managed? At the present, no guideline-directed recommendations exist. This gap in the scientific and clinical evidence base has led to increased calls for research directed at expanding diagnostic tools to address the full spectrum of IHD affecting patients, which is facilitating a transformation in how CVD is defined. Previously important distinctions, such as “primary” versus “secondary” prevention, are becoming obsolete, and the outsized role of CMD on the persistence of symptoms and prognosis of CVD is more apparent.
Figure 1: Schematic of the Epicardial Coronary Arteries and the Full Coronary Circulation.
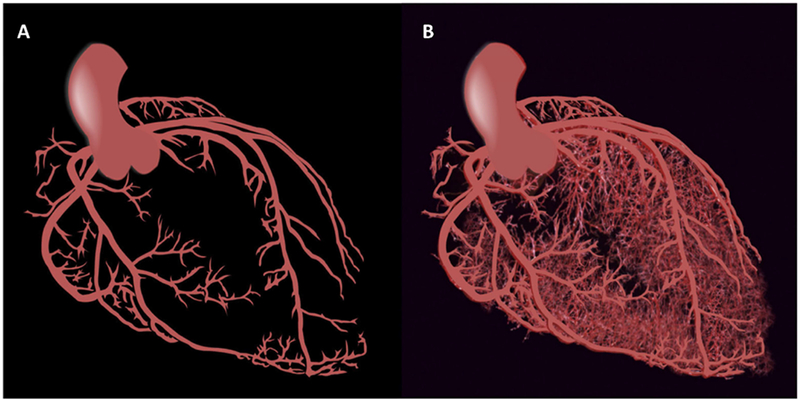
(A) Macrocirculation. (B) Macro- and microcirculation.
Despite pioneering efforts, confusion surrounding CMD remains (8–13). First, there is the challenge of nomenclature. Variously described in the past, including with the nonspecific term coronary syndrome X, CMD risks losing meaning for the clinical provider if it is not objectively diagnosed. Second, because it is too small to be directly imaged in patients in vivo, the coronary microcirculation represents a complex series of compartments that must be evaluated indirectly through standardized methods designed to perturb its function in regulating myocardial blood flow. Third, pathology affecting the microcirculation does not exist in a vacuum, but is intricately linked to that of the macrocirculation via direct physical as well as humoral factors. Contemporary evidence (14–16) strongly supports the coexistence of CMD with atherosclerosis in most affected patients, and this consideration represents a critical differentiator for what may constitute appropriate therapeutic strategies in CMD. The interaction of macrovessel atherosclerosis with microvessel ischemia is fundamental to the effective diagnosis and management of disease and represents a central thesis of this review. Here, we summarize what is known about the pathophysiology of CMD, provide an update of diagnostic testing strategies, and classify CMD into phenotypes according to severity and coexistence with atherosclerosis. We examine emerging data highlighting the significance of CMD in specific populations, including obesity and insulin resistance, myocardial injury and heart failure with preserved ejection fraction, and nonobstructive and obstructive coronary artery disease. Finally, we discuss the role of CMD as a potential target for novel interventions beyond conventional approaches.
PATHOPHYSIOLOGY OF CMD: STRUCTURAL AND FUNCTIONAL ABNORMALITIES
The coronary arterial system represents a continuous network of functionally distinct vessel segments of decreasing size (Central Illustration) (9,10). The proximal large epicardial coronary arteries (>400 μm) give way to small pre-arterioles (100 to 400 μm) and smaller intramural arterioles (<100 μm) that interface directly with the coronary capillary bed (<10 μm). The epicardial arteries have a primary conductance function and exhibit minimal resistance to coronary flow under normal conditions, with their diameters regulated by shear stress and endothelial function. In contrast, the pre-arterioles and arterioles make up most of the resistance circuit of the heart and are responsible for regulation and distribution of blood flow to match the dynamic needs of local tissue metabolism via the coronary capillaries (9,10).
Central Illustration: Normal and Abnormal Structure and Function of the Coronary Macro- and Microcirculation.
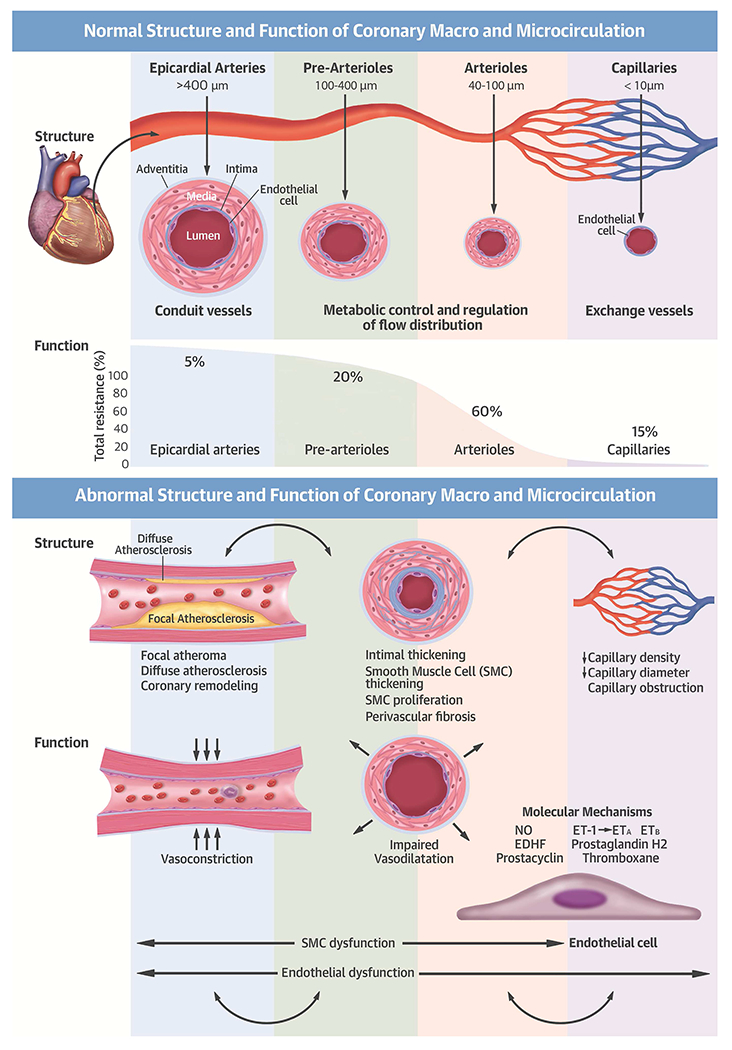
Ca2+ = calcium; EDHF = endothelium-derived hyperpolarizing factor; EnNac = endothelial sodium channel; eNOS = endothelial nitric oxide synthase; ET-1 = endothelin-1, ETA/ETB = endothelin receptors; NO – nitrous oxide; SKCa= small-conductance Ca2+-activated potassium channels SMC = smooth muscle cell; T. = tunica.
Microvascular regulation of coronary blood flow and myocardial perfusion
In healthy vessels, coronary blood flow and myocardial perfusion are regulated by coronary arteriolar tone (Central Illustration). Coronary blood flow remains constant over a wide range of coronary perfusion pressures through dynamic changes in resistance vessel tone. These dynamic changes result from a series of partially redundant mechanisms, including adrenergic stimuli, changes in local oxygen tension, and the response to changes in transmural pressure (10,17). Such redundant control of coronary blood flow helps to mitigate myocardial ischemia during the development and progression of epicardial atherosclerosis. Because myocardial oxygen extraction is near-maximal at rest, myocardial oxygen delivery is almost completely dependent on coronary blood flow. Consequently, an increase in myocardial oxygen demand must be matched by a proportional increase in coronary blood flow to prevent myocardial ischemia. CMD attenuates coronary flow augmentation in response to stress and, if severe enough to lead to demand-supply mismatch, may lead to subclinical or clinical myocardial ischemia.
SPECTRUM OF VASCULAR ABNORMALITIES IN CMD.
Structural abnormalities.
A number of structural changes, or microvascular remodeling, have been associated with CMD (Central Illustration). This spectrum of abnormalities does not include atheroma, which occurs in epicardial arteries, but is nonetheless likely magnified by the presence of atherosclerosis, particularly in patients with CVD risk factors. These changes lead to microvascular obstruction with luminal narrowing of the intramural arterioles and capillaries, and capillary rarefaction, often in the context of increased LV mass. Evidence of microvascular remodeling has been reported in some (18–22) but not all (23,24) studies, and is consistently linked to risk factors including diabetes, hypertension and renal impairment, or evidence of diffuse epicardial atherosclerosis (18–22).
Functional abnormalities.
Ample evidence exists that endothelial dysfunction in resistance coronary vessels is an important contributor to CMD. The vascular endothelium plays a pivotal role in modulating smooth muscle function by releasing vasoactive substances, including nitric oxide. In the presence of normal endothelial function, acetylcholine and physiological stimuli (e.g., exercise) produce vasodilation of the coronary epicardial and microvascular circulation, thereby resulting in increased coronary blood flow and myocardial perfusion. However, with the development of CVD risk factors and atherosclerosis, the vascular endothelium becomes dysfunctional and the vasodilator response to pharmacological and physiological interventions is attenuated, resulting in blunted coronary blood flow augmentation or vasoconstriction with frank reduction in blood flow (25).
There is evidence that functional abnormalities of smooth muscle cells regulating arteriolar tone are also present in many patients with CMD. Attenuated vasodilator responses to papaverine, adenosine, or dipyridamole, which are largely mediated by vascular smooth muscle relaxation of resistive vessels, have been documented in patients with diabetes and metabolic syndrome (26–28), dyslipidemia (29,30), hypertension (31,32), obesity (33), smoking (34), renal impairment (35,36), and cardiomyopathy (37,38). Coronary spasm is part of the spectrum of vasomotor abnormalities seen in patients with CMD and affects large and small coronary arteries. Acetylcholine-induced epicardial or microvascular spasm has been reported in approximately 50% of patients with stable chest pain and no evidence of obstructive CAD (39). The pathogenesis of coronary spasm appears to be closely linked to atherosclerosis and endothelial dysfunction (13).
ADDITIONAL ROLE OF DIFFUSE NONOBSTRUCTIVE ATHEROSCLEROSIS.
Diffuse nonobstructive atherosclerosis in the epicardial coronary arteries is a common finding in the majority of symptomatic patients with CMD. In studies involving patients with chest pain and no obstructive CAD, nearly 70% to 80% of patients showed evidence of diffuse nonobstructive atherosclerosis by intravascular ultrasound (14,40) or coronary arterial calcifications (15). The association of nonobstructive atherosclerosis with CMD has important clinical implications. First, there is evidence that more than one-half of atherosclerotic coronary arteries without focally obstructive stenoses display a significant longitudinal pressure gradient (41) affecting coronary blood flow and myocardial perfusion (42), which can contribute to myocardial ischemia and symptoms. This may help to explain the discrepancy between ischemic symptoms and the low frequency of obstructive CAD in large registries of invasive coronary angiography (2–4) and recent diagnostic clinical trials with cardiac computed tomography angiography (43,44). Second, the recognition that symptoms in patients with nonobstructive CAD may be related to CMD offers an opportunity for directing additional investigations to improving diagnosis and management.
CLINICAL PRESENTATION AND DIAGNOSIS OF CMD: ROLE OF NONINVASIVE AND INVASIVE TESTING
The clinical presentation and diagnosis of IHD has long been influenced by the practice of defining heart disease as obstructive CAD and tailoring diagnostic (and treatment) strategies to this subset of patients. The accuracy of standard noninvasive diagnostic testing for ischemia, however, can vary significantly when evaluated against a gold standard of finding anatomic obstructive CAD. This poses a particular challenge for patients whose symptoms are less likely to be explained by findings on coronary angiography and whose abnormal stress tests in the absence of obstructive CAD are more likely to be interpreted as “false positives.” The recent application of modern diagnostic tools is changing the paradigm of disease diagnosis, broadening the definitions of CAD and ischemia to better reflect prevalent pathological phenotypes including CMD.
CLINICAL MANIFESTATIONS OF CMD.
Cardinal manifestations of CMD include angina, exertional dyspnea, and possibly heart failure. The combination of symptoms is common. The natural history of CMD has a relatively long asymptomatic phase during which patients may be identified only incidentally. Angina occurs in approximately 30% to 60% of patients with CMD (15,45–48). It is indistinguishable from angina caused by obstructive CAD in that it is commonly precipitated by exertion and relieved by rest. Atypical angina, including episodes of chest pain at rest, is also common. Patients may also manifest with a gradual decrease in exercise tolerance or dyspnea on exertion (12,15,45–49). Exertional dyspnea may represent an ischemic equivalent caused by left ventricular (LV) diastolic dysfunction, with an excessive rise in end-diastolic pressure leading to cardiopulmonary congestion. Exertional symptoms may also result from a limited ability to increase cardiac output with exercise.
LIMITATIONS OF CONVENTIONAL DIAGNOSTIC TESTING.
Many patients with CMD have normal findings on physical examination. The single best clue to diagnosis may be the detailed clinical history, including ascertainment of risk factors, which overlap significantly with those of atherosclerotic CVD. In patients presenting with heart failure, the typical signs of elevated filling pressure, including jugular venous distention, rales, and pedal edema, may be present. The resting electrocardiogram is normal or nondiagnostic in most patients.
Exercise stress testing for detection of myocardial ischemia is a Class I recommendation for patients with suspected CAD (50). A positive exercise stress test has been traditionally used as a requirement for the diagnosis of CMD (12). However, large studies including women and men with chest pain and nonobstructive CAD found that a positive exercise stress test was neither sensitive nor specific for CMD (46,47). Stress imaging tests, such as with echocardiography or nuclear scintigraphy, are frequently normal but can occasionally show regional abnormalities that may not follow typical vascular distributions. As such, conventional stress testing with or without imaging is neither sensitive nor specific for detecting CMD and thus has a limited role in its diagnosis.
Because the coronary microcirculation is beyond the resolution of invasive or noninvasive coronary angiography, direct interrogation of coronary microvascular function is necessary to establish the diagnosis of CMD (12). There are several noninvasive and invasive approaches for the evaluation of coronary vasomotor dysfunction (Table 1), each with advantages and limitations.
Table 1.
Strengths and Limitations of Select Diagnostic Techniques for the Evaluation of CMD
| Accuracy | Reproducibility | Diagnostic Threshold | Prognostic Validation | Availability | Cost | |
|---|---|---|---|---|---|---|
| Noninvasive* | ||||||
| PET | ++++ | ++++ | CFR < 2 | ++++ | ++ | $$$ |
| CMR | +++ | +++ | MPRI < 2 | ++ | ++ | $$$ |
| Doppler echocardiography | ++ | +++ | CFVR < 2 | +++ | ++++ | $ |
| Invasive* | ||||||
| CFR | ++++ | ++++ | < 2.3 | +++ | ++++ | $$$$ |
| IMR | ++++ | +++ | > 25 U | ++ | ++ | $$$$ |
| WIA | +++ | Limited data | −4.8 | ++ | ++ | $$$$ |
Assumes exclusion of obstructive coronary artery disease
CFR = coronary flow reserve; CFVR = coronary flow velocity reserve; CMD = coronary microvascular disease; CMR = cardiac magnetic resonance; CT = computed tomography; IMR = index of microvascular resistance; MPRI = myocardial perfusion reserve index; PET = positron emission tomography; WIA = wave intensity analysis
NONINVASIVE TECHNIQUES FOR DIAGNOSING CMD.
Noninvasive diagnosis relies on interrogation of coronary vasomotor function by measuring regional and global myocardial blood flow at rest and during stress, microvascular resistance, and coronary flow reserve (CFR). CFR, calculated as the ratio of hyperemic to rest absolute myocardial blood flow, is a measure of coronary vasomotor dysfunction that integrates the hemodynamic effects of focal, diffuse, and small-vessel disease on myocardial tissue perfusion. CMD can be defined practically as impaired CFR in the absence of epicardial flow-limiting (obstructive) CAD, reflecting downstream vasomotor dysfunction. As discussed later, whereas some patients may have coexistence of focal obstructive CAD and CMD, and others may manifest CMD in the absence of detectable atherosclerosis, it is becoming clear that a large proportion of patients demonstrate CMD in the presence of diffuse nonobstructive CAD. Although quantification of regional myocardial ischemia and regional CFR play a critical role in the assessment of focally obstructive CAD, for the purposes of this review, we focus on the use of global measurements of myocardial blood flow and CFR for diagnostic and prognostic assessments of CMD affecting the collective health of the whole left ventricle.
Positron emission tomography (PET) is the most validated and accurate noninvasive approach for the quantitative assessment of coronary vasomotor function. Technical advances have enabled these measurements to be incorporated into routine PET myocardial perfusion stress testing (51). The imaging protocol consists of a rest and vasodilator-stress myocardial perfusion study, each following the injection of a blood flow radiotracer (82Rubidium and 13N-ammonia are Food and Drug Administration–approved for this application). Post-processing of the rest and stress images allows for the quantification of regional and global myocardial blood flow (in ml/min/g of myocardium) and calculation of CFR (as the ratio of stress over rest myocardial blood flow). Recent data support that coronary vascular dysfunction, as quantified by reduced CFR, is highly prevalent among patients with known or suspected CAD (15), increases the severity of inducible myocardial ischemia (beyond the effects of epicardial coronary obstruction) and subclinical myocardial injury (52), and identifies patients at high risk for MACE, including cardiac death (53–55). The accuracy of PET for quantitative noninvasive measurement of myocardial blood flow and CFR has been extensively validated in experimental animals and humans, and the reproducibility of this technique is well-established (51).
Cardiac magnetic resonance (CMR) can also be used to quantify myocardial perfusion in a similar manner as PET (51), although post-processing is technically demanding and time-consuming. Like PET, the imaging protocol consists of a rest and vasodilator-stress first-pass myocardial perfusion study, each following the injection of a gadolinium-based contrast agent (51). Post-processing of the rest and stress images allows for the quantification of regional and global myocardial perfusion using semiquantitative (myocardial perfusion reserve index) or fully quantitative (CFR) models. A gadolinium-free stress CMR approach using T1 mapping has also been recently proposed for diagnosis of myocardial ischemia with and without obstructive CAD (56). Advantages of CMR are high spatial resolution, allowing transmural characterization of myocardial blood flow, and the lack of ionizing radiation, along with the ability to perform a comprehensive assessment of cardiovascular structure and function. A reduced myocardial perfusion reserve index has been used to evaluate CMD and was shown to predict prognosis (57), but data remain limited.
Doppler echocardiography of the left anterior descending coronary artery can also be used to quantify coronary blood flow velocity at rest and during vasodilator stress. Coronary flow velocities are measured by pulsed-wave Doppler and assessed as diastolic peak flow velocities at rest and at peak hyperemia. Coronary flow velocity reserve is calculated as the ratio of hyperemic coronary flow velocity to rest coronary flow velocity. The advantages of this technique are its low cost, lack of ionizing radiation, and potentially broad access. Nonetheless, this technique is highly operator-dependent and requires echocardiographic visualization of the proximal coronary arteries, a significant challenge in obese adults. There is a growing body of evidence that a reduced coronary flow velocity reserve index helps to identify CMD and allows risk stratification (58–60).
Dynamic myocardial perfusion CT can also be used to estimate myocardial blood flow in a manner similar to CMR perfusion imaging. Dynamic CT scanning is performed after injection of an iodinated contrast agent with prospective electrocardiographic triggering to capture the first pass of the contrast medium through the heart (51). These dynamic image sets are then used to produce estimates of myocardial blood flow using methodology developed for CMR. The major advantages of this technique are the superior spatial resolution of CT and the opportunity to perform accurate anatomic and functional assessments of both the myocardium and the coronary arteries within one examination. However, these benefits come at the price of a higher radiation dose to the patient.
INVASIVE TECHNIQUES FOR DIAGNOSING CMD.
Invasive coronary angiography, by combining the ability to exclude obstructive CAD with complementary catheter-based techniques to probe epicardial and microvascular coronary physiology, is an attractive approach to evaluate patients with CMD. Various catheter-based measures are available and described in the following sections.
Invasive coronary flow reserve (iCFR) is assessed most commonly using an intracoronary Doppler-tipped guidewire or thermodilution techniques to measure coronary blood flow velocity at rest and in response to adenosine (endothelium-independent) or acetylcholine (endothelium-dependent) vasodilation (25). Microvascular angina in the setting of CMD is defined as symptoms of myocardial ischemia, absence of obstructive CAD, and abnormal iCFR or microvascular spasm to acetycholine (12). In patients with suspected ischemia, reductions in adenosine-stimulated iCFR have been associated with increased risk of MACE (11).
Index of microvascular resistance (IMR) is calculated as the distal coronary pressure divided by the inverse of the mean transit time during maximal hyperemia. This requires the use of a combined pressure-temperature sensor-tipped coronary guidewire, which allows for simultaneous measurement of coronary pressure and hyperemic flow. In patients with stable nonobstructive atherosclerosis, abnormal IMR was associated with worse CVD outcomes (61).
Fractional flow reserve (FFR) provides a surrogate measure of flow limitation and stenosis-level physiological obstruction and is calculated as the ratio between coronary pressure distal to a stenosis and aortic pressure during hyperemia. Evidence from randomized controlled trials supports that the use of FFR to guide clinical decisions regarding coronary revascularization leads to reduced cardiac events relative to an anatomic (62) or conservative strategy alone (63). Based on these findings, the use of FFR is now incorporated into guidelines regarding management of patients with stable IHD (50). Because FFR represents a pressure ratio across a stenosis during hyperemia, it is a relative index of epicardial conductance. Thus, it is influenced by limits to maximal achievable blood flow caused by CMD and diffuse atherosclerosis. However, FFR is not primarily used to measure CMD. The presence of CMD increases microvascular resistance and may pseudonormalize FFR (64).
Instantaneous wave-free ratio (iFR) uses wave intensity analysis to identify the mid-late diastolic instantaneous wave-free interval over which the iFR ratio is computed. iFR is a pressure-derived index of stenosis severity, which can be obtained during diastole without administration of adenosine. This trans-stenotic pressure gradient is an accurate measure of the severity of a coronary stenosis and is closely correlated with FFR. In clinical trials of patients with stable angina and acute coronary syndromes, an iFR-guided revascularization strategy (for iFR <0.89) was noninferior to an FFR-guided revascularization approach (for FFR <0.80) with respect to the risk of adverse events at one year (65,66). iFR shares some of the same limitations as FFR in the setting of CMD.
Wave-intensity analysis (WIA) may also be useful in the evaluation of coronary microvascular function (67). The key measurement is the augmentation of coronary blood flow that results from the re-expansion of the capillary bed during early diastole (67). Because capillary density is a significant determinant of the capacity of the intramyocardial vasculature and the backward suction that accelerates flow during diastole, quantification of microcirculatory filling in early diastole with WIA may provide a measure of myocardial capillary rarefaction (68).
DIAGNOSTIC TESTING STRATEGY FOR CMD.
Figure 2 illustrates a conceptual framework for diagnostic testing strategies in suspected IHD, which underscores the value of evaluating the macro- and microcirculations.
Figure 2: Extended Algorithm of Diagnostic Testing Strategies in Suspected IHD.
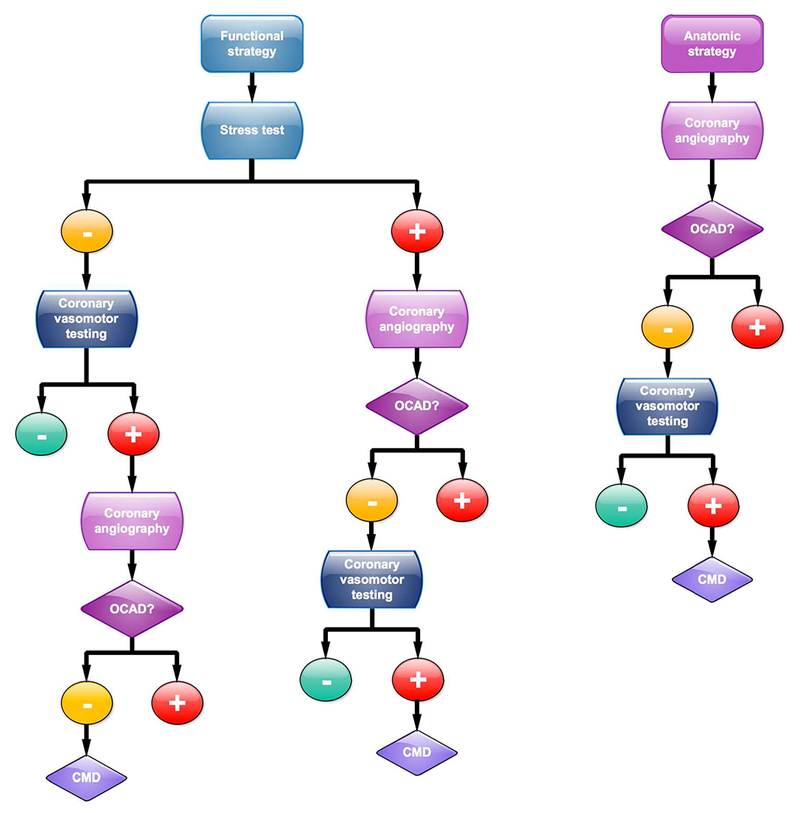
Whether a functional or anatomic strategy is pursued first, unexplained symptoms and findings merit consideration for testing of coronary microvascular disease (CMD). Please refer to the section on the Diagnostic Testing Strategy for CMD for a more detailed explanation of this conceptual diagnostic algorithm. IHD = ischemic heart disease; oCAD = obstructive coronary artery disease
Initial functional strategy:
This represents a common diagnostic approach recommended by guidelines and begins with conventional stress testing with or without imaging. A positive stress test is generally assumed to reflect obstructive CAD, and many patients are referred for coronary angiography for definitive anatomic diagnosis. If obstructive CAD is identified, those patients may be considered for revascularization, depending on symptom burden and the extent and severity of myocardial ischemia and angiographic disease. However, when nonobstructive CAD is identified, the result of the stress test is typically interpreted as a “false positive.” Although in some instances this may be accurate, the diagnosis of CMD may represent an important alternative diagnosis, especially in the presence of symptoms and positive stress testing. Those patients should be considered further for direct assessments of coronary vasomotor function via either invasive or noninvasive techniques. Although there are differences in accuracy and levels of validation between the different techniques (Table 1), the ultimate choice may depend on local availability, clinical expertise, and patient preference. Alternatively, if conventional stress testing is negative or equivocal, but the clinical suspicion for CMD remains high, patients could be referred for coronary vasomotor reactivity testing. If the test is positive, coronary angiography can then be used to exclude obstructive CAD.
Initial anatomic strategy:
In selected cases, an anatomic strategy with noninvasive cardiac CT angiography or direct invasive coronary angiography may be the initial strategy. When severe obstructive CAD is confirmed, patients can be considered for revascularization, depending on the symptom burden, extent and severity of angiographic disease, and presence of lesion-specific myocardial ischemia (e.g., via FFR, iFR). As in the initial functional strategy, the identification of nonobstructive CAD in a symptomatic patient should trigger consideration of CMD with referral for specialized testing as outlined.
SPECTRUM OF CMD PHENOTYPES: INTERACTIONS BETWEEN ATHEROSCLEROSIS, ISCHEMIA, AND OUTCOMES
CMD is prevalent in a broad spectrum of CVDs. In 2007, Camici and Crea (9) proposed categories of CMD that have been useful in describing clinical settings in which CMD may occur. However, there is growing evidence that many of these conditions overlap in their clinical manifestations and pathogenesis. There also appears to be a relationship between the underlying severity of CMD and clinical risk of MACE, where coronary atherosclerosis is neither necessary nor sufficient but is commonly present and interacts with CMD to mediate worse outcomes.
SIMPLIFIED CLASSIFICATION OF CMD
As such, the clinical spectrum of CMD may be contemporaneously conceptualized in terms of 3 important features of this disease with diagnostic and therapeutic implications, including the documentation of atherosclerosis (none, nonobstructive, or obstructive), the severity of CMD, and any associated clinical risk (Figure 3). This approach is simple and practical, and embeds CMD within the modern concept of distinguishing CAD risk at both the high and low range of disease severity. The interplay of CMD, CAD, and risk is clinically relevant and represents a critical differentiator for what may constitute appropriate therapeutic strategies in CMD.
Figure 3: Interplay of CMD, CAD, and Clinical Risk Across Thresholds of Severity.
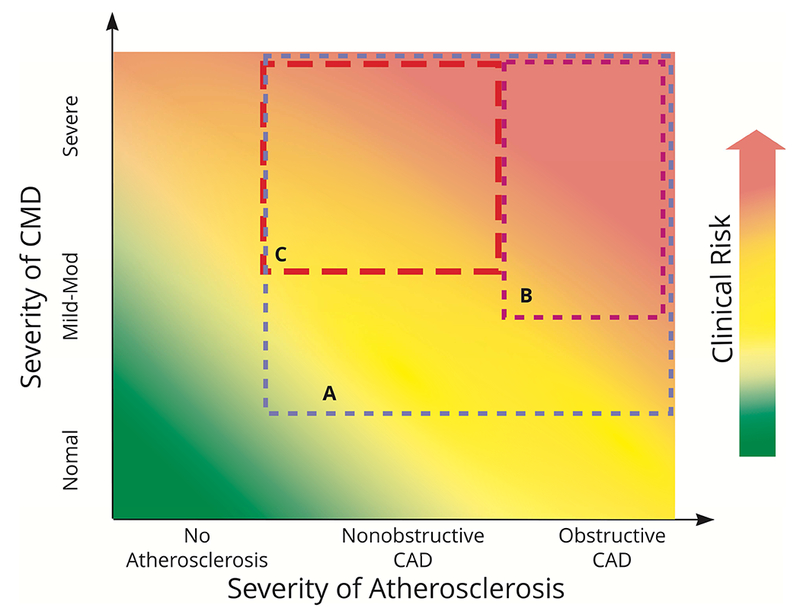
Boxes represent different CMD phenotypes of particular therapeutic interest. (A) CMD with CAD; (B) moderate-severe CMD with obstructive CAD; (C) moderate-severe CMD with nonobstructive CAD. CAD = coronary artery disease; CMD = coronary microvascular disease.
CMD WITHOUT ATHEROSCLEROSIS.
CMD is prevalent in a number of clinical conditions where atherosclerosis plays little or no role in its pathogenesis. For example, there is extensive published data documenting the presence of CMD in patients with arterial hypertension (31,32,69), aortic stenosis (70), and nonischemic cardiomyopathies. The latter group includes idiopathic (37,38), hypertrophic (71,72), infiltrative (73) and stress (74,75) cardiomyopathies. Whether CMD in nonischemic cardiomyopathies is a cause or effect of the underlying myopathic process is unknown. However, in all of these conditions, severe CMD has been implicated in the pathophysiology of subendocardial ischemia and increased myocardial stress, subclinical myocardial injury and diffuse interstitial fibrosis (76), worsening systolic and diastolic function, heart failure, arrhythmias (77), and adverse cardiovascular events (37,38,72– 75).
CMD WITH NONOBSTRUCTIVE ATHEROSCLEROSIS.
Most patients undergoing evaluation for CMD have some degree of atherosclerosis, even if no obstructive lesions are found. This phenomenon, although expected given the high prevalence of CVD comorbidities in this population, represents a fundamental change from previous approaches for the classification of CMD. Some patients may present with severe ischemia or acute MI before being diagnosed with no obstructive CAD (recently described as ischemia and no obstructive coronary artery disease [INOCA] (8) and myocardial infarction with nonobstructive coronary artery disease [MINOCA] (78), respectively), but the majority of these patients have atherosclerosis. It is becoming clear that CMD without obstructive CAD is not a synonym for CMD with no CAD. Rather, it represents a separate entity with unique prognostic and therapeutic implications. This phenotype is quite prevalent and identifies patients at risk for MACE (Figure 4).
Figure 4: Prevalence, Severity, and Clinical Risk Associated With CMD.
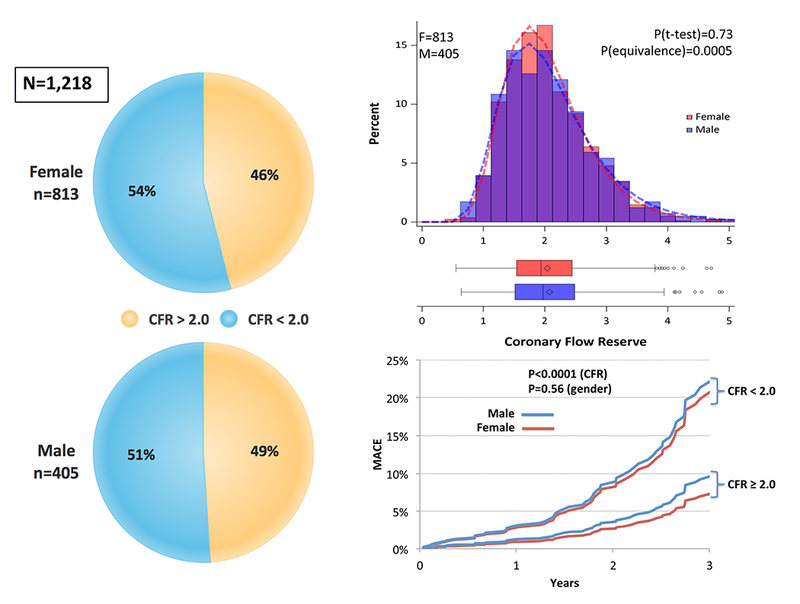
CMD is defined by cardiac PET/CT coronary flow reserve (CFR) <2 in patients without overt obstructive coronary artery disease. Reprinted, with permission, from Murthy et al. (15). CMD = coronary microvascular disease; MACE = major adverse cardiovascular events.
Indeed, this subgroup represents the largest cohort of patients with CMD and includes patients with cardiometabolic disease (i.e., obesity, metabolic syndrome, and diabetes mellitus), chronic kidney disease, and HFpEF, and is disproportionately female. In obese patients, CMD is prevalent (79,80), increases in severity with increasing body mass index, and serves as a better discriminator of future CVD risk than body mass index or traditional risk factors (81). Although patients with diabetes and metabolic syndrome are at a markedly increased risk of future atherosclerotic and heart failure complications, this excess risk is incompletely explained by obstructive CAD or LV dysfunction, and is significantly higher in women with diabetes than in men with diabetes (82). Mounting evidence suggests that diabetes and prediabetic states contribute to important alterations in the regulation of coronary vascular tone before they present with obstructive CAD (26–28). Patients with diabetes show a range of structural and functional microvascular abnormalities (as depicted in the Central Illustration), which vary in extent and severity across cardiometabolic states. Symptomatic patients with diabetes, even without known CAD, demonstrated a variable risk of events when stratified by severity of coronary vasomotor dysfunction (83), and those with metabolic syndrome and diabetes, respectively, demonstrated a stepwise increase in rates of CMD and CVD events (84). CVD is also highly prevalent in patients with chronic kidney disease (CKD), accounting for more than one-half of their associated mortality, which is not fully explained by the presence of obstructive CAD. Consistent evidence demonstrates a stepwise increase in CMD severity with decreasing glomerular filtration rate (35,85), which was detectable in early CKD and led to significant reclassification of CVD mortality risk across the spectrum of kidney function (35).
Recent evidence supports that CMD associated with cardiomyocyte injury and myocardial stiffness likely play an important role in the pathophysiology of HFpEF (49,52). In patients with stable CAD and preserved LV ejection fraction, chronic circulating levels of high-sensitivity troponins are common in patients with LV hypertrophy, diabetes, and CKD, and are associated with increased incidence of cardiovascular death and heart failure (86). In symptomatic, otherwise low-risk patients with minimally-elevated troponin, only those with CMD demonstrated a significant risk of MACE (52). Moreover, CMD was independently associated with worsening diastolic dysfunction, and only in the presence of CMD was a detectable troponin significantly associated with diastolic dysfunction (49). Strikingly, patients with CMD and diastolic dysfunction demonstrated a greater than 5-fold risk of HFpEF hospitalization (49) (Figure 5). With increased oxygen demand, factors tipping the balance toward cardiomyocyte injury in patients with existing CMD may worsen myocardial mechanics and increase the risk of HFpEF outcomes, even absent overt structural abnormalities or obstructive CAD (49,87–89). In particular, microvascular endothelial dysfunction, decreased nitric oxide bioavailability, and increased inflammatory cytokine signaling may contribute to reduced coronary microvascular density, or rarefaction, and increased myocardial fibrosis observed in HFpEF (90,91).
Figure 5: Relationship Between CMD, Diastolic Dysfunction, and Rate of Hospitalization for HFpEF.
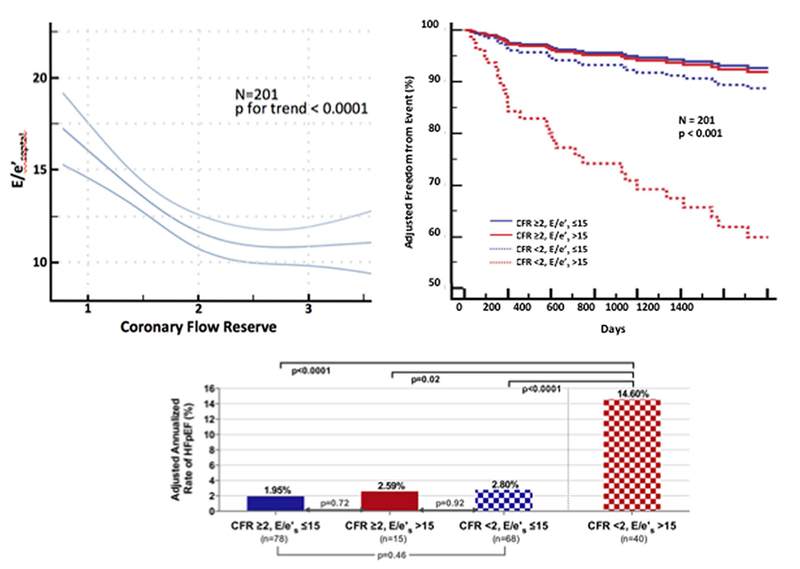
CMD is defined by cardiac PET/CT coronary flow reserve, CFR, <2 in patients without overt obstructive coronary artery disease, Diastolic dysfunction was measured by transthoracic echocardiography E/e’. Modified, with permission, from Taqueti et al. (49).
These findings are particularly prevalent in the comorbid population of elderly women with cardiometabolic risk factors, and highlight important sex differences in the prevalence of CMD, especially among patients with nonobstructive atherosclerosis. A robust evidence base (2,11,92–95) supports that the pathophysiology of IHD in women varies from that in men. Women present with a higher burden of symptoms and comorbidities as compared with men and experience similar or worse outcomes (1), but are less likely to manifest anatomic obstructive CAD, regardless of whether they present with stable IHD (2,95) or acute coronary syndromes (92,94). A major contributor to this apparent paradox is CMD, which often coexists with diffuse, nonobstructive atherosclerosis (11,15,95). CMD increases cardiovascular risk in both women and men (15), but may constitute an especially malignant phenotype in a subset of severely affected women (95). Over the lifespan, female-specific factors may modify the development of CAD in susceptible patients into a diffuse pattern with more contribution from CMD than focal obstruction (96). Although not a uniquely female disorder, this pattern of abnormalities may be more prognostically useful in women (97), in whom it often occurs without concomitant obstructive CAD (95), and may be especially relevant in patients with diabetes or metabolic syndrome, INOCA, MINOCA, and HFpEF.
CMD WITH OBSTRUCTIVE ATHEROSCLEROSIS.
CMD is also prevalent in patients with obstructive CAD. This finding is not surprising because endothelial and coronary vasomotor dysfunction represents an early manifestation of atherosclerosis, which may long precede the development of obstructive stenosis. In patients with stable CAD, reductions in microcirculatory reserve exacerbate the functional significance of upstream coronary stenosis and may magnify the severity of inducible myocardial ischemia. From a clinical perspective, the presence of CMD in patients with stable obstructive CAD has several important diagnostic, prognostic, and management implications.
First, the variability in the severity of CMD can have a significant impact on the evaluation of the physiological significance of a coronary stenosis using trans-stenosis pressure gradients or noninvasive imaging for ischemia. In the presence of CMD, FFR and iFR values measured for any given stenosis are higher (and potentially pseudonormal) than when coronary microvascular resistance is normal, which can lead to underestimation of the physiological severity of a stenosis. This may help, in part, to explain discrepancies observed between obstructive lesion severity on invasive angiography and the extent and severity of myocardial ischemia (4).
Second, emerging data from noninvasive imaging studies have consistently shown that reduced CFR measurements by PET, reflecting the combined hemodynamic effects of obstructive stenosis, diffuse atherosclerosis, and CMD, can identify patients at high risk for MACE, independent of angiographic disease severity (16,53–55).
Third, the severity of CMD has important implications for patient management. For example, patients with normal FFR but abnormal CFR who had revascularization deferred on the basis of FFR experienced increased adverse events, suggesting a vital role for CMD in revascularization outcomes (98). The prevalence of severe CMD in patients in who have revascularization deferred based on FFR is unknown. Some studies have estimated that this phenomenon may affect up to one-third of patients with normal FFR (63,98). This suggests that interrogation of CMD in patients with obstructive CAD could potentially identify circumstances in which mixed abnormalities from upstream stenoses and the microcirculation synergize to alter the functional significance of a focal stenosis. A recent study suggested that measurements of global CFR modified the effect of revascularization such that only patients with severely reduced global CFR appeared to benefit from revascularization, and only if the revascularization included coronary artery bypass graft (16). This supports that sensitive measures of diffuse atherosclerosis and downstream CMD may be able to guide therapeutic benefit derived from more complete revascularization with coronary artery bypass graft, especially in comorbid populations with increased cardiometabolic risk. Furthermore, residual CMD may also account for the high frequency of angina at 1 and 2 years post-randomization in clinical trials of stable IHD (62,99–102), and underscores the need to optimize medical therapy to treat residual CMD that would not be improved with revascularization.
Finally, CMD also plays a role in the pathogenesis of myocardial injury in patients with acute coronary syndromes with atherothrombotic plaque rupture, even post-reperfusion. A serious complication in these patients involves microvascular obstruction, which occurs to varying degrees in approximately 50% of patients after primary percutaneous coronary intervention following ST-segment elevation MI and is associated with adverse LV remodeling and even death (103). The mechanisms involved in the pathogenesis of microvascular obstruction include the preexistence of CMD from atherosclerosis and a series of pathological changes that collectively lead to obstruction of the microvessels (104).
An additional special condition includes cardiac allograft vasculopathy, which remains one of the leading causes of death in long-term orthotopic heart transplant survivors and the principal cause of retransplantation after 1 year. The pathogenesis of cardiac allograft vasculopathy includes intimal proliferation in the epicardial coronary arteries and medial thickening in the small intramyocardial coronary vessels, resulting in focal epicardial coronary stenoses, diffuse distal vessel tapering, obstructive microvasculopathy, and reduced capillary density. Recent studies have described significant associations between markers of graft dysfunction, measures of coronary vasomotor dysfunction (67,105), and increased cardiovascular risk (67,105,106) in these patients.
CMD AS POTENTIAL TARGET FOR NOVEL INTERVENTIONS
ROLE OF CONVENTIONAL THERAPIES.
The treatment of CMD has so far been empirical because its pathophysiology is multifactorial, with overlapping phenotypes that often coexist (Figure 6A). CMD may be the primary abnormality in some patients and a secondary pathological feature in others. There are no effective therapies specifically targeting CMD. The results of available therapeutic trials have been limited by variable patient selection stemming from the lack of a standardized diagnosis, and inadequate designs with small sample size and insufficient demonstration of clinical improvement (107). Nonetheless, the conventional approach to management of patients with CMD includes a number of strategies described in the following sections.
Figure 6: Conceptual Illustration of Overlapping Phenotypes in CMD and Potential Therapeutic Strategies.
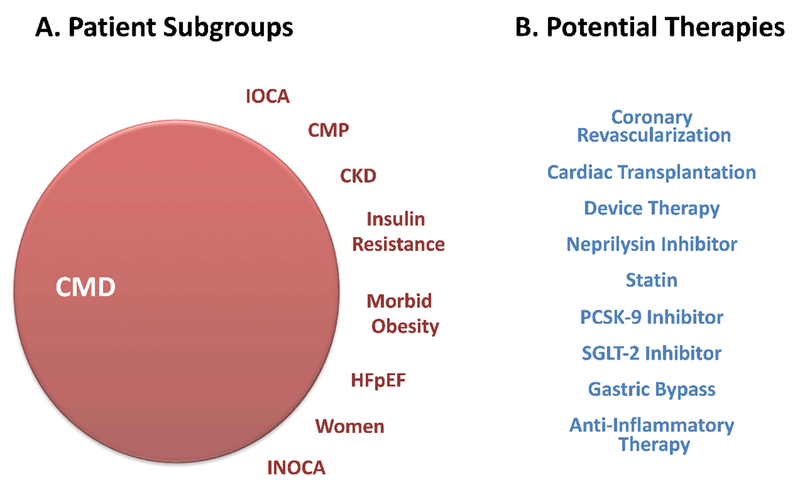
(A) CMD phenotypes. (B) Potential therapeutic strategies. CMP = cardiomyopathy; CKD = chronic kidney disease; GLP-1 = glucagon-like peptide-1; HFpEF = heart failure with preserved ejection fraction; INOCA = ischemia and no obstructive CAD; IOCA = ischemia and obstructive CAD; PCSK-9 = pro-protein convertase subtilisin/kexin type 9; SGLT-2 = sodium-glucose cotransporter-2.
MANAGEMENT OF UNDERLYING CVD RISK FACTORS.
Because of the close association of CVD risk factors and atherosclerosis in the majority of patients with CMD (Figure 3, Box A), aggressive management of comorbidities is an important goal of treatment. This includes smoking cessation, weight loss, adequate control of blood pressure, diabetes and related metabolic abnormalities, lipid management, improved nutrition, and regular exercise. Optimized medical therapy has been associated with reductions in myocardial ischemia in patients with stable CAD (108), far exceeding regression in atherosclerotic plaque burden (109), and is likely related to improved coronary microcirculatory function. Surgical weight loss has also been associated with improvements in microvascular function (110), which also may be related to improved risk factor profiles.
ANTIPLATELET AND LIPID-LOWERING THERAPY.
Because of the strong association between atherosclerosis and CMD, low-dose aspirin (or alternative antiplatelet drugs in case of aspirin intolerance) remains an important component of patient management, even in patients with nonobstructive CAD (50). Similarly, initiation or intensification of statin therapy in patients with documented atherosclerosis, especially in the setting of CMD (Figure 3, Box A), should be strongly considered (50). There is evidence that statin therapy improves myocardial ischemia and CMD (111).
ANTI-ISCHEMIC THERAPY.
Traditional anti-ischemic drugs, including beta-blockers and short-acting nitrates, should be considered first-line in symptom management of CMD (50). Calcium antagonists and long-acting nitrates may be helpful when used in addition to beta-blockers in cases of insufficient symptom control, and are preferred when increased vasomotor tone or spasm is the presumed mechanism for symptoms (50). Angiotensin-converting enzyme inhibitors (and possibly angiotensin receptor blockers) may improve coronary microvascular function by blocking the powerful vasoconstrictor effects of angiotensin II (50). Coronary revascularization of obstructive lesions also has a potential role in improvement of symptoms and outcomes in patients with coexistent CMD (Figure 3, Box B), especially in the setting of extensive myocardial ischemia or objective documentation of flow-limiting CAD (50).
ANTI-ANGINAL THERAPY.
Multiple drugs that primarily reduce angina, including ivabradine, ranolazine, mibefradil, nicorandil, and trimetazidine, have been evaluated in patients with CMD (45,48,112). Ranolazine, one of the best studied of these drugs, is thought to improve myocardial perfusion by decreasing sodium and calcium overload, thereby improving myocyte relaxation and diastolic stiffness. However, recent randomized placebo-controlled crossover trials of ranolazine in patients with CMD showed no significant benefit in symptoms or change in coronary microvascular function in patients with CMD (45,48).
POTENTIAL ROLE OF EMERGING THERAPIES: NOVEL AGENTS AND INTERVENTIONS.
A number of novel therapeutic strategies have recently demonstrated improved outcomes over conventional therapies in patients with elevated CVD risk and are now Food and Drug Administration–approved (113–116). Although none of these specifically target or have been tested explicitly in patients with CMD, the evidence reviewed previously suggests that one or more of these approaches may be beneficial in CMD (Figure 6B). Patients with severe CMD and nonobstructive CAD (Figure 3, Box C) represent a potentially large number of patients with a clinically unmet need and an especially appealing phenotype for this approach (117). A common mechanism underlying IHD risk across patient subsets may involve inflammation, endothelial dysfunction, and increased cardiomyocyte oxygen demand with ensuing microvascular ischemia, myocardial injury, and impaired cardiac mechanics. Thus, a clearer understanding of the relationship between CMD and CAD comorbid conditions, including insulin resistance and HFpEF, may guide development of novel systemic therapies to harness the benefit of more “complete revascularization” in a manner not defined by anatomy alone (Figure 6B).
CONCLUSIONS
CMD represents a combination of structural and functional abnormalities in the coronary microcirculation, is prevalent across a broad spectrum of cardiovascular risk factors and diseases, and is associated with increased risk of MACE. Contemporary evidence supports that most patients with CMD have coexisting obstructive or nonobstructive atherosclerosis, with important implications for their prognosis. The interaction of CMD, CAD, and adverse outcomes, including heart failure, likely holds a key to novel therapeutic strategies for cardiovascular health promotion.
Acknowledgments
Disclosures: This work was supported by K23HL135438 to Dr. Taqueti and R01HL132021 to Dr. Di Carli. Dr. Taqueti has reported that she has no relationships relevant to the contents of this paper to disclose. Dr. Di Carli has reported consulting fees from Sanofi and General Electric and a research grant from SpectrumDynamics.
ABBREVIATIONS AND ACRONYMS
- CFR
coronary flow reserve
- CKD
chronic kidney disease
- CMD
coronary microvascular disease
- CT
computed tomography
- HFpEF
heart failure with preserved ejection fraction
- IHD
ischemic heart disease
- INOCA
ischemia and no obstructive coronary artery disease
- LV
left ventricular
- MACE
major adverse cardiovascular events
- MI
myocardial infarction
- MINOCA
myocardial infarction with nonobstructive coronary artery disease
- PET
positron emission tomography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benjamin EJ, Virani SS, Callaway CW, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2018 update: a report from the American Heart Association [Published correction appears in Circulation 2018;137:e493]. Circulation 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 2.Jespersen L, Hvelplund A, Abildstrøm SZ, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J 2012;33:734–44. [DOI] [PubMed] [Google Scholar]
- 3.Maddox TM, Stanislawski MA, Grunwald GK, et al. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA 2014;312:1754–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel MR, Peterson ED, Dai D, et al. Low diagnostic yield of elective coronary angiography [Published correction appears in N Engl J Med 2010;363:498]. N Engl J Med 2010;362:886–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rozanski A, Gransar H, Hayes SW, et al. Temporal trends in the frequency of inducible myocardial ischemia during cardiac stress testing: 1991 to 2009. J Am Coll Cardiol 2013;61:1054–65. [DOI] [PubMed] [Google Scholar]
- 6.Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med 2010;362:2155–65. [DOI] [PubMed] [Google Scholar]
- 7.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006;355:251–9. [DOI] [PubMed] [Google Scholar]
- 8.Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and No Obstructive Coronary Artery Disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation 2017;135:1075–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med 2007;356:830–40. [DOI] [PubMed] [Google Scholar]
- 10.Chilian WM. Coronary microcirculation in health and disease. Summary of an NHLBI workshop. Circulation 1997;95:522–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pepine CJ, Anderson RD, Sharaf BL, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia: results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol 2010;55:2825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ong P, Camici PG, Beltrame JF, et al. ; Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for microvascular angina. Int J Cardiol 2018;250:16–20. [DOI] [PubMed] [Google Scholar]
- 13.Shimokawa H 2014 Williams Harvey Lecture: importance of coronary vasomotion abnormalities-from bench to bedside. Eur Heart J 2014;35:3180–93. [DOI] [PubMed] [Google Scholar]
- 14.Khuddus MA, Pepine CJ, Handberg EM, et al. An intravascular ultrasound analysis in women experiencing chest pain in the absence of obstructive coronary artery disease: a substudy from the National Heart, Lung and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). J Interv Cardiol 2010;23:511–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murthy VL, Naya M, Taqueti VR, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation 2014;129:2518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taqueti VR, Hachamovitch R, Murthy VL, et al. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation 2015;131:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farias M III, Gorman MW, Savage MV, Feigl EO. Plasma ATP during exercise: possible role in regulation of coronary blood flow. Am J Physiol Heart Circ Physiol 2005;288:H1586–90. [DOI] [PubMed] [Google Scholar]
- 18.Amann K, Wiest G, Zimmer G, Gretz N, Ritz E, Mall G. Reduced capillary density in the myocardium of uremic rats--a stereological study. Kidney Int 1992;42:1079–85. [DOI] [PubMed] [Google Scholar]
- 19.Asghar O, Al-Sunni A, Khavandi K, et al. Diabetic cardiomyopathy. Clin Sci (Lond) 2009;116:741–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mosseri M, Schaper J, Admon D, et al. Coronary capillaries in patients with congestive cardiomyopathy or angina pectoris with patent main coronary arteries. Ultrastructural morphometry of endomyocardial biopsy samples. Circulation 1991;84:203–10. [DOI] [PubMed] [Google Scholar]
- 21.Mosseri M, Yarom R, Gotsman MS, Hasin Y. Histologic evidence for small-vessel coronary artery disease in patients with angina pectoris and patent large coronary arteries. Circulation 1986;74:964–72. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki H, Takeyama Y, Koba S, Suwa Y, Katagiri T. Small vessel pathology and coronary hemodynamics in patients with microvascular angina. Int J Cardiol 1994;43:139–50. [DOI] [PubMed] [Google Scholar]
- 23.Opherk D, Zebe H, Weihe E, et al. Reduced coronary dilatory capacity and ultrastructural changes of the myocardium in patients with angina pectoris but normal coronary arteriograms. Circulation 1981;63:817–25. [DOI] [PubMed] [Google Scholar]
- 24.Richardson PJ, Livesley B, Oram S, Olsen EG, Armstrong P. Angina pectoris with normal coronary arteries. Transvenous myocardial biopsy in diagnosis. Lancet 1974;2:677–80. [DOI] [PubMed] [Google Scholar]
- 25.Egashira K, Inou T, Hirooka Y, et al. Impaired coronary blood flow response to acetylcholine in patients with coronary risk factors and proximal atherosclerotic lesions. J Clin Invest 1993;91:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Carli MF, Charytan D, McMahon GT, Ganz P, Dorbala S, Schelbert HR. Coronary circulatory function in patients with the metabolic syndrome. J Nucl Med 2011;52:1369–77. [DOI] [PubMed] [Google Scholar]
- 27.Di Carli MF, Janisse J, Grunberger G, Ager J. Role of chronic hyperglycemia in the pathogenesis of coronary microvascular dysfunction in diabetes. J Am Coll Cardiol 2003;41:1387–93. [DOI] [PubMed] [Google Scholar]
- 28.Yokoyama I, Momomura S, Ohtake T, et al. Reduced myocardial flow reserve in non-insulin-dependent diabetes mellitus. J Am Coll Cardiol 1997;30:1472–7. [DOI] [PubMed] [Google Scholar]
- 29.Dayanikli F, Grambow D, Muzik O, Mosca L, Rubenfire M, Schwaiger M. Early detection of abnormal coronary flow reserve in asymptomatic men at high risk for coronary artery disease using positron emission tomography. Circulation 1994;90:808–17. [DOI] [PubMed] [Google Scholar]
- 30.Pitkänen OP, Raitakari OT, Niinikoski H, et al. Coronary flow reserve is impaired in young men with familial hypercholesterolemia. J Am Coll Cardiol 1996;28:1705–11. [DOI] [PubMed] [Google Scholar]
- 31.Hamasaki S, Al Suwaidi J, Higano ST, Miyauchi K, Holmes DR Jr., Lerman A. Attenuated coronary flow reserve and vascular remodeling in patients with hypertension and left ventricular hypertrophy. J Am Coll Cardiol 2000;35:1654–60. [DOI] [PubMed] [Google Scholar]
- 32.Laine H, Raitakari OT, Niinikoski H, et al. Early impairment of coronary flow reserve in young men with borderline hypertension. J Am Coll Cardiol 1998;32:147–53. [DOI] [PubMed] [Google Scholar]
- 33.Quercioli A, Pataky Z, Montecucco F, et al. Coronary vasomotor control in obesity and morbid obesity: contrasting flow responses with endocannabinoids, leptin, and inflammation. J Am Coll Cardiol Img 2012;5:805–15. [DOI] [PubMed] [Google Scholar]
- 34.Campisi R, Czernin J, Schöder H, et al. Effects of long-term smoking on myocardial blood flow, coronary vasomotion, and vasodilator capacity. Circulation 1998;98:119–25. [DOI] [PubMed] [Google Scholar]
- 35.Charytan DM, Skali H, Shah NR, et al. Coronary flow reserve is predictive of the risk of cardiovascular death regardless of chronic kidney disease stage. Kidney Int 2018;93:501–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah NR, Charytan DM, Murthy VL, et al. Prognostic Value of coronary flow reserve in patients with dialysis-dependent ESRD. J Am Soc Nephrol 2016;27:1823–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Majmudar MD, Murthy VL, Shah RV, et al. Quantification of coronary flow reserve in patients with ischaemic and non-ischaemic cardiomyopathy and its association with clinical outcomes. Eur Heart J Cardiovasc Imaging 2015;16:900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neglia D, Michelassi C, Trivieri MG, et al. Prognostic role of myocardial blood flow impairment in idiopathic left ventricular dysfunction. Circulation 2002;105:186–93. [DOI] [PubMed] [Google Scholar]
- 39.Ong P, Athanasiadis A, Borgulya G, Mahrholdt H, Kaski JC, Sechtem U. High prevalence of a pathological response to acetylcholine testing in patients with stable angina pectoris and unobstructed coronary arteries. The ACOVA Study (Abnormal COronary VAsomotion in patients with stable angina and unobstructed coronary arteries). J Am Coll Cardiol 2012;59:655–62. [DOI] [PubMed] [Google Scholar]
- 40.Lee BK, Lim HS, Fearon WF, et al. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation 2015;131:1054–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Bruyne B, Hersbach F, Pijls NH, et al. Abnormal epicardial coronary resistance in patients with diffuse atherosclerosis but “Normal” coronary angiography. Circulation 2001;104:2401–6. [DOI] [PubMed] [Google Scholar]
- 42.Gould KL, Nakagawa Y, Nakagawa K, et al. Frequency and clinical implications of fluid dynamically significant diffuse coronary artery disease manifest as graded, longitudinal, base-to-apex myocardial perfusion abnormalities by noninvasive positron emission tomography. Circulation 2000;101:1931–9. [DOI] [PubMed] [Google Scholar]
- 43.Hoffmann U, Ferencik M, Udelson JE, et al. ; PROMISE Investigators. Prognostic value of noninvasive cardiovascular testing in patients with stable chest pain: insights from the PROMISE Trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation 2017;135:2320–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.SCOT-HEART investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial [Published correction appears in Lancet 2015;385:2354]. Lancet 2015;385:2383–91. [DOI] [PubMed] [Google Scholar]
- 45.Bairey Merz CN, Handberg EM, Shufelt CL, et al. A randomized, placebo-controlled trial of late Na current inhibition (ranolazine) in coronary microvascular dysfunction (CMD): impact on angina and myocardial perfusion reserve. Eur Heart J 2016;37:1504–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mygind ND, Michelsen MM, Pena A, et al. Coronary microvascular function and cardiovascular risk factors in women with angina pectoris and no obstructive coronary artery disease: the iPOWER Study. J Am Heart Assoc 2016;5:e003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, Lerman A. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. J Am Coll Cardiol Intv 2015;8:1445–53. [DOI] [PubMed] [Google Scholar]
- 48.Shah NR, Cheezum MK, Veeranna V, et al. Ranolazine in symptomatic diabetic patients without obstructive coronary artery disease: impact on microvascular and diastolic function. J Am Heart Assoc 2017;6;e005027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taqueti VR, Solomon SD, Shah AM, et al. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J 2018;39:840–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Task Force Members, Montalescot G, Sechtem U, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 51.Feher A, Sinusas AJ. Quantitative assessment of coronary microvascular function: dynamic single-photon emission computed tomography, positron emission tomography, ultrasound, computed tomography, and magnetic resonance imaging. Circ Cardiovasc Imaging 2017;10;e006427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taqueti VR, Everett BM, Murthy VL, et al. Interaction of impaired coronary flow reserve and cardiomyocyte injury on adverse cardiovascular outcomes in patients without overt coronary artery disease. Circulation 2015;131:528–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gupta A, Taqueti VR, van de Hoef TP, et al. Integrated noninvasive physiological assessment of coronary circulatory function and impact on cardiovascular mortality in patients with stable coronary artery disease. Circulation 2017;136:2325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herzog BA, Husmann L, Valenta I, et al. Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Am Coll Cardiol 2009;54:150–6. [DOI] [PubMed] [Google Scholar]
- 55.Ziadi MC, Dekemp RA, Williams KA, et al. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol 2011;58:740–8. [DOI] [PubMed] [Google Scholar]
- 56.Liu A, Wijesurendra RS, Liu JM, et al. Diagnosis of microvascular angina using cardiac magnetic resonance. J Am Coll Cardiol 2018;71:969–79. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Doyle M, Weinberg N, Pohost GM, et al. Prognostic value of global MR myocardial perfusion imaging in women with suspected myocardial ischemia and no obstructive coronary disease: results from the NHLBI-sponsored WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol Img 2010;3:1030–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cortigiani L, Rigo F, Gherardi S, et al. Additional prognostic value of coronary flow reserve in diabetic and nondiabetic patients with negative dipyridamole stress echocardiography by wall motion criteria. J Am Coll Cardiol 2007;50:1354–61. [DOI] [PubMed] [Google Scholar]
- 59.Gan LM, Svedlund S, Wittfeldt A, et al. Incremental value of transthoracic Doppler echocardiography-assessed coronary flow reserve in patients with suspected myocardial ischemia undergoing myocardial perfusion scintigraphy. J Am Heart Assoc 2017;6:e004875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rigo F, Sicari R, Gherardi S, Djordjevic-Dikic A, Cortigiani L, Picano E. Prognostic value of coronary flow reserve in medically treated patients with left anterior descending coronary disease with stenosis 51% to 75% in diameter. Am J Cardiol 2007;100:1527–31. [DOI] [PubMed] [Google Scholar]
- 61.Lee JM, Jung JH, Hwang D, et al. Coronary flow reserve and microcirculatory resistance in patients with intermediate coronary stenosis. J Am Coll Cardiol 2016;67:1158–69. [DOI] [PubMed] [Google Scholar]
- 62.Tonino PA, De Bruyne B, Pijls NH, et al. ; FAME Study Investigators. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 2009;360:213–24. [DOI] [PubMed] [Google Scholar]
- 63.De Bruyne B, Pijls NH, Kalesan B, et al. ; FAME 2 Trial Investigators. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012;367:991–1001. [DOI] [PubMed] [Google Scholar]
- 64.Echavarria-Pinto M, Escaned J, Macías E, et al. Disturbed coronary hemodynamics in vessels with intermediate stenoses evaluated with fractional flow reserve: a combined analysis of epicardial and microcirculatory involvement in ischemic heart disease. Circulation 2013;128:2557–66. [DOI] [PubMed] [Google Scholar]
- 65.Davies JE, Sen S, Escaned J. Instantaneous wave-free ratio versus fractional flow reserve. N Engl J Med 2017;377:1597–8. [DOI] [PubMed] [Google Scholar]
- 66.Götberg M, Christiansen EH, Gudmundsdottir IJ, et al. ; iFR-SWEDEHEART Investigators. Instantaneous wave-free ratio versus fractional flow reserve to guide PCI. N Engl J Med 2017;376:1813–23. [DOI] [PubMed] [Google Scholar]
- 67.Sen S, Petraco R, Mayet J, Davies J. Wave intensity analysis in the human coronary circulation in health and disease. Curr Cardiol Rev 2014;10:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Broyd CJ, Hernández-Pérez F, Segovia J, et al. Identification of capillary rarefaction using intracoronary wave intensity analysis with resultant prognostic implications for cardiac allograft patients. Eur Heart J 2018;39:1807–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brush JE Jr., Cannon RO III, Schenke WH et al. Angina due to coronary microvascular disease in hypertensive patients without left ventricular hypertrophy. N Engl J Med 1988;319:1302–7. [DOI] [PubMed] [Google Scholar]
- 70.Rajappan K, Rimoldi OE, Camici PG, Bellenger NG, Pennell DJ, Sheridan DJ. Functional changes in coronary microcirculation after valve replacement in patients with aortic stenosis. Circulation 2003;107:3170–5. [DOI] [PubMed] [Google Scholar]
- 71.Bravo PE, Zimmerman SL, Luo HC, et al. Relationship of delayed enhancement by magnetic resonance to myocardial perfusion by positron emission tomography in hypertrophic cardiomyopathy. Circ Cardiovasc Imaging 2013;6:210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cecchi F, Olivotto I, Gistri R, Lorenzoni R, Chiriatti G, Camici PG. Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. N Engl J Med 2003;349:1027–35. [DOI] [PubMed] [Google Scholar]
- 73.Dorbala S, Vangala D, Bruyere J Jr., et al. Coronary microvascular dysfunction is related to abnormalities in myocardial structure and function in cardiac amyloidosis. J Am Coll Cardiol HF 2014;2:358–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pelliccia F, Kaski JC, Crea F, Camici PG. Pathophysiology of Takotsubo syndrome. Circulation 2017;135:2426–41. [DOI] [PubMed] [Google Scholar]
- 75.Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med 2015;373:929–38. [DOI] [PubMed] [Google Scholar]
- 76.Dweck MR, Boon NA, Newby DE. Calcific aortic stenosis: a disease of the valve and the myocardium. J Am Coll Cardiol 2012;60:1854–63. [DOI] [PubMed] [Google Scholar]
- 77.Dilsizian V, Bonow RO, Epstein SE, Fananapazir L. Myocardial ischemia detected by thallium scintigraphy is frequently related to cardiac arrest and syncope in young patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 1993;22:796–804. [DOI] [PubMed] [Google Scholar]
- 78.Pasupathy S, Tavella R, Beltrame JF. Myocardial infarction with nonobstructive coronary arteries (MINOCA): the past, present, and future management. Circulation 2017;135:1490–3. [DOI] [PubMed] [Google Scholar]
- 79.Schindler TH, Cardenas J, Prior JO, et al. Relationship between increasing body weight, insulin resistance, inflammation, adipocytokine leptin, and coronary circulatory function. J Am Coll Cardiol 2006;47:1188–95. [DOI] [PubMed] [Google Scholar]
- 80.Tona F, Serra R, Di Ascenzo L, et al. Systemic inflammation is related to coronary microvascular dysfunction in obese patients without obstructive coronary disease. Nutr Metab Cardiovasc Dis 2014;24:447–53. [DOI] [PubMed] [Google Scholar]
- 81.Bajaj NS, Osborne MT, Gupta A, et al. Coronary microvascular dysfunction and cardiovascular risk in obese patients. J Am Coll Cardiol 2018;72:707–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peters SA, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia 2014;57:1542–51. [DOI] [PubMed] [Google Scholar]
- 83.Murthy VL, Naya M, Foster CR, et al. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation 2012;126:1858–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Osborne MT, Bajaj NS, Taqueti VR, et al. Coronary microvascular dysfunction identifies patients at high risk of adverse events across cardiometabolic diseases. J Am Coll Cardiol 2017;70:2835–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chade AR, Brosh D, Higano ST, Lennon RJ, Lerman LO, Lerman A. Mild renal insufficiency is associated with reduced coronary flow in patients with non-obstructive coronary artery disease. Kidney Int 2006;69:266–71. [DOI] [PubMed] [Google Scholar]
- 86.Everett BM, Cook NR, Magnone MC, et al. Sensitive cardiac troponin T assay and the risk of incident cardiovascular disease in women with and without diabetes mellitus: the Women’s Health Study. Circulation 2011;123:2811–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dryer K, Gajjar M, Narang N, et al. Coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Am J Physiol Heart Circ Physiol 2018;314:H1033–H1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kato S, Saito N, Kirigaya H, et al. Impairment of coronary flow reserve evaluated by phase contrast cine-magnetic resonance imaging in patients with heart failure with preserved ejection fraction. J Am Heart Assoc 2016;5:e002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Srivaratharajah K, Coutinho T, deKemp R, et al. Reduced myocardial flow in heart failure patients with preserved ejection fraction. Circ Heart Fail 2016;9:e002562. [DOI] [PubMed] [Google Scholar]
- 90.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 2015;131:550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013;62:263–71. [DOI] [PubMed] [Google Scholar]
- 92.Arbustini E, Dal Bello B, Morbini P, et al. Plaque erosion is a major substrate for coronary thrombosis in acute myocardial infarction. Heart 1999;82:269–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bairey Merz CN, Shaw LJ, Reis SE, et al. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol 2006;47:S21–9. [DOI] [PubMed] [Google Scholar]
- 94.Hochman JS, Tamis JE, Thompson TD, et al. ; Global Use of Strategies to Open Occluded Coronary Arteries in Acute Coronary Syndromes IIb Investigators. Sex, clinical presentation, and outcome in patients with acute coronary syndromes. N Engl J Med 1999;341:226–32. [DOI] [PubMed] [Google Scholar]
- 95.Taqueti VR, Shaw LJ, Cook NR, et al. Excess cardiovascular risk in women relative to men referred for coronary angiography is associated with severely impaired coronary flow reserve, not obstructive disease. Circulation 2017;135:566–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Taqueti VR. Sex differences in the coronary system. Adv Exp Med Biol 2018;1065:257–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Taqueti VR, Dorbala S, Wolinsky D, et al. Myocardial perfusion imaging in women for the evaluation of stable ischemic heart disease-state-of-the-evidence and clinical recommendations. J Nucl Cardiol 2017;24:1402–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.van de Hoef TP, van Lavieren MA, Damman P, et al. Physiological basis and long-term clinical outcome of discordance between fractional flow reserve and coronary flow velocity reserve in coronary stenoses of intermediate severity. Circ Cardiovasc Interv 2014;7:301–11. [DOI] [PubMed] [Google Scholar]
- 99.Abdallah MS, Wang K, Magnuson EA, et al. ; FREEDOM Trial Investigators. Quality of life after PCI vs CABG among patients with diabetes and multivessel coronary artery disease: a randomized clinical trial. JAMA 2013;310:1581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dagenais GR, Lu J, Faxon DP, et al. ; Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Study Group. Effects of optimal medical treatment with or without coronary revascularization on angina and subsequent revascularizations in patients with type 2 diabetes mellitus and stable ischemic heart disease. Circulation 2011;123:1492–500. [DOI] [PubMed] [Google Scholar]
- 101.Pijls NH, Fearon WF, Tonino PA, et al. ; FAME Study Investigators. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2-year follow-up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) study. J Am Coll Cardiol 2010;56:177–84. [DOI] [PubMed] [Google Scholar]
- 102.Weintraub WS, Spertus JA, Kolm P, et al. ; COURAGE Trial Research Group. Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med 2008;359:677–87. [DOI] [PubMed] [Google Scholar]
- 103.van Kranenburg M, Magro M, Thiele H, et al. Prognostic value of microvascular obstruction and infarct size, as measured by CMR in STEMI patients. J Am Coll Cardiol Img 2014;7:930–9. [DOI] [PubMed] [Google Scholar]
- 104.Bulluck H, Foin N, Tan JW, Low AF, Sezer M, Hausenloy DJ. Invasive assessment of the coronary microcirculation in reperfused ST-segment-elevation myocardial infarction patients: where do we stand? Circ Cardiovasc Interv 2017;10:e004373. [DOI] [PubMed] [Google Scholar]
- 105.Bravo PE, Bergmark BA, Vita T, et al. Diagnostic and prognostic value of myocardial blood flow quantification as non-invasive indicator of cardiac allograft vasculopathy. Eur Heart J 2018;39:316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mc Ardle BA, Davies RA, Chen L, et al. Prognostic value of rubidium-82 positron emission tomography in patients after heart transplant. Circ Cardiovasc Imaging 2014;7:930–7. [DOI] [PubMed] [Google Scholar]
- 107.Marinescu MA, Löffler AI, Ouellette M, Smith L, Kramer CM, Bourque JM. Coronary microvascular dysfunction, microvascular angina, and treatment strategies. J Am Coll Cardiol Img 2015;8:210–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shaw LJ, Berman DS, Maron DJ, et al. ; COURAGE Investigators. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation 2008;117:1283–91. [DOI] [PubMed] [Google Scholar]
- 109.Gould KL, Ornish D, Scherwitz L, et al. Changes in myocardial perfusion abnormalities by positron emission tomography after long-term, intense risk factor modification. JAMA 1995;274:894–901. [DOI] [PubMed] [Google Scholar]
- 110.Quercioli A, Montecucco F, Pataky Z, et al. Improvement in coronary circulatory function in morbidly obese individuals after gastric bypass-induced weight loss: relation to alterations in endocannabinoids and adipocytokines. Eur Heart J 2013;34:2063–73. [DOI] [PubMed] [Google Scholar]
- 111.Guethlin M, Kasel AM, Coppenrath K, Ziegler S, Delius W, Schwaiger M. Delayed response of myocardial flow reserve to lipid-lowering therapy with fluvastatin. Circulation 1999;99:475–81. [DOI] [PubMed] [Google Scholar]
- 112.Villano A, Di Franco A, Nerla R, et al. Effects of ivabradine and ranolazine in patients with microvascular angina pectoris. Am J Cardiol 2013;112:8–13. [DOI] [PubMed] [Google Scholar]
- 113.McMurray JJ, Packer M, Desai AS, et al. ; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 114.Ridker PM, Everett BM, Thuren T, et al. ; CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–31. [DOI] [PubMed] [Google Scholar]
- 115.Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–22. [DOI] [PubMed] [Google Scholar]
- 116.Zinman B, Wanner C, Lachin JM, et al. ; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–28. [DOI] [PubMed] [Google Scholar]
- 117.Schmaier AA, Taqueti VR. A lack of reserve: recognizing the large impact of small vessels in the heart. Circulation 2018;138:424–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


